Identification of Metabolomic Markers in Frozen or Formalin-Fixed and Paraffin-Embedded Samples of Diffuse Glioma from Adults
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
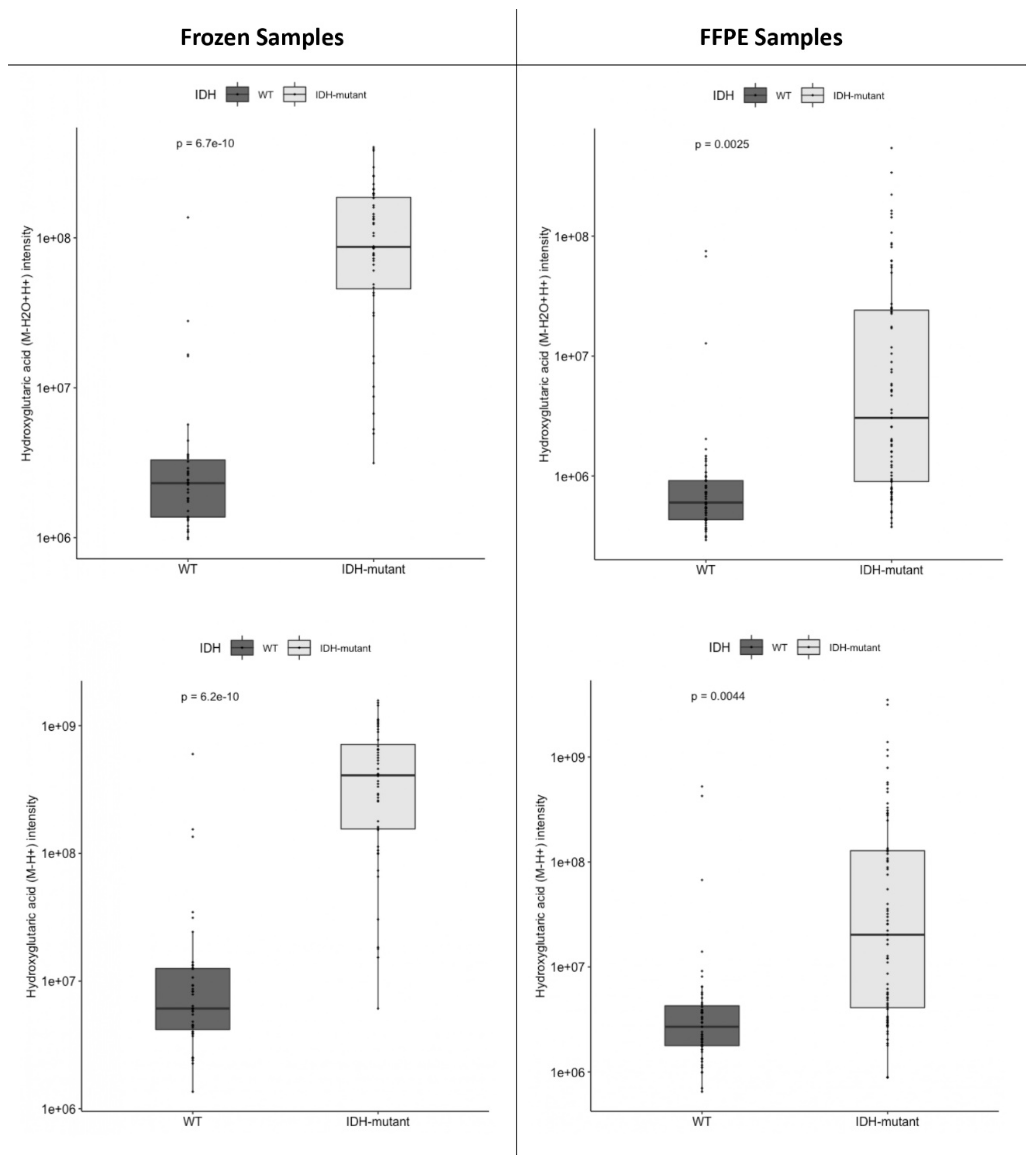
2.1. Metabolomic Markers of the Gliomagenesis Pathway: IDH-Mutant versus IDH-Wild-Type Tumors
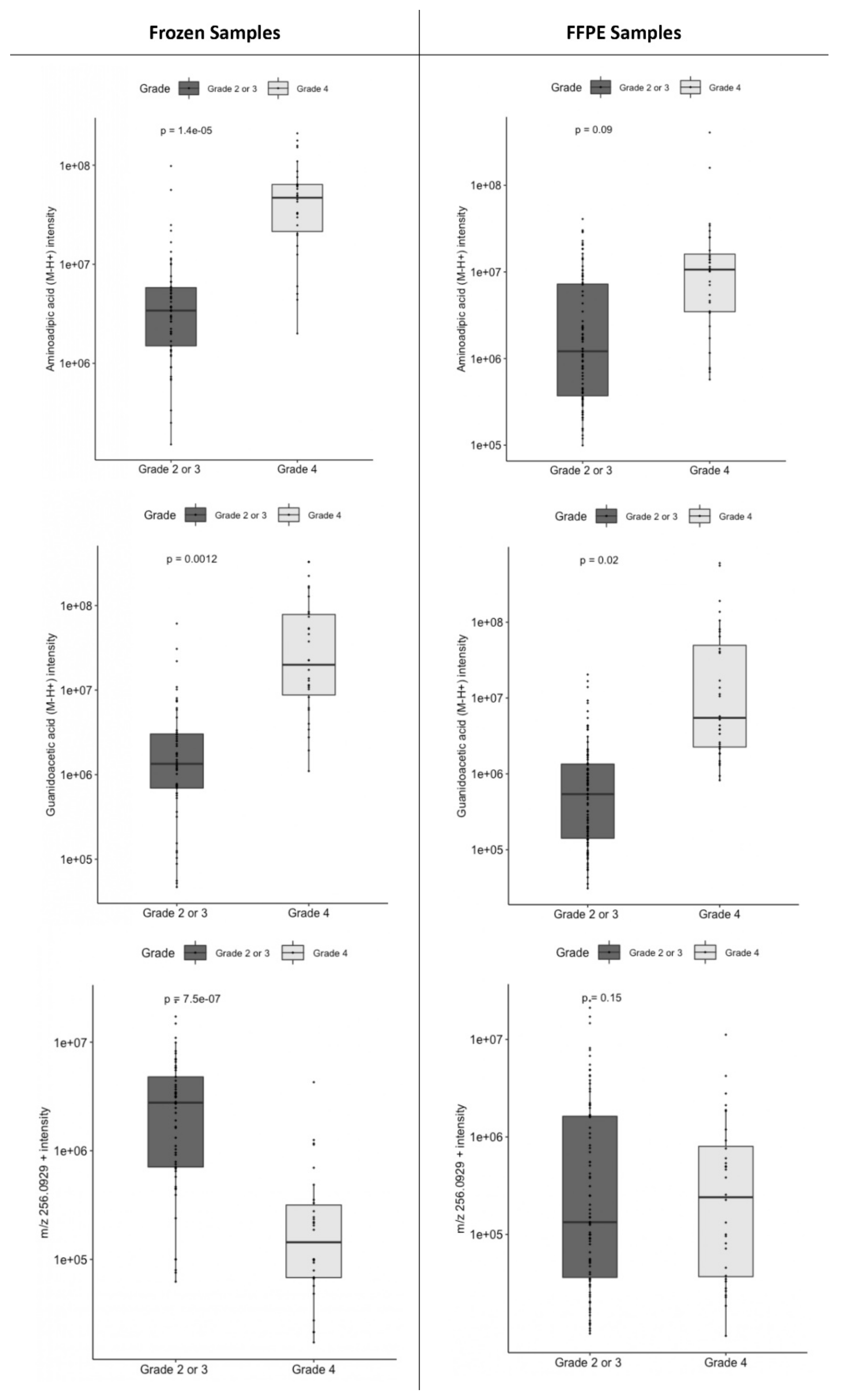
2.2. Metabolomic Markers of Grade: Grade 4 versus Lower Grades
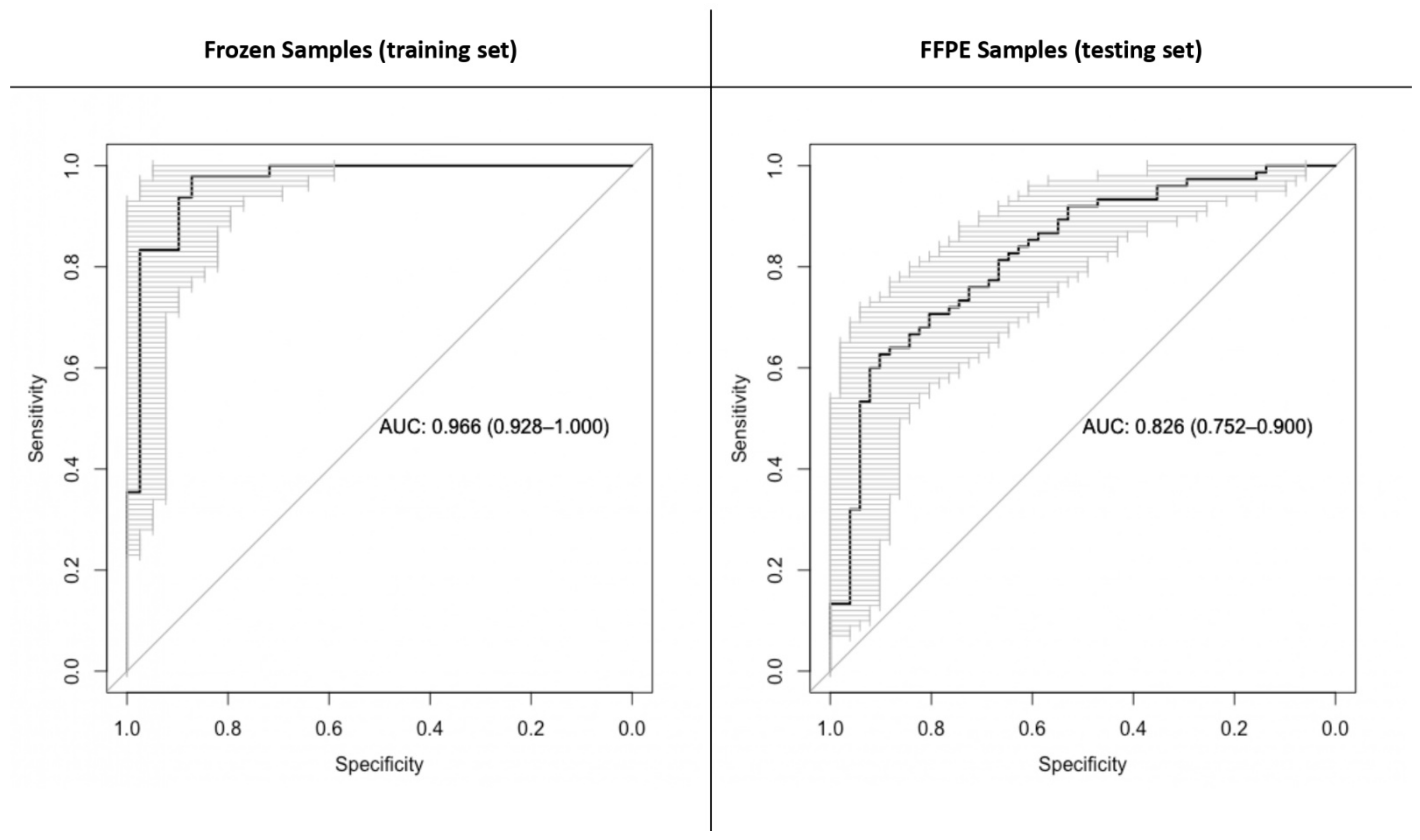
2.3. A First Analysis Included Both IDH-Mutant and IDH-Wild-Type Tumors
2.4. In an Additional Step, IDH-Mutant and IDH-Wild-Type Astrocytomas Were Separately Analyzed
2.4.1. Concerning IDH-Mutant Astrocytomas
2.4.2. Concerning IDH-Wild-Type Astrocytomas
3. Discussion
- We filtered the initial raw data, removing peaks of lower intensities. This step aims to denoise the data but could also discard metabolites of interest with low concentrations.
- We used a constrained statistical method, the LASSO penalized logistic regression, which is robust to correlated features and to overfitting.
- We systematically verified the identification of the selected ions by comparing the obtained mass/charge ratios with mass spectrometry databases and by comparing the corresponding MS/MS spectrums to spectrum databases.
- We verified the results obtained using the frozen sample cohort using the FFPE sample cohort, which we used as a validation dataset.
