Aerococcus viridans Phage Lysin AVPL Had Lytic Activity against Streptococcus suis in a Mouse Bacteremia Model
Abstract
:1. Introduction
2. Results
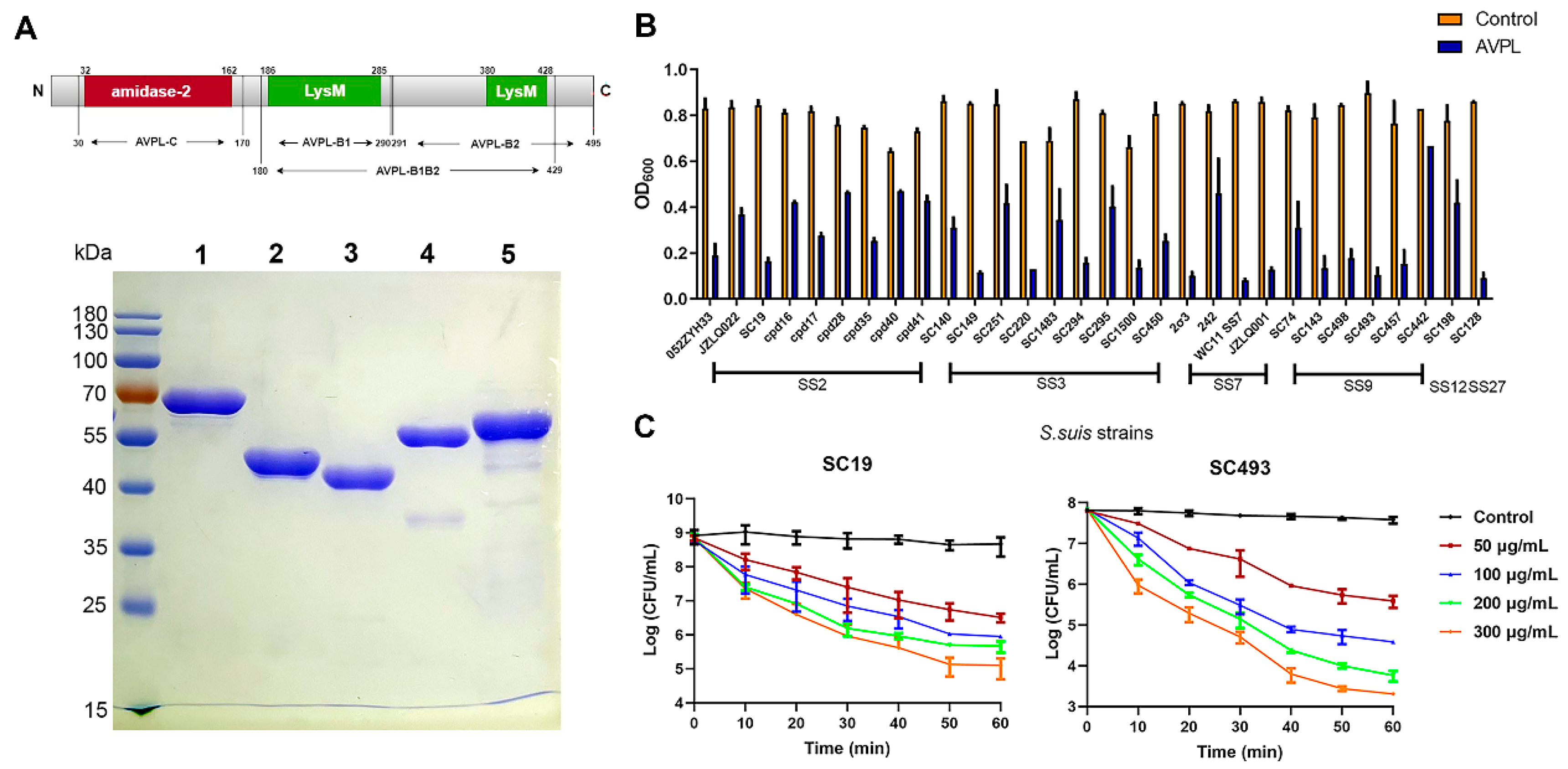
2.1. The Lytic Activity of AVPL against Different Serotypes of S. suis
2.2. Binding Activity of the Truncated Fragments of AVPL to S. suis
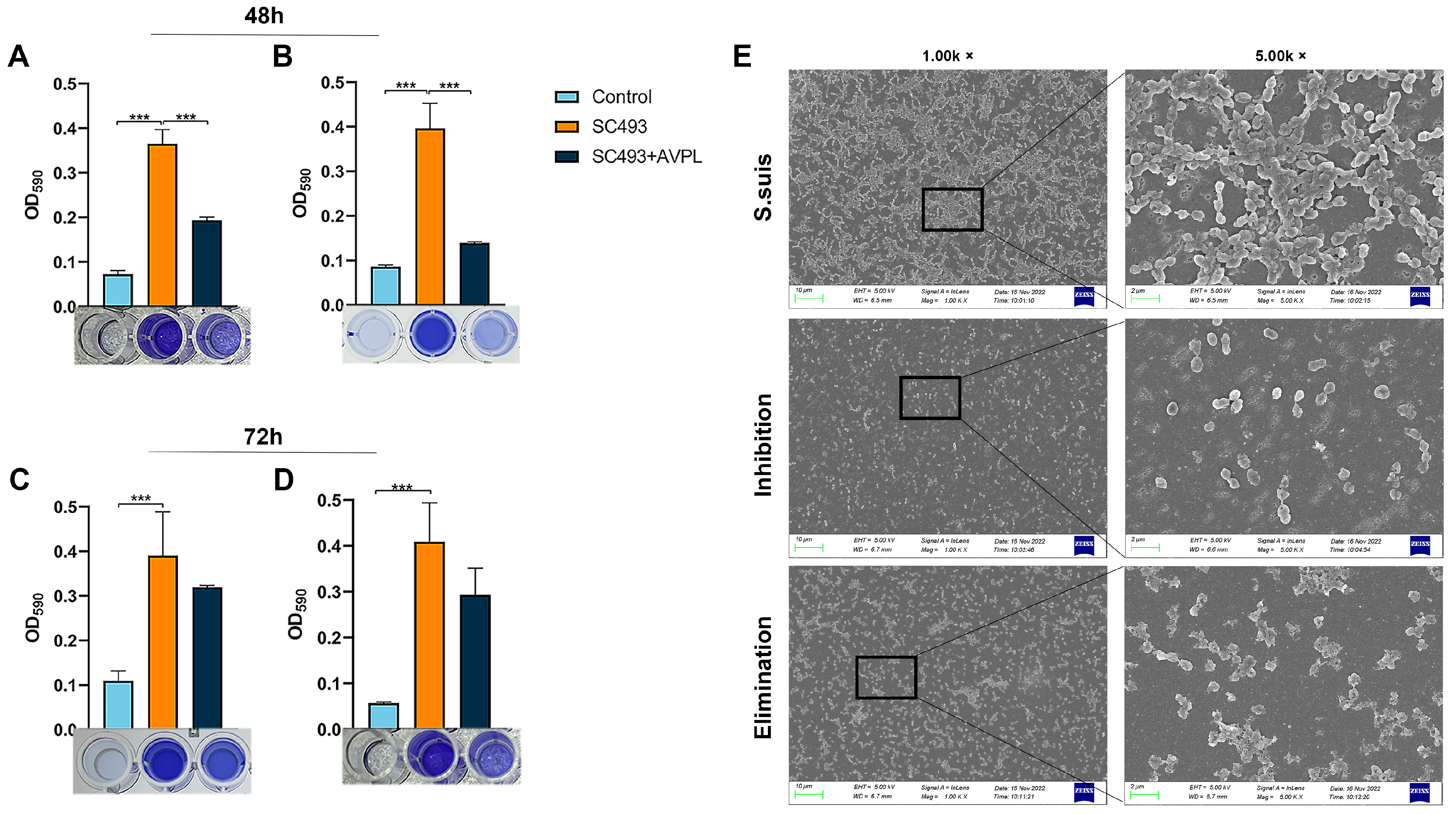
2.3. Antibiofilm Activity of AVPL
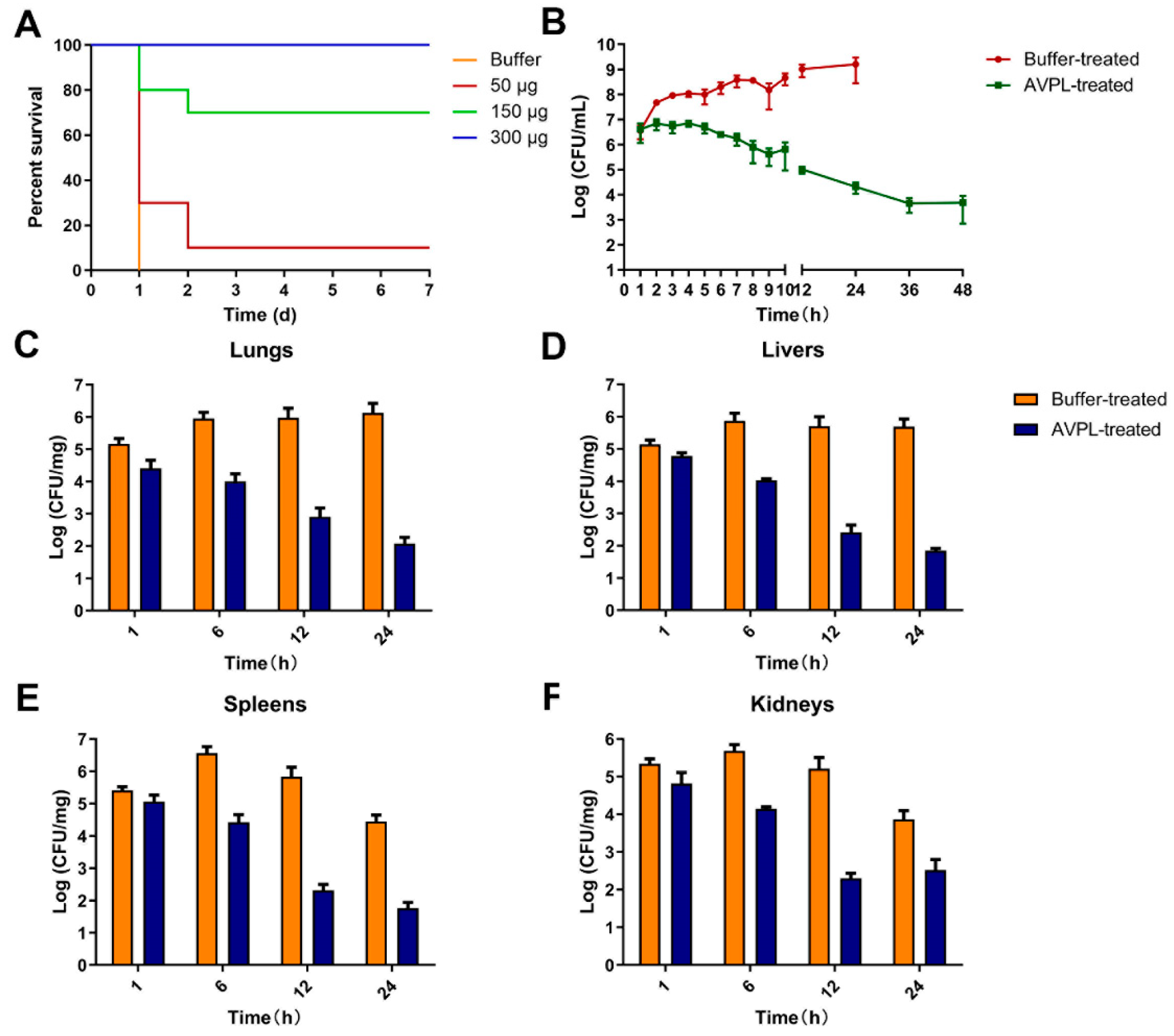
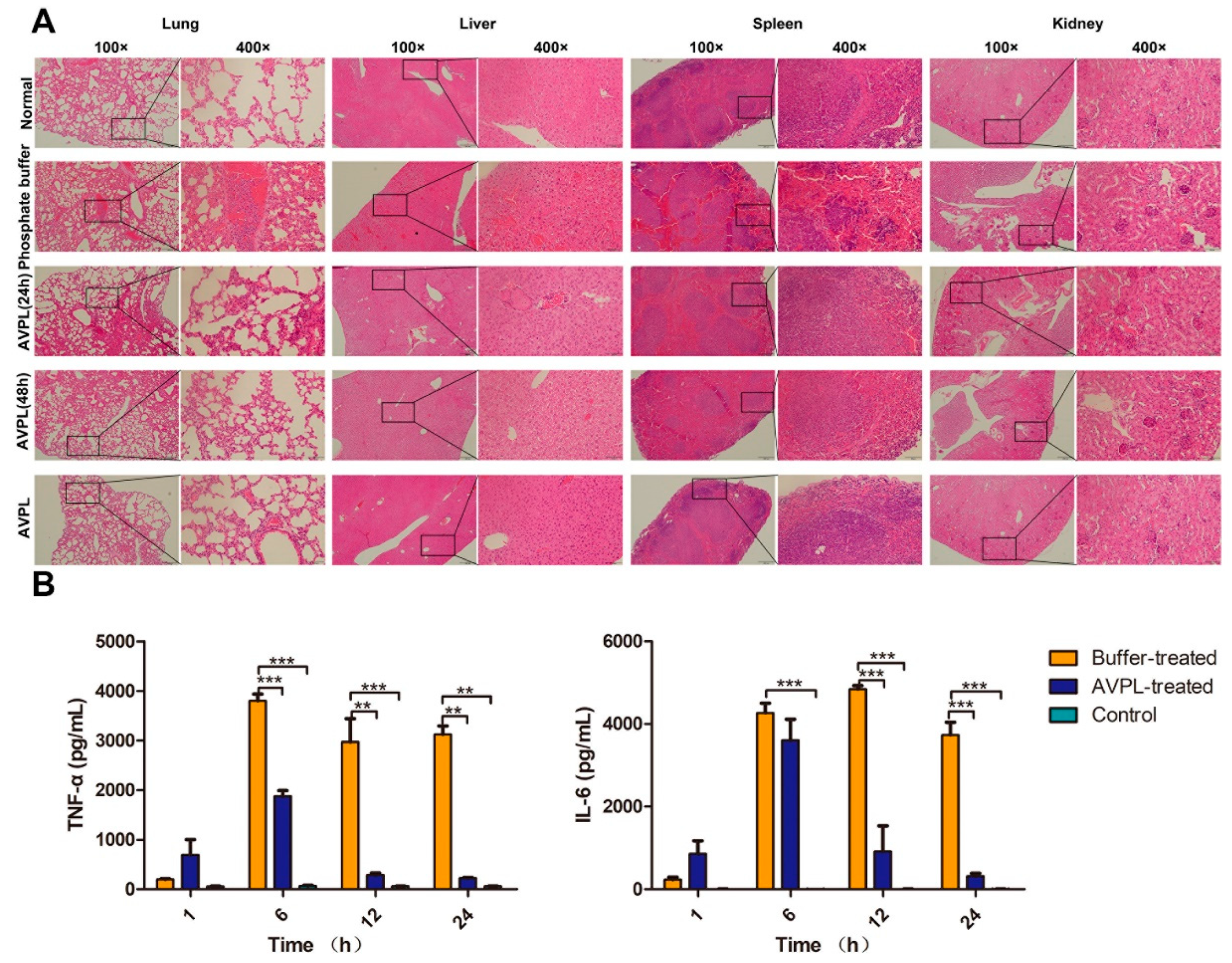
2.4. AVPL Protects Mice against Lethal S. suis Infection
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain Culture
4.2. Constructs, Protein Expression, and Purification
4.3. Bactericidal Assessment of AVPL
4.4. Determination of the Biofilm-Forming Ability of S. suis
4.5. The Activity of AVPL against S. suis Biofilms
4.6. Observation of Biofilms Using Scanning Electron Microscopy (SEM)
4.7. Binding Activity of Different Domains of AVPL
4.8. The Therapeutic Effect of AVPL In Vivo
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.H.; Ma, J.L.; Shang, K.X.; Hu, X.; Liang, Y.; Li, D.W.; Wu, Z.W.; Dai, L.; Chen, L.; Wang, L.P. Evolution and Diversity of the Antimicrobial Resistance Associated Mobilome in Streptococcus suis: A Probable Mobile Genetic Elements Reservoir for Other Streptococci. Front. Cell. Infect. Microbiol. 2016, 6, 118. [Google Scholar] [CrossRef]
- Yu, H.J.; Jing, H.Q.; Chen, Z.H.; Zheng, H.; Zhu, X.P.; Wang, H.; Wang, S.W.; Liu, L.G.; Zu, R.Q.; Luo, L.Z.; et al. Human Streptococcus suis outbreak, Sichuan, China. Emerg. Infect. Dis. 2006, 12, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Goyette-Desjardins, G.; Auger, J.P.; Xu, J.; Segura, M.; Gottschalk, M. Streptococcus suis, an important pig pathogen and emerging zoonotic agent-an update on the worldwide distribution based on serotyping and sequence typing. Emerg. Microbes Infect. 2014, 3, e45. