Changes of Ex Vivo Cervical Epithelial Cells Due to Electroporation with JMY
Abstract
1. Introduction
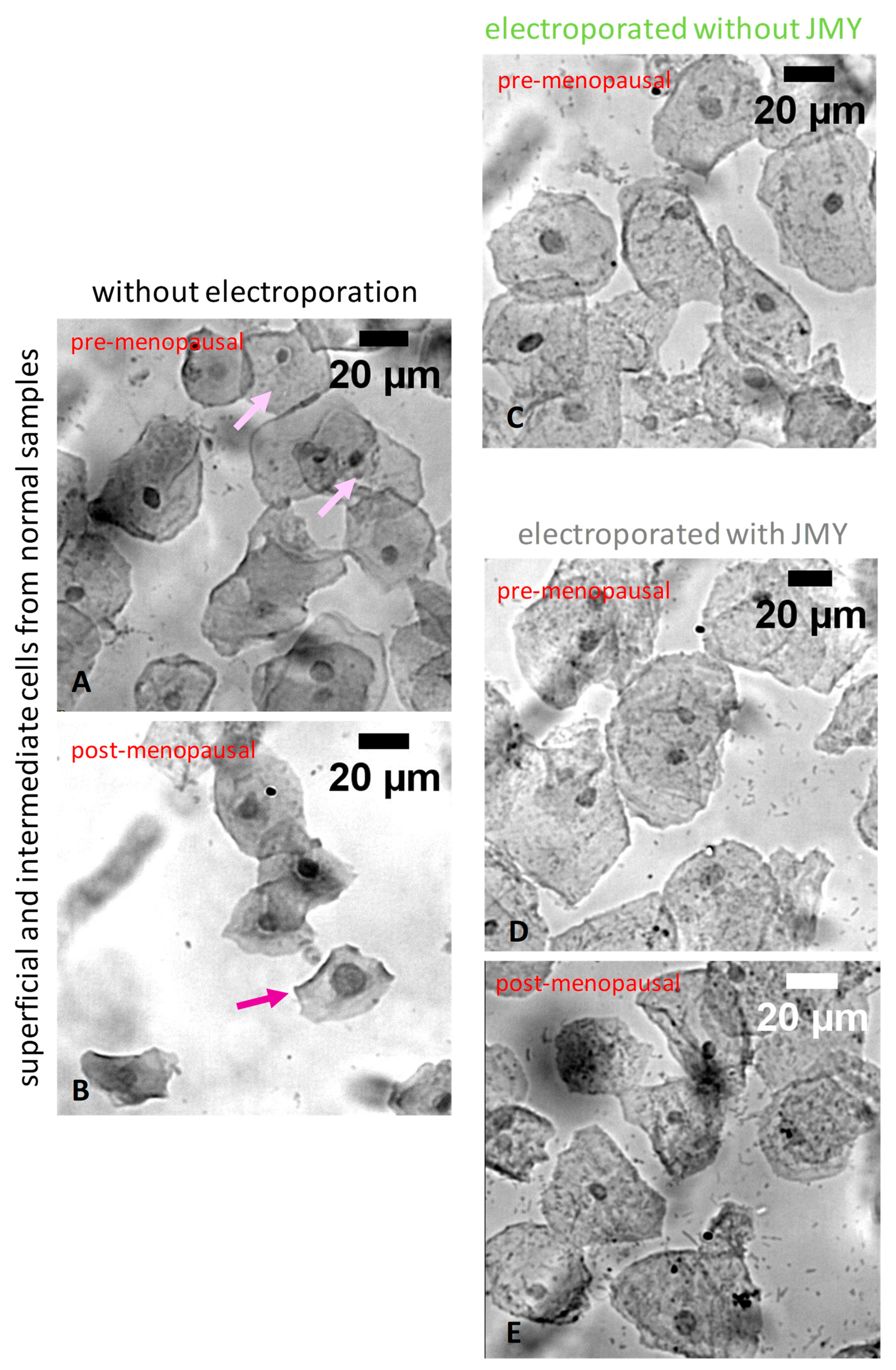
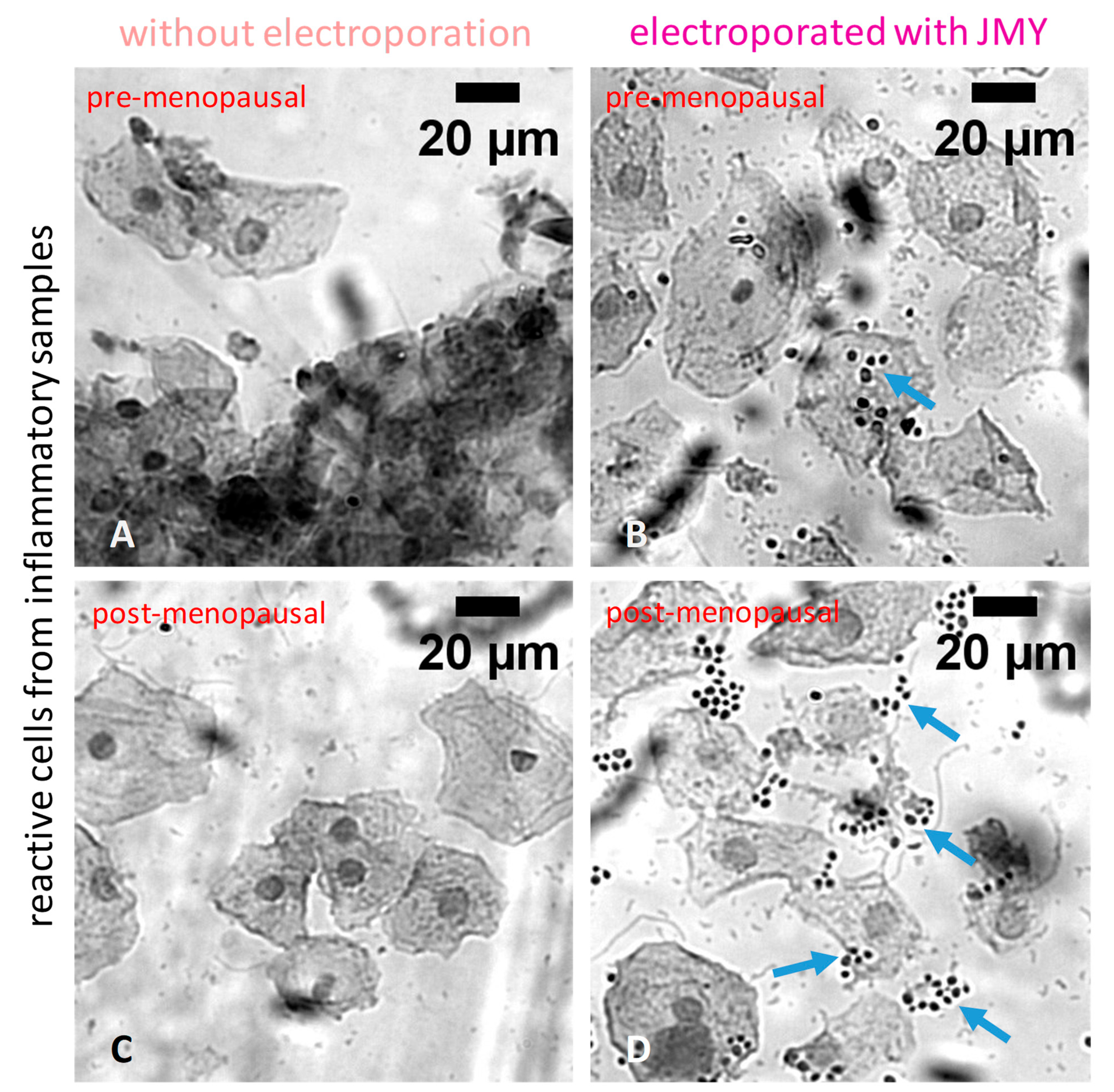
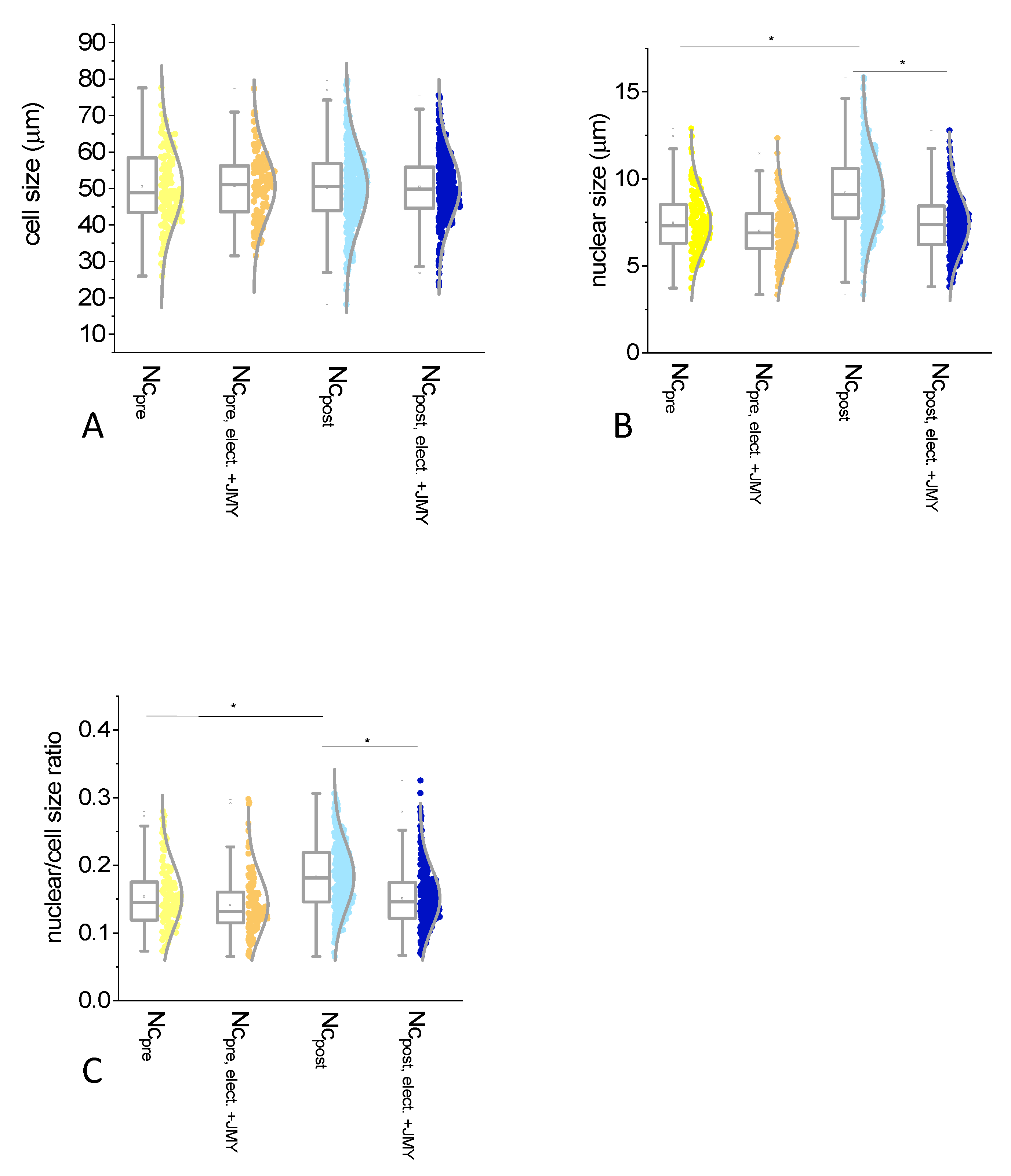
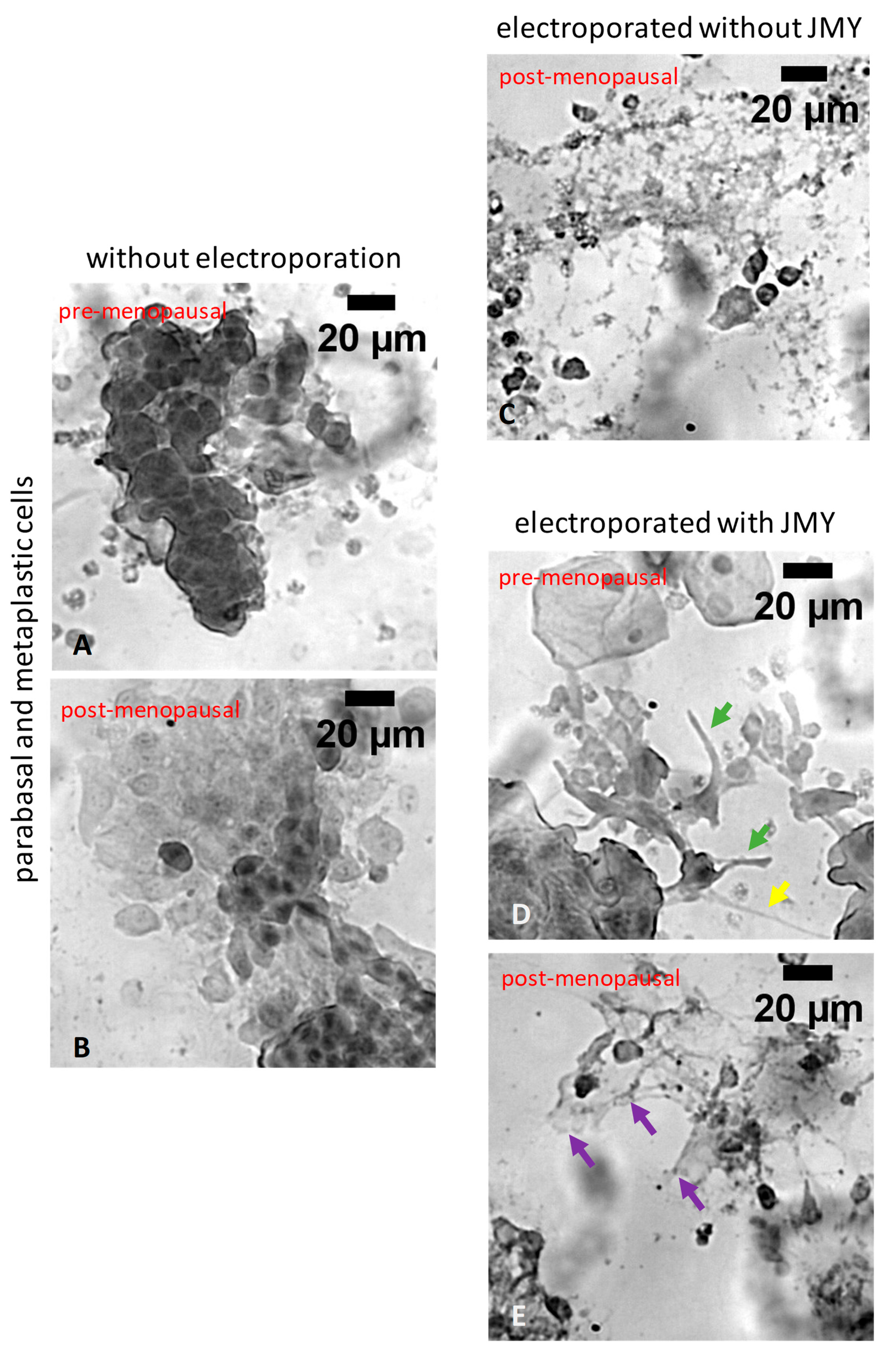
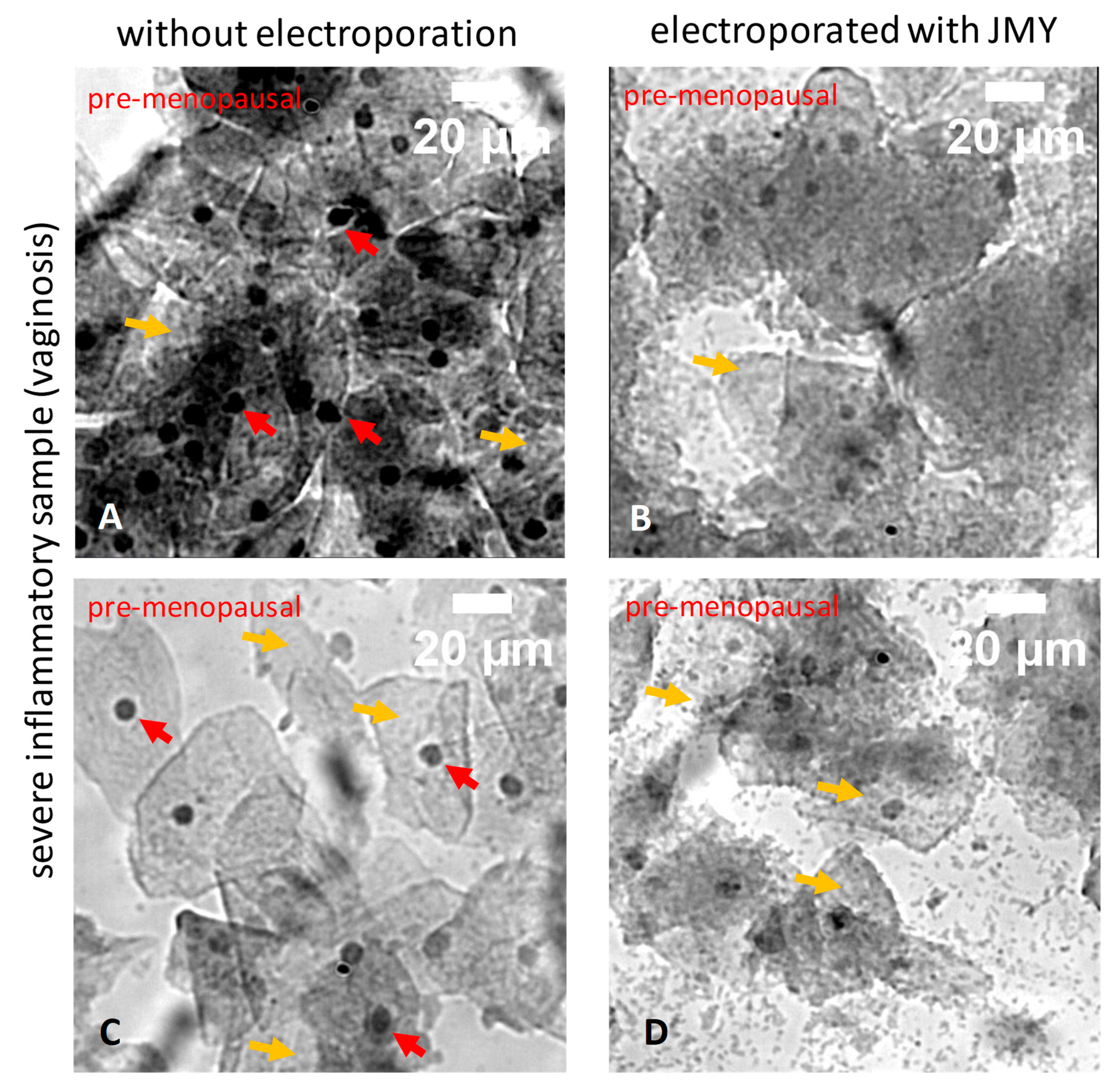
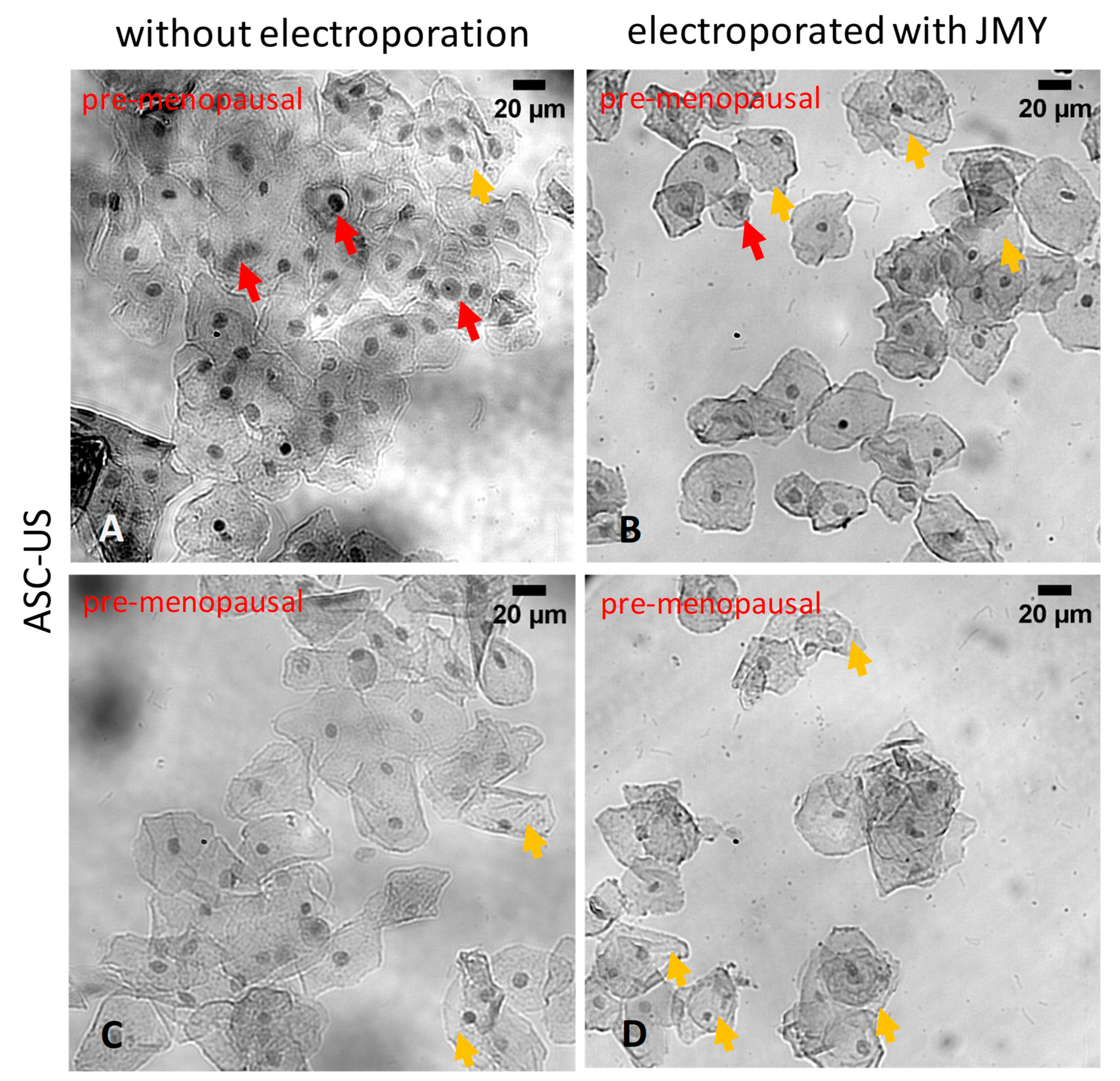
2. Results
3. Discussion
4. Materials and Methods
4.1. Proteins
4.2. Ex Vivo Epithelial Cells
4.3. Low-Voltage Electroporation to Increase the Intracellular Level of JMY
4.4. Microscopy
4.5. Image Analysis
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atif, R.M.; Shahid, L.; Waqas, M.; Ali, B.; Rashid, M.A.R.; Azeem, F.; Nawaz, M.A.; Wani, S.H.; Chung, G. Insights on Calcium-Dependent Protein Kinases (CPKs) Signaling for Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 5298. [Google Scholar] [CrossRef]
- Welch, W.J. How cells respond to stress. Sci. Am. 1993, 268, 56–64. [Google Scholar] [CrossRef]
- Amaya, M.J.; Oliveira, A.G.; Guimaraes, E.S.; Casteluber, M.C.; Carvalho, S.M.; Andrade, L.M.; Pinto, M.C.; Mennone, A.; Oliveira, C.A.; Resende, R.R.; et al. The insulin receptor translocates to the nucleus to regulate cell proliferation in liver. Hepatology 2014, 59, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Arantes, L.A.; Aguiar, C.J.; Amaya, M.J.; Figueiro, N.C.; Andrade, L.M.; Rocha-Resende, C.; Resende, R.R.; Franchini, K.G.; Guatimosim, S.; Leite, M.F. Nuclear inositol 1,4,5-trisphosphate is a necessary and conserved signal for the induction of both pathological and physiological cardiomyocyte hypertrophy. J. Mol. Cell. Cardiol. 