Impact Assessment of Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) and Hemostatic Sponge on Vascular Anastomosis Regeneration in Rats
Abstract
:1. Introduction
2. Results
2.1. General Observations
2.2. Microcirculation
2.3. Hematological and Hemorheological Changes
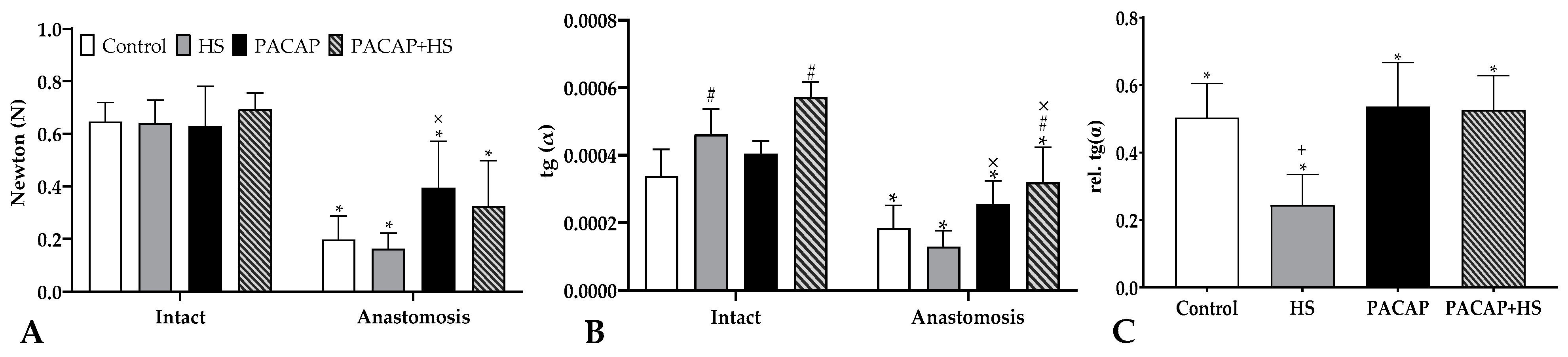
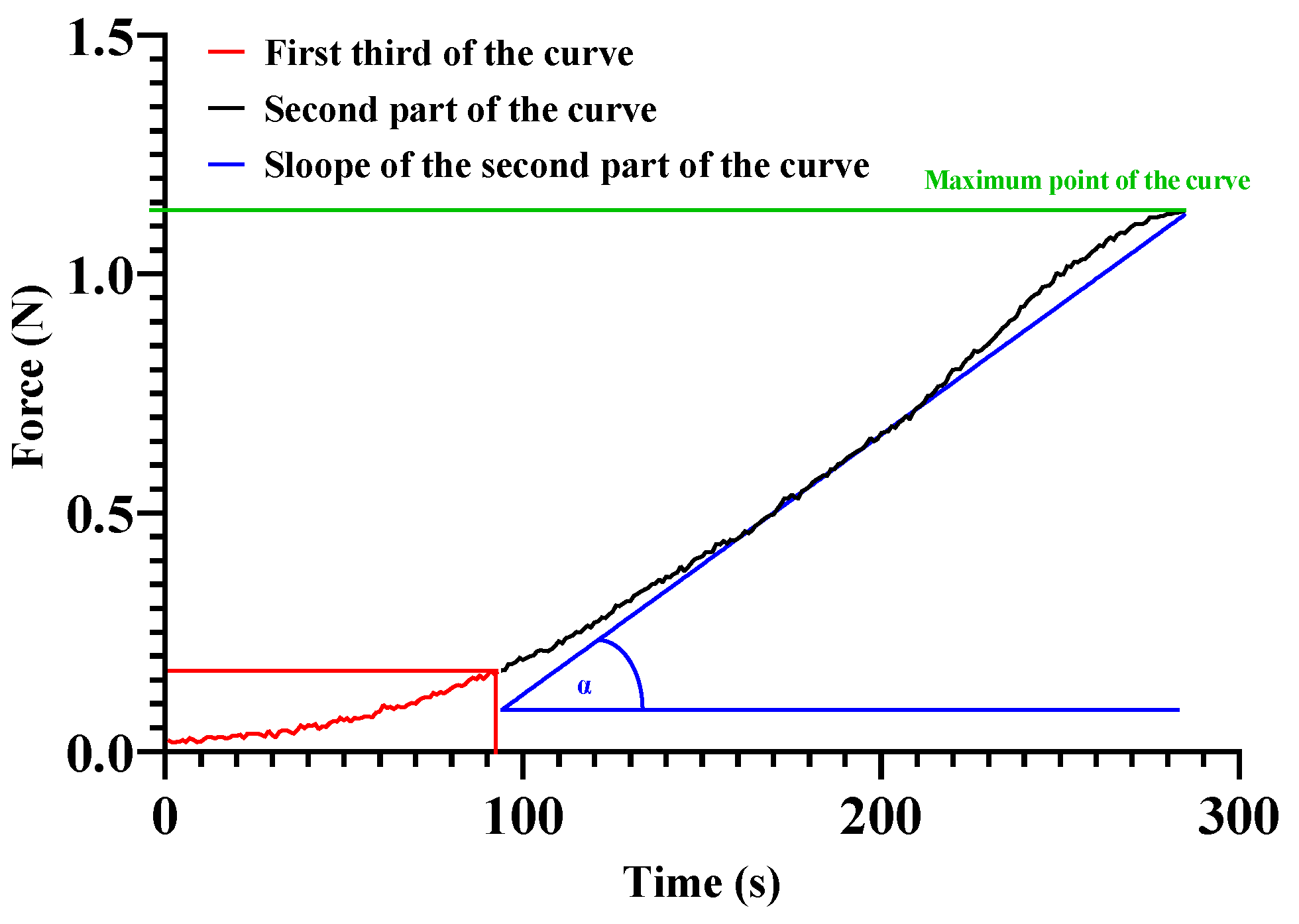
2.4. Tensile Strength
2.5. Histology, Molecular Biology
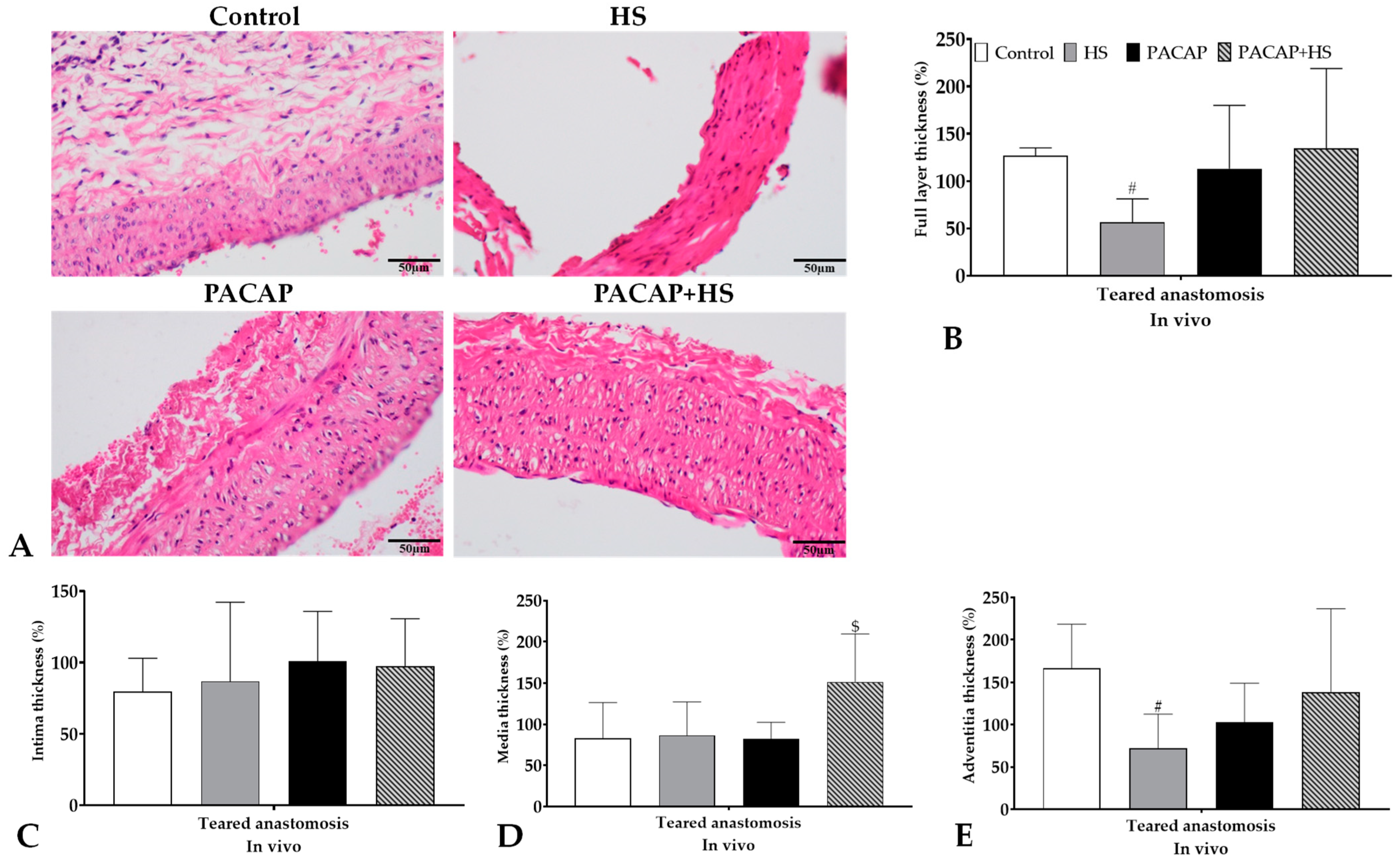
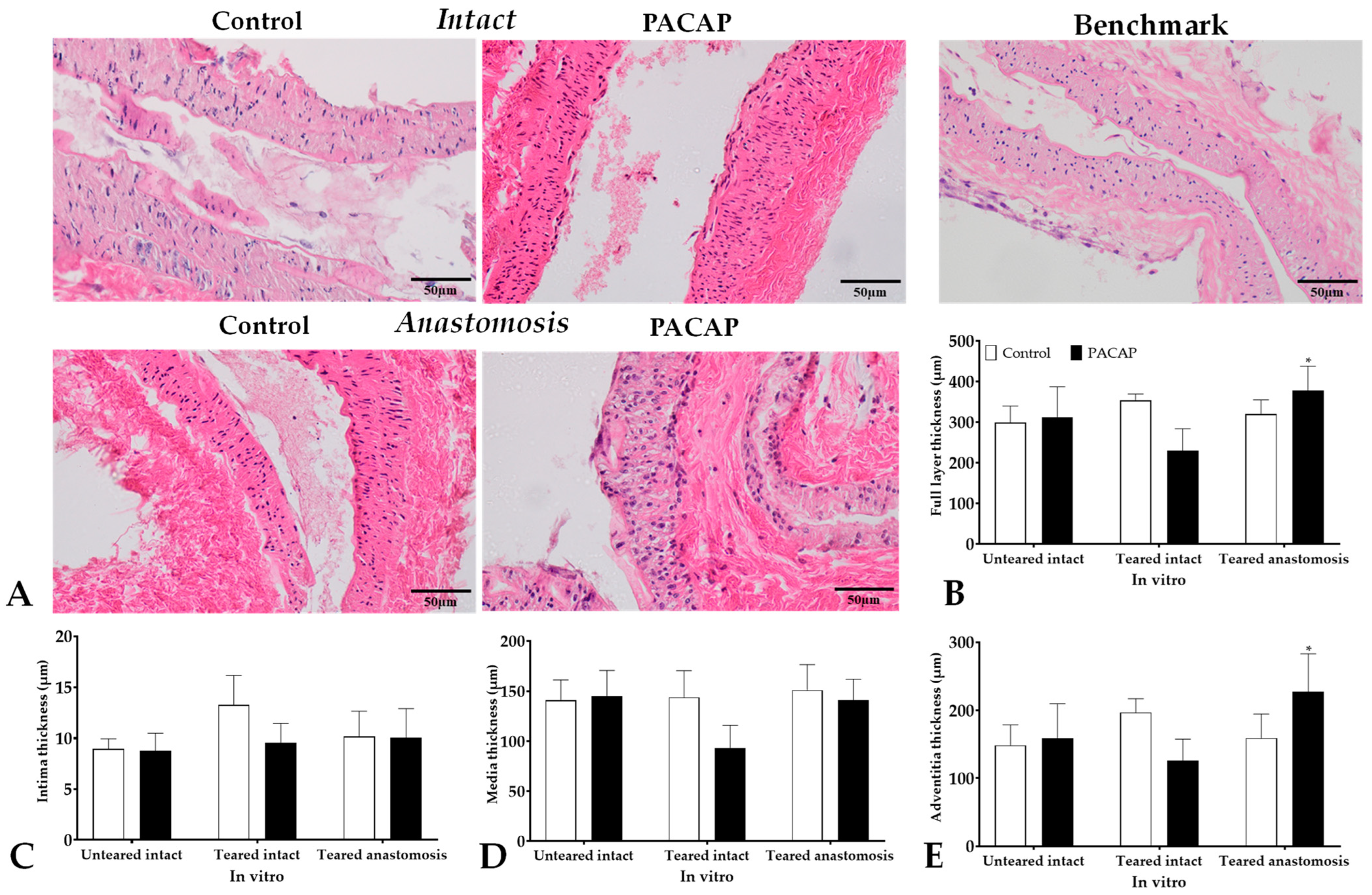
2.5.1. Thickness of Vessel Wall Layer
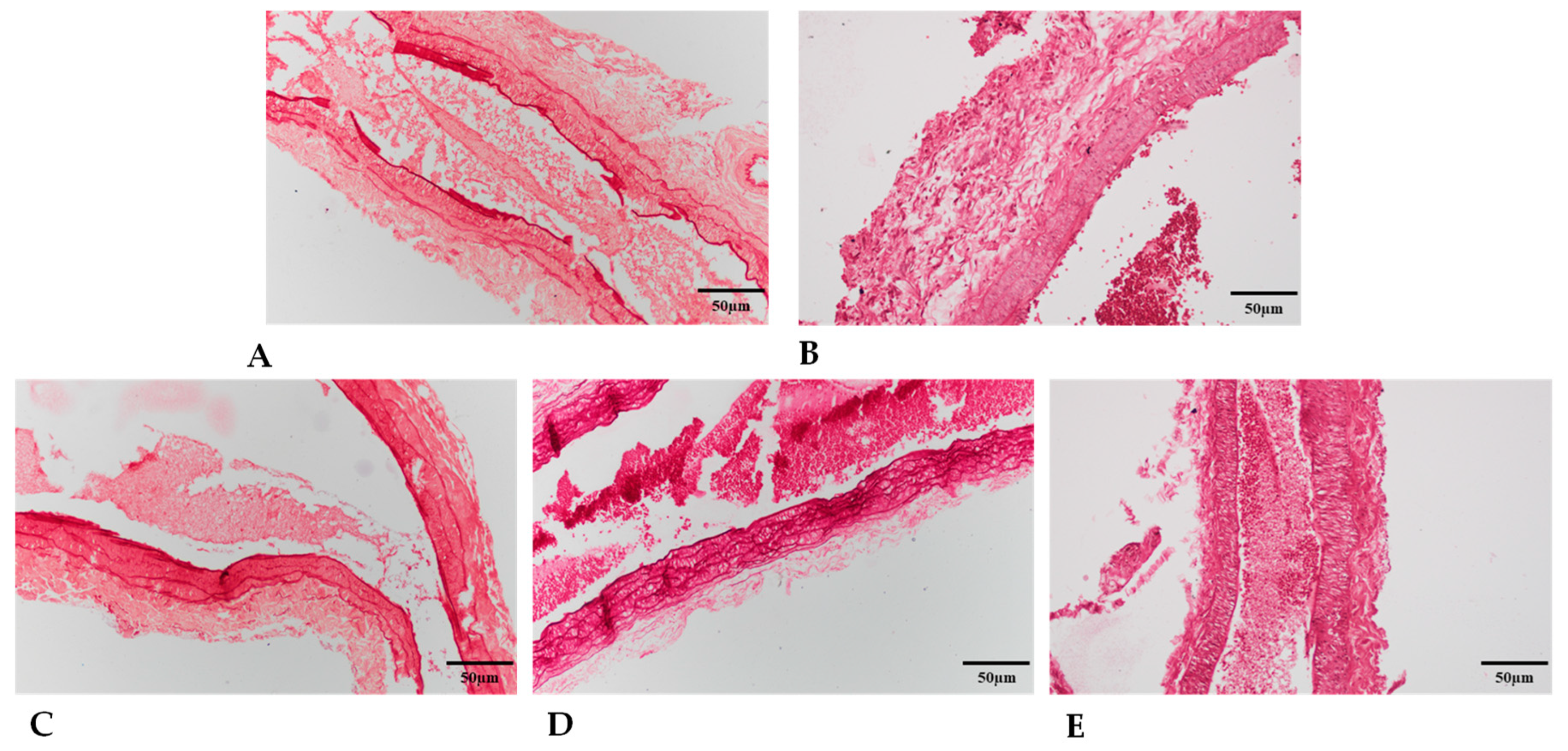
2.5.2. Elastin Expression
2.5.3. Collagen Type I Expression
2.5.4. Granulomatosus Tissue around the Cannula
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Drugs Used
4.2. Operative Techniques, Experimental Groups and Sampling Protocol
4.3. Laboratory Tests
4.4. Microcirculatory Measurements
4.5. Tensile Strength Measurement of the Anastomoses
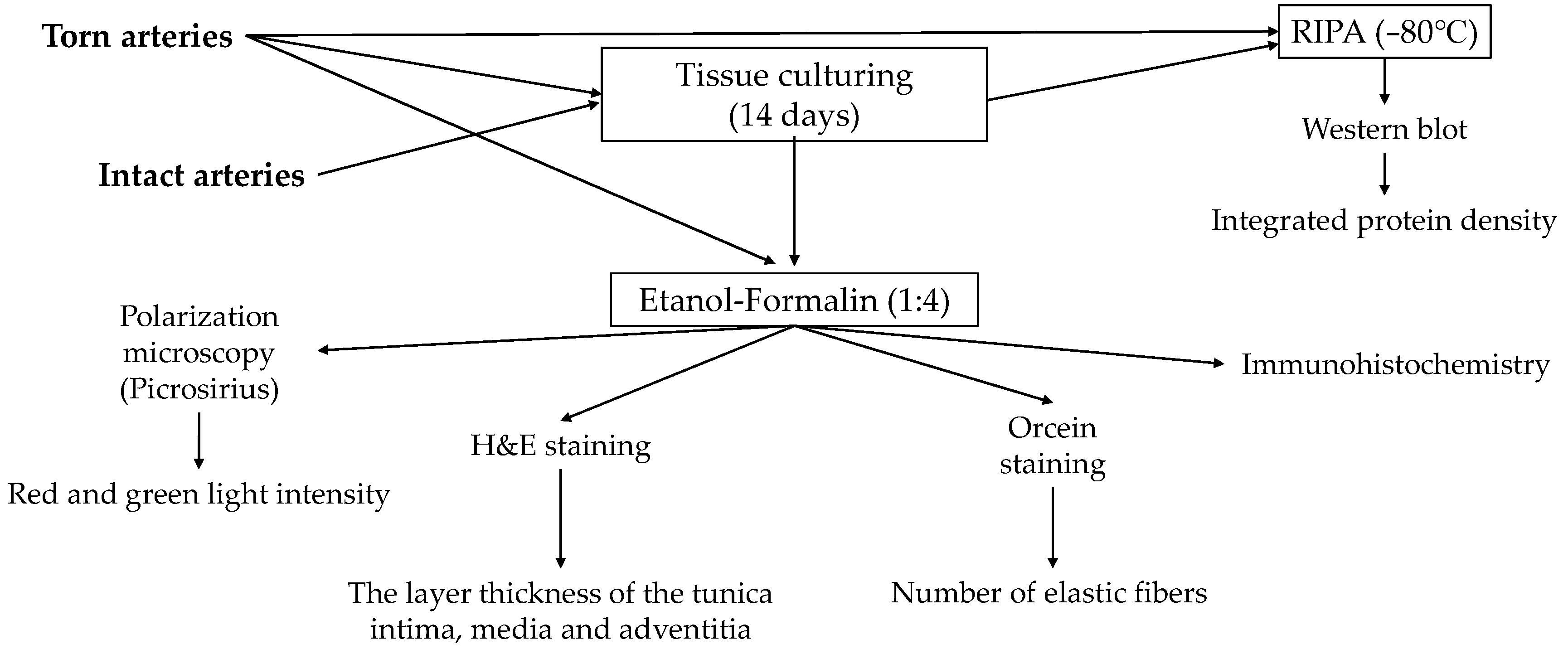
4.6. Tissue Cultures
4.7. Light Microscopical Morphology
4.8. Immunohistochemistry
4.9. Western Blot Analysis
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BFU | blood flux unit |
| CNS | central nervous system |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DPP4 | dipeptidyl peptidase-4 inhibitor |
| EImax | maximal elongation index |
| GHRH | growth hormone-releasing hormone |
| H&E | hematoxilin eosin |
| Hct | hematocrit |
| Hgb | hemoglobin concentration |
| HS | hemostatic sponge |
| M 10s | erythrocyte aggregation index values at the 10th second of the process at 0 s−1 shear rate |
| M 5s | erythrocyte aggregation index values at the 5th second of the process at 0 s−1 shear rate |
| M1 10s | erythrocyte aggregation index values at the 10th second of the process at 3 s−1 shear rate |
| M1 5s | erythrocyte aggregation index values at the 5th second of the process at 3 s−1 shear rate |
| MCV | mean corpuscular volume |
| N | newton |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| Plt | platelet count |
| RBC | red blood cell |
| SD | standard deviation |
| SS1/2 | shear stress at half EImax |
| VIP | vasoactive intestinal polypeptide |
| WBC | white blood cell count |
References
- Lizana, J.; Montemurro, N.; Aliaga, N.; Marani, W.; Tanikawa, R. From textbook to patient: A practical guide to train the end-to-side microvascular anastomosis. Br. J. Neurosurg. 2023, 37, 116–120. [Google Scholar] [CrossRef]
- Burton, H.; Pafitanis, G. The tale of the “unlucky” 13 errors in the “journey” towards an outstanding end-product in microvascular anastomosis training. J. Plast. Reconstr. Aesthet. Surg. 2023, 76, 1–3. [Google Scholar] [CrossRef]
- Moritz, W.R.; Raman, S.; Pessin, S.; Martin, C.; Li, X.; Westman, A.; Sacks, J.M. The history and innovations of blood vessel anastomosis. Bioengineering 2022, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Huber, T.S.; Berceli, S.A.; Scali, S.T.; Neal, D.; Anderson, E.M.; Allon, M.; Cheung, A.K.; Dember, L.M.; Himmelfarb, J.; Roy-Chaudhury, P.; et al. Arteriovenous fistula maturation, functional patency, and intervention rates. JAMA Surg. 2021, 156, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, W.B.; Naresse, L.E.; Angeleli, A.Y.O.; Lastoria, S.; Defaveri, J.; Curi, P.R.; Rodrigues, A. The relationship between suture number and the healing process of end-to-end arterial anastomosis. Acta Cir. Bras. 1997, 12, 89–93. [Google Scholar] [CrossRef]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Miyamoto, S.; Kayano, S.; Fujiki, M.; Chuman, H.; Kawai, A.; Sakuraba, M. Early mobilization after free-flap transfer to the lower extremities. Plast. Reconstr. Surg. Glob. Open 2014, 2, e127. [Google Scholar] [CrossRef]
- Szabo, B.; Fazekas, L.; Ghanem, S.; Godo, Z.A.; Madar, J.; Apro, A.; Nemeth, N. Biomechanical comparison of microvascular anastomoses prepared by various suturing techniques. Injury 2020, 51, 2866–2873. [Google Scholar] [CrossRef]