4. Materials and Methods
4.1. Sample Collection
4.2. Sample Preparation
4.3. LC-MS/MS Analysis
4.4. Metabolomic Profiling
4.5. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chinnaiyan, P.; Kensicki, E.; Bloom, G.; Prabhu, A.; Sarcar, B.; Kahali, S.; Eschrich, S.; Qu, X.; Forsyth, P.; Gillies, R. The metabolomic signature of malignant glioma reflects accelerated anabolic metabolism. Cancer Res. 2012, 72, 5878–5888. [Google Scholar] [CrossRef] [PubMed]
- Reitman, Z.J.; Jin, G.; Karoly, E.D.; Spasojevic, I.; Yang, J.; Kinzler, K.W.; He, Y.; Bigner, D.D.; Vogelstein, B.; Yan, H. Profiling the effects of isocitrate dehydrogenase 1 and 2 mutations on the cellular metabolome. Proc. Natl. Acad. Sci. USA 2011, 108, 3270–3275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, Z.; Hu, C.; Zhang, C.; Kovatcheva-Datchary, P.; Yu, D.; Liu, S.; Ren, F.; Wang, X.; Li, Y.; et al. Integrated Metabolomics and Lipidomics Analyses Reveal Metabolic Reprogramming in Human Glioma with IDH1 Mutation. J. Proteome Res. 2019, 18, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Cacciatore, S.; Zadra, G.; Bango, C.; Penney, K.L.; Tyekucheva, S.; Yanes, O.; Loda, M. Metabolic Profiling in Formalin-Fixed and Paraffin-Embedded Prostate Cancer Tissues. Mol. Cancer Res. 2017, 15, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Kelly, A.D.; Breitkopf, S.B.; Yuan, M.; Goldsmith, J.; Spentzos, D.; Asara, J.M. Metabolomic profiling from formalin-fixed, paraffin-embedded tumor tissue using targeted LC/MS/MS: Application in sarcoma. PLoS ONE 2011, 6, e25357. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013, 15 (Suppl. S2), ii1–ii56. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Brat, D.J.; Aldape, K.; Colman, H.; Holland, E.C.; Louis, D.N.; Jenkins, R.B.; Kleinschmidt-DeMasters, B.; Perry, A.; Reifenberger, G.; Stupp, R.; et al. cIMPACT-NOW Update 3: Recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018, 136, 805–810. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2009, 462, 739–744. [Google Scholar] [CrossRef]
- Mörén, L.; Bergenheim, A.T.; Ghasimi, S.; Brännström, T.; Johansson, M.; Antti, H. Metabolomic Screening of Tumor Tissue and Serum in Glioma Patients Reveals Diagnostic and Prognostic Information. Metabolites 2015, 5, 502–520. [Google Scholar] [CrossRef]
- Lee, J.E.; Jeun, S.S.; Kim, S.H.; Yoo, C.Y.; Baek, H.-M.; Yang, S.H. Metabolic profiling of human gliomas assessed with NMR. J. Clin. Neurosci. 2019, 68, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Capper, D.; Pusch, S.; Balss, J.; Koch, A.; Langhans, C.; Okun, J.G.; von Deimling, A. Detection of 2-Hydroxyglutarate in Formalin-Fixed Paraffin-Embedded Glioma Specimens by Gas Chromatography/Mass Spectrometry. Brain Pathol. 2011, 22, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Dannhorn, A.; Swales, J.G.; Hamm, G.; Strittmatter, N.; Kudo, H.; Maglennon, G.; Goodwin, R.J.A.; Takats, Z. Evaluation of Formalin-Fixed and FFPE Tissues for Spatially Resolved Metabolomics and Drug Distribution Studies. Pharmaceuticals 2022, 15, 1307. [Google Scholar] [CrossRef] [PubMed]
- Arima, K.; Lau, M.C.; Zhao, M.; Haruki, K.; Kosumi, K.; Mima, K.; Gu, M.; Väyrynen, J.P.; Twombly, T.S.; Baba, Y.; et al. Metabolic Profiling of Formalin-Fixed Paraffin-Embedded Tissues Discriminates Normal Colon from Colorectal Cancer. Mol. Cancer Res. 2020, 18, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, J.; Mazurie, A.; Tokmina-Lukaszewska, M.; Beilman, G.J.; Bothner, B. Application of support vector machines to metabolomics experiments with limited replicates. Metabolomics 2014, 10, 1121–1128. [Google Scholar] [CrossRef]
