Bisphenol A Effects on Neurons’ Neurochemical Character in the Urinary Bladder Intramural Ganglia of Domestic Pigs
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogala, D.; Kulik-Kupka, K.; Spychała, A.; Śnieżek, E.; Janicka, A.; Moskalenko, O. Bisfenol A—niebezpieczny związek ukryty w tworzywach sztucznych. Probl. Hig. Epidemiol. 2016, 97, 213–219. [Google Scholar]
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Ishikawa, K.; Sugiyama, K.; Furuta, H.; Nishimura, F. Content and release of bisphenol A from polycarbonate dental products. Dent. Mater. J. 2000, 19, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Czarczyńska-Goślińska, B.; Zgoła-Grześkowiak, A.; Jeszka-Skowron, M.; Frankowski, R.; Grześkowiak, T. Detection of bisphenol A, cumylphenol and parabens in surface waters of Greater Poland Voivodeship. J. Environ. Manag. 2017, 15, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Aloisi, A.M.; Della Seta, D.; Rendo, C.; Ceccarelli, I.; Scaramuzzino, A.; Farabollini, F. Exposure to the estrogenic pollutant bisphenol A affects pain behavior induced by subcutaneous formalin injection in male and female rats. Brain Res. 2002, 937, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bosch, R.J.; Quiroga, B.; Muñoz-Moreno, C.; Olea-Herrero, N.; Arenas, M.I.; González-Santander, M.; Reventún, P.; Zaragoza, C.; de Arriba, G.; Saura, M. Bisphenol A: An environmental factor implicated in renal vascular damage. Nefrologia 2016, 36, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Braniste, V.; Audebert, M.; Zalko, D.; Houdeau, E. Bisphenol A in the Gut: Another Break in the Wall? In Multi-System Endocrine Disruption, Research and Perspectives in Endocrine Interactions; Bourguignon, J.-P., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 127–144. [Google Scholar]
- MacLusky, N.J.; Hajszan, T.; Leranth, C. The environmental estrogen bisphenol A inhibits estradiol-induced hippocampal synaptogenesis. Environ. Health Perspect. 2005, 113, 675–679. [Google Scholar] [CrossRef]
- Sakazaki, H.; Ueno, H.; Nakamuro, K. Estrogen receptor alpha in mouse splenic lymphocytes: Possible involvement in immunity. Toxicol. Lett. 2002, 133, 221–229. [Google Scholar] [CrossRef]
- Nakamura, K.; Itoh, K.; Dai, H.; Han, L.; Wang, X.; Kato, S.; Sugimoto, T.; Fushiki, S. Prenatal and lactational exposure to low-doses of bisphenol A alters adult mice behavior. Brain Dev. 2011, 34, 57–63. [Google Scholar] [CrossRef]