- Rossi, L.F.; Ramos, R.R.; de Medeiros Kestering, D.; da Silveira Soldi, M.; Ely, J.B.; d’Acampora, A.J. Tensile strength study of the abdominal wall following laparotomy synthesis using three types of surgical wires in Wistar rats. Acta Cir. Bras. 2008, 23, 73–77. [Google Scholar] [CrossRef]
- Duraes, L.C.; Duraes, E.F.; Lobato, L.F.; Oliveira, P.G.; Sousa, J.B. Correlation between bursting pressure and breaking strength in colonic anastomosis. Acta Cir. Bras. 2013, 28, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, C.H.M.; Santos Filho, K.G. dos; Cassino, P.C.; Chiquetti, C.V.; Mello, A.P. de; Dourado, D.M. Differences between polydioxanone and poliglactin in intestinal anastomoses—A comparative study of intestinal anastomoses. J. Coloproctol. 2017, 37, 263–267. [Google Scholar] [CrossRef]
- Jeon, A.; Nagappan, L. Postoperative complications and disposition for vascular surgery. Anaesth. Intensive Care 2023, 51, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Bennett, K.M.; Kent, K.C.; Schumacher, J.; Greenberg, C.C.; Scarborough, J.E. Targeting the most important complications in vascular surgery. J. Vasc. Surg. 2017, 65, 793–803. [Google Scholar] [CrossRef]
- Janvikul, W.; Uppanan, P.; Thavornyutikarn, B.; Krewraing, J.; Prateepasen, R. In vitro comparative hemostatic studies of chitin, chitosan, and their derivatives. J. Appl. Polym. Sci. 2006, 102, 445–451. [Google Scholar] [CrossRef]
- Zhong, Y.; Hu, H.; Min, N.; Wei, Y.; Li, X.; Li, X. Application and outlook of topical hemostatic materials: A narrative review. Ann. Transl. Med. 2021, 9, 577. [Google Scholar] [CrossRef]
- Kang, B.S.; Na, Y.C.; Jin, Y.W. Comparison of the wound healing effect of cellulose and gelatin: An in vivo study. Arch. Plast. Surg. 2012, 39, 317–321. [Google Scholar] [CrossRef]
- Lei, T.; Liu, X.; Cao, J.; Sun, Y.; Li, G.; Huang, H. Intracranial foreign body granuloma caused by gelatin sponge: A case report and literature review. Int. J. Clin. Exp. Med. 2017, 10, 3996–4000. [Google Scholar]
- Wachol-Drewek, Z.; Pfeiffer, M.; Scholl, E. Comparative investigation of drug delivery of collagen implants saturated in antibiotic solutions and a sponge containing gentamicin. Biomaterials 1996, 17, 1733–1738. [Google Scholar] [CrossRef]
- Anisha, B.S.; Biswas, R.; Chennazhi, K.P.; Jayakumar, R. Chitosan–hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef]
- Szabo, B.; Gasz, B.; Fazekas, L.A.; Varga, A.; Kiss-Papai, L.; Matolay, O.; Rezsabek, Z.; Al-Smadi, M.W.; Nemeth, N. Heterogeneous maturation of arterio-venous fistulas and loop-shaped venous interposition grafts: A histological and 3D flow simulation comparison. Biomedicines 2022, 10, 1508. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.P.; Meng, S. Vascular regeneration in peripheral artery disease. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1627–1634. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Nawaz, M.I. Molecular mechanism of VEGF and its role in pathological angiogenesis. J. Cell Biochem. 2022, 123, 1938–1965. [Google Scholar] [CrossRef] [PubMed]
- Dudley, A.C.; Griffioen, A.W. The modes of angiogenesis: An updated perspective. Angiogenesis 2023, 26, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Wautier, J.L.; Wautier, M.P. Vascular permeability in diseases. Int. J. Mol. Sci. 2022, 23, 3645. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Hu, H.; Isaji, T.; Dardik, A. Molecular identity of arteries, veins, and lymphatics. J. Vasc. Surg. 2019, 69, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.C.; Hashimoto, H.; Galas, L.; et al. Pituitary adenylate cyclase-activating polypeptide and its receptors: 20 years after the discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Juhasz, T.; Szentleleky, E.; Somogyi, C.; Takacs, R.; Dobrosi, N.; Engler, M.; Tamas, A.; Reglodi, D.; Zakany, R. Pituitary adenylate cyclase activating polypeptide (PACAP) pathway is induced by mechanical load and reduces the activity of hedgehog signaling in chondrogenic micromass cell cultures. Int. J. Mol. Sci. 2015, 16, 17344–17367. [Google Scholar] [CrossRef]
