Influence of Topography and Composition of Commercial Titanium Dental Implants on Cell Adhesion of Human Gingiva-Derived Mesenchymal Stem Cells: An In Vitro Study
Abstract
:1. Introduction
2. Results
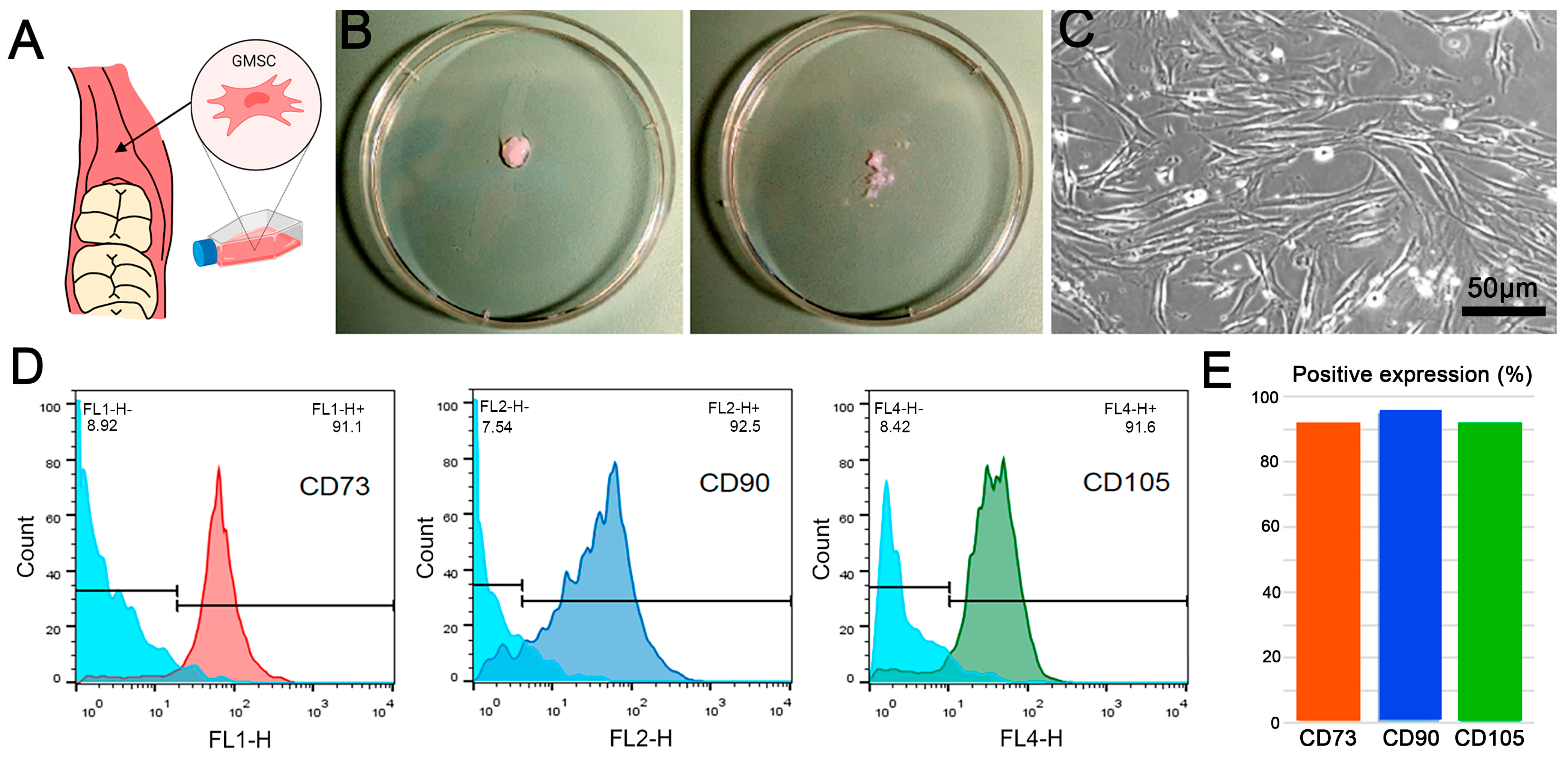
2.1. GMSC Characterization
2.2. Morphological and Textural Characterization
2.2.1. Macrogeometry and Surface Microstructure
2.2.2. Roughness
2.3. Composition
2.4. Protein Adsorption
2.5. Cell Adhesion
2.5.1. Assessment of Cell Adhesion Using Scanning Electron Microscopy
2.5.2. Quantification of Cell Adhesion to Different Implant Surfaces
3. Discussion
3.1. Characterization of Implant Topography and Composition
3.2. Protein Adsorption and Cell Adhesion Assays
3.3. Concluding Remarks and Future Outlook
4. Materials and Methods
4.1. Surface Property Characterization
4.1.1. Studied Implants
4.1.2. SEM/EDX Analysis
4.1.3. AFM Surface Roughness Measurement
4.2. Biological Assessment
4.2.1. Ethics Statement
4.2.2. Cell Culture
4.2.3. Identification and Characterization of GMSCs via Flow Cytometry
4.2.4. The Protein Adsorption Assay
4.2.5. Cell Culture on Dental Implants
4.2.6. Analysis of Cell Morphology and Adhesion via Confocal and SEM Microscopy
4.2.7. Cell Nucleus Detection and Quantification
4.3. Data and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Bernabe, E.; Marcenes, W.; Kassebaum, N.J.; Hernandez, C.R.; Bailey, J.; Hay, S.I.; James, S.L.; Mokdad, A.H.; Nixon, M.R.; Abreu, L.G.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L.; Cicciù, M.; Tozum, T.F.; Saccucci, M.; Orlando, C.; Romano, G.L.; D’amico, C.; Cervino, G. Endosseous Dental Implant Materials and Clinical Outcomes of Different Alloys: A Systematic Review. Materials 2022, 15, 1979. [Google Scholar] [CrossRef] [PubMed]
- Losic, D. Advancing of titanium medical implants by surface engineering: Recent progress and challenges. Expert Opin. Drug Deliv. 2021, 18, 1355–1378. [Google Scholar] [CrossRef]
- Sidambe, A.T. Biocompatibility of Advanced Manufactured Titanium Implants—A Review. Materials 2014, 7, 8168–8188. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Tsoi, J.K.; Matinlinna, J.P. Binary titanium alloys as dental implant materials—A review. Regen. Biomater. 2017, 4, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, N.; Weber, D.K. Dental Implants; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Osman, R.B.; Swain, M.V. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Fiorillo, L.; D’Amico, C.; Campagna, P.; Terranova, A.; Militi, A. Dental materials implant alloys: A X-ray fluorescence analysis on FDS76®. Minerva Dent. Oral Sci. 2020, 69, 370–376. [Google Scholar] [CrossRef]
- Silva, R.C.S.; Agrelli, A.; Andrade, A.N.; Mendes-Marques, C.L.; Arruda, I.R.S.; Santos, L.R.L.; Vasconcelos, N.F.; Machado, G. Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections. Materials 2022, 15, 3150. [Google Scholar] [CrossRef]
- Guglielmotti, M.B.; Olmedo, D.G.; Cabrini, R.L. Research on implants and osseointegration. Periodontology 2019, 79, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. BioMed Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [PubMed]
