Mitochondrial Cation Signalling in the Control of Inflammatory Processes
Abstract
1. Introduction
2. Mitochondrial Cation Homeostasis
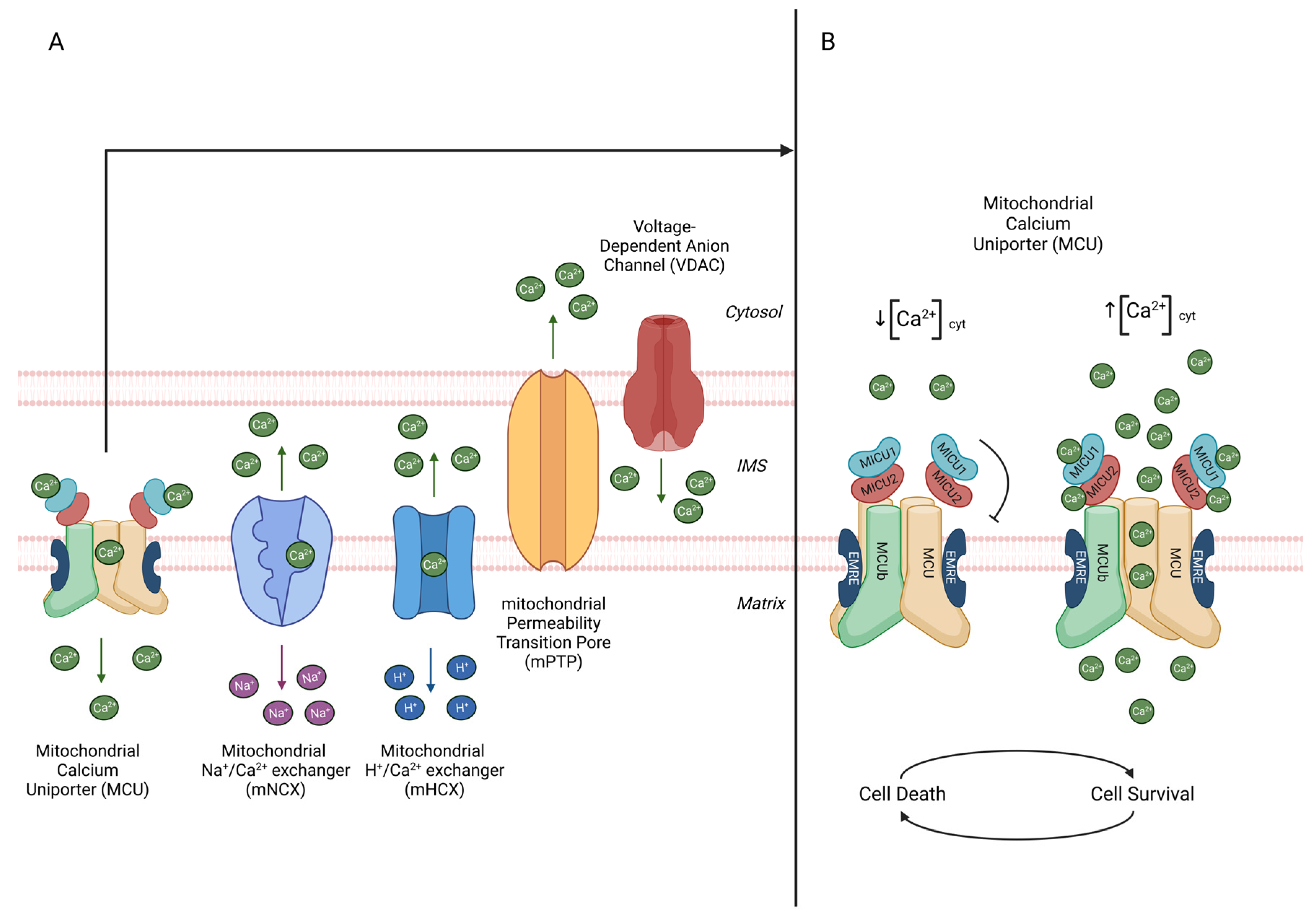
2.1. Mitochondrial Ca2+ Signalling
2.1.1. Mitochondrial Ca2+ Uniporter Complex
2.1.2. Mitochondrial Ca2+ Efflux
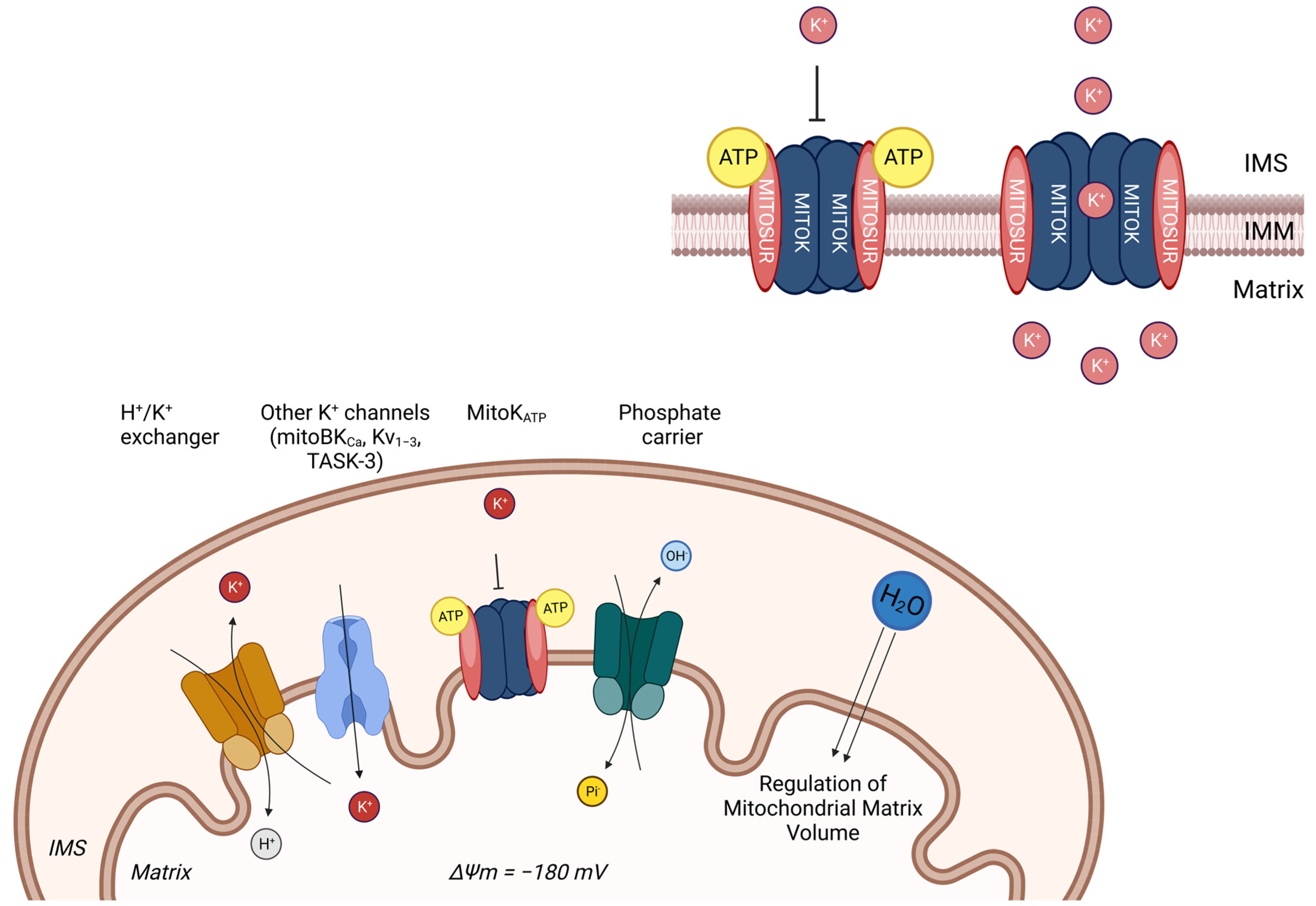
2.2. Mitochondrial K+ Signalling
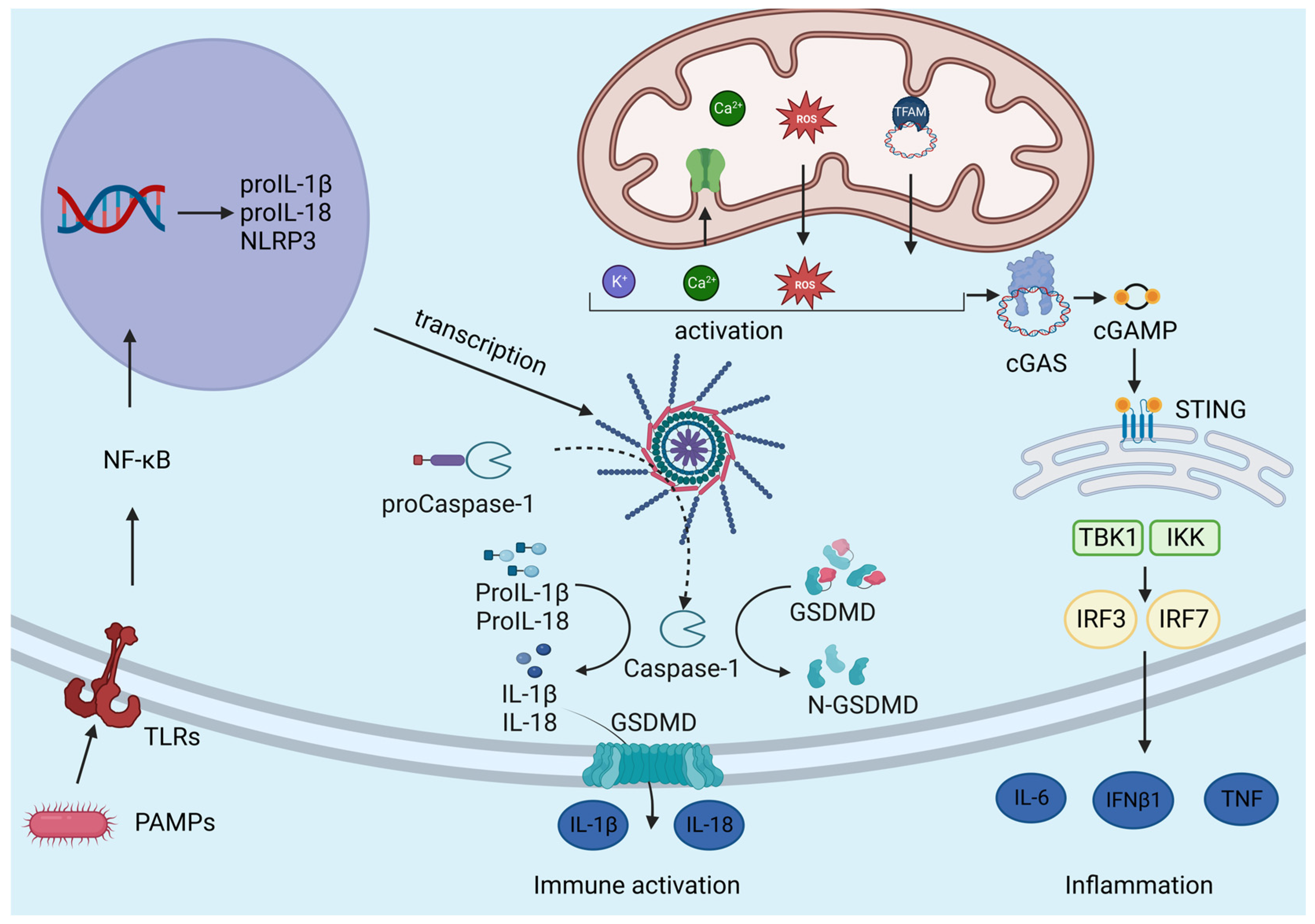
3. Mitochondrial Control of Inflammation
3.1. Innate Immunity Receptors
3.2. NLRP3 Inflammasome
3.3. Role of Mitochondrial Ca2+ Uptake in Inflammation
3.4. Role of Mitochondrial K+ Fluxes in Inflammation
3.5. mtDAMP
3.6. cGAS-STING Pathway
4. Diseases Associated with NLRP3 Inflammasome Dysfunction
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitchell, P. Chemiosmotic Coupling in Oxidative and Photosynthetic Phosphorylation. Biol. Rev. 1966, 41, 445–501. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; Nowikovsky, K. LETM1: Essential for Mitochondrial Biology and Cation Homeostasis? Trends Biochem. Sci. 2019, 44, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; Yamazaki, T.; Galluzzi, L. Mitochondrial Control of Inflammation. Nat. Rev. Immunol. 2023, 23, 159. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s That Spur Autophagy and Immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef] [PubMed]
- Nakahira, K.; Hisata, S.; Choi, A.M.K. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal. 2015, 23, 1329–1350. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Larson-Casey, J.L.; Carter, A.B. Macrophages Utilize the Mitochondrial Calcium Uniporter for Profibrotic Polarization. FASEB J. 2017, 31, 3072–3083. [Google Scholar] [CrossRef]
- Muñoz-Planillo, R.; Kuffa, P.; Martínez-Colón, G.; Smith, B.L.; Rajendiran, T.M.; Núñez, G. K+ Efflux Is the Common Trigger of NLRP3 Inflammasome Activation by Bacterial Toxins and Particulate Matter. Immunity 2013, 38, 1142–1153. [Google Scholar] [CrossRef]
- Berridge, M.J.; Bootman, M.D.; Roderick, H.L. Calcium Signalling: Dynamics, Homeostasis and Remodelling. Nat. Rev. Mol. Cell Biol. 2003, 4, 517–529. [Google Scholar] [CrossRef]
- Rizzuto, R.; De Stefani, D.; Raffaello, A.; Mammucari, C. Mitochondria as Sensors and Regulators of Calcium Signalling. Nat. Rev. Mol. Cell Biol. 2012, 13, 566–578. [Google Scholar] [CrossRef]
- Meldolesi, J.; Pozzan, T. Pathways of Ca2+ Influx at the Plasma Membrane: Voltage-, Receptor-, and Second Messenger-Operated Channels. Exp. Cell Res. 1987, 171, 271–283. [Google Scholar] [CrossRef]
- Emrich, S.M.; Yoast, R.E.; Trebak, M. Physiological Functions of CRAC Channels. Annu. Rev. Physiol. 2022, 84, 355–379. [Google Scholar] [CrossRef] [PubMed]
- Lanner, J.T.; Georgiou, D.K.; Joshi, A.D.; Hamilton, S.L. Ryanodine Receptors: Structure, Expression, Molecular Details, and Function in Calcium Release. Cold Spring Harb. Perspect. Biol. 2010, 2, a003996. [Google Scholar] [CrossRef] [PubMed]
- Philipson, K.D.; Nicoll, D.A. Sodium-Calcium Exchange: A Molecular Perspective. Annu. Rev. Physiol. 2000, 62, 111–133. [Google Scholar] [CrossRef] [PubMed]
- Colombini, M. The VDAC Channel: Molecular Basis for Selectivity. Biochim. Biophys. Acta 2016, 1863, 2498–2502. [Google Scholar] [CrossRef] [PubMed]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Robert Bao, X.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative Genomics Identifies MCU as an Essential Component of the Mitochondrial Calcium Uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabò, I.; Rizzuto, R. A Forty-Kilodalton Protein of the Inner Membrane Is the Mitochondrial Calcium Uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Palty, R.; Silverman, W.F.; Hershfinkel, M.; Caporale, T.; Sensi, S.L.; Parnis, J.; Nolte, C.; Fishman, D.; Shoshan-Barmatz, V.; Herrmann, S.; et al. NCLX Is an Essential Component of Mitochondrial Na+/Ca2+ Exchange. Proc. Natl. Acad. Sci. USA 2010, 107, 436–441. [Google Scholar] [CrossRef]
- Carafoli, E.; Tiozzo, R.; Lugli, G.; Crovetti, F.; Kratzing, C. The Release of Calcium from Heart Mitochondria by Sodium. J. Mol. Cell Cardiol. 1974, 6, 361–371. [Google Scholar] [CrossRef]
- Rizzuto, R.; Brini, M.; Murgia, M.; Pozzan, T. Microdomains with High Ca2+ Close to IP3-Sensitive Channels That Are Sensed by Neighboring Mitochondria. Science 1993, 262, 744–747. [Google Scholar] [CrossRef]
- McCormack, J.G.; Halestrap, A.P.; Denton, R.M. Role of Calcium Ions in Regulation of Mammalian Intramitochondrial Metabolism. Physiol. Rev. 1990, 70, 391–425. [Google Scholar] [CrossRef]