- Jozsa, G.; Szegeczki, V.; Palfi, A.; Kiss, T.; Helyes, Z.; Fulop, B.; Cserhati, C.; Daroczi, L.; Tamas, A.; Zakany, R.; et al. Signalling alterations in bones of pituitary adenylate cyclase activating polypeptide (PACAP) gene deficient mice. Int. J. Mol. Sci. 2018, 19, 2538. [Google Scholar] [CrossRef]
- Ivic, I.; Balasko, M.; Fulop, B.D.; Hashimoto, H.; Toth, G.; Tamas, A.; Juhasz, T.; Koller, A.; Reglodi, D.; Solymar, M. VPAC1 receptors play a dominant role in PACAP-induced vasorelaxation in female mice. PLoS ONE 2019, 14, e0211433. [Google Scholar] [CrossRef] [PubMed]
- Ivic, I.; Fulop, B.D.; Juhasz, T.; Reglodi, D.; Toth, G.; Hashimoto, H.; Tamas, A.; Koller, A. Backup mechanisms maintain PACAP/VIP-induced arterial relaxations in pituitary adenylate cyclase-activating polypeptide-deficient mice. J. Vasc. Res. 2017, 54, 180–192. [Google Scholar] [CrossRef]
- Juhasz, T.; Matta, C.; Katona, E.; Somogyi, C.; Takacs, R.; Gergely, P.; Csernoch, L.; Panyi, G.; Toth, G.; Reglodi, D.; et al. Pituitary adenylate cyclase activating polypeptide (PACAP) signalling exerts chondrogenesis promoting and protecting effects: Implication of calcineurin as a downstream target. PLoS ONE 2014, 9, e91541. [Google Scholar] [CrossRef] [PubMed]
- Shioda, S.; Nakamachi, T. PACAP as a neuroprotective factor in ischemic neuronal injuries. Peptides 2015, 72, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Dejda, A.; Jolivel, V.; Bourgault, S.; Seaborn, T.; Fournier, A.; Vaudry, H.; Vaudry, D. Inhibitory effect of PACAP on caspase activity in neuronal apoptosis: A better understanding towards therapeutic applications in neurodegenerative diseases. J. Mol. Neurosci. 2008, 36, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Roth, E.; Weber, G.; Kiss, P.; Horvath, G.; Toth, G.; Gasz, B.; Ferencz, A.; Gallyas, F.; Reglodi, D.; Racz, B. Effects of PACAP and preconditioning against ischemia/reperfusion-induced cardiomyocyte apoptosis in vitro. Ann. N. Y. Acad. Sci. 2009, 1163, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Seaborn, T.; Masmoudi-Kouli, O.; Fournier, A.; Vaudry, H.; Vaudry, D. Protective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) against apoptosis. Curr. Pharm. Des. 2011, 17, 204–214. [Google Scholar] [CrossRef]
- Ye, D.; Shi, Y.; Xu, Y.; Huang, J. PACAP attenuates optic nerve crush-induced retinal ganglion cell apoptosis via activation of the CREB-Bcl-2 pathway. J. Mol. Neurosci. 2019, 68, 475–484. [Google Scholar] [CrossRef]
- Tamas, A.; Reglodi, D.; Farkas, O.; Kovesdi, E.; Pal, J.; Povlishock, J.T.; Schwarcz, A.; Czeiter, E.; Szanto, Z.; Doczi, T.; et al. Effect of PACAP in central and peripheral nerve injuries. Int. J. Mol. Sci. 2012, 13, 8430–8448. [Google Scholar] [CrossRef]
- Lerner, U.H.; Lundberg, P.; Persson, P.; Ransjö, M.; Håkanson, R. Helodermin, helospectin, and PACAP stimulate cyclic AMP formation in intact bone, isolated osteoblasts, and osteoblastic cell lines. Calcif Tissue Int. 1994, 54, 284–289. [Google Scholar] [CrossRef]
- Szentleleky, E.; Szegeczki, V.; Karanyicz, E.; Hajdu, T.; Tamas, A.; Toth, G.; Zakany, R.; Reglodi, D.; Juhasz, T. Pituitary adenylate cyclase activating polypeptide (PACAP) reduces oxidative and mechanical stress-evoked matrix degradation in chondrifying cell cultures. Int. J. Mol. Sci. 2019, 20, 168. [Google Scholar] [CrossRef] [PubMed]