- Thakral, G.; Thakral, R.; Sharma, N.; Seth, J.; Vashisht, P. Nanosurface—The future of implants. J. Clin. Diagn. Res. 2014, 8, Ze07–Ze10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, Z.; Xing, Y.; Jia, S.; Mo, Z.; Gong, H. Effect of microtopography on osseointegration of implantable biomaterials and its modification strategies. Front. Bioeng. Biotechnol. 2022, 10, 981062. [Google Scholar] [CrossRef]
- Sakornwimon, N.; Leevailoj, C. Clinical marginal fit of zirconia crowns and patients’ preferences for impression techniques using intraoral digital scanner versus polyvinyl siloxane material. J. Prosthet. Dent. 2017, 118, 386–391. [Google Scholar] [CrossRef]
- Zhang, Y.; Gulati, K.; Li, Z.; Di, P.; Liu, Y. Dental Implant Nano-Engineering: Advances, Limitations and Future Directions. Nanomaterials 2021, 11, 2489. [Google Scholar] [CrossRef]
- Zhou, Q.; Su, X.; Wu, J.; Zhang, X.; Su, R.; Ma, L.; Sun, Q.; He, R. Additive Manufacturing of Bioceramic Implants for Restoration Bone Engineering: Technologies, Advances, and Future Perspectives. ACS Biomater. Sci. Eng. 2023, 9, 1164–1189. [Google Scholar] [CrossRef]
- Dong, H.; Liu, H.; Zhou, N.; Li, Q.; Yang, G.; Chen, L.; Mou, Y. Surface Modified Techniques and Emerging Functional Coating of Dental Implants. Coatings 2020, 10, 1012. [Google Scholar] [CrossRef]
- Vetrone, F.; Variola, F.; de Oliveira, P.T.; Zalzal, S.F.; Yi, J.-H.; Sam, J.; Bombonato-Prado, K.F.; Sarkissian, A.; Perepichka, D.F.; Wuest, J.D.; et al. Nanoscale Oxidative Patterning of Metallic Surfaces to Modulate Cell Activity and Fate. Nano Lett. 2009, 9, 659–665. [Google Scholar] [CrossRef]
- Goené, R.J.; Testori, T.; Trisi, P. Influence of a nanometer-scale surface enhancement on de novo bone formation on titanium implants: A histomorphometric study in human maxillae. Int. J. Periodontics Restor. Dent. 2007, 27, 211–219. [Google Scholar]
- López-Valverde, N.; Flores-Fraile, J.; Ramírez, J.M.; de Sousa, B.M.; Herrero-Hernández, S.; López-Valverde, A. Bioactive Surfaces vs. Conventional Surfaces in Titanium Dental Implants: A Comparative Systematic Review. J. Clin. Med. 2020, 9, 2047. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Cerqueira, A.; Romero-Gavilán, F.; García-Arnáez, I.; Martinez-Ramos, C.; Ozturan, S.; Azkargorta, M.; Elortza, F.; Gurruchaga, M.; Goñi, I.; et al. Influence of calcium ion-modified implant surfaces in protein adsorption and implant integration. Int. J. Implant. Dent. 2021, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Sartoretto, S.C.; Calasans-Maia, J.; Resende, R.; Câmara, E.; Ghiraldini, B.; Bezerra, F.J.B.; Granjeiro, J.M.; Calasans-Maia, M.D. The Influence of Nanostructured Hydroxyapatite Surface in the Early Stages of Osseointegration: A Multiparameter Animal Study in Low-Density Bone. Int. J. Nanomed. 2020, 15, 8803–8817. [Google Scholar] [CrossRef] [PubMed]