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.J.; Wang, M.L.; Gottschalk, M.; Vela, A.I.; Estrada, A.A.; Wang, J.P.; Du, P.C.; Luo, M.; Zheng, H.; Wu, Z.F. Genomic and pathogenic investigations of Streptococcus suis serotype 7 population derived from a human patient and pigs. Emerg. Microbes Infect. 2021, 10, 1960–1974. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, M.; Xu, J.G.; Calzas, C.; Segura, M. Streptococcus suis: A new emerging or an old neglected zoonotic pathogen? Future Microbiol. 2010, 5, 371–391. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, C.; Varaldo, P.E.; Facinelli, B. Streptococcus suis, an emerging drug-resistant animal and human pathogen. Front. Microbiol. 2011, 2, 235. [Google Scholar] [CrossRef]
- Huang, J.H.; Liang, Y.; Guo, D.W.; Shang, K.X.; Ge, L.; Kashif, J.; Wang, L.P. Comparative Genomic Analysis of the ICESa2603 Family ICEs and Spread of erm(B)- and tet(O)-Carrying Transferable 89K-Subtype ICEs in Swine and Bovine Isolates in China. Front. Microbiol. 2016, 7, 53. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.X.; Sun, L.Y.; Grenier, D.; Yi, L. The LuxS/AI-2 system of Streptococcus suis. Appl. Microbiol. Biotechnol. 2018, 102, 7231–7238. [Google Scholar] [CrossRef]
- Allen, H.K.; Trachsel, J.; Looft, T.; Casey, T.A. Finding alternatives to antibiotics. Ann. N. Y. Acad. Sci. 2014, 1323, 91–100. [Google Scholar] [CrossRef]
- Gilmer, D.B.; Schmitz, J.E.; Euler, C.W.; Fischetti, V.A. Novel Bacteriophage Lysin with Broad Lytic Activity Protects against Mixed Infection by Streptococcus pyogenes and Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2013, 57, 2743–2750. [Google Scholar] [CrossRef]
- Bae, J.Y.; Jun, K.I.; Kang, C.K.; Song, K.H.; Choe, P.G.; Bang, J.H.; Kim, E.S.; Park, S.W.; Kim, H.B.; Kim, N.J.; et al. Efficacy of Intranasal Administration of the Recombinant Endolysin SAL200 in a Lethal Murine Staphylococcus aureus Pneumonia Model. Antimicrob. Agents. Chemother. 2019, 63, e02009-18. [Google Scholar] [CrossRef]