- Sharifpour, M.; Bittner, E.A. Critical care of the vascular surgery patient. Anesthesiol. Clin. 2022, 40, 775–790. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, M.D.; Markovic, D.Z.; Vukovic, A.Z.; Dinic, V.D.; Nikolic, A.N.; Maricic, T.G.; Jankovic, R.J. Enhanced recovery after vascular surgery. Front. Med. 2018, 5, 2. [Google Scholar] [CrossRef] [PubMed]
- Khanh, L.N.; Helenowski, I.B.; Hoel, A.W.; Ho, K.J. The comorbidity-polypharmacy score is an objective and practical predictor of outcomes and mortality after vascular surgery. Ann. Vasc. Surg. 2020, 69, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R.P.; Marcovici, A.; Suarez, M.; Goodrich, J.T. Foreign body granuloma mimicking intracranial meningioma: Case report and review of the literature. Neurosurgery 1999, 44, 855–858. [Google Scholar] [CrossRef] [PubMed]
- Tanghe, K.K.; Chalmers, B.P.; Blevins, J.L.; Figgie, M.P.; Carli, A.V.; Agrusa, C.J.; Sculco, P.K.; Gausden, E.B. Hemostatic agents in orthopedic surgery. HSS J. 2023, 19, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Charlesworth, T.M.; Agthe, P.; Moores, A.; Anderson, D.M. The use of haemostatic gelatin sponges in veterinary surgery. J. Small Anim. Pract. 2012, 53, 51–56. [Google Scholar] [CrossRef]
- Raunio, H.; Taavitsainen, P.; Honkakoski, P.; Juvonen, R.; Pelkonen, O. In vitro methods in the prediction of kinetics of drugs: Focus on drug metabolism. Altern. Lab. Anim. 2004, 32, 425–430. [Google Scholar] [CrossRef]
- Martinez, C.; Delgado, M.; Pozo, D.; Leceta, J.; Calvo, J.R.; Ganea, D.; Gomariz, R.P. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide modulate endotoxin-induced IL-6 production by murine peritoneal macrophages. J. Leukoc. Biol. 1998, 63, 591–601. [Google Scholar] [CrossRef]
- Delgado, M.; Ganea, D. Inhibition of endotoxin-induced macrophage chemokine production by vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide in vitro and in vivo. J. Immunol. 2001, 167, 966–975. [Google Scholar] [CrossRef]
- Lamine, A.; Letourneau, M.; Doan, N.D.; Maucotel, J.; Couvineau, A.; Vaudry, H.; Chatenet, D.; Vaudry, D.; Fournier, A. Characterizations of a synthetic pituitary adenylate cyclase-activating polypeptide analog displaying potent neuroprotective activity and reduced in vivo cardiovascular side effects in a Parkinson’s disease model. Neuropharmacology 2016, 108, 440–450. [Google Scholar] [CrossRef]
- Reglodi, D.; Atlasz, T.; Jungling, A.; Szabo, E.; Kovari, P.; Manavalan, S.; Tamas, A. Alternative routes of administration of the neuroprotective pituitary adenylate cyclase activating polypeptide. Curr. Pharm. Des. 2018, 24, 3892–3904. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Tamvakopoulos, C.; Xie, D.; Dragovic, J.; Shen, X.; Fenyk-Melody, J.E.; Schmidt, K.; Bagchi, A.; Griffin, P.R.; Thornberry, N.A.; et al. The role of dipeptidyl peptidase IV in the cleavage of glucagon family peptides: In vivo metabolism of pituitary adenylate cyclase activating polypeptide-(1-38). J. Biol. Chem. 2003, 278, 22418–22423. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.J.; Christensen, A.V. Subarachnoidal administration of the 5-HT uptake inhibitor citalopram points to the spinal role of 5-HT in morphine antinociception. Pain 1982, 14, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, T.; Shibato, J.; Kimura, A.; Yamashita, M.; Takenoya, F.; Shioda, S. Potential therapeutic role of pituitary adenylate cyclase-activating polypeptide for dry eye disease. Int. J. Mol. Sci. 2022, 23, 664. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Ren, R.; Shi, H.; Huang, L.; Lenahan, C.; Lu, Q.; Tang, L.; Huang, Y.; Tang, J.; Zhang, J.; et al. Pituitary adenylate cyclase-activating polypeptide: A promising neuroprotective peptide in stroke. Aging Dis. 2020, 11, 1496–1512. [Google Scholar] [CrossRef]