- Bernardi, P.; Vassanelli, S.; Veronese, P.; Colonna, R.; Szabo, I.; Zoratti, M. Modulation of the Mitochondrial Permeability Transition Pore. Effect of Protons and Divalent Cations. J. Biol. Chem. 1992, 267, 2934–2939. [Google Scholar] [CrossRef] [PubMed]
- Solesio, M.E.; Garcia del Molino, L.C.; Elustondo, P.A.; Diao, C.; Chang, J.C.; Pavlov, E.V. Inorganic Polyphosphate Is Required for Sustained Free Mitochondrial Calcium Elevation, Following Calcium Uptake. Cell Calcium. 2020, 86, 102127. [Google Scholar] [CrossRef] [PubMed]
- Solesio, M.E.; Demirkhanyan, L.; Zakharian, E.; Pavlov, E.V. Contribution of Inorganic Polyphosphate towards Regulation of Mitochondrial Free Calcium. Biochim. Biophys. Acta 2016, 1860, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, D.G. The Regulation of Extramitochondrial Free Calcium Ion Concentration by Rat Liver Mitochondria. Biochem. J. 1978, 176, 463–474. [Google Scholar] [CrossRef]
- Bernardi, P.; Carraro, M.; Lippe, G. The Mitochondrial Permeability Transition: Recent Progress and Open Questions. FEBS J. 2022, 289, 7051–7074. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, G.; Monticelli, H.; Rizzuto, R.; Mammucari, C. The Mitochondrial Ca2+ Uptake and the Fine-Tuning of Aerobic Metabolism. Front. Physiol. 2020, 11, 554904. [Google Scholar] [CrossRef]
- Bick, A.G.; Calvo, S.E.; Mootha, V.K. Evolutionary Diversity of the Mitochondrial Calcium Uniporter. Science 2012, 336, 886. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zheng, J.; Jia, Z. Structural Characterization of the Mitochondrial Ca2+ Uniporter Provides Insights into Ca2+ Uptake and Regulation. iScience 2021, 24, 102895. [Google Scholar] [CrossRef]
- De Stefani, D.; Rizzuto, R.; Pozzan, T. Enjoy the Trip: Calcium in Mitochondria Back and Forth. Annu. Rev. Biochem. 2016, 85, 161–192. [Google Scholar] [CrossRef]
- Pan, X.; Liu, J.; Nguyen, T.; Liu, C.; Sun, J.; Teng, Y.; Fergusson, M.M.; Rovira, I.I.; Allen, M.; Springer, D.A.; et al. The Physiological Role of Mitochondrial Calcium Revealed by Mice Lacking the Mitochondrial Calcium Uniporter. Nat. Cell Biol. 2013, 15, 1464–1472. [Google Scholar] [CrossRef]
- Murphy, E.; Pan, X.; Nguyen, T.; Liu, J.; Holmström, K.M.; Finkel, T. Unresolved Questions from the Analysis of Mice Lacking MCU Expression. Biochem. Biophys. Res. Commun. 2014, 449, 384–385. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, G.; De Mario, A.; Mammucari, C. The Mitochondrial Calcium Homeostasis Orchestra Plays Its Symphony: Skeletal Muscle Is the Guest of Honor. Int. Rev. Cell Mol. Biol. 2021, 362, 209–259. [Google Scholar] [CrossRef] [PubMed]
- Raffaello, A.; De Stefani, D.; Sabbadin, D.; Teardo, E.; Merli, G.; Picard, A.; Checchetto, V.; Moro, S.; Szabò, I.; Rizzuto, R. The Mitochondrial Calcium Uniporter Is a Multimer That Can Include a Dominant-Negative Pore-Forming Subunit. EMBO J. 2013, 32, 2362–2376. [Google Scholar] [CrossRef] [PubMed]
- Fieni, F.; Bae Lee, S.; Nung Jan, Y.; Kirichok, Y. Activity of the Mitochondrial Calcium Uniporter Varies Greatly between Tissues. Nat. Commun. 2012, 3, 1317. [Google Scholar] [CrossRef]
- Sancak, Y.; Markhard, A.L.; Kitami, T.; Kovács-Bogdán, E.; Kamer, K.J.; Udeshi, N.D.; Carr, S.A.; Chaudhuri, D.; Clapham, D.E.; Li, A.A.; et al. EMRE Is an Essential Component of the Mitochondrial Calcium Uniporter Complex. Science 2013, 342, 1379–1382. [Google Scholar] [CrossRef]
- Kovaćs-Bogdán, E.; Sancak, Y.; Kamer, K.J.; Plovanich, M.; Jambhekar, A.; Huber, R.J.; Myre, M.A.; Blower, M.D.; Mootha, V.K. Reconstitution of the Mitochondrial Calcium Uniporter in Yeast. Proc. Natl. Acad. Sci. USA 2014, 111, 8985–8990. [Google Scholar] [CrossRef]
- Patron, M.; Checchetto, V.; Raffaello, A.; Teardo, E.; VecellioReane, D.; Mantoan, M.; Granatiero, V.; Szabò, I.; DeStefani, D.; Rizzuto, R. MICU1 and MICU2 Finely Tune the Mitochondrial Ca2+ Uniporter by Exerting Opposite Effects on MCU Activity. Mol. Cell. 2014, 53, 726–737. [Google Scholar] [CrossRef]
- Liu, J.C.; Syder, N.C.; Ghorashi, N.S.; Willingham, T.B.; Parks, R.J.; Sun, J.; Fergusson, M.M.; Liu, J.; Holmström, K.M.; Menazza, S.; et al. EMRE Is Essential for Mitochondrial Calcium Uniporter Activity in a Mouse Model. JCI Insight 2020, 5, e134063. [Google Scholar] [CrossRef]
- Perocchi, F.; Gohil, V.M.; Girgis, H.S.; Bao, X.R.; McCombs, J.E.; Palmer, A.E.; Mootha, V.K. MICU1 Encodes a Mitochondrial EF Hand Protein Required for Ca2+ Uptake. Nature 2010, 467, 291. [Google Scholar] [CrossRef]