- Eichenseher, F.; Herpers, B.L.; Badoux, P.; Leyva-Castillo, J.M.; Geha, R.S.; van der Zwart, M.; McKellar, J.; Janssen, F.; de Rooij, B.; Selvakumar, L.; et al. Linker-Improved Chimeric Endolysin Selectively Kills Staphylococcus aureus In Vitro, on Reconstituted Human Epidermis, and in a Murine Model of Skin Infection. Antimicrob. Agents. Chemother. 2022, 66, e02273-21. [Google Scholar] [CrossRef] [PubMed]
- Harhala, M.A.; Gembara, K.; Nelson, D.C.; Miernikiewicz, P.; Dabrowska, K. Immunogenicity of Endolysin PlyC. Antibiotics 2022, 11, 966. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V.A. Bacteriophage endolysins: A novel anti-infective to control Gram-positive pathogens. Int. J. Med. Microbiol. 2010, 300, 357–362. [Google Scholar] [CrossRef]
- Young, R. Phage lysis: Do we have the hole story yet? Curr. Opin. Microbiol. 2013, 16, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Xi, H.Y.; Dai, J.X.; Tong, Y.G.; Cheng, M.J.; Zhao, F.Y.; Fan, H.; Li, X.W.; Cai, R.P.; Ji, Y.L.; Sun, C.J.; et al. The Characteristics and Genome Analysis of vB_AviM_AVP, the First Phage Infecting Aerococcus viridans. Viruses 2019, 11, 104. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Wilson, M.E.; Wertheim, H.F.; Nghia, H.D.; Taylor, W.; Schultsz, C. Streptococcus suis:An Emerging Human Pathogen. Clin. Infect. Dis. 2009, 48, 617–625. [Google Scholar]
- Gajdács, M.; Németh, A.; Knausz, M.; Barrak, I.; Stájer, A.; Mestyán, G.; Melegh, S.; Nyul, A.; Tóth, Á.; Ágoston, Z.; et al. Streptococcus suis: An Underestimated Emerging Pathogen in Hungary? Microorganisms 2020, 8, 1292. [Google Scholar] [CrossRef]
- Huang, J.; Sun, J.; Wu, Y.; Chen, L.; Duan, D.; Lv, X.; Wang, L. Identification and pathogenicity of an XDR Streptococcus suis isolate that harbours the phenicol-oxazolidinone resistance genes optrA and cfr, and the bacitracin resistance locus bcrABDR. Int. J. Antimicrob. Agents 2019, 54, 43–48. [Google Scholar] [CrossRef]
- Du, F.; Lv, X.; Duan, D.; Wang, L.; Huang, J. Characterization of a Linezolid- and Vancomycin-Resistant Streptococcus suis Isolate That Harbors optrA and vanG Operons. Front. Microbiol. 2019, 10, 2026. [Google Scholar] [CrossRef]