- Viberg, H.; Fredriksson, A.; Buratovic, S.; Eriksson, P. Dose-dependent behavioral disturbances after a single neonatal bisphenol A dose. Toxicology 2011, 290, 187–194. [Google Scholar] [CrossRef]
- Priego, A.R.; Parra, E.G.; Mas, S.; Morgado-Pascual, J.L.; Ruiz-Ortega, M.; Rayego-Mateos, S. Bisphenol A Modulates Autophagy and Exacerbates Chronic Kidney Damage in Mice. Int. J. Mol. Sci. 2021, 22, 7189. [Google Scholar] [CrossRef] [PubMed]
- Kataria, A.; Trasande, L.; Trachtman, H. The effects of environmental chemicals on renal function. Nat. Rev. Nephrol. 2015, 11, 610–625. [Google Scholar] [CrossRef]
- Moreno-Gómez-Toledano, R.; Arenas, M.I.; Vélez-Vélez, E.; Coll, E.; Quiroga, B.; Bover, J.; Bosch, R.J. Bisphenol a Exposure and Kidney Diseases: Systematic Review, Meta-Analysis, and NHANES 03-16 Study. Biomolecules 2021, 11, 1046. [Google Scholar] [CrossRef] [PubMed]
- Tam, N.N.; Zhang, X.; Xiao, H.; Song, D.; Levin, L.; Meller, J.; Ho, S.M. Increased susceptibility of estrogen-induced bladder outlet obstruction in a novel mouse model. Lab. Investig. 2015, 95, 546–560. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Panadero, E.; Mas, S.; Civantos, E.; Abaigar, P.; Camarero, V.; Ruiz-Priego, A.; Ortiz, A.; Egido, J.; González-Parra, E. Bisphenol A is an exogenous toxin that promotes mitochondrial injury and death in tubular cells. Environ. Toxicol. 2017, 33, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Yokosuka, M.; Ohtani-Kaneko, R.; Yamashita, K.; Muraoka, D.; Kuroda, Y.; Watanabe, C. Estrogen and environmental estrogenic chemicals exert developmental effects on rat hypothalamic neurons and glias. Toxicol. Vitr. 2008, 22, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hajszan, T.; Leranth, C. Bisphenol A interferes with synaptic remodeling. Front. Neuroendocrinol. 2010, 31, 519–530. [Google Scholar] [CrossRef]
- Seki, S.; Aoki, M.; Hosokawa, T.; Saito, T.; Masuma, R.; Komori, M.; Kurasaki, M. Bisphenol-A suppresses neurite extension due to inhibition of phosphorylation of mitogenactivated protein kinase in PC12 cells. Chem. Biol. Interact. 2011, 194, 23–30. [Google Scholar] [CrossRef]
- Makowska, K.; Szymańska, K.; Palus, K.; Gonkowski, S.; Całka, J. Influence of bisfenol A on chemical coding of the nerve fibers of the cardiac apex in the domestic pig. Med. Wet. 2017, 73, 572–578. [Google Scholar] [CrossRef]
- Viberg, H.; Lee, I. A single exposure to bisphenol A alters the levels of important neuroproteins in adult male and female mice. Neurotoxicology 2012, 33, 1390–1395. [Google Scholar] [CrossRef]