- Tsai, C.-W.; Liu, T.-Y.; Chao, F.-Y.; Tu, Y.-C.; Rodriguez, M.X.; Van Keuren, A.M.; Ma, Z.; Bankston, J.; Tsai, M.-F. Evidence Supporting the MICU1 Occlusion Mechanism and against the Potentiation Model in the Mitochondrial Calcium Uniporter Complex. Proc. Natl. Acad. Sci. USA 2023, 120, e2217665120. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Suzuki, J.; Paranjpe, I.; Unsulangi, T.; Boyman, L.; Milescu, L.S.; Jonathan Lederer, W.; Kirichok, Y. The Mechanism of MICU-Dependent Gating of the Mitochondrial Ca2+uniporter. eLife 2021, 10, e69312. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, J.; Tsai, C.W.; Orlando, B.J.; Rodriguez, M.; Xu, Y.; Liao, M.; Tsai, M.F.; Feng, L. Structure and Mechanism of the Mitochondrial Ca2+ Uniporter Holocomplex. Nature 2020, 582, 129. [Google Scholar] [CrossRef] [PubMed]
- Vecellio Reane, D.; Vallese, F.; Checchetto, V.; Acquasaliente, L.; Butera, G.; De Filippis, V.; Szabò, I.; Zanotti, G.; Rizzuto, R.; Raffaello, A. A MICU1 Splice Variant Confers High Sensitivity to the Mitochondrial Ca2+ Uptake Machinery of Skeletal Muscle. Mol. Cell. 2016, 64, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Plovanich, M.; Bogorad, R.L.; Sancak, Y.; Kamer, K.J.; Strittmatter, L.; Li, A.A.; Girgis, H.S.; Kuchimanchi, S.; De Groot, J.; Speciner, L.; et al. MICU2, a Paralog of MICU1, Resides within the Mitochondrial Uniporter Complex to Regulate Calcium Handling. PLoS ONE 2013, 8, e55785. [Google Scholar] [CrossRef]
- Patron, M.; Granatiero, V.; Espino, J.; Rizzuto, R.; De Stefani, D. MICU3 Is a Tissue-Specific Enhancer of Mitochondrial Calcium Uptake. Cell Death Differ. 2019, 26, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, G.; de Juan-Sanz, J.; Farrell, R.J.; Ryan, T.A. Molecular Tuning of the Axonal Mitochondrial Ca2+ Uniporter Ensures Metabolic Flexibility of Neurotransmission. Neuron 2020, 105, 678–687.e5. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.W.; Baysal, K.; Brierley, G.P. The Sodium-Calcium Antiport of Heart Mitochondria Is Not Electroneutral. J. Biol. Chem. 1995, 270, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Gunter, T.E.; Chace, J.H.; Puskin, J.S.; Gunter, K.K. Mechanism of Sodium Independent Calcium Efflux from Rat Liver Mitochondria. Biochemistry 1983, 22, 6341–6351. [Google Scholar] [CrossRef]
- Jiang, D.; Zhao, L.; Clapham, D.E. Genome-Wide RNAi Screen Identifies Letm1 as a Mitochondrial Ca2+/H+ Antiporter. Science 2009, 326, 144–147. [Google Scholar] [CrossRef]
- Nowikovsky, K.; Bernardi, P.; Forte, M.; Tsai, M.F.; Jiang, D.; Clapham, L. LETM1 in Mitochondrial Cation Transport A Commentary on Functional Reconstitution of the Mito-Chondrial Ca2+/H+ Antiporter Letm1. J. Gen. Physiol. 2014, 143, 67–73. [Google Scholar] [CrossRef][Green Version]
- Austin, S.; Mekis, R.; Mohammed, S.E.M.; Scalise, M.; Wang, W.; Galluccio, M.; Pfeiffer, C.; Borovec, T.; Parapatics, K.; Vitko, D.; et al. TMBIM5 Is the Ca2+/H+ Antiporter of Mammalian Mitochondria. EMBO Rep. 2022, 23, e54978. [Google Scholar] [CrossRef] [PubMed]
- Patron, M.; Tarasenko, D.; Nolte, H.; Kroczek, L.; Ghosh, M.; Ohba, Y.; Lasarzewski, Y.; Ahmadi, Z.A.; Cabrera-Orefice, A.; Eyiama, A.; et al. Regulation of Mitochondrial Proteostasis by the Proton Gradient. EMBO J. 2022, 41, e110476. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Dietsche, F.; Seitaj, B.; Rojas-Charry, L.; Latchman, N.; Tomar, D.; Wüst, R.C.; Nickel, A.; Frauenknecht, K.B.; Schoser, B.; et al. TMBIM5 Loss of Function Alters Mitochondrial Matrix Ion Homeostasis and Causes a Skeletal Myopathy. Life Sci. Alliance 2022, 5, e202201478. [Google Scholar] [CrossRef] [PubMed]
- Garlid, K.D.; Paucek, P. Mitochondrial Potassium Transport: The K+ Cycle. Biochim. Biophys. Acta Bioenerg. 2003, 1606, 23–41. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, B. Evidence for Mitochondrial K+ Channels and Their Role in Cardioprotection. Circ. Res. 2004, 94, 420–432. [Google Scholar] [CrossRef]
- Rusznák, Z.; Bakondi, G.; Kosztka, L.; Pocsai, K.; Dienes, B.; Fodor, J.; Telek, A.; Gönczi, M.; Szűcs, G. Mitochondrial Expression of the Two-Pore Domain TASK-3 Channels in Malignantly Transformed and Non-Malignant Human Cells. Virchows Arch. 2008, 452, 415–426. [Google Scholar] [CrossRef]
- Siemen, D.; Loupatatzis, C.; Borecky, J.; Gulbins, E.; Lang, F. Ca2+-Activated K Channel of the BK-Type in the Inner Mitochondrial Membrane of a Human Glioma Cell Line. Biochem. Biophys. Res. Commun. 1999, 257, 549–554. [Google Scholar] [CrossRef]