- Hoy, S.M. Contezolid: First Approval. Drugs 2021, 81, 1587–1591. [Google Scholar] [CrossRef]
- Sharma, U.; Vipra, A.; Channabasappa, S. Phage-derived lysins as potential agents for eradicating biofilms and persisters. Drug Discov. Today 2018, 23, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Osei, E.K.; Mahony, J.; Kenny, J.G. From Farm to Fork: Streptococcus suis as a Model for the Development of Novel Phage-Based Biocontrol Agents. Viruses 2022, 14, 1996. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, J.H.; Lu, C.P. Purified Recombinant Phage Lysin LySMP: An Extensive Spectrum of Lytic Activity for Swine Streptococci. Curr. Microbiol. 2009, 58, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Oechslin, F.; Daraspe, J.; Giddey, M.; Moreillon, P.; Resch, G. In Vitro Characterization of PlySK1249, a Novel Phage Lysin, and Assessment of Its Antibacterial Activity in a Mouse Model of Streptococcus agalactiae Bacteremia. Antimicrob. Agents Chemother. 2013, 57, 6276–6283. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Luo, D.H.; Gondil, V.S.; Gong, Y.J.; Jia, M.H.; Yan, D.Z.; He, J.; Hu, S.C.; Yang, H.; Wei, H.P. Construction and characterization of a chimeric lysin ClyV with improved bactericidal activity against Streptococcus agalactiae in vitro and in vivo. Appl. Microbiol. Biotechnol. 2020, 104, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Linden, S.B.; Wang, J.; Yu, J.P.; Nelson, D.C.; Wei, H.P. A chimeolysin with extended-spectrum streptococcal host range found by an induced lysis-based rapid screening method. Sci. Rep. 2015, 5, 17257. [Google Scholar] [CrossRef]
- Vander Elst, N.; Linden, S.B.; Lavigne, R.; Meyer, E.; Briers, Y.; Nelson, D.C. Characterization of the Bacteriophage-Derived Endolysins PlySs2 and PlySs9 with In Vitro Lytic Activity against Bovine Mastitis Streptococcus uberis. Antibiotics 2020, 9, 621. [Google Scholar] [CrossRef]