- Yoshiyama, M.; de Groat, W.C. The role of vasoactive intestinal polypeptide and pituitary adenylate cyclase-activating polypeptide in the neural pathways controlling the lower urinary tract. J. Mol. Neurosci. 2008, 36, 227–2240. [Google Scholar] [CrossRef] [PubMed]
- Pirker, M.E.; Montedonico, S.; Rolle, U.; Austvoll, H.; Puri, P. Regional differences in nitrergic neuronal density in the developing porcine urinary bladder. Pediatr. Surg. Int. 2005, 21, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Mazur, U.; Lepiarczyk, E.; Janikiewicz, P.; Bossowska, A. Somatostatin immunoreactivity within the urinary bladder nerve fibers and paracervical ganglion urinary bladder projecting neurons in the female pig. J. Chem. Neuroanat. 2021, 117, 102007. [Google Scholar] [CrossRef]
- Janikiewicz, P.; Wasilewska, B.; Mazur, U.; Franke-Radowiecka, A.; Majewski, M.; Bossowska, A. The Influence of an Adrenergic Antagonist Guanethidine (GUA) on the Distribution Pattern and Chemical Coding of Dorsal Root Ganglia (DRG) Neurons Supplying the Porcine Urinary Bladder. Int. J. Mol. Sci. 2021, 22, 13399. [Google Scholar] [CrossRef] [PubMed]
- Smith-Anttila, C.J.A.; Morrison, V.; Keast, J.R. Spatiotemporal mapping of sensory and motor innervation of the embryonic and postnatal mouse urinary bladder. Dev. Biol. 2021, 476, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Pidsudko, Z. Immunohistochemical characteristics and distribution of sensory dorsal root Ganglia neurons supplying the urinary bladder in the male pig. J. Mol. Neurosci. 2014, 52, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.S.; Gilpin, S.A.; Gilpin, C.J.; Gosling, J.A. Intramural ganglia of the human urinary bladder. Br. J. Urol. 1983, 55, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.S.; Jen, P.Y.; Gosling, J.A. A double-label immunohistochemical study of intramural ganglia from the human male urinary bladder neck. J. Anat. 1997, 190, 125–134. [Google Scholar] [CrossRef]
- Pidsudko, Z. Distribution and chemical coding of neurons in intramural ganglia of the porcine urinary bladder trigone. Folia Histochem. Cytobiol. 2004, 42, 3–11. [Google Scholar]
- Pidsudko, Z. Immunohistochemical characteristics and distribution of neurons in the intramural ganglia supplying the urinary bladder in the male pig. Pol. J. Vet. Sci. 2013, 16, 629–638. [Google Scholar] [CrossRef]