2012, 53, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Resende, R.R.; Andrade, L.M.; Oliveira, A.G.; Guimaraes, E.S.; Guatimosim, S.; Leite, M.F. Nucleoplasmic calcium signaling and cell proliferation: Calcium signaling in the nucleus. Cell Commun. Signal. CCS 2013, 11, 14. [Google Scholar] [CrossRef]
- Oliveira, A.G.; Guimaraes, E.S.; Andrade, L.M.; Menezes, G.B.; Fatima Leite, M. Decoding calcium signaling across the nucleus. Physiology 2014, 29, 361–368. [Google Scholar] [CrossRef][Green Version]
- Heeg, U.; Dienes, H.P.; Muller, S.; Falke, D. Involvement of actin-containing microfilaments in HSV-induced cytopathology and the influence of inhibitors of glycosylation. Arch. Virol. 1986, 91, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-G.; Niu, J.-T.; Wu, H.-W.; Si, X.-L.; Zhang, S.-J.; Li, D.-H.; Bian, T.-T.; Li, Y.-F.; Yan, X.-K. Actin-Binding Proteins as Potential Biomarkers for Chronic Inflammation-Induced Cancer Diagnosis and Therapy. Anal. Cell. Pathol. 2021, 2021, 6692811. [Google Scholar] [CrossRef]
- Klages-Mundt, N.L.; Kumar, A.; Zhang, Y.; Kapoor, P.; Shen, X. The Nature of Actin-Family Proteins in Chromatin-Modifying Complexes. Front. Genet. 2018, 9, 398. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.J.; McMahon, H.T. Mediation, modulation, and consequences of membrane-cytoskeleton interactions. Annu. Rev. Biophys. 2008, 37, 65–95. [Google Scholar] [CrossRef]