- Szabò, I.; Bock, J.; Jekle, A.; Soddemann, M.; Adams, C.; Lang, F.; Zoratti, M.; Gulbins, E. A Novel Potassium Channel in Lymphocyte Mitochondria. J. Biol. Chem. 2005, 280, 12790–12798. [Google Scholar] [CrossRef]
- Garlid, K.D.; Halestrap, A.P. The Mitochondrial K ATP Channel—Fact or Fiction? J. Mol. Cell. Cardiol. 2012, 52, 578–583. [Google Scholar] [CrossRef]
- Paggio, A.; Checchetto, V.; Campo, A.; Menabò, R.; Di Marco, G.; Di Lisa, F.; Szabo, I.; Rizzuto, R.; De Stefani, D. Identification of an ATP-Sensitive Potassium Channel in Mitochondria. Nature 2019, 572, 609–613. [Google Scholar] [CrossRef]
- Kroemer, G.; Galassi, C.; Zitvogel, L.; Galluzzi, L. Immunogenic Cell Stress and Death. Nat. Immunol. 2022, 23, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.M.; Weinberg, S.E.; Chandel, N.S. Mitochondrial Control of Immunity: Beyond ATP. Nat. Rev. Immunol. 2017, 17, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Cloonan, S.M.; Choi, A.M.K. Mitochondria: Sensors and Mediators of Innate Immune Receptor Signaling. Curr. Opin. Microbiol. 2013, 16, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, M. Pattern Recognition Receptors in Health and Diseases. Signal. Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and Adaptive Immune Cells in the Tumor Microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.K.; Wen, H.; P-Y Ting, J. IY29CH23-Ting ARI: The Inflammasome NLRs in Immunity, Inflammation, and Associated Diseases. Annu. Rev. Immunol. 2011, 29, 707–735. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of ProIL-1beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kanneganti, T.D. NLRP3 Inflammasome in Cancer and Metabolic Diseases. Nat. Immunol. 2021, 22, 550–559. [Google Scholar] [CrossRef]
- Agostini, L.; Martinon, F.; Burns, K.; Mcdermott, M.F.; Hawkins, P.N.; Rg Tschopp, J. NALP3 Forms an IL-1-Processing Inflammasome with Increased Activity in Muckle-Wells Autoinflammatory Disorder. Immunity 2004, 20, 319–325. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by Inflammatory Caspases Determines Pyroptotic Cell Death. Nature 2015, 526, 660–665. [Google Scholar] [CrossRef]
- Ding, J.; Wang, K.; Liu, W.; She, Y.; Sun, Q.; Shi, J.; Sun, H.; Wang, D.-C.; Shao, F. Pore-Forming Activity and Structural Autoinhibition of the Gasdermin Family. Nature 2016, 535, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Taabazuing, C.Y.; Griswold, A.R.; Bachovchin, D.A. The NLRP1 and CARD8 Inflammasomes. Immunol. Rev. 2020, 297, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Hewton, K.; Monack, D.M.; Vucic, D.; French, D.M.; Lee, W.P.; Roose-Girma, M.; Erickson, S.; Dixit, V.M. Differential Activation of the Inflammasome by Caspase-1 Adaptors ASC and Ipaf. Nature 2004, 430, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R.; Rauch, I. The NAIP/NLRC4 Inflammasome in Infection and Pathology. Mol. Asp. Med. 2020, 76, 100863. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory Caspases Are Innate Immune Receptors for Intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 Inflammasome: Molecular Activation and Regulation to Therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Malik, A.; Kanneganti, T.D. Inflammasome Activation and Assembly at a Glance. J. Cell. Sci. 2017, 130, 3955–3963. [Google Scholar] [CrossRef]
- Mathur, A.; Hayward, J.A.; Man, S.M. Molecular Mechanisms of Inflammasome Signaling. J. Leukoc. Biol. 2018, 103, 233–257. [Google Scholar] [CrossRef]
- Boucher, D.; Monteleone, M.; Coll, R.C.; Chen, K.W.; Ross, C.M.; Teo, J.L.; Gomez, G.A.; Holley, C.L.; Bierschenk, D.; Stacey, K.J.; et al. Caspase-1 Self-Cleavage Is an Intrinsic Mechanism to Terminate Inflammasome Activity. J. Exp. Med. 2018, 215, 827–840. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, Y.; Li, X.; Zhan, X.; Tang, M.; Fina, M.; Su, L.; Pratt, D.; Bu, C.H.; Hildebrand, S.; et al. NLRP3 Activation and Mitosis Are Mutually Exclusive Events Coordinated by NEK7, a New Inflammasome Component. Nat. Immunol. 2016, 17, 250–258. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zeng, M.; Yang, D.; Benny, M.; Núñez, G. NEK7 Is an Essential Mediator of NLRP3 Activation Downstream of Potassium Efflux. Nature 2016, 530, 354–357. [Google Scholar] [CrossRef]
- Juliana, C.; Fernandes-Alnemri, T.; Kang, S.; Farias, A.; Qin, F.; Alnemri, E.S. Non-Transcriptional Priming and Deubiquitination Regulate NLRP3 Inflammasome Activation. J. Biol. Chem. 2012, 287, 36617–36622. [Google Scholar] [CrossRef] [PubMed]