- Pesce, P.; Menini, M.; Santori, G.; De Giovanni, E.; Bagnasco, F.; Canullo, L. Photo and Plasma Activation of Dental Implant Titanium Surfaces. A Systematic Review with Meta-Analysis of Pre-Clinical Studies. J. Clin. Med. 2020, 9, 2817. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, P.; Guerra, F.; Reis, R.; Krafft, T.; Benz, K.; Jackowski, J. 10-year outcomes with immediate and early loaded implants with a chemically modified SLA surface. Quintessence Int. 2019, 50, 114–124. [Google Scholar] [CrossRef]
- Kellesarian, S.V.; Malignaggi, V.R.; Kellesarian, T.V.; Ahmed, H.B.; Javed, F. Does incorporating collagen and chondroitin sulfate matrix in implant surfaces enhance osseointegration? A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 241–251. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Kamel, A.H.; Hashem, H.M.; Bary, E.M.A. Drug delivery systems between metal, liposome, and polymer-based nanomedicine: A review. Eur. Chem. Bull. 2020, 9, 91–102. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Apostolova, M.D. Advances in Multifunctional Bioactive Coatings for Metallic Bone Implants. Materials 2022, 16, 183. [Google Scholar] [CrossRef]
- Bonilla-Represa, V.; Abalos-Labruzzi, C.; Herrera-Martinez, M.; Guerrero-Pérez, M.O. Nanomaterials in Dentistry: State of the Art and Future Challenges. Nanomaterials 2020, 10, 1770. [Google Scholar] [CrossRef]
- Radunovic, M.; Pavic, A.; Ivanovic, V.; Milivojevic, M.; Radovic, I.; Di Carlo, R.; Pilato, S.; Fontana, A.; Piattelli, A.; Petrovic, S. Biocompatibility and antibiofilm activity of graphene-oxide functionalized titanium discs and collagen membranes. Dent. Mater. 2022, 38, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Stoilov, M.; Stoilov, L.; Enkling, N.; Stark, H.; Winter, J.; Marder, M.; Kraus, D. Effects of Different Titanium Surface Treatments on Adhesion, Proliferation and Differentiation of Bone Cells: An In Vitro Study. J. Funct. Biomater. 2022, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar]
- Amengual-Peñafiel, L.; Córdova, L.A.; Jara-Sepúlveda, M.C.; Brañes-Aroca, M.; Marchesani-Carrasco, F.; Cartes-Velásquez, R. Osteoimmunology drives dental implant osseointegration: A new paradigm for implant dentistry. Jpn. Dent. Sci. Rev. 2021, 57, 12–19. [Google Scholar] [CrossRef]
- Mavrogenis, A.F.; Dimitriou, R.; Parvizi, J.; Babis, G.C. Biology of implant osseointegration. J. Musculoskelet. Neuronal Interact 2009, 9, 61–71. [Google Scholar] [PubMed]
- Pandey, C.; Rokaya, D.; Bhattarai, B.P. Contemporary Concepts in Osseointegration of Dental Implants: A Review. BioMed Res. Int. 2022, 2022, 6170452. [Google Scholar] [CrossRef]
- Ratajczak, J.; Bronckaers, A.; Dillen, Y.; Gervois, P.; Vangansewinkel, T.; Driesen, R.B.; Wolfs, E.; Lambrichts, I.; Hilkens, P. The Neurovascular Properties of Dental Stem Cells and Their Importance in Dental Tissue Engineering. Stem Cells Int. 2016, 2016, 9762871. [Google Scholar] [CrossRef]
- Parisi, L.; Toffoli, A.; Cutrera, M.; Bianchi, M.; Lumetti, S.; Bussolati, O.; Macaluso, G. Plasma Proteins at the Interface of Dental Implants Modulate Osteoblasts Focal Adhesions Expression and Cytoskeleton Organization. Nanomaterials 2019, 9, 1407. [Google Scholar] [CrossRef]