- Zhou, Y.; Ling, E.A. Nitric oxide synthase—Its distribution and alteration in the intramural ganglia of the urinary bladder in normal and urethra-obstructed guinea pigs. Ann. Acad. Med. Singap. 1999, 28, 49–61. [Google Scholar]
- Arrighi, S.; Bosi, G.; Cremonesi, F.; Domeneghini, C. Immunohistochemical study of the pre- and postnatal innervation of the dog lower urinary tract: Morphological aspects at the basis of the consolidation of the micturition reflex. Vet. Res. Commun. 2008, 32, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Lech, P.; Majewski, M.; Rychlik, A.; Gonkowski, S. Bisphenol A affects vipergic nervous structures in the porcine urinary bladder trigone. Sci. Rep. 2021, 11, 12147. [Google Scholar] [CrossRef] [PubMed]
- Zvarova, K.; Vizzard, M.A. Distribution and fate of cocaine- and amphetamine-regulated transcript peptide (CARTp)-expressing cells in rat urinary bladder: A developmental study. J. Comp. Neurol. 2005, 489, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Yanai, Y.; Hashitani, H.; Hayase, M.; Sasaki, S.; Suzuki, H.; Kohri, K. Role of nitric oxide/cyclic GMP pathway in regulating spontaneous excitations in detrusor smooth muscle of the guinea-pig bladder. Neurourol. Urodyn. 2008, 27, 446–453. [Google Scholar] [CrossRef]
- Honda, M.; Yoshimura, N.; Inoue, S.; Hikita, K.; Muraoka, K.; Saito, M.; Chancellor, M.B.; Takenaka, A. Inhibitory role of the spinal galanin system in the control of micturition. Urology 2013, 82, 1188.e9–1188.e13. [Google Scholar] [CrossRef]
- Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 2011, 11, 776–793. [Google Scholar] [CrossRef]
- Lepiarczyk, E.; Majewski, M.; Bossowska, A. The influence of intravesical administration of resiniferatoxin (RTX) on the chemical coding of sympathetic chain ganglia (SChG) neurons supplying the porcine urinary bladder. Histochem. Cell Biol. 2015, 144, 479–489. [Google Scholar] [CrossRef]
- Bossowska, A.; Majewski, M. Tetrodotoxin induced changes in the chemical coding of dorsal root ganglion neurons supplying the porcine urinary bladder. Pol. J. Vet. Sci. 2012, 15, 355–363. [Google Scholar] [CrossRef]
- Vesela, R.; Aronsson, P.; Andersson, M.; Wsol, V.; Tobin, G. The potential of non-adrenergic, non-cholinergic targets in the treatment of interstitial cystitis/painful bladder syndrome. J. Physiol. Pharmacol. 2012, 63, 209–216. [Google Scholar]
- Lepiarczyk, E.; Bossowska, A.; Kaleczyc, J.; Skowrońska, A.; Majewska, M.; Majewski, M. The Influence of Resiniferatoxin (RTX) and Tetrodotoxin (TTX) on the Distribution, Relative Frequency, and Chemical Coding of Noradrenergic and Cholinergic Nerve Fibers Supplying the Porcine Urinary Bladder Wall. Toxins 2017, 9, 310. [Google Scholar] [CrossRef] [PubMed]