- Gurung, P.; Subbarao Malireddi, R.K.; Anand, P.K.; Demon, D.; Vande Walle, L.; Liu, Z.; Vogel, P.; Lamkanfi, M.; Kanneganti, T.D. Toll or Interleukin-1 Receptor (TIR) Domain-Containing Adaptor Inducing Interferon-β (TRIF)-Mediated Caspase-11 Protease Production Integrates Toll-like Receptor 4 (TLR4) Protein- and Nlrp3 Inflammasome-Mediated Host Defense against Enteropathogens. J. Biol. Chem. 2012, 287, 34474–34483. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, F.G.; Horvath, G.; Stutz, A.; Alnemri, E.S.; MacDonald, K.; Speert, D.; Fernandes-Alnemri, T.; Wu, J.; Monks, B.G.; Fitzgerald, K.A.; et al. Cutting Edge: NF-KappaB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. J. Immunol. 2009, 183, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Paik, S.; Kim, J.K.; Silwal, P.; Sasakawa, C.; Jo, E.K. An Update on the Regulatory Mechanisms of NLRP3 Inflammasome Activation. Cell. Mol. Immunol. 2021, 18, 1141–1160. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Wang, Y. Recent Advances and the Mechanism of Astaxanthin in Ophthalmological Diseases. J. Ophthalmol. 2022, 2022, 8071406. [Google Scholar] [CrossRef]
- Putney, J.W.; Tomita, T. Phospholipase C Signaling and Calcium Influx. Adv. Biol. Regul. 2011, 52, 152–164. [Google Scholar] [CrossRef]
- Hogan, P.G.; Rao, A. Store-Operated Calcium Entry: Mechanisms and Modulation. Biochem. Biophys. Res. Commun. 2015, 460, 40–49. [Google Scholar] [CrossRef]
- Shimada, K.; Crother, T.R.; Karlin, J.; Dagvadorj, J.; Chiba, N.; Chen, S.; Ramanujan, V.K.; Wolf, A.J.; Vergnes, L.; Ojcius, D.M.; et al. Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome during Apoptosis. Immunity 2012, 36, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Gris, D.; Lei, Y.; Jha, S.; Zhang, L.; Huang, M.T.H.; Brickey, W.J.; Ting, J.P.Y. Fatty Acid-Induced NLRP3-ASC Inflammasome Activation Interferes with Insulin Signaling. Nat. Immunol. 2011, 12, 408–415. [Google Scholar] [CrossRef]
- Sebag, S.C.; Koval, O.M.; Paschke, J.D.; Winters, C.J.; Comellas, A.P.; Grumbach, I.M. Inhibition of the Mitochondrial Calcium Uniporter Prevents IL-13 and Allergen-Mediated Airway Epithelial Apoptosis and Loss of Barrier Function. Exp. Cell. Res. 2018, 362, 400–411. [Google Scholar] [CrossRef]
- Rimessi, A.; Bezzerri, V.; Patergnani, S.; Marchi, S.; Cabrini, G.; Pinton, P. Mitochondrial Ca2+-Dependent NLRP3 Activation Exacerbates the Pseudomonas Aeruginosa-Driven Inflammatory Response in Cystic Fibrosis. Nat. Commun. 2015, 6, 6201. [Google Scholar] [CrossRef] [PubMed]
- Rimessi, A.; Pozzato, C.; Carparelli, L.; Rossi, A.; Ranucci, S.; De Fino, I.; Cigana, C.; Talarico, A.; Wieckowski, M.R.; Ribeiro, C.M.P.; et al. Pharmacological modulation of mitochondrial calcium uniporter controls lung inflammation in cystic fibrosis. Sci. Adv. 2020, 6, eaax9093. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Scattolini, V.; Albiero, M.; Bortolozzi, M.; Avogaro, A.; Cignarella, A.; Fadini, G.P. Mitochondrial Calcium Uptake Is Instrumental to Alternative Macrophage Polarization and Phagocytic Activity. Int. J. Mol. Sci. 2019, 20, 4966. [Google Scholar] [CrossRef]
- Feno, S.; Munari, F.; Vecellio Reane, D.; Gissi, R.; Hoang, D.-H.; Castegna, A.; Chazaud, B.; Viola, A.; Rizzuto, R.; Raffaello, A. The Dominant-Negative Mitochondrial Calcium Uniporter Subunit MCUb Drives Macrophage Polarization during Skeletal Muscle Regeneration. Sci. Signal. 2021, 14, eabf3838. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, B.; Chen, J.; Liu, Z.; Li, X.; Li, L.; Wen, H. Mitochondrial Calcium Uniporter Promotes Phagocytosis-Dependent Activation of the NLRP3 Inflammasome. Proc. Natl. Acad. Sci. USA 2022, 119, e2123247119. [Google Scholar] [CrossRef]
- Skowyra, M.L.; Schlesinger, P.H.; Naismith, T.V.; Hanson, P.I. Triggered Recruitment of ESCRT Machinery Promotes Endolysosomal Repair. Science 2018, 360, eaar5078. [Google Scholar] [CrossRef]
- Raiborg, C.; Stenmark, H. The ESCRT Machinery in Endosomal Sorting of Ubiquitylated Membrane Proteins. Nature 2009, 458, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Lang, X.; Xu, C.; Wang, X.; Gong, T.; Yang, Y.; Cui, J.; Bai, L.; Wang, J.; Jiang, W.; et al. CLICs-Dependent Chloride Efflux Is an Essential and Proximal Upstream Event for NLRP3 Inflammasome Activation. Nat. Commun. 2017, 8, 202. [Google Scholar] [CrossRef] [PubMed]