- Cai, K.; Bossert, J.; Jandt, K.D. Does the nanometre scale topography of titanium influence protein adsorption and cell proliferation? Colloids Surf. B Biointerfaces 2006, 49, 136–144. [Google Scholar] [CrossRef]
- Abdallah, M.-N.; Abughanam, G.; Tran, S.D.; Sheikh, Z.; Mezour, M.A.; Basiri, T.; Xiao, Y.; Cerruti, M.; Siqueira, W.L.; Tamimi, F. Comparative adsorption profiles of basal lamina proteome and gingival cells onto dental and titanium surfaces. Acta Biomater. 2018, 73, 547–558. [Google Scholar] [CrossRef]
- Barberi, J.; Spriano, S. Titanium and Protein Adsorption: An Overview of Mechanisms and Effects of Surface Features. Materials 2021, 14, 1590. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Ghezzi, B.; Bianchi, M.G.; Toffoli, A.; Rossi, F.; Bussolati, O.; Macaluso, G.M. Titanium dental implants hydrophilicity promotes preferential serum fibronectin over albumin competitive adsorption modulating early cell response. Mater. Sci. Eng. C 2020, 117, 111307. [Google Scholar] [CrossRef] [PubMed]
- Siebers, M.; ter Brugge, P.; Walboomers, X.; Jansen, J. Integrins as linker proteins between osteoblasts and bone replacing materials. A critical review. Biomaterials 2005, 26, 137–146. [Google Scholar] [CrossRef]
- Stewart, S.; Darwood, A.; Masouros, S.; Higgins, C.; Ramasamy, A. Mechanotransduction in osteogenesis. Bone Jt. Res. 2020, 9, 1–14. [Google Scholar] [CrossRef]
- Dalby, M.J.; Gadegaard, N.; Oreffo, R.O.C. Harnessing nanotopography and integrin–matrix interactions to influence stem cell fate. Nat. Mater. 2014, 13, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chan, Y.; Li, X.; Xu, R.; Zhang, Z.; Hu, X.; Wu, F.; Deng, F.; Yu, X. Multi-omics analysis of oral bacterial biofilm on titanium oxide nanostructure modified implant surface: In vivo sequencing-based pilot study in beagle dogs. Mater. Today Bio 2022, 15, 100275. [Google Scholar] [CrossRef] [PubMed]
- Luke Yeo, I.-S. Dental Implants: Enhancing Biological Response Through Surface Modifications. Dent. Clin. N. Am. 2022, 66, 627–642. [Google Scholar] [CrossRef]
- Makary, C.; Menhall, A.; Lahoud, P.; An, H.-W.; Park, K.-B.; Traini, T. Nanostructured Calcium-Incorporated Surface Compared to Machined and SLA Dental Implants—A Split-Mouth Randomized Case/Double-Control Histological Human Study. Nanomaterials 2023, 13, 357. [Google Scholar] [CrossRef]
- Mansoor, A.; Khurshid, Z.; Khan, M.T.; Mansoor, E.; Butt, F.A.; Jamal, A.; Palma, P.J. Medical and Dental Applications of Titania Nanoparticles: An Overview. Nanomaterials 2022, 12, 3670. [Google Scholar] [CrossRef]
- Babuska, V.; Palan, J.; Dobra, J.K.; Kulda, V.; Duchek, M.; Cerny, J.; Hrusak, D. Proliferation of Osteoblasts on Laser-Modified Nanostructured Titanium Surfaces. Materials 2018, 11, 1827. [Google Scholar] [CrossRef]
- Bressan, E.; Sbricoli, L.; Guazzo, R.; Tocco, I.; Roman, M.; Vindigni, V.; Stellini, E.; Gardin, C.; Ferroni, L.; Sivolella, S.; et al. Nanostructured Surfaces of Dental Implants. Int. J. Mol. Sci. 2013, 14, 1918–1931. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, R.; Barhoum, A.; Uludag, H. A review of nanostructured surfaces and materials for dental implants: Surface coating, patterning and functionalization for improved performance. Biomater. Sci. 2018, 6, 1312–1338. [Google Scholar] [CrossRef] [PubMed]