- Tang, F.; Li, D.Z.; Wang, H.J.; Ma, Z.; Lu, C.P.; Dai, J.J. Prophage Lysin Ply30 Protects Mice from Streptococcus suis and Streptococcus equi subsp zooepidemicus Infections. Appl. Environ. Microbiol. 2015, 81, 7377–7384. [Google Scholar] [CrossRef]
- Ji, W.H.; Huang, Q.Q.; Sun, L.; Wang, H.G.; Yan, Y.X.; Sun, J.H. A novel endolysin disrupts Streptococcus suis with high efficiency. FEMS Microbiol. Lett. 2015, 362, fnv25. [Google Scholar] [CrossRef]
- Briers, Y.; Lavigne, R. Breaking barriers: Expansion of the use of endolysins as novel antibacterials against Gram-negative bacteria. Future Microbiol. 2015, 10, 377–390. [Google Scholar] [CrossRef]
- Sanz-Gaitero, M.; van Raaij, M.J. Crystallographic Structure Determination of Bacteriophage Endolysins. Curr. Issues Mol. Biol. 2021, 40, 165–188. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Nang, S.C.; Chan, H.K.; Li, J. Novel antimicrobial agents for combating antibiotic-resistant bacteria. Adv. Drug Deliv. Rev. 2022, 187, 114378. [Google Scholar] [CrossRef]
- Nelson, D.; Loomis, L.; Fischetti, V.A. Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc. Natl. Acad. Sci. USA 2001, 98, 4107–4112. [Google Scholar] [CrossRef]
- Linden, S.B.; Alreja, A.B.; Nelson, D.C. Application of bacteriophage-derived endolysins to combat streptococcal disease: Current State and perspectives. Curr. Opin. Biotechnol. 2021, 68, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Yang, H.; Yu, J.P.; Wei, H.P. Molecular dissection of phage lysin PlySs2: Integrity of the catalytic and cell wall binding domains is essential for its broad lytic activity. Virol. Sin. 2015, 30, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.X.; Sun, L.Y.; Grenier, D.; Yi, L. Streptococcus suis biofilm: Regulation, drug-resistance mechanisms, and disinfection strategies. Appl. Microbiol. Biotechnol. 2018, 102, 9121–9129. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; Fan, Q.Y.; Jin, M.Y.; Mao, C.L.; Zhang, H.; Zhang, X.L.; Sun, L.Y.; Grenier, D.; Yi, L.; Hou, X.G.; et al. Paeoniflorin reduce luxS/AI-2 system-controlled biofilm formation and virulence in Streptococcus suis. Virulence 2021, 12, 3062–3073. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.H.; Tang, H.Y.; Zhang, S.M.; Ren, H.Y.; Dai, J.; Lai, L.Y.; Lu, C.P.; Yao, H.C.; Fan, H.J.; Wu, Z.F. Streptococcus suis small RNA rss04 contributes to the induction of meningitis by regulating capsule synthesis and by inducing biofilm formation in a mouse infection model. Vet. Microbiol. 2017, 199, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Gao, X.P.; Xiao, G.H.; Lu, C.P.; Yao, H.C.; Fan, H.J.; Wu, Z.F. Intracranial Subarachnoidal Route of Infection for Investigating Roles of Streptococcus suis Biofilms in Meningitis in a Mouse Infection Model. JoVE J. Vis. Exp. 2018, 137, 57658. [Google Scholar]
- Lakkitjaroen, N.; Takamatsu, D.; Okura, M.; Sato, M.; Osaki, M.; Sekizaki, T. Loss of capsule among Streptococcus suis isolates from porcine endocarditis and its biological significance. J. Med. Microbiol. 2011, 60, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Soto, I.; McTiernan, C.; Gonzalez-Gomez, M.; Ross, A.; Gupta, K.; Suuronen, E.J.; Mah, T.F.; Griffith, M.; Alarcon, E.I. Mimicking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. iScience 2021, 24, 102443. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Wu, Z.F.; Lu, C.P. Reduced virulence is an important characteristic of biofilm infection of Streptococcus suis. FEMS Microbiol. Lett. 2011, 316, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.P.; Shi, Y.B.; Ji, W.H.; Meng, X.L.; Zhang, J.; Wang, H.G.; Lu, C.P.; Sun, J.H.; Yan, Y.X. Application of a Bacteriophage Lysin To Disrupt Biofilms Formed by the Animal Pathogen Streptococcus suis. Appl. Environ. Microbiol. 2011, 77, 8272–8279. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, R.; Domenech, M.; Iglesias-Bexiga, M.; Menendez, M.; Garcia, P. Csl2, a novel chimeric bacteriophage lysin to fight infections caused by Streptococcus suis, an emerging zoonotic pathogen. Sci. Rep. 2017, 7, 16506. [Google Scholar] [CrossRef]