- Horvath, G.; Reglodi, D.; Fabian, E.; Opper, B. Effects of pituitary adenylate cyclase activating polypeptide on cell death. Int. J. Mol. Sci. 2022, 23, 4953. [Google Scholar] [CrossRef]
- Baskurt, O.K. Mechanisms of blood rheology alterations. In Handbook of Hemorheology and Hemodynamics; Baskurt, O.K., Hardeman, M.R., Rampling, M.W., Meiselman, H.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2007; pp. 170–190. [Google Scholar]
- Nemeth, N.; Deak, A.; Szentkereszty, Z.; Peto, K. Effects and influencing factors on hemorheological variables taken into consideration in surgical pathophysiology research. Clin. Hemorheol. Microcirc. 2018, 69, 133–140. [Google Scholar] [CrossRef]
- Nemeth, N.; Peto, K.; Magyar, Z.; Klarik, Z.; Varga, G.; Oltean, M.; Mantas, A.; Czigany, Z.; Tolba, R.H. Hemorheological and microcirculatory factors in liver ischemia-reperfusion injury—An update on pathophysiology, molecular mechanisms and protective strategies. Int. J. Mol. Sci. 2021, 22, 1864. [Google Scholar] [CrossRef]
- Abellan, D.; Nart, J.; Pascual, A.; Cohen, R.E.; Sanz-Moliner, J.D. Physical and mechanical evaluation of five suture materials on three knot configurations: An in vitro study. Polymers 2016, 8, 147. [Google Scholar] [CrossRef]
- Anushya, P.; Ganesh, S.B.; Jayalakshmi, S. Evaluation of tensile strength of surgical absorbable and nonabsorbable suture materials after immersion in different fruit juices: An in vitro study. J. Adv. Pharm. Technol. Res. 2022, 13 (Suppl. 1), S108–S111. [Google Scholar] [CrossRef] [PubMed]
- Yun, X.; Gardner, L. Stress-strain curves for hot-rolled steels. J. Construct. Steel Res. 2017, 133, 36–46. [Google Scholar] [CrossRef]
- Ebrahimi, A.P. Mechanical properties of normal and diseased cerebrovascular system. J. Vasc. Interv. Neurol. 2009, 2, 155–162. [Google Scholar] [PubMed]
- Millereau, P.; Ducrot, E.; Clough, J.M.; Wiseman, M.E.; Brown, H.R.; Sijbesma, R.P.; Creton, C. Mechanics of elastomeric molecular composites. Proc. Natl. Acad. Sci. USA. 2018, 115, 9110–9115. [Google Scholar] [CrossRef] [PubMed]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Li, W.J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef]
- Svensson, R.B.; Smith, S.T.; Moyer, P.J.; Magnusson, S.P. Effects of maturation and advanced glycation on tensile mechanics of collagen fibrils from rat tail and Achilles tendons. Acta Biomater. 2018, 70, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Vidal, L.; Murdica, V.; Venegoni, C.; Pederzoli, F.; Bandini, M.; Necchi, A.; Salonia, A.; Alfano, M. Causal contributors to tissue stiffness and clinical relevance in urology. Commun. Biol. 2021, 4, 1011. [Google Scholar] [CrossRef]
- Park, J.H.; Jo, S.B.; Lee, J.H.; Lee, H.H.; Knowles, J.C.; Kim, H.W. Materials and extracellular matrix rigidity highlighted in tissue damages and diseases: Implication for biomaterials design and therapeutic targets. Bioact. Mater. 2022, 20, 381–403. [Google Scholar] [CrossRef]
- Gauthier, V.; Kyriazi, M.; Nefla, M.; Pucino, V.; Raza, K.; Buckley, C.D.; Alsaleh, G. Fibroblast heterogeneity: Keystone of tissue homeostasis and pathology in inflammation and ageing. Front. Immunol. 2023, 14, 1137659. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Amico, A.G.; Saccone, S.; Federico, C.; Rasa, D.M.; Caltabiano, R.; Broggi, G.; Giunta, S.; Musumeci, G.; D’Agata, V. Effect of PACAP on hypoxia-induced angiogenesis and epithelial-mesenchymal transition in glioblastoma. Biomedicines 2021, 9, 965. [Google Scholar] [CrossRef]