- Ebata, T.; Hirata, H.; Kawauchi, K. Functions of the Tumor Suppressors p53 and Rb in Actin Cytoskeleton Remodeling. BioMed Res. Int. 2016, 2016, 9231057. [Google Scholar] [CrossRef]
- Antonangeli, F.; Grimsholm, O.; Rossi, M.N.; Velotti, F. Editorial: Cellular Stress and Inflammation: How the Immune System Drives Tissue Homeostasis. Front. Immunol. 2021, 12, 668876. [Google Scholar] [CrossRef]
- Wacquier, B.; Combettes, L.; Dupont, G. Cytoplasmic and Mitochondrial Calcium Signaling: A Two-Way Relationship. Cold Spring Harb. Perspect. Biol. 2019, 11, a035139. [Google Scholar] [CrossRef]
- Lechuga, S.; Ivanov, A.I. Actin cytoskeleton dynamics during mucosal inflammation: A view from broken epithelial barriers. Curr. Opin. Physiol. 2021, 19, 10–16. [Google Scholar] [CrossRef]
- Adighibe, O.; Pezzella, F. The Role of JMY in p53 Regulation. Cancers 2018, 10, 173. [Google Scholar] [CrossRef]
- Hencz, A.; Szabo-Meleg, E.; Dayo, M.Y.; Bilibani, A.; Barko, S.; Nyitrai, M.; Szatmari, D. The p53 and Calcium Regulated Actin Rearrangement in Model Cells. Int. J. Mol. Sci. 2022, 23, 9078. [Google Scholar] [CrossRef] [PubMed]
- Coutts, A.S.; Weston, L.; La Thangue, N.B. A transcription co-factor integrates cell adhesion and motility with the p53 response. Proc. Natl. Acad. Sci. USA 2009, 106, 19872–19877. [Google Scholar] [CrossRef] [PubMed]