- Lepiarczyk, E.; Dudek, A.; Kaleczyc, J.; Majewski, M.; Markiewicz, W.; Radziszewski, P.; Bossowska, A. The influence of resiniferatoxin on the chemical coding of caudal mesenteric ganglion neurons supplying the urinary bladder in the pig. J. Physiol. Pharmacol. 2016, 67, 625–632. [Google Scholar] [PubMed]
- Zacharko-Siembida, A.; Arciszewski, M. Cocaine- and amphetamine-regulated transcript-like immunoreactivity (CART-LI) in intramural ganglia of porcine urinary bladder trigone. Med. Wet. 2014, 70, 594–598. [Google Scholar]
- Steinhoff, M.S.; von Mentzer, B.; Geppetti, P.; Pothoulakis, C.; Bunnett, N.W. Tachykinins and their receptors: Contributions to physiological control and the mechanisms of disease. Physiol. Rev. 2014, 94, 265–301. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhao, C.; Zhong, H.; Zhang, S.; Xia, Y.; Cai, Z. Bisphenol S induced epigenetic and transcriptional changes in human breast cancer cell line MCF-7. Environ. Pollut. 2019, 246, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, S.; Manabe, N.; Nishizawa, H.; Morita, M.; Sugimoto, M.; Iwahori, M.; Miyamoto, H. Effects of oral exposure of bisphenol A on mRNA expression of nuclear receptors in murine placentae assessed by DNA microarray. J. Reprod. Dev. 2003, 49, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.; Mulligan, K. Does Bisphenol A Confer Risk of Neurodevelopmental Disorders? What We Have Learned from Developmental Neurotoxicity Studies in Animal Models. Int. J. Mol. Sci. 2022, 23, 2894. [Google Scholar] [CrossRef] [PubMed]
- Soriano, S.; Ripoll, C.; Alonso-Magdalena, P.; Fuentes, E.; Quesada, I.; Nadal, A.; Martinez-Pinna, J. Effects of Bisphenol A on ion channels: Experimental evidence and molecular mechanisms. Steroids 2016, 111, 12–20. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, Z.; Ji, W. Bisphenol A induces apoptosis, oxidative stress and inflammatory response in colon and liver of mice in a mitochondria-dependent manner. Biomed. Pharmacother. 2019, 117, 109182. [Google Scholar] [CrossRef]
- Vasina, V.; Barbara, G.; Talamonti, L.; Stanghellini, V.; Corinaldesi, R.; Tonini, M.; De Ponti, F.; De Giorgio, R. Enteric neuroplasticity evoked by inflammation. Auton. Neurosci. 2006, 126–127, 264–272. [Google Scholar] [CrossRef]