- Lauretta, G.; Ravalli, S.; Szychlinska, M.A.; Castorina, A.; Maugeri, G.; D’Amico, A.G.; D’Agata, V.; Musumeci, G. Current knowledge of pituitary adenylate cyclase activating polypeptide (PACAP) in articular cartilage. Histol Histopathol. 2020, 35, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Balamuthusamy, S.; Khan, A.M.; Maderdrut, J.L.; Simon, E.E.; Batuman, V. Pituitary adenylate cyclase-activating polypeptide prevents cisplatin-induced renal failure. J. Mol. Neurosci. 2011, 43, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Nakamachi, T.; Ohtaki, H.; Yofu, S.; Sato, A.; Endo, K.; Iso, Y.; Suzuki, H.; Takeyama, Y.; Shintani, N.; et al. Cardioprotective effect of endogenous pituitary adenylate cyclase-activating polypeptide on Doxorubicin-induced cardiomyopathy in mice. Circ. J. 2010, 74, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, L.; Yi, P.Q.; Xu, C.; Su, J.; Chen, P.Z.; Li, M.; Chen, J.Y. Pituitary adenylate cyclase-activating polypeptide ameliorates radiation-induced cardiac injury. Am. J. Transl. Res. 2019, 11, 6585–6599. [Google Scholar] [CrossRef] [PubMed]
- Moody, T.W.; Nuche-Berenguer, B.; Jensen, R.T. Vasoactive intestinal peptide/pituitary adenylate cyclase activating polypeptide, and their receptors and cancer. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 38–47. [Google Scholar] [CrossRef]
- Green, C.J.; Knight, J.; Precious, S.; Simpkin, S. Ketamine alone and combined with diazepam or xylazine in laboratory animals: A 10-year experience. Lab. Anim. 1981, 15, 163–170. [Google Scholar] [CrossRef]
- Cannon, C.Z.; Kissling, G.E.; Hoenerhoff, M.J.; King-Herbert, A.P.; Blankenship-Paris, T. Evaluation of dosages and routes of administration of tramadol analgesia in rats using hot-plate and tail-flick tests. Lab. Anim. 2010, 39, 342–351. [Google Scholar] [CrossRef]
- Pandya, A.N.; Vaingankar, N.; Grant, I.; James, N.K. End-to-side venous anastomoses.... a patency test. Br. J. Plast. Surg. 2003, 56, 810–811. [Google Scholar] [CrossRef]
- Hardeman, M.; Goedhart, P.; Shin, S. Methods in hemorheology. In Handbook of Hemorheology and Hemodynamics; Baskurt, O.K., Hardeman, M.R., Rampling, M.W., Meiselman, H.J., Eds.; IOS Press: Amsterdam, The Netherlands, 2007; pp. 242–266. [Google Scholar]
- Leahy, M.J. Microcirculation Imaging; John Wiley & Sons: Hoboken, NJ, USA, 2012; ISBN 978-3-527-32894-9. [Google Scholar]
- Baskurt, O.K.; Boynard, M.; Cokelet, G.C.; Connes, P.; Cooke, B.M.; Forconi, S.; Liao, F.; Hardeman, M.R.; Jung, F.; Meiselman, H.J.; et al. New guidelines for hemorheological laboratory techniques. Clin. Hemorheol. Microcirc. 2009, 42, 75–97. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Hardeman, M.R.; Uyuklu, M.; Ulker, P.; Cengiz, M.; Nemeth, N.; Shin, S.; Alexy, T.; Meiselman, H.J. Parameterization of red blood cell elongation index–shear stress curves obtained by ektacytometry. Scand. J. Clin. Lab. Investig. 2009, 69, 777–788. [Google Scholar] [CrossRef]
- Godo, Z.A.; Apro, A.; Madar, J.; Szabo, B.; Nemeth, N. Microcontroller-based vein testing system. In Proceedings of the 2017 9th International Conference on Electronics, Computers and Artificial Intelligence (ECAI), Targoviste, Romania, 9 June–1 July 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–6. [Google Scholar] [CrossRef]
- Mead, R. The Design of Experiments: Statistical Principles for Practical Applications; Cambridge University Press: New York, NY, USA, 1988. [Google Scholar]
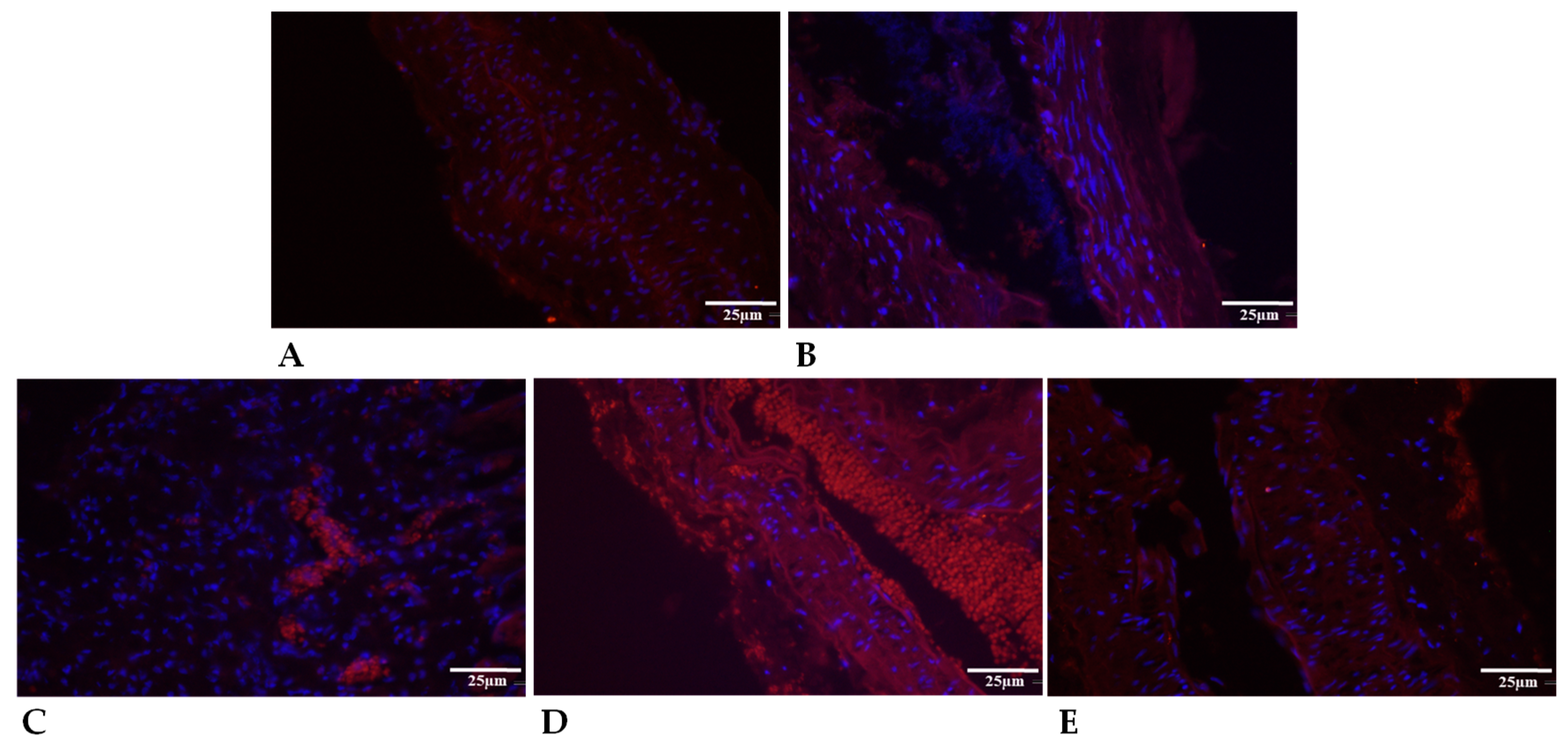
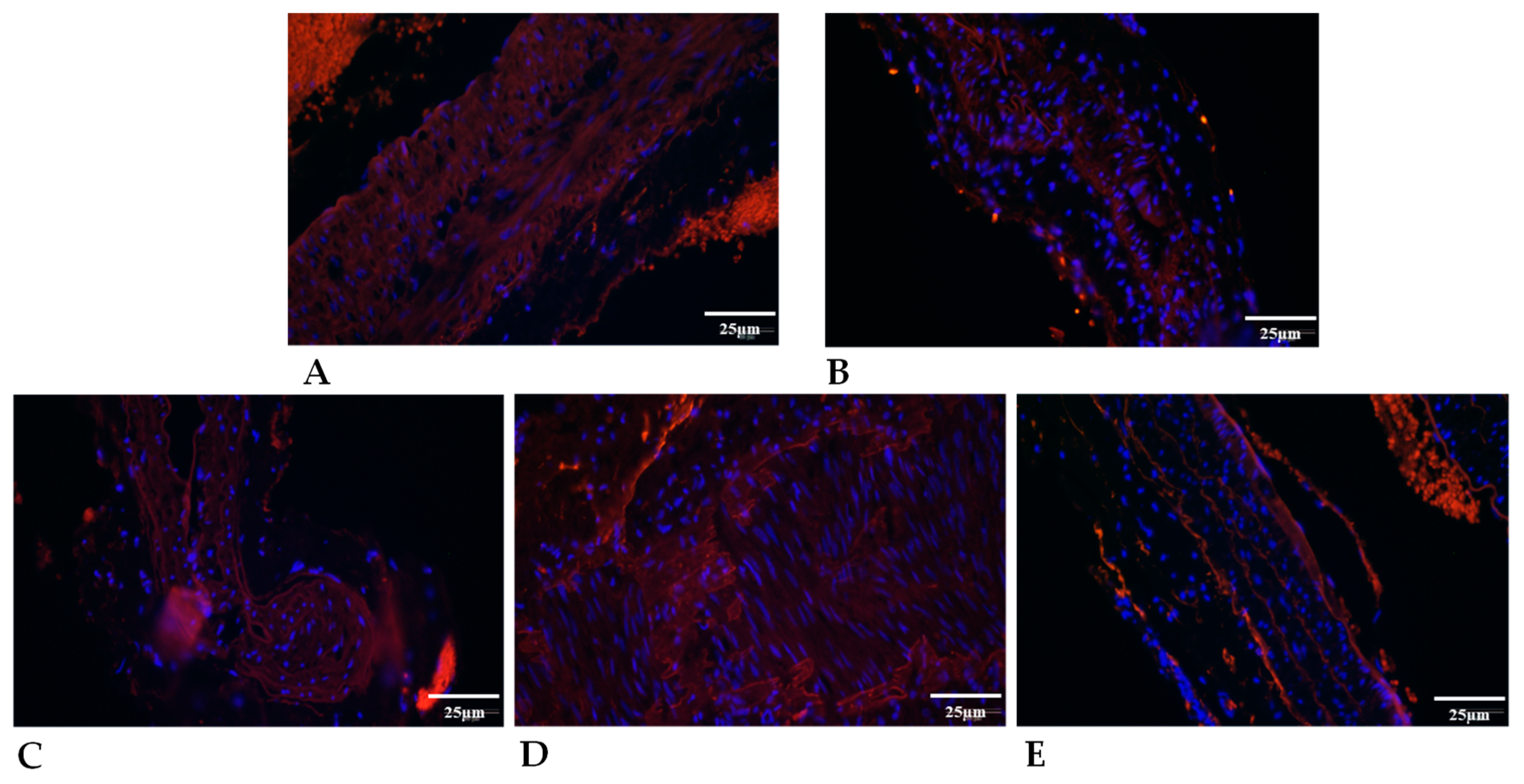

| Variable | Group | Base | 7th p.o. Day | 14th p.o. Day | 21st P.O. Day |
|---|---|---|---|---|---|
| WBC [109/L] | Control | 11.95 ± 3.63 | 18.92 ± 7.94 * | 18.89 ± 3.18 * | 13.82 ± 4.84 |
| HS | 10.88 ± 40 | 17.47 ± 6.37 * | 17.79 ± 3.15 * | 15.88 ± 5.07 | |
| PACAP | 11.11 ± 4.65 | 16.31 ± 2.43 * | 21.95 ± 6.78 * | 18.81 ± 5.67 * | |
| PACAP + HS | 10.04 ± 3.58 | 15.29 ± 4.04 * | 16.13 ± 4.12 *& | 13.38 ± 2.72 *& | |
| RBC [1012/L] | Control | 7.59 ± 0.48 | 6.84 ± 0.81 | 7.02 ± 0.34 * | 7.44 ± 0.37 |
| HS | 7.57 ± 0.44 | 7.51 ± 0.34 # | 7.35 ± 0.29 # | 7.22 ± 0.61 * | |