- Coutts, A.S.; Boulahbel, H.; Graham, A.; La Thangue, N.B. Mdm2 targets the p53 transcription cofactor JMY for degradation. EMBO Rep. 2007, 8, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Zuchero, J.B.; Coutts, A.S.; Quinlan, M.E.; Thangue, N.B.; Mullins, R.D. p53-cofactor JMY is a multifunctional actin nucleation factor. Nat. Cell Biol. 2009, 11, 451–459. [Google Scholar] [CrossRef]
- Thompson, C.C.; Ashcroft, F.J.; Patel, S.; Saraga, G.; Vimalachandran, D.; Prime, W.; Campbell, F.; Dodson, A.; Jenkins, R.E.; Lemoine, N.R.; et al. Pancreatic cancer cells overexpress gelsolin family-capping proteins, which contribute to their cell motility. Gut 2007, 56, 95–106. [Google Scholar] [CrossRef]
- Shieh, D.B.; Godleski, J.; Herndon, J.E., 2nd; Azuma, T.; Mercer, H.; Sugarbaker, D.J.; Kwiatkowski, D.J. Cell motility as a prognostic factor in Stage I nonsmall cell lung carcinoma: The role of gelsolin expression. Cancer 1999, 85, 47–57. [Google Scholar] [CrossRef]
- Visapaa, H.; Bui, M.; Huang, Y.; Seligson, D.; Tsai, H.; Pantuck, A.; Figlin, R.; Rao, J.Y.; Belldegrun, A.; Horvath, S.; et al. Correlation of Ki-67 and gelsolin expression to clinical outcome in renal clear cell carcinoma. Urology 2003, 61, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Li, W.X.; Yang, M.X.; Hong, X.Q.; Dong, T.G.; Yi, T.; Lin, S.L.; Qin, X.Y.; Niu, W.X. Overexpression of gelsolin reduces the proliferation and invasion of colon carcinoma cells. Mol. Med. Rep. 2016, 14, 3059–3065. [Google Scholar] [CrossRef]
- Stock, A.M.; Klee, F.; Edlund, K.; Grinberg, M.; Hammad, S.; Marchan, R.; Cadenas, C.; Niggemann, B.; Zanker, K.S.; Rahnenfuhrer, J.; et al. Gelsolin Is Associated with Longer Metastasis-free Survival and Reduced Cell Migration in Estrogen Receptor-positive Breast Cancer. Anticancer Res. 2015, 35, 5277–5285. [Google Scholar] [PubMed]