- Bartel, J.; Krumsiek, J.; Theis, F.J. Statistical Methods for the Analysis of High-Throughput Metabolomics Data. Comput. Struct. Biotechnol. J. 2013, 4, e201301009. [Google Scholar] [CrossRef]
- Broadhurst, D.I.; Kell, D.B. Statistical strategies for avoiding false discoveries in metabolomics and related experiments. Metabolomics 2006, 2, 171–196. [Google Scholar] [CrossRef]
- Bujak, R.; Daghir-Wojtkowiak, E.; Kaliszan, R.; Markuszewski, M.J. PLS-Based and Regularization-Based Methods for the Selection of Relevant Variables in Non-targeted Metabolomics Data. Front. Mol. Biosci. 2016, 3, 35. [Google Scholar] [CrossRef]
- Hallen, A.; Jamie, J.F.; Cooper, A.J.L. Lysine metabolism in mammalian brain: An update on the importance of recent discoveries. Amino Acids 2013, 45, 1249–1272. [Google Scholar] [CrossRef]
- Bellance, N.; Pabst, L.; Allen, G.; Rossignol, R.; Nagrath, D. Oncosecretomics coupled to bioenergetics identifies α-amino adipic acid, isoleucine and GABA as potential biomarkers of cancer: Differential expression of c-Myc, Oct1 and KLF4 coordinates metabolic changes. Biochim. Biophys. Acta 2012, 1817, 2060–2071. [Google Scholar] [CrossRef]
- Locasale, J.W.; Melman, T.; Song, S.; Yang, X.; Swanson, K.D.; Cantley, L.C.; Wong, E.T.; Asara, J.M. Metabolomics of human cerebrospinal fluid identifies signatures of malignant glioma. Mol. Cell. Proteom. 2012, 11, M111.014688. [Google Scholar] [CrossRef]
- Rosi, A.; Ricci-Vitiani, L.; Biffoni, M.; Grande, S.; Luciani, A.M.; Palma, A.; Runci, D.; Cappellari, M.; De Maria, R.; Guidoni, L.; et al. (1) H NMR spectroscopy of glioblastoma stem-like cells identifies alpha-aminoadipate as a marker of tumor aggressiveness. NMR Biomed. 2015, 28, 317–326. [Google Scholar] [CrossRef]
- Björkblom, B.; Wibom, C.; Eriksson, M.; Bergenheim, A.T.; Sjöberg, R.L.; Jonsson, P.; Brännström, T.; Antti, H.; Sandström, M.; Melin, B. Distinct metabolic hallmarks of WHO classified adult glioma subtypes. Neuro-Oncol. 2022, 24, 1454–1468. [Google Scholar] [CrossRef]
- Goryńska, P.Z.; Chmara, K.; Kupcewicz, B.; Goryński, K.; Jaroch, K.; Paczkowski, D.; Furtak, J.; Harat, M.; Bojko, B. Metabolomic Phenotyping of Gliomas: What Can We Get with Simplified Protocol for Intact Tissue Analysis? Cancers 2022, 14, 312. [Google Scholar] [CrossRef]
- Jung, K.; Reszka, R.; Kamlage, B.; Bethan, B.; Stephan, C.; Lein, M.; Kristiansen, G. Tissue metabolite profiling identifies differentiating and prognostic biomarkers for prostate carcinoma. Int. J. Cancer 2013, 133, 2914–2924. [Google Scholar] [CrossRef]
- McBean, G.J. Inhibition of the glutamate transporter and glial enzymes in rat striatum by the gliotoxin, alpha aminoadipate. Br. J. Pharmacol. 1994, 113, 536–540. [Google Scholar] [CrossRef]
- da Silva, J.C.; Amaral, A.U.; Cecatto, C.; Wajner, A.; Dos Santos Godoy, K.; Ribeiro, R.T.; de Mello Gonçalves, A.; Zanatta, Â.; da Rosa, M.S.; Loureiro, S.O.; et al. α-Ketoadipic Acid and α-Aminoadipic Acid Cause Disturbance of Glutamatergic Neurotransmission and Induction of Oxidative Stress In Vitro in Brain of Adolescent Rats. Neurotox. Res. 2017, 32, 276–290. [Google Scholar] [CrossRef]