- Jhang, J.J.; Cheng, Y.T.; Ho, C.Y.; Yen, G.C. Monosodium Urate Crystals Trigger Nrf2- and Heme Oxygenase-1-Dependent Inflammation in THP-1 Cells. Cell. Mol. Immunol. 2015, 12, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Schorn, C.; Frey, B.; Lauber, K.; Janko, C.; Strysio, M.; Keppeler, H.; Gaipl, U.S.; Voll, R.E.; Springer, E.; Munoz, L.E.; et al. Sodium Overload and Water Influx Activate the NALP3 Inflammasome. J. Biol. Chem. 2011, 286, 35–41. [Google Scholar] [CrossRef]
- Zhou, F.; Yao, H.H.; Wu, J.Y.; Ding, J.H.; Sun, T.; Hu, G. Opening of Microglial K(ATP) Channels Inhibits Rotenone-Induced Neuroinflammation. J. Cell. Mol. Med. 2008, 12, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Yu, Y.; Li, B.; Gu, X.; Xie, K.; Wang, G.; Yu, Y. Protective Effects of Hydrogen-rich Saline against Experimental Diabetic Peripheral Neuropathy via Activation of the Mitochondrial ATP-sensitive Potassium Channel Channels in Rats. Mol. Med. Rep. 2020, 21, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wu, D.; Jiang, Z.; Ling, W.; Qian, G. Protective Effect of Nicorandil on Cardiac Microvascular Injury: Role of Mitochondrial Integrity. Oxid. Med. Cell Longev. 2021, 2021, 4665632. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Duarte, E.; Trujillo, X.; Cortés-Rojo, C.; Saavedra-Molina, A.; Camargo, G.; Hernández, L.; Huerta, M.; Montoya-Pérez, R. Nicorandil Improves Post-Fatigue Tension in Slow Skeletal Muscle Fibers by Modulating Glutathione Redox State. J. Bioenerg. Biomembr. 2017, 49, 159–170. [Google Scholar] [CrossRef]
- Pain, T.; Yang, X.M.; Critz, S.D.; Yue, Y.; Nakano, A.; Liu, G.S.; Heusch, G.; Cohen, M.V.; Downey, J.M. Opening of Mitochondrial K(ATP) Channels Triggers the Preconditioned State by Generating Free Radicals. Circ. Res. 2000, 87, 460–466. [Google Scholar] [CrossRef]
- Wenceslau, C.F.; McCarthy, C.G.; Szasz, T.; Spitler, K.; Goulopoulou, S.; Webb, R.C. Mitochondrial Damage-Associated Molecular Patterns and Vascular Function. Eur. Heart J. 2014, 35, 1172–1177. [Google Scholar] [CrossRef]
- Kong, C.; Song, W.; Fu, T. Systemic Inflammatory Response Syndrome Is Triggered by Mitochondrial Damage (Review). Mol. Med. Rep. 2022, 25, 147. [Google Scholar] [CrossRef] [PubMed]
- Forni, L.G.; McKinnon, W.; Lord, G.A.; Treacher, D.F.; Peron, J.-M.R.; Hilton, P.J. Circulating Anions Usually Associated with the Krebs Cycle in Patients with Metabolic Acidosis. Crit. Care 2005, 9, R591. [Google Scholar] [CrossRef] [PubMed]
- Hobert, J.A.; Mester, J.L.; Moline, J.; Eng, C. Elevated Plasma Succinate in PTEN, SDHB, and SDHD Mutation-Positive Individuals. Genet. Med. 2012, 14, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, J.; Müller, D.C.; Müller, S.C.; Hauser, S.; Heukamp, L.C.; von Ruecker, A.; Bastian, P.J.; Walgenbach-Brunagel, G. Circulating Mitochondrial DNA in Serum: A Universal Diagnostic Biomarker for Patients with Urological Malignancies. Urol. Oncol. 2012, 30, 509–515. [Google Scholar] [CrossRef]
- Kohler, C.; Radpour, R.; Barekati, Z.; Asadollahi, R.; Bitzer, J.; Wight, E.; Bürki, N.; Diesch, C.; Holzgreve, W.; Zhong, X.Y. Levels of Plasma Circulating Cell Free Nuclear and Mitochondrial DNA as Potential Biomarkers for Breast Tumors. Mol. Cancer 2009, 8, 105. [Google Scholar] [CrossRef]
- Zachariah, R.R.; Schmid, S.; Buerki, N.; Radpour, R.; Holzgreve, W.; Zhong, X. Levels of Circulating Cell-Free Nuclear and Mitochondrial DNA in Benign and Malignant Ovarian Tumors. Obstet. Gynecol. 2008, 112, 843–850. [Google Scholar] [CrossRef]
- Burnstock, G. Pathophysiology and Therapeutic Potential of Purinergic Signaling. Pharmacol. Rev. 2006, 58, 58–86. [Google Scholar] [CrossRef]
- Deli, T.; Csernoch, L. Extracellular ATP and Cancer: An Overview with Special Reference to P2 Purinergic Receptors. Pathol. Oncol. Res. 2008, 14, 219–231. [Google Scholar] [CrossRef]
- Gui, X.; Yang, H.; Li, T.; Tan, X.; Shi, P.; Li, M.; Du, F.; Chen, Z.J. Autophagy Induction via STING Trafficking Is a Primordial Function of the CGAS Pathway. Nature 2019, 567, 262–266. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, L.; Chen, Z.J. Regulation and Function of the CGAS-STING Pathway of Cytosolic DNA Sensing. Nat. Immunol. 2016, 17, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Xian, H.; Watari, K.; Sanchez-Lopez, E.; Offenberger, J.; Onyuru, J.; Sampath, H.; Ying, W.; Hoffman, H.M.; Shadel, G.S.; Karin, M. Oxidized DNA Fragments Exit Mitochondria via MPTP- and VDAC-Dependent Channels to Activate NLRP3 Inflammasome and Interferon Signaling. Immunity 2022, 55, 1370–1385.e8. [Google Scholar] [CrossRef] [PubMed]