- Guo, Y.; Zhang, H.; Xing, X.; Wang, L.; Zhang, J.; Yan, L.; Zheng, X.; Zhang, M. Gelsolin regulates proliferation, apoptosis and invasion in natural killer/T-cell lymphoma cells. Biol. Open 2018, 7, bio027557. [Google Scholar]
- Winston, J.S.; Asch, H.L.; Zhang, P.J.; Edge, S.B.; Hyland, A.; Asch, B.B. Downregulation of gelsolin correlates with the progression to breast carcinoma. Breast Cancer Res. Treat. 2001, 65, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Dosaka-Akita, H.; Hommura, F.; Fujita, H.; Kinoshita, I.; Nishi, M.; Morikawa, T.; Katoh, H.; Kawakami, Y.; Kuzumaki, N. Frequent loss of gelsolin expression in non-small cell lung cancers of heavy smokers. Cancer Res. 1998, 58, 322–327. [Google Scholar] [PubMed]
- Tanaka, H.; Shirkoohi, R.; Nakagawa, K.; Qiao, H.; Fujita, H.; Okada, F.; Hamada, J.; Kuzumaki, S.; Takimoto, M.; Kuzumaki, N. siRNA gelsolin knockdown induces epithelial-mesenchymal transition with a cadherin switch in human mammary epithelial cells. Int. J. Cancer 2006, 118, 1680–1691. [Google Scholar] [CrossRef]
- Rao, J.; Seligson, D.; Visapaa, H.; Horvath, S.; Eeva, M.; Michel, K.; Pantuck, A.; Belldegrun, A.; Palotie, A. Tissue microarray analysis of cytoskeletal actin-associated biomarkers gelsolin and E-cadherin in urothelial carcinoma. Cancer 2002, 95, 1247–1257. [Google Scholar] [CrossRef]