| PACAP | 7.81 ± 0.34 | 7.63 ± 0.33 # | 7.29 ± 0.49 * | 7.63 ± 0.21 | |
| PACAP + HS | 8.01 ± 0.34 + | 7.33 ± 0.55 * | 7.40 ± 0.23 *# | 7.50 ± 0.46 * | |
| Hct [%] | Control | 44.66 ± 2.79 | 39.23 ± 3.05 * | 39.75 ± 1.92 * | 41.76 ± 2.26 * |
| HS | 44.38 ± 1.8 | 42.34 ± 2.40 *# | 41.06 ± 2.35 * | 40.06 ± 3.50 * | |
| PACAP | 45.60 ± 1.85 | 43.16 ± 2.52 *# | 40.54 ± 2.26 * | 42.19 ± 2.28 * | |
| PACAP + HS | 46.15 ± 1.87 | 40.75 ± 2.96 * | 41.04 ± 1.58 * | 41.54 ± 2.20 * | |
| Hgb [g/L] | Control | 14.9 ± 1.01 | 13.35 ± 1.43 * | 13.14 ± 0.75 * | 13.61 ± 0.81 * |
| HS | 15.09 ± 0.69 | 14.34 ± 0.88 * | 13.51 ± 0.76 * | 12.96 ± 1.07 * | |
| PACAP | 15.40 ± 0.61 | 14.56 ± 0.92 * | 13.38 ± 0.84 * | 13.70 ± 0.87 * | |
| PACAP + HS | 15.53 ± 0.45 | 13.71 ± 0.98 * | 13.43 ± 0.41 * | 13.36 ± 0.81 * | |
| MCV [fL] | Control | 58.90 ± 2.13 | 57.76 ± 4.59 | 56.69 ± 2.77 * | 56.19 ± 1.78 * |
| HS | 58.73 ± 1.69 | 56.33 ± 2.04 * | 55.82 ± 2.09 * | 55.56 ± 1.89 * | |
| PACAP | 58.41 ± 1.44 | 56.56 ± 1.73 * | 55.70 ± 1.76 * | 55.24 ± 2.07 * | |
| PACAP + HS | 57.51 ± 10 | 55.59 ± 1.08 * | 55.48 ± 0.98 * | 55.43 ± 1.11 * | |
| Plt [109/L] | Control | 718.43 ± 236.20 | 900 ± 140.97 | 990.93 ± 181.6 * | 835.86 ± 129.69 |
| HS | 647.14 ± 209.38 | 877.93 ± 232.37 | 719.79 ± 375.57 | 598.93 ± 216.68 # | |
| PACAP | 736.50 ± 115.93 | 899.63 ± 254.41 | 819.81 ± 266.16 | 751.06 ± 196.66 | |
| PACAP + HS | 682.19 ± 220.62 | 1025.63 ± 173.21 * | 1031.31 ± 236.01 * | 917.31 ± 135.92 *+& |
| Variable | Group | Base | 7th p.o. Day | 14th p.o. Day | 21st p.o. Day |
|---|---|---|---|---|---|
| EImax | Control | 0.57 ± 0.01 | 0.55 ± 0.03 | 0.57 ± 0.01 | 0.58 ± 0.02 |
| HS | 0.58 ± 0.02 | 0.57 ± 0.01 | 0.58 ± 0.01 | 0.58 ± 0.02 | |
| PACAP | 0.58 ± 0.02 | 0.57 ± 0.02 * | 0.57 ± 0.02 | 0.58 ± 0.01 | |
| PACAP + HS | 0.59 ± 0.01 # | 0.56 ± 0.01 * | 0.57 ± 0.01 * | 0.59 ± 0.01 +& | |
| SS1/2 [Pa] | Control | 1.51 ± 0.20 | 1.50 ± 0.12 | 1.58 ± 0.18 | 1.54 ± 0.22 |
| HS | 1.47 ± 0.20 | 1.56 ± 0.14 | 1.67 ± 0.21 | 1.54 ± 0.15 | |
| PACAP | 1.47 ± 0.19 | 1.45 ± 0.13 | 1.63 ± 0.32 | 1.48 ± 0.23 | |
| PACAP + HS | 1.61 ± 0.13 | 1.61 ± 0.18 & | 1.46 ± 0.17 *+ | 1.59 ± 0.16 | |
| M 5s | Control | 2.64 ± 1.04 | 3.87 ± 1.46 * | 3.14 ± 0.82 | 2.78 ± 1.01 |
| HS | 3 ± 1.35 | 4.44 ± 1.21 * | 3.90 ± 1.55 | 3.88 ± 2.16 | |
| PACAP | 3.62 ± 1.34 # | 4.97 ± 1.83 * | 4.51 ± 1.37 *# | 5.15 ± 1.67 *# | |
| PACAP + HS | 3.09 ± 1.05 | 4.56 ± 1.63 * | 4.67 ± 1.31 *# | 3.68 ± 0.98 #& | |
| M 10s | Control | 6.39 ± 3.81 | 8.31 ± 2.42 | 7.84 ± 2.04 | 6.87 ± 1.55 |
| HS | 7.45 ± 2.70 | 10.38 ± 3.81 *# | 9.17 ± 4.12 | 9.75 ± 2.41 *# | |
| PACAP | 8.89 ± 2.59 # | 11.24 ± 3.58 *# | 9.10 ± 2.60 | 9.22 ± 2.53 # | |
| PACAP + HS | 9.32 ± 2.41 # | 9.53 ± 3.02 | 9.16 ± 2.04 # | 8.60 ± 1.47 # | |
| M1 5s | Control | 2.41 ± 1.41 | 3.01 ± 1.51 | 2.93 ± 0.95 | 2.14 ± 1.09 |
| HS | 2.48 ± 1.02 | 4.08 ± 1.56 * | 2.90 ± 0.95 | 3.03 ± 1.63 | |
| PACAP | 3.43 ± 1.30 #+ | 4.08 ± 1.37 *# | 3.32 ± 1.29 | 4.05 ± 1.16 *#+ | |
| PACAP + HS | 3.20 ± 1.08 + | 3.78 ± 1.53 | 3.69 ± 1.18 #+ | 3.29 ± 0.98 #& | |
| M1 10s | Control | 6.06 ± 3.62 | 7.83 ± 3.64 | 7.22 ± 2.21 | 5.89 ± 1.37 |
| HS | 6.90 ± 3.11 | 9.82 ± 4.43 * | 8.92 ± 4.13 | 8.23 ± 2.82 # | |
| PACAP | 9.30 ± 2.66 #+ | 10.82 ± 3.95 # | 8.47 ± 2.86 | 9.67 ± 2.61 # | |
| PACAP + HS | 9.02 ± 20 #+ | 9.15 ± 3.36 | 9.77 ± 2.89 # | 8.39 ± 2.81 # |
| Variable | Group | Control Side | Anastomosis |
|---|---|---|---|
| Freshly excised arteries | |||
| Integrated green light density [number of pixels] median (Q1–Q3) | Control | 13,501.88 (13,205.36–197,852.10) | 14,731.83 (13,597.90–224,739.80) |
| HS | 81,904.64 (13,576.89–168,056.55) | 1587.68 (1117.11–14,289.80) | |
| PACAP | 15,613.63 (14,051.40–149,313.40) | 13,520.22 (13,218.87–14,900.47) | |
| PACAP + HS | 13,912.72 (13,437.50–106,210.71) | 13,516.40 (13,218.87–14,149.79) | |