- Prodanov, L.; Lamers, E.; Domanski, M.; Luttge, R.; Jansen, J.A.; Walboomers, X.F. The effect of nanometric surface texture on bone contact to titanium implants in rabbit tibia. Biomaterials 2013, 34, 2920–2927. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Maher, S.; Findlay, D.M.; Losic, D. Titania nanotubes for orchestrating osteogenesis at the bone–implant interface. Nanomedicine 2016, 11, 1847–1864. [Google Scholar] [CrossRef]
- Laino, L.; La Noce, M.; Fiorillo, L.; Cervino, G.; Nucci, L.; Russo, D.; Herford, A.S.; Crimi, S.; Bianchi, A.; Biondi, A.; et al. Dental Pulp Stem Cells on Implant Surface: An In Vitro Study. BioMed Res. Int. 2021, 2021, 3582342. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Lei, T.; Dusevich, V.; Yao, X.; Liu, Y.; Walker, M.P.; Wang, Y.; Ye, L. Surface characteristics and cell adhesion: A comparative study of four commercial dental implants. J. Prosthodont. Dent. Implant. 2013, 22, 641–651. [Google Scholar] [CrossRef]
- Subirà-Pifarré, C.; Masuet-Aumatell, C.; Alonso, C.R.; Madrid, R.M.; Galletti, C. Assessment of Dental Implants with Modified Calcium-Phosphate Surface in a Multicenter, Prospective, Non-Interventional Study: Results up to 50 Months of Follow-Up. J. Funct. Biomater. 2019, 10, 5. [Google Scholar] [CrossRef]
- Farronato, D.; Mangano, F.; Briguglio, F.; Iorio-Siciliano, V.; Riccitiello, F.; Guarnieri, R. Influence of Laser-Lok Surface on Immediate Functional Loading of Implants in Single-Tooth Replacement: A 2-Year Prospective Clinical Study. Int. J. Periodontics Restor. Dent. 2014, 34, 79–89. [Google Scholar] [CrossRef]
- Annunziata, M.; Oliva, A.; Buosciolo, A.; Giordano, M.; Guida, A.; Guida, L. Bone marrow mesenchymal stem cell response to nano-structured oxidized and turned titanium surfaces. Clin. Oral Implant. Res. 2011, 23, 733–740. [Google Scholar] [CrossRef]
- Li, D.; Yang, L.; Deng, H.; Li, T.; Zhang, Z. Optimized titanium dioxide nanotubes for dental implants: Estimation of mechanical properties and effects on the biological behaviors of human gingival fibroblasts and oral bacteria. J. Mech. Behav. Biomed. Mater. 2023, 144, 105988. [Google Scholar] [CrossRef]
- Al-Wahadni, A.; Barakat, M.S.; Abu Afifeh, K.; Khader, Y. Dentists’ Most Common Practices when Selecting an Implant System. J. Prosthodont. 2017, 27, 250–259. [Google Scholar] [CrossRef]
- Covarrubias, C.; Mattmann, M.; Von Marttens, A.; Caviedes, P.; Arriagada, C.; Valenzuela, F.; Rodríguez, J.P.; Corral, C. Osseointegration properties of titanium dental implants modified with a nanostructured coating based on ordered porous silica and bioactive glass nanoparticles. Appl. Surf. Sci. 2016, 363, 286–295. [Google Scholar] [CrossRef]