- Shieh, D.B.; Chen, I.W.; Wei, T.Y.; Shao, C.Y.; Chang, H.J.; Chung, C.H.; Wong, T.Y.; Jin, Y.T. Tissue expression of gelsolin in oral carcinogenesis progression and its clinicopathological implications. Oral Oncol. 2006, 42, 599–606. [Google Scholar] [CrossRef]
- D’Souza-Schorey, C. Disassembling adherens junctions: Breaking up is hard to do. Trends Cell Biol. 2005, 15, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Jeanes, A.; Gottardi, C.J.; Yap, A.S. Cadherins and cancer: How does cadherin dysfunction promote tumor progression? Oncogene 2008, 27, 6920–6929. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.H.; Tang, V.W.; Brieher, W.M. Actin protrusions push at apical junctions to maintain E-cadherin adhesion. Proc. Natl. Acad. Sci. USA 2020, 117, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Adighibe, O.; Turley, H.; Leek, R.; Harris, A.; Coutts, A.S.; La Thangue, N.; Gatter, K.; Pezzella, F. JMY protein, a regulator of P53 and cytoplasmic actin filaments, is expressed in normal and neoplastic tissues. Virchows Arch. Int. J. Pathol. 2014, 465, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Wilbur, D.C.; Nayar, R. Bethesda 2014: Improving on a paradigm shift. Cytopathology 2015, 26, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Nayar, R.; Wilbur, D.C. The Bethesda System for Reporting Cervical Cytology: A Historical Perspective. Acta Cytol. 2017, 61, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Alrajjal, A.; Pansare, V.; Choudhury, M.S.R.; Khan, M.Y.A.; Shidham, V.B. Squamous intraepithelial lesions (SIL: LSIL, HSIL, ASCUS, ASC-H, LSIL-H) of Uterine Cervix and Bethesda System. CytoJournal 2021, 18, 16. [Google Scholar] [CrossRef]