- Riviere-Cazaux, C.; Neth, B.J.; Hoplin, M.D.; Wessel, B.; Miska, J.; Kizilbash, S.H.; Burns, T.C. Glioma Metabolic Feedback In Situ: A First-In-Human Pharmacodynamic Trial of Difluoromethylornithine + AMXT-1501 Through High-Molecular Weight Microdialysis. Neurosurgery 2023, 93, 932–938. [Google Scholar] [CrossRef]
- Bera, S.; Wallimann, T.; Ray, S.; Ray, M. Enzymes of creatine biosynthesis, arginine and methionine metabolism in normal and malignant cells. FEBS J. 2008, 275, 5899–5909. [Google Scholar] [CrossRef]
- Bogusławska, J.; Popławski, P.; Alseekh, S.; Koblowska, M.; Iwanicka-Nowicka, R.; Rybicka, B.; Kędzierska, H.; Głuchowska, K.; Hanusek, K.; Tański, Z.; et al. MicroRNA-Mediated Metabolic Reprograming in Renal Cancer. Cancers 2019, 11, 1825. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Z.; Yan, H.; Wang, W.; Wu, Z.; Zhang, F.; Zhang, Q.; Shi, G.; Du, J.; Cai, H.; et al. Creatine promotes cancer metastasis through activation of Smad2/3. Cell Metab. 2021, 33, 1111–1123.e4. [Google Scholar] [CrossRef]
- Yang, J.; Ren, B.; Ren, J.; Yang, G.; Fang, Y.; Wang, X.; Zhou, F.; You, L.; Zhao, Y. Epigenetic reprogramming-induced guanidinoacetic acid synthesis promotes pancreatic cancer metastasis and transcription-activating histone modifications. J. Exp. Clin. Cancer Res. 2023, 42, 155. [Google Scholar] [CrossRef]
- Choi, C.; Ganji, S.K.; DeBerardinis, R.J.; Hatanpaa, K.J.; Rakheja, D.; Kovacs, Z.; Yang, X.-L.; Mashimo, T.; Raisanen, J.M.; Marin-Valencia, I.; et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated glioma patients. Nat. Med. 2012, 18, 624–629. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.; Lee, H.H.; Heo, H. In-Vivo Proton Magnetic Resonance Spectroscopy of 2-Hydroxyglutarate in Isocitrate Dehydrogenase-Mutated Gliomas: A Technical Review for Neuroradiologists. Korean J. Radiol. 2016, 17, 620–632. [Google Scholar] [CrossRef]
- Suh, C.H.; Kim, H.S.; Paik, W.; Choi, C.; Ryu, K.H.; Kim, D.; Woo, D.-C.; Park, J.E.; Jung, S.C.; Choi, C.G.; et al. False-Positive Measurement at 2-Hydroxyglutarate MR Spectroscopy in Isocitrate Dehydrogenase Wild-Type Glioblastoma: A Multifactorial Analysis. Radiology 2019, 291, 752–762. [Google Scholar] [CrossRef]
- Di Stefano, A.L.; Nichelli, L.; Berzero, G.; Valabregue, R.; Touat, M.; Capelle, L.; Pontoizeau, C.; Bielle, F.; Lerond, J.; Giry, M.; et al. In Vivo 2-Hydroxyglutarate Monitoring with Edited MR Spectroscopy for the Follow-up of IDH-Mutant Diffuse Gliomas: The IDASPE Prospective Study. Neurology 2023, 100, e94–e106. [Google Scholar] [CrossRef]
- Van Dijken, B.R.J.; Jeltema, H.-R.; Kłos, J.; van Laar, P.J.; Enting, R.H.; Maatman, R.G.H.J.; Bijsterveld, K.; Den Dunnen, W.F.A.; Dierckx, R.A.; Sijens, P.E.; et al. The Correlation of In Vivo MR Spectroscopy and Ex Vivo 2-Hydroxyglutarate Concentration for the Prediction of Isocitrate Dehydrogenase Mutation Status in Diffuse Glioma. Diagnostics 2023, 13, 2791. [Google Scholar] [CrossRef]
- Righi, V.; Cavallini, N.; Valentini, A.; Pinna, G.; Pavesi, G.; Rossi, M.C.; Puzzolante, A.; Mucci, A.; Cocchi, M. A metabolomic data fusion approach to support gliomas grading. NMR Biomed. 2020, 33, e4234. [Google Scholar] [CrossRef]
- Ensenauer, R.; Thiel, T.; Schwab, K.O.; Tacke, U.; Stöckler-Ipsiroglu, S.; Schulze, A.; Hennig, J.; Lehnert, W. Guanidinoacetate methyltransferase deficiency: Differences of creatine uptake in human brain and muscle. Mol. Genet. Metab. 2004, 82, 208–213. [Google Scholar] [CrossRef]