- Al-Tarawneh, S.; Thalji, G.; Cooper, L. Macrogeometric Differentiation of Dental Implant Primary Stability: An In Vitro Study. Int. J. Oral Maxillofac. Implants 2022, 37, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chang, C.; Guo, W.; Wu, Y.; Zhou, W.; Yu, D. Primary implant stability based on alternative site preparation techniques: A systematic review and meta-analysis. Clin. Implant. Dent. Relat. Res. 2022, 24, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implant. Res. 2009, 20 (Suppl. 4), 172–184. [Google Scholar] [CrossRef] [PubMed]
- Kournetas, N.; Spintzyk, S.; Schweizer, E.; Sawada, T.; Said, F.; Schmid, P.; Geis-Gerstorfer, J.; Eliades, G.; Rupp, F. Comparative evaluation of topographical data of dental implant surfaces applying optical interferometry and scanning electron microscopy. Dent. Mater. 2017, 33, e317–e327. [Google Scholar] [CrossRef] [PubMed]
- Rompen, E.; Domken, O.; Degidi, M.; Pontes, A.E.F.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implant. Res. 2006, 17 (Suppl. 2), 55–67. [Google Scholar] [CrossRef]
- Rosa, M.B.; Albrektsson, T.; Francischone, C.E.; Filho, H.O.S.; Wennerberg, A. The influence of surface treatment on the implant roughness pattern. J. Appl. Oral Sci. 2012, 20, 550–555. [Google Scholar] [CrossRef]
- Wiskott, H.W.A.; Belser, U.C. Lack of integration of smooth titanium surfaces: A working hypothesis based on strains generated in the surrounding bone. Clin. Oral Implant. Res. 1999, 10, 429–444. [Google Scholar] [CrossRef]
- Kang, B.; Sul, Y.; Johansson, C.B.; Oh, S.; Lee, H.; Albrektsson, T. The effect of calcium ion concentration on the bone response to oxidized titanium implants. Clin. Oral Implant. Res. 2012, 23, 690–697. [Google Scholar] [CrossRef]
- Wexell, C.L.; Thomsen, P.; Aronsson, B.-O.; Tengvall, P.; Rodahl, M.; Lausmaa, J.; Kasemo, B.; Ericson, L.E. Bone Response to Surface-Modified Titanium Implants: Studies on the Early Tissue Response to Implants with Different Surface Characteristics. Int. J. Biomater. 2013, 2013, 412482. [Google Scholar] [CrossRef]
- Piao, H.; McIntyre, N.S. Adventitious carbon growth on aluminium and gold-aluminium alloy surfaces. Surf. Interface Anal. 2002, 33, 591–594. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Vazquez, L.; Park, Y.J.; Sammartino, G.; Bernard, J.P. Identification Card and Codification of the Chemical and Morphological Characteristics of 14 Dental Implant Surfaces. J. Oral Implantol. 2011, 37, 525–542. [Google Scholar] [CrossRef]
- Murphy, M.; Walczak, M.; Thomas, A.; Silikas, N.; Berner, S.; Lindsay, R. Toward optimizing dental implant performance: Surface characterization of Ti and TiZr implant materials. Dent. Mater. 2017, 33, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Greczynski, G.; Hultman, L. The same chemical state of carbon gives rise to two peaks in X-ray photoelectron spectroscopy. Sci. Rep. 2021, 11, 11195. [Google Scholar] [CrossRef]