- Chang, L.; Chen, Y.; Li, J.; Liu, Z.; Wang, Z.; Chen, J.; Cao, W.; Xu, Y. Cocaine-and amphetamine-regulated transcript modulates peripheral immunity and protects against brain injury in experimental stroke. Brain Behav. Immun. 2011, 25, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Fang, P.; Yu, M.; Wan, D.; Zhang, L.; Han, L.; Shen, Z.; Shi, M.; Zhu, Y.; Zhang, Z.; Bo, P. Regulatory effects of galanin system on development of several age-related chronic diseases. Exp. Gerontol. 2017, 95, 88–97. [Google Scholar] [CrossRef]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S. Vasoactive Intestinal Polypeptide in the Carotid Body-A History of Forty Years of Research. A Mini Review. Int. J. Mol. Sci. 2020, 21, 4692. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Gajęcka, M.; Makowska, K. Mycotoxins and the Enteric Nervous System. Toxins 2020, 12, 461. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Matsuyama, H.; Shiina, T.; Takewaki, T.; Furness, J.B. Tachykinins and their functions in the gastrointestinal tract. Cell. Mol. Life Sci. 2008, 65, 295–311. [Google Scholar] [CrossRef]
- Adamus, M. Wpływ Substancji P na komórki krwi [Substance P as a regulatory peptide of hematopoiesis and blood cell functions]. Postep. Hig. Med. Dosw. (Online) 2009, 63, 106–113. (In Polish) [Google Scholar]
- Suvas, S. Role of substance P neuropeptide in inflammation, wound healing, and tissue homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef]
- Koller, A.; Bianchini, R.; Schlager, S.; Münz, C.; Kofler, B.; Wiesmayr, S. The neuropeptide galanin modulates natural killer cell function. Neuropeptides 2017, 64, 109–115. [Google Scholar] [CrossRef]
- Su, Y.; Ganea, D.; Peng, X.; Jonakait, G.M. Galanin down-regulates microglial tumor necrosis factor-alpha production by a post-transcriptional mechanism. J. Neuroimmunol. 2003, 134, 52–60. [Google Scholar] [CrossRef]
- Palmieri, E.M.; McGinity, C.; Wink, D.A.; McVicar, D.W. Nitric Oxide in Macrophage Immunometabolism: Hiding in Plain Sight. Metabolites 2020, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, K.; Calka, J.; Gonkowski, S. Nitric oxide as an active substance in the enteric neurons of the porcine digestive tract in physiological conditions and under intoxication with bisphenol A (BPA). Nitric Oxide 2018, 80, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, K.; Makowska, K.; Gonkowski, S. The Influence of High and Low Doses of Bisphenol A (BPA) on the Enteric Nervous System of the Porcine Ileum. Int. J. Mol. Sci. 2018, 19, 917. [Google Scholar] [CrossRef]