- Liu, B.X.; Guo, Q.C.; Li, Z.; Guo, X.X.; Liu, X.C. Bacteriophage Endolysin: A Powerful Weapon to Control Bacterial Biofilms. Protein J. 2023, 42, 463–476. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Briers, Y.; Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; Lavigne, R.; García, P. Role of the Pre-neck Appendage Protein (Dpo7) from Phage vB_SepiS-phiIPLA7 as an Anti-biofilm Agent in Staphylococcal Species. Front. Microbiol. 2015, 6, 1315. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, M.; Zhang, H.; Dai, J.; Guo, Z.; Li, X.; Ji, Y.; Cai, R.; Xi, H.; Wang, X.; et al. Antibacterial Effects of Phage Lysin LysGH15 on Planktonic Cells and Biofilms of Diverse Staphylococci. Appl. Environ. Microbiol. 2018, 84, e00886-18. [Google Scholar] [CrossRef]
- Poonacha, N.; Nair, S.; Desai, S.; Tuppad, D.; Hiremath, D.; Mohan, T.; Vipra, A.; Sharma, U. Efficient Killing of Planktonic and Biofilm-Embedded Coagulase-Negative Staphylococci by Bactericidal Protein P128. Antimicrob. Agents Chemother. 2017, 61, e00457-17. [Google Scholar] [CrossRef]
- Oliveira, H.; Drulis-Kawa, Z.; Azeredo, J. Exploiting phage-derived carbohydrate depolymerases for combating infectious diseases. Trends Microbiol. 2022, 30, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, M. Aerococci and aerococcal infections. J. Infect. 2013, 66, 467–474. [Google Scholar] [CrossRef]
- Uh, Y.; Son, J.S.; Jang, I.H.; Yoon, K.J.; Hong, S.K. Penicillin-resistant Aerococcus viridans bacteremia associated with granulocytopenia. J. Korean Med. Sci. 2002, 17, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.H.; Ma, Y.; Ma, J.L.; Dong, W.Y.; Yao, H.C. Acute meningitis of piglets and mice caused by co-infected with Streptococcus suis and Aerococcus viridans. Microb. Pathog. 2017, 106, 60–64. [Google Scholar] [CrossRef]