- Yang, S.-M.; Park, J.-B.; Ko, Y. Use of confocal microscopy for quantification of plastic remnants on rough titanium after instrumentation and evaluation of efficacy of removal. Int. J. Oral Maxillofac. Implants 2015, 30, 519–525. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A review on the wettability of dental implant surfaces II: Biological and clinical aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Choi, S.; Jo, Y.-H.; Yeo, I.-S.L.; Yoon, H.-I.; Lee, J.-H.; Han, J.-S. The effect of surface material, roughness and wettability on the adhesion and proliferation of Streptococcus gordonii, Fusobacterium nucleatum and Porphyromonas gingivalis. J. Dent. Sci. 2023, 18, 517–525. [Google Scholar] [CrossRef]
- Cortez, C.; Real, F.; Yoshida, N. Lysosome biogenesis/scattering increases host cell susceptibility to invasion by Trypanosoma cruzi metacyclic forms and resistance to tissue culture trypomastigotes. Cell. Microbiol. 2016, 18, 748–760. [Google Scholar] [CrossRef]
- Vicencio, E.; Cordero, E.M.; Cortés, B.I.; Palominos, S.; Parra, P.; Mella, T.; Henrríquez, C.; Salazar, N.; Monasterio, G.; Cafferata, E.A.; et al. Aggregatibacter actinomycetemcomitans Induces Autophagy in Human Junctional Epithelium Keratinocytes. Cells 2020, 9, 1221. [Google Scholar] [CrossRef]
- Ioanid, N.; Musca, G. Functional surface area in biomechanical integration of endosseous implant. In Proceedings of the 2013 E-Health and Bioengineering Conference (EHB), Iasi, Romania, 21–23 November 2013. [Google Scholar]
- Toro, M.; Leitao, J.; Sánchez, G.; Díaz, L.; Gil, A.C.; Fernández, E. Simplified method to quantify the osseointegration area of dental implants. Rev. Cuba. De Investig. Biomédicas 2020, 39, 1–10. [Google Scholar]






| Control | INNO | BioHorizonsTM | Biounite® | Zimmer | |
|---|---|---|---|---|---|
| Sa (μm) | 0.28 ± 0.02 | 1.34 ± 0.36 | 1.36 ± 0.04 | 1.13 ± 0.20 | 0.78 ± 0.17 |
| Sq (μm) | 0.33 ± 0.03 | 1.66 ± 0.37 | 1.59 ± 0.06 | 1.35 ± 0.24 | 0.90 ± 0.19 |
| Sp (μm) | 0.79 ± 0.11 | 5.45 ± 0.62 | 2.97 ± 0.05 | 3.02 ± 0.85 | 2.22 ± 0.42 |
| Sv (μm) | 0.77 ± 0.12 | 4.19 ± 0.56 | 4.35 ± 0.40 | 2.92 ± 0.39 | 1.89 ± 0.17 |
| Sz (μm) | 1.56 ± 0.17 | 9.64 ± 1.08 | 7.32 ± 0.46 | 5.94 ± 1.01 | 4.11 ± 0.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos-Bijit, V.; Inostroza, N.C.; Orellana, R.; Rivera, A.; Von Marttens, A.; Cortez, C.; Covarrubias, C. Influence of Topography and Composition of Commercial Titanium Dental Implants on Cell Adhesion of Human Gingiva-Derived Mesenchymal Stem Cells: An In Vitro Study. Int. J. Mol. Sci. 2023, 24, 16686. https://doi.org/10.3390/ijms242316686
Campos-Bijit V, Inostroza NC, Orellana R, Rivera A, Von Marttens A, Cortez C, Covarrubias C. Influence of Topography and Composition of Commercial Titanium Dental Implants on Cell Adhesion of Human Gingiva-Derived Mesenchymal Stem Cells: An In Vitro Study. International Journal of Molecular Sciences. 2023; 24(23):16686. https://doi.org/10.3390/ijms242316686
Chicago/Turabian StyleCampos-Bijit, Vanessa, Nicolás Cohn Inostroza, Rocío Orellana, Alejandro Rivera, Alfredo Von Marttens, Cristian Cortez, and Cristian Covarrubias. 2023. "Influence of Topography and Composition of Commercial Titanium Dental Implants on Cell Adhesion of Human Gingiva-Derived Mesenchymal Stem Cells: An In Vitro Study" International Journal of Molecular Sciences 24, no. 23: 16686. https://doi.org/10.3390/ijms242316686