- Dixit, D.; Singh, S.K.; Tiwari, A.K.; Mandal, M.B. Effects of chronic ingestion of Bisphenol A on gut contractility in rats. J. Physiol. Pharm. Pharmacol. 2017, 7, 1109–1115. [Google Scholar] [CrossRef]
- Gupta, H.; Deshpande, S.B. Bisphenol A decreases the spontaneous contractions of rat uterus in vitro through a nitrergic mechanism. J. Basic Clin. Physiol. Pharmacol. 2018, 29, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, K.; Tarafder, P.; Nath, P.P.; Mondal, M.; Paul, G. Bisphenol A inhibits the motor function of duodenal smooth muscle in rat. GSTF J. Adv. Med. Res. 2018, 1, 34–38. [Google Scholar] [CrossRef]
- Kabakçi, R.; Macun, H.C.; Polat, İ.M.; Yıldırım, E. Inhibitory effect of Bisphenol A on in vitro feline uterine contractions. Anim. Reprod. Sci. 2019, 205, 27–33. [Google Scholar] [CrossRef]
- Zygmuntowicz, A.; Markiewicz, W.; Grabowski, T.; Jaroszewski, J. Effects of Bisphenol A and Bisphenol F on Porcine Uterus Contractility. J. Vet. Res. 2022, 66, 257–265. [Google Scholar] [CrossRef]
- An, B.S.; Ahn, H.J.; Kang, H.S.; Jung, E.M.; Yang, H.; Hong, E.J.; Jeung, E.B. Effects of estrogen and estrogenic compounds, 4-tert-octylphenol, and bisphenol A on the uterine contraction and contraction-associated proteins in rats. Mol. Cell. Endocrinol. 2013, 375, 27–34. [Google Scholar] [CrossRef]
- Sarkar, K.; Tarafder, P.; Paul, G. Bisphenol A inhibits duodenal movement ex vivo of rat through nitric oxide-mediated soluble guanylyl cyclase and α-adrenergic signaling pathways. J. Appl. Toxicol. 2016, 36, 131–139. [Google Scholar] [CrossRef]
- Van Geldre, L.A.; Lefebvre, R.A. Interaction of NO and VIP in gastrointestinal smooth muscle relaxation. Curr. Pharm. Des. 2004, 10, 2483–2497. [Google Scholar] [CrossRef] [PubMed]
- Opinion of the Scientific Panel on food additives, flavorings, processing aids and materials in contact with food on a request from the Commission to 2,2-bis(4-hydroxyphenyl) propane (Bisphenol A). EFSA J. 2006, 428, 1–75.
- Bisphenol A: EFSA Draft Opinion Proposes Lowering the Tolerable Daily Intake. 2021. Available online: https://www.efsa.europa.eu/en/news/bisphenol-efsa-draft-opinion-proposes-lowering-tolerable-daily-intake (accessed on 28 March 2022).
- Almeida, S.; Raposo, A.; Almeida-Gonzales, M.; Carrascosa, C. Bisphenol A: Food exposure and impact on human health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef]
- Fleisch, A.F.; Sheffield, P.E.; Chinn, C.; Edelstein, B.L.; Landrigan, P.J. Bisphenol A and related compounds in dental materials. Pediatrics 2010, 126, 760–768. [Google Scholar] [CrossRef]