- Isberg, O.G.; Xiang, Y.; Bodvarsdottir, S.K.; Jonasson, J.G.; Thorsteinsdottir, M.; Takats, Z. The effect of sample age on the metabolic information extracted from formalin-fixed and paraffin embedded tissue samples using desorption electrospray ionization mass spectrometry imaging. J. Mass Spectrom. Adv. Clin. Lab. 2021, 22, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Myers, O.D.; Sumner, S.J.; Li, S.; Barnes, S.; Du, X. One Step Forward for Reducing False Positive and False Negative Compound Identifications from Mass Spectrometry Metabolomics Data: New Algorithms for Constructing Extracted Ion Chromatograms and Detecting Chromatographic Peaks. Anal. Chem. 2017, 89, 8696–8703. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Burke, J.R.; Guijas, C.; Siuzdak, G. METLIN: A Tandem Mass Spectral Library of Standards. Methods Mol. Biol. 2020, 2104, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Bach, F.R. Bolasso: Model consistent Lasso estimation through the bootstrap. In Proceedings of the 25th International Conference on Machine Learning, Helsinki, Finland, 5–9 July 2008; Association for Computing Machinery: New York, NY, USA, 2008; pp. 33–40. [Google Scholar]

| Histological Subtypes | IDH Status | WHO 2021 Grade | WHO 2007 Grade | Frozen Samples n = 87 (%) | FFPE Samples n = 126 (%) |
|---|---|---|---|---|---|
| Oligodendroglioma | Mutant | 2 | II | 7 (8.0%) | 15 (11.9%) |
| Astrocytoma | Mutant | 2 | II | 19 (21.8%) | 29 (23.0%) |
| Astrocytoma | Mutant | 3 | III | 10 (11.5%) | 15 (11.9%) |
| Astrocytoma | Mutant | 4 | IV | 12 (13.8%) | 16 (12.7%) |
| Astrocytoma * | Wild type | 4 | II | 9 (10.3%) | 15 (11.9%) |
| Astrocytoma * | Wild type | 4 | III | 12 (13.8%) | 20 (15.9%) |
| Glioblastoma | Wild type | 4 | IV | 18 (20.7%) | 16 (12.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chardin, D.; Jing, L.; Chazal-Ngo-Mai, M.; Guigonis, J.-M.; Rigau, V.; Goze, C.; Duffau, H.; Virolle, T.; Pourcher, T.; Burel-Vandenbos, F. Identification of Metabolomic Markers in Frozen or Formalin-Fixed and Paraffin-Embedded Samples of Diffuse Glioma from Adults. Int. J. Mol. Sci. 2023, 24, 16697. https://doi.org/10.3390/ijms242316697
Chardin D, Jing L, Chazal-Ngo-Mai M, Guigonis J-M, Rigau V, Goze C, Duffau H, Virolle T, Pourcher T, Burel-Vandenbos F. Identification of Metabolomic Markers in Frozen or Formalin-Fixed and Paraffin-Embedded Samples of Diffuse Glioma from Adults. International Journal of Molecular Sciences. 2023; 24(23):16697. https://doi.org/10.3390/ijms242316697
Chicago/Turabian StyleChardin, David, Lun Jing, Mélanie Chazal-Ngo-Mai, Jean-Marie Guigonis, Valérie Rigau, Catherine Goze, Hugues Duffau, Thierry Virolle, Thierry Pourcher, and Fanny Burel-Vandenbos. 2023. "Identification of Metabolomic Markers in Frozen or Formalin-Fixed and Paraffin-Embedded Samples of Diffuse Glioma from Adults" International Journal of Molecular Sciences 24, no. 23: 16697. https://doi.org/10.3390/ijms242316697
APA StyleChardin, D., Jing, L., Chazal-Ngo-Mai, M., Guigonis, J.-M., Rigau, V., Goze, C., Duffau, H., Virolle, T., Pourcher, T., & Burel-Vandenbos, F. (2023). Identification of Metabolomic Markers in Frozen or Formalin-Fixed and Paraffin-Embedded Samples of Diffuse Glioma from Adults. International Journal of Molecular Sciences, 24(23), 16697. https://doi.org/10.3390/ijms242316697