- Gaidt, M.M.; Ebert, T.S.; Chauhan, D.; Ramshorn, K.; Pinci, F.; Zuber, S.; O’Duill, F.; Schmid-Burgk, J.L.; Hoss, F.; Buhmann, R.; et al. The DNA Inflammasome in Human Myeloid Cells Is Initiated by a STING-Cell Death Program Upstream of NLRP3. Cell 2017, 171, 1110–1124.e18. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.R.; Whitefoot-Keliin, K.M.; Palmatier, E.M.; Mahon, A.R.; Greenlee-Wacker, M.C. Extracellular Vesicles from A23187-Treated Neutrophils Cause CGAS-STING-Dependent IL-6 Production by Macrophages. Front. Immunol. 2022, 13, 949451. [Google Scholar] [CrossRef] [PubMed]
- Barnett, K.C.; Xie, Y.; Asakura, T.; Song, D.; Liang, K.; Taft-Benz, S.A.; Guo, H.; Yang, S.; Okuda, K.; Gilmore, R.C.; et al. An Epithelial-Immune Circuit Amplifies Inflammasome and IL-6 Responses to SARS-CoV-2. Cell Host Microbe 2023, 31, 243–259.e6. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Huang, Y.; Chen, M.; Yang, Y.; Li, X.; Zhang, W. Mitochondrial DNA in NLRP3 Inflammasome Activation. Int. Immunopharmacol. 2022, 108, 108719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, G.; Luo, R.; Lei, J.; Song, Y.; Wang, B.; Ma, L.; Liao, Z.; Ke, W.; Liu, H.; et al. Cytosolic Escape of Mitochondrial DNA Triggers CGAS-STING-NLRP3 Axis-Dependent Nucleus Pulposus Cell Pyroptosis. Exp. Mol. Med. 2022, 54, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Seok, J.K.; Kang, H.C.; Cho, Y.Y.; Lee, H.S.; Lee, J.Y. Therapeutic Regulation of the NLRP3 Inflammasome in Chronic Inflammatory Diseases. Arch. Pharm. Res. 2021, 44, 16–35. [Google Scholar] [CrossRef]
- Donovan, C.; Liu, G.; Shen, S.; Marshall, J.E.; Kim, R.Y.; Alemao, C.A.; Budden, K.F.; Choi, J.P.; Kohonen-Corish, M.; El-Omar, E.M.; et al. The Role of the Microbiome and the NLRP3 Inflammasome in the Gut and Lung. J. Leukoc. Biol. 2020, 108, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.Y.; Pinkerton, J.W.; Essilfie, A.T.; Robertson, A.A.B.; Baines, K.J.; Brown, A.C.; Mayall, J.R.; Ali, M.K.; Starkey, M.R.; Hansbro, N.G.; et al. Role for NLRP3 Inflammasome-Mediated, IL-1β-Dependent Responses in Severe, Steroid-Resistant Asthma. Am. J. Respir. Crit. Care Med. 2017, 196, 283–297. [Google Scholar] [CrossRef]
- Baines, K.J.; Simpson, J.L.; Wood, L.G.; Scott, R.J.; Gibson, P.G. Transcriptional Phenotypes of Asthma Defined by Gene Expression Profiling of Induced Sputum Samples. J. Allergy Clin. Immunol. 2011, 127, 153–160.e6. [Google Scholar] [CrossRef]
- Wu, Y.P.; Cao, C.; Wu, Y.F.; Li, M.; Lai, T.W.; Zhu, C.; Wang, Y.; Ying, S.M.; Chen, Z.H.; Shen, H.H.; et al. Activating Transcription Factor 3 Represses Cigarette Smoke-Induced IL6 and IL8 Expression via Suppressing NF-ΚB Activation. Toxicol. Lett. 2017, 270, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Jiang, Y.X.; Yang, Y.C.; Liu, J.Y.; Huo, C.; Ji, X.L.; Qu, Y.Q. Cigarette Smoke Extract Induces Pyroptosis in Human Bronchial Epithelial Cells through the ROS/NLRP3/Caspase-1 Pathway. Life Sci. 2021, 269, 119090. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Zhang, H. NLRP3 Inflammasome and Inflammatory Bowel Disease. Front. Immunol. 2019, 10, 276. [Google Scholar] [CrossRef] [PubMed]
- Goyette, P.; Labbé, C.; Trinh, T.T.; Xavier, R.J.; Rioux, J.D. Molecular Pathogenesis of Inflammatory Bowel Disease: Genotypes, Phenotypes and Personalized Medicine. Ann. Med. 2007, 39, 177–199. [Google Scholar] [CrossRef] [PubMed]
- Khatri, V.; Kalyanasundaram, R. Therapeutic Implications of Inflammasome in Inflammatory Bowel Disease. FASEB J. 2021, 35, e21439. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhao, Y.; Ma, Y.; Wang, Z.; Rong, L.; Wang, B.; Zhang, N. Biological Functions of NLRP3 Inflammasome: A Therapeutic Target in Inflammatory Bowel Disease. Cytokine Growth Factor Rev. 2021, 60, 61–75. [Google Scholar] [CrossRef]
- Tourkochristou, E.; Aggeletopoulou, I.; Konstantakis, C.; Triantos, C. Role of NLRP3 Inflammasome in Inflammatory Bowel Diseases. World J. Gastroenterol. 2019, 25, 4796–4804. [Google Scholar] [CrossRef]
- Glocker, E.-O.; Kotlarz, D.; Boztug, K.; Gertz, E.M.; Schäffer, A.A.; Noyan, F.; Perro, M.; Diestelhorst, J.; Allroth, A.; Murugan, D.; et al. Inflammatory Bowel Disease and Mutations Affecting the Interleukin-10 Receptor. N. Engl. J. Med. 2009, 361, 2033–2045. [Google Scholar] [CrossRef]
- Zaki, M.H.; Boyd, K.L.; Vogel, P.; Kastan, M.B.; Lamkanfi, M.; Kanneganti, T.D. The NLRP3 Inflammasome Protects against Loss of Epithelial Integrity and Mortality during Experimental Colitis. Immunity 2010, 32, 379–391. [Google Scholar] [CrossRef]