- Staples, C.A.; Dorn, P.B.; Klecka, G.M.; O’Block, S.T.; Harris, L.R. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 1998, 36, 2149–2173. [Google Scholar] [CrossRef]
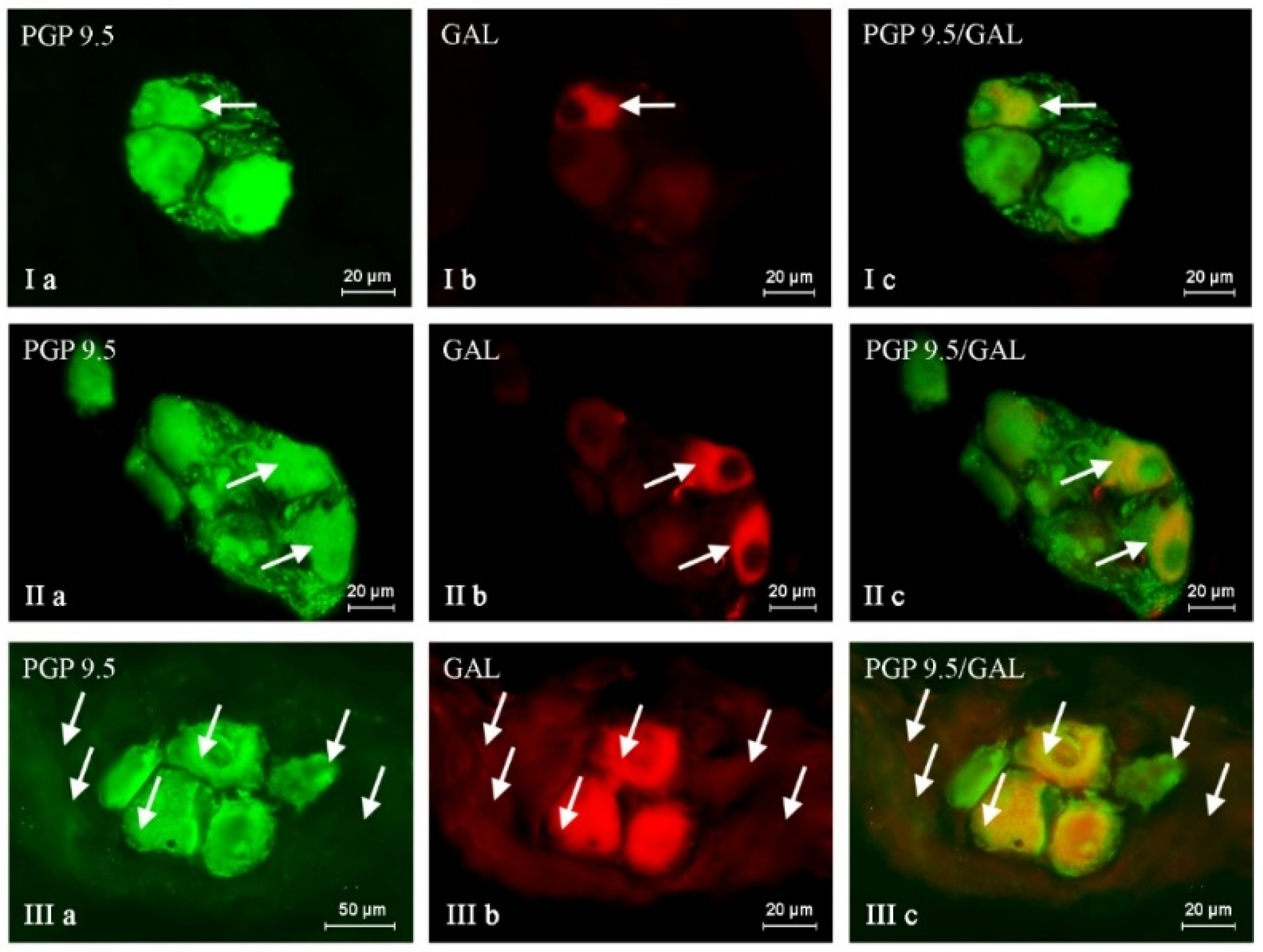
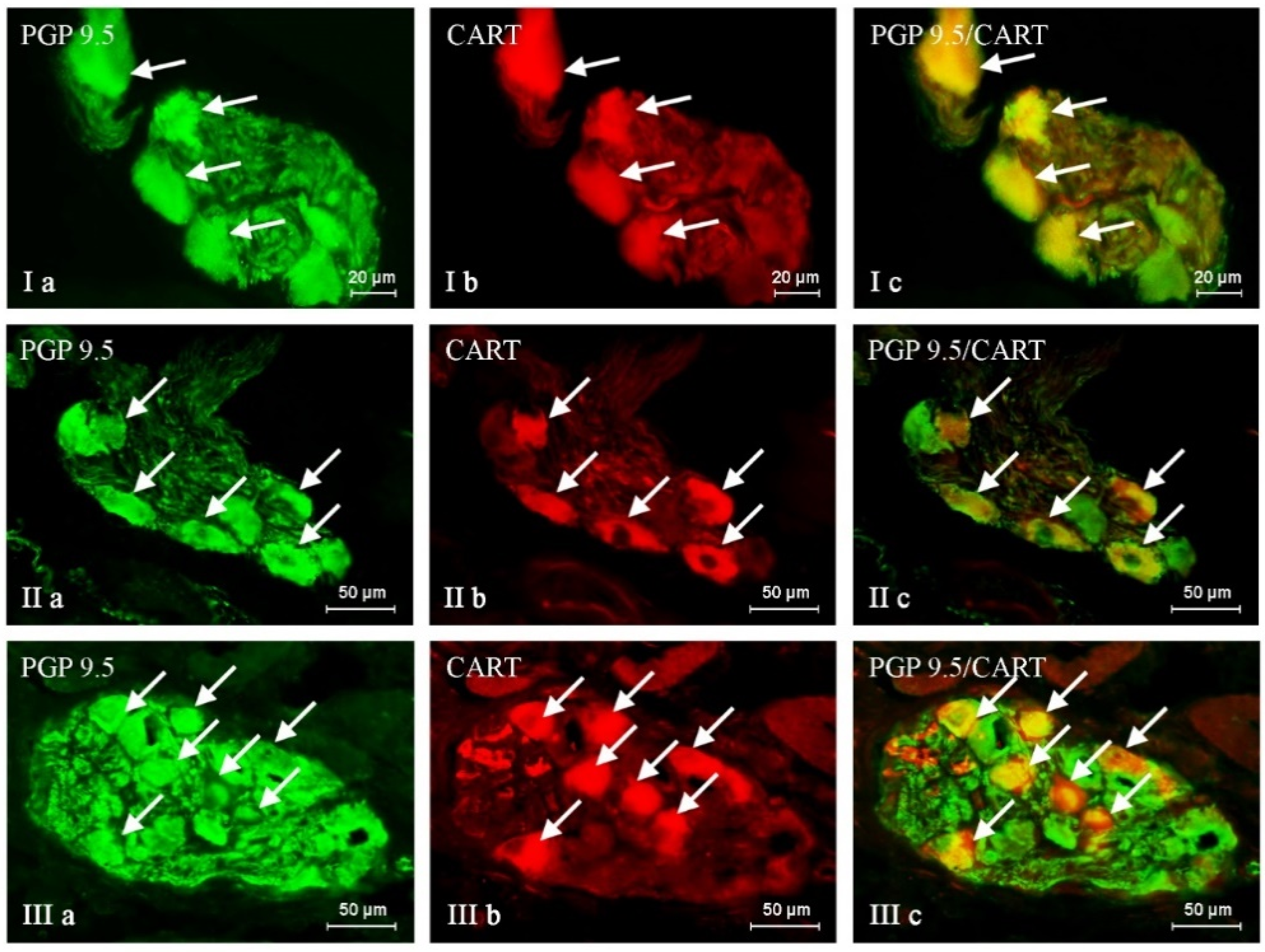
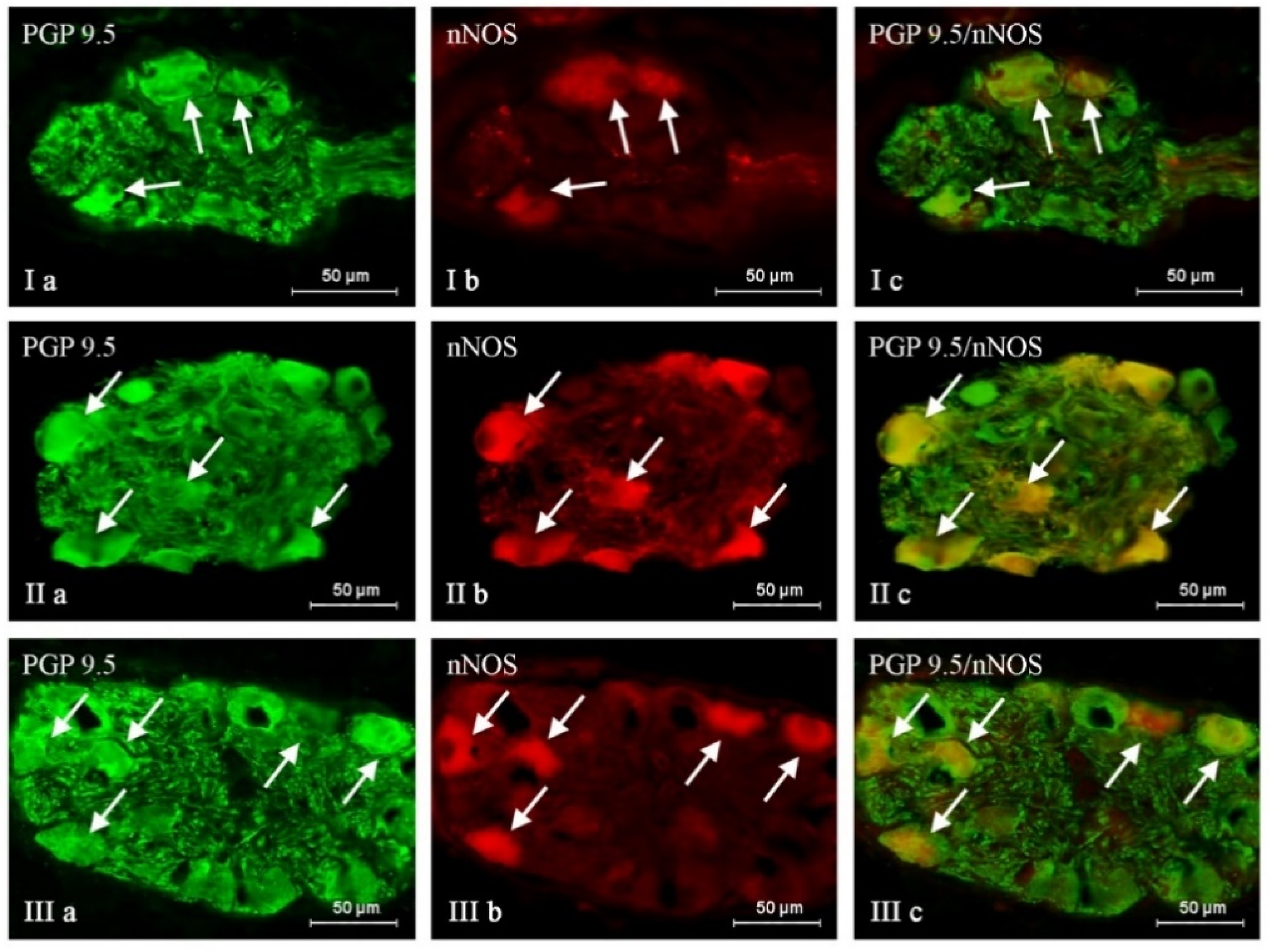

| PGP/SP | PGP/GAL | PGP/nNOS | PGP/CART | |
|---|---|---|---|---|
| C | 20.70 ± 0.82 | 26.16 ± 0.74 | 43.72 ± 1.20 | 38.65 ± 0.30 |
| BPA 1 | 23.86 ± 0.40 * | 35.29 ± 1.16 * | 47.49 ± 0.30 * | 44.95 ± 0.57 * |
| BPA 2 | 34.83 ± 0.64 * | 39.57 ± 0.48 * | 49.60 ± 1.08 * | 49.05 ± 1.70 * |
| PGP/SP | PGP/GAL | PGP/nNOS | PGP/CART | |
|---|---|---|---|---|
| BPA 1 | +3.16 | +9.13 | +3.77 | +6.3 |
| BPA 2 | +14.13 | +13.41 | +5.88 | +10.4 |
| Primary Antibodies | ||||
|---|---|---|---|---|
| Antigen | Code | Species | Working Dilution | Supplier |
| PGP 9,5 | 7863-2004 | Mouse | 1:1000 | Biogenesis Ltd., Poole, UK |
| SP | 8450-0505 | Rat | 1:1000 | Bio-Rad (AbD Serotec), Kidlington, UK |
| CART | 1-003-61 | Rabbit | 1:8000 | Phoenix Pharmaceuticals, INC, Belmont, CA, USA |
| GAL | T-5036 | Guinea Pig | 1:2000 | Peninsula, New York, NY, USA |
| nNOS | AB5380 | Rabbit | 1:6000 | MercMillipore, DEU, İzmir, Turkey |
| Secondary antibodies | ||||
| Reagents | Working dilution | Supplier | ||
| Alexa fluor 488 donkey anti-mouse IgG | 1:1000 | Invitrogen, Carlsbad, CA, USA | ||
| Alexa fluor 546 donkey anti-rabbit IgG | 1:1000 | Invitrogen | ||
| Alexa fluor 546 donkey anti-rat IgG | 1:1000 | Invitrogen | ||
| Alexa fluor 546 donkey anti-guinea pig IgG | 1:1000 | Invitrogen | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makowska, K.; Lech, P.; Gonkowski, S. Bisphenol A Effects on Neurons’ Neurochemical Character in the Urinary Bladder Intramural Ganglia of Domestic Pigs. Int. J. Mol. Sci. 2023, 24, 16792. https://doi.org/10.3390/ijms242316792
Makowska K, Lech P, Gonkowski S. Bisphenol A Effects on Neurons’ Neurochemical Character in the Urinary Bladder Intramural Ganglia of Domestic Pigs. International Journal of Molecular Sciences. 2023; 24(23):16792. https://doi.org/10.3390/ijms242316792
Chicago/Turabian StyleMakowska, Krystyna, Piotr Lech, and Sławomir Gonkowski. 2023. "Bisphenol A Effects on Neurons’ Neurochemical Character in the Urinary Bladder Intramural Ganglia of Domestic Pigs" International Journal of Molecular Sciences 24, no. 23: 16792. https://doi.org/10.3390/ijms242316792