- Liang, W.G.; Ouyang, S.Y.; Shaw, N.; Joachimiak, A.; Zhang, R.G.; Liu, Z.J. Conversion of D-ribulose 5-phosphate to D-xylulose 5-phosphate: New insights from structural and biochemical studies on human RPE. FASEB J. 2011, 25, 497–504. [Google Scholar] [CrossRef]
- Gu, J.M.; Feng, Y.G.; Feng, X.; Sun, C.J.; Lei, L.C.; Ding, W.; Niu, F.F.; Jiao, L.Y.; Yang, M.; Li, Y.; et al. Structural and Biochemical Characterization Reveals LysGH15 as an Unprecedented “EF-Hand-Like” Calcium-Binding Phage Lysin. PLoS Pathog. 2014, 10, e1004109. [Google Scholar] [CrossRef]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, Y.; Li, X.; Liang, J.; Hu, L.; Gong, P.; Zhang, L.; Cai, R.; Zhang, H.; Ge, J.; et al. Endolysin LysEF-P10 shows potential as an alternative treatment strategy for multidrug-resistant Enterococcus faecalis infections. Sci. Rep. 2017, 7, 10164. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, H.; Fu, Y.; Chen, C.; Feng, X.; Han, W.; Gu, J.; Ji, Y. Aerococcus viridans Phage Lysin AVPL Had Lytic Activity against Streptococcus suis in a Mouse Bacteremia Model. Int. J. Mol. Sci. 2023, 24, 16670. https://doi.org/10.3390/ijms242316670
Xi H, Fu Y, Chen C, Feng X, Han W, Gu J, Ji Y. Aerococcus viridans Phage Lysin AVPL Had Lytic Activity against Streptococcus suis in a Mouse Bacteremia Model. International Journal of Molecular Sciences. 2023; 24(23):16670. https://doi.org/10.3390/ijms242316670
Chicago/Turabian StyleXi, Hengyu, Yao Fu, Chong Chen, Xin Feng, Wenyu Han, Jingmin Gu, and Yalu Ji. 2023. "Aerococcus viridans Phage Lysin AVPL Had Lytic Activity against Streptococcus suis in a Mouse Bacteremia Model" International Journal of Molecular Sciences 24, no. 23: 16670. https://doi.org/10.3390/ijms242316670
APA StyleXi, H., Fu, Y., Chen, C., Feng, X., Han, W., Gu, J., & Ji, Y. (2023). Aerococcus viridans Phage Lysin AVPL Had Lytic Activity against Streptococcus suis in a Mouse Bacteremia Model. International Journal of Molecular Sciences, 24(23), 16670. https://doi.org/10.3390/ijms242316670