| Number of elastic membranes [number/field] means ± S.D. | Control | 3.50 ± 0.58 | 4 ± 1.41 |
| HS | 4.20 ± 0.44 | 3 ± 1 | |
| PACAP | 4.86 ± 1.21 | 5.40 ± 1.34 | |
| PACAP + HS | 5.00 ± 1.58 | 3.33 ± 1.15 | |
| Western blot integrated pixel density [number of pixels] means ± S.D. | Control | 1 ± 0 | 0.6 ± 0.0 |
| HS | 1 ± 0 | 1.05 ± 0.07 | |
| PACAP | 1 ± 0 | 1.6 ± 0.0 | |
| PACAP + HS | 1 ± 0 | 2.6 ± 0.0 | |
| Tissue cultured arteries | |||
| Integrated green light density [number of pixels] median (Q1–Q3) | Control | 13,850.36 (13,566.50–48,305.46) | 13,871.32 (13,486.03–166,405.70) |
| PACAP | 13,912.72 (13,396.37–14,600.46) | 14,511.32 (14,076.57–185,590.30) | |
| Number of elastic membranes [number/field] means ± S.D. | Control | 3.50 ± 0.55 | 3.75 ± 0.96 |
| PACAP | 4.80 ± 1.30 | 4.67 ± 0.58 | |
| Variable | Group | Control Side | Anastomosis |
|---|---|---|---|
| Freshly excised arteries | |||
| Red light intensity [number of pixels] median (Q1–Q3) | Control | 13,825.44 (9343.24–20,063.81) | 9291.28 (7364.89–14,288.00) |
| HS | 9322.30 (7509.55–22,460.15) | 7120.21 (5930.57–7931.24) | |
| PACAP | 12,484.11 (8666.51–13,373.92) | 10,621.18 (8903.27–11,105.26) | |
| PACAP + HS | 13,198.04 (11,975.60–13,240.30) | 9597.01 (7794.21–10,650.40) | |
| Green light intensity [number of pixels] median (Q1–Q3) | Control | 1473.62 (1270.92–8565.78) | 3799.73 (1564.56–6101.55) |
| HS | 4389.07 (4042.19–9744.79) | 3677.36 (2871.04–4479.74) | |
| PACAP | 2787.34 (2182.58–4455.78) | 3223.48 (2049.68–3928.61) | |
| PACAP + HS | 4632.54 (1583.69–4829.60) | 1979.14 (1846.70–2104.08) | |
| Western blot integrated pixel density [number/field] means ± S.D. | Control | 1 ± 0 | 0.4 ± 0 |
| HS | 1 ± 0 | 1 ± 0 | |
| PACAP | 1 ± 0 | 0.2 ± 0 | |
| PACAP + HS | 1 ± 0 | 0.5 ± 0 | |
| Tissue cultured arteries | |||
| Red light intensity [number of pixels] median (Q1–Q3) | Control | 41,106.71 (22,098.34–43,538.714) | 21,036.31 (8323.01–21,060.99) |
| PACAP | 65,156.94 (6218.90–77,448.39) | 12,360.17 (12,281.60–12,473.94) | |
| Green light intensity [number of pixels] median (Q1-Q3) | Control | 4767.25 (4491.69–5700.38) | 5973.45 (2862.03–6410.84) |
| PACAP | 3199.41 (1657.89–8563.06) | 2092.74 (1686.29–5685.69) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazekas, L.A.; Szabo, B.; Szegeczki, V.; Filler, C.; Varga, A.; Godo, Z.A.; Toth, G.; Reglodi, D.; Juhasz, T.; Nemeth, N. Impact Assessment of Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) and Hemostatic Sponge on Vascular Anastomosis Regeneration in Rats. Int. J. Mol. Sci. 2023, 24, 16695. https://doi.org/10.3390/ijms242316695
Fazekas LA, Szabo B, Szegeczki V, Filler C, Varga A, Godo ZA, Toth G, Reglodi D, Juhasz T, Nemeth N. Impact Assessment of Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) and Hemostatic Sponge on Vascular Anastomosis Regeneration in Rats. International Journal of Molecular Sciences. 2023; 24(23):16695. https://doi.org/10.3390/ijms242316695
Chicago/Turabian StyleFazekas, Laszlo Adam, Balazs Szabo, Vince Szegeczki, Csaba Filler, Adam Varga, Zoltan Attila Godo, Gabor Toth, Dora Reglodi, Tamas Juhasz, and Norbert Nemeth. 2023. "Impact Assessment of Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) and Hemostatic Sponge on Vascular Anastomosis Regeneration in Rats" International Journal of Molecular Sciences 24, no. 23: 16695. https://doi.org/10.3390/ijms242316695
APA StyleFazekas, L. A., Szabo, B., Szegeczki, V., Filler, C., Varga, A., Godo, Z. A., Toth, G., Reglodi, D., Juhasz, T., & Nemeth, N. (2023). Impact Assessment of Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) and Hemostatic Sponge on Vascular Anastomosis Regeneration in Rats. International Journal of Molecular Sciences, 24(23), 16695. https://doi.org/10.3390/ijms242316695