- Solomon, D.; Davey, D.; Kurman, R.; Moriarty, A.; O'Connor, D.; Prey, M.; Raab, S.; Sherman, M.; Wilbur, D.; Wright, T., Jr.; et al. The 2001 Bethesda System: Terminology for reporting results of cervical cytology. JAMA 2002, 287, 2114–2119. [Google Scholar] [CrossRef]
- IARC. Histopathology of the Uterine Cervix-Digital Atlas. WHO, IARC. 2023. Available online: https://screening.iarc.fr/atlascopyright.php (accessed on 15 August 2023).
- Papa, L.; Djedaini, M.; Hoffman, R. Ex vivo HSC expansion challenges the paradigm of unidirectional human hematopoiesis. Ann. N. Y. Acad. Sci. 2020, 1466, 39–50. [Google Scholar] [CrossRef]
- Schopow, N.; Kallendrusch, S.; Gong, S.; Rapp, F.; Korfer, J.; Gericke, M.; Spindler, N.; Josten, C.; Langer, S.; Bechmann, I. Examination of ex-vivo viability of human adipose tissue slice culture. PLoS ONE 2020, 15, e0233152. [Google Scholar] [CrossRef]
- Daviaud, N.; Garbayo, E.; Sindji, L.; Martinez-Serrano, A.; Schiller, P.C.; Montero-Menei, C.N. Survival, differentiation, and neuroprotective mechanisms of human stem cells complexed with neurotrophin-3-releasing pharmacologically active microcarriers in an ex vivo model of Parkinson's disease. Stem Cells Transl. Med. 2015, 4, 670–684. [Google Scholar] [CrossRef] [PubMed]