- Ranson, N.; Veldhuis, M.; Mitchell, B.; Fanning, S.; Cook, A.L.; Kunde, D.; Eri, R. NLRP3-Dependent and -Independent Processing of Interleukin (IL)-1β in Active Ulcerative Colitis. Int. J. Mol. Sci. 2018, 20, 10057. [Google Scholar] [CrossRef]
- Özsoy, M.; Stummer, N.; Zimmermann, F.A.; Feichtinger, R.G.; Sperl, W.; Weghuber, D.; Schneider, A.M. Role of Energy Metabolism and Mitochondrial Function in Inflammatory Bowel Disease. Inflamm. Bowel. Dis. 2022, 28, 1443–1450. [Google Scholar] [CrossRef]
- Xue, X.; Bredell, B.X.; Anderson, E.R.; Martin, A.; Mays, C.; Nagao-Kitamoto, H.; Huang, S.; Győrffy, B.; Greenson, J.K.; Hardiman, K.; et al. Quantitative Proteomics Identifies STEAP4 as a Critical Regulator of Mitochondrial Dysfunction Linking Inflammation and Colon Cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9608–E9617. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.E.; Vincent, G.; Sodhi, C.P.; Novak, E.A.; Ranganathan, S.; Egan, C.E.; Stolz, D.B.; Rogers, M.B.; Firek, B.; Morowitz, M.J.; et al. Peroxisome Proliferator-Activated Receptor-γ Coactivator 1-α (PGC1α) Protects against Experimental Murine Colitis. J. Biol. Chem. 2016, 291, 10184–10200. [Google Scholar] [CrossRef] [PubMed]
- Heller, S.; Penrose, H.M.; Cable, C.; Biswas, D.; Nakhoul, H.; Baddoo, M.; Flemington, E.; Crawford, S.E.; Savkovic, S.D. Reduced Mitochondrial Activity in Colonocytes Facilitates AMPKa2-Dependent Inflammation. FASEB J. 2017, 31, 2013. [Google Scholar] [CrossRef]
- Boyapati, R.K.; Dorward, D.A.; Tamborska, A.; Kalla, R.; Ventham, N.T.; Doherty, M.K.; Whitfield, P.D.; Gray, M.; Loane, J.; Rossi, A.G.; et al. Mitochondrial DNA Is a Pro-Inflammatory Damage-Associated Molecular Pattern Released During Active IBD. Inflamm. Bowel. Dis. 2018, 24, 2113–2122. [Google Scholar] [CrossRef] [PubMed]
- Sifroni, K.G.; Damiani, C.R.; Stoffel, C.; Cardoso, M.R.; Ferreira, G.K.; Jeremias, I.C.; Rezin, G.T.; Scaini, G.; Schuck, P.F.; Dal-Pizzol, F.; et al. Mitochondrial Respiratory Chain in the Colonic Mucosal of Patients with Ulcerative Colitis. Mol. Cell. Biochem. 2010, 342, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Bär, F.; Bochmann, W.; Widok, A.; Von Medem, K.; Pagel, R.; Hirose, M.; Yu, X.; Kalies, K.; König, P.; Böhm, R.; et al. Mitochondrial Gene Polymorphisms That Protect Mice from Colitis. Gastroenterology 2013, 145, 1055–1063.e6. [Google Scholar] [CrossRef] [PubMed]
- Stienstra, R.; Van Diepen, J.A.; Tack, C.J.; Zaki, M.H.; Van De Veerdonk, F.L.; Perera, D.; Neale, G.A.; Hooiveld, G.J.; Hijmans, A.; Vroegrijk, I.; et al. Inflammasome Is a Central Player in the Induction of Obesity and Insulin Resistance. Proc. Natl. Acad. Sci. USA 2011, 108, 15324–15329. [Google Scholar] [CrossRef]
- Stienstra, R.; Joosten, L.A.B.; Koenen, T.; Van Tits, B.; Van Diepen, J.A.; Van Den Berg, S.A.A.; Rensen, P.C.N.; Voshol, P.J.; Fantuzzi, G.; Hijmans, A.; et al. The Inflammasome-Mediated Caspase-1 Activation Controls Adipocyte Differentiation and Insulin Sensitivity. Cell. Metab. 2010, 12, 593–605. [Google Scholar] [CrossRef]
- Vandanmagsar, B.; Youm, Y.H.; Ravussin, A.; Galgani, J.E.; Stadler, K.; Mynatt, R.L.; Ravussin, E.; Stephens, J.M.; Dixit, V.D. The NLRP3 Inflammasome Instigates Obesity-Induced Inflammation and Insulin Resistance. Nat. Med. 2011, 17, 179–189. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Dong, Y.Q.; Wang, P.; Zhang, X.; Yan, Y.; Sun, L.; Liu, B.; Zhang, D.; Zhang, H.; Liu, H.; et al. Adipocyte-Derived Lysophosphatidylcholine Activates Adipocyte and Adipose Tissue Macrophage Nod-Like Receptor Protein 3 Inflammasomes Mediating Homocysteine-Induced Insulin Resistance. EBioMedicine 2018, 31, 202–216. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pain, P.; Spinelli, F.; Gherardi, G. Mitochondrial Cation Signalling in the Control of Inflammatory Processes. Int. J. Mol. Sci. 2023, 24, 16724. https://doi.org/10.3390/ijms242316724
Pain P, Spinelli F, Gherardi G. Mitochondrial Cation Signalling in the Control of Inflammatory Processes. International Journal of Molecular Sciences. 2023; 24(23):16724. https://doi.org/10.3390/ijms242316724
Chicago/Turabian StylePain, Pampa, Francesca Spinelli, and Gaia Gherardi. 2023. "Mitochondrial Cation Signalling in the Control of Inflammatory Processes" International Journal of Molecular Sciences 24, no. 23: 16724. https://doi.org/10.3390/ijms242316724
APA StylePain, P., Spinelli, F., & Gherardi, G. (2023). Mitochondrial Cation Signalling in the Control of Inflammatory Processes. International Journal of Molecular Sciences, 24(23), 16724. https://doi.org/10.3390/ijms242316724