- Halasz, H.; Ghadaksaz, A.R.; Madarasz, T.; Huber, K.; Harami, G.; Toth, E.A.; Osteikoetxea-Molnar, A.; Kovacs, M.; Balogi, Z.; Nyitrai, M.; et al. Live cell superresolution-structured illumination microscopy imaging analysis of the intercellular transport of microvesicles and costimulatory proteins via nanotubes between immune cells. Methods Appl. Fluoresc. 2018, 6, 045005. [Google Scholar] [CrossRef]
- McRae, M.P.; Kerr, A.R.; Janal, M.N.; Thornhill, M.H.; Redding, S.W.; Vigneswaran, N.; Kang, S.K.; Niederman, R.; Christodoulides, N.J.; Trochesset, D.A.; et al. Nuclear F-actin Cytology in Oral Epithelial Dysplasia and Oral Squamous Cell Carcinoma. J. Dent. Res. 2021, 100, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Smitha, A.; Rao, K.; Umadevi, H.S.; Smitha, T.; Sheethal, H.S.; Vidya, M.A. Immunohistochemical study of alpha-smooth muscle actin expression in oral leukoplakia and oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2019, 23, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Chung, H.J.; Bhawan, J. Primary Cutaneous Spindle Cell Squamous Carcinoma Expressing Smooth Muscle Actin: Diagnostic Pitfalls. Am. J. Dermatopathol. 2018, 40, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Brand, A.C.; Morrison, E.; Milne, S.; Gonia, S.; Gale, C.A.; Gow, N.A. Cdc42 GTPase dynamics control directional growth responses. Proc. Natl. Acad. Sci. USA 2014, 111, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Bahar, E.; Kim, H.; Yoon, H. ER Stress-Mediated Signaling: Action Potential and Ca(2+) as Key Players. Int. J. Mol. Sci. 2016, 17, 1558. [Google Scholar] [CrossRef]
- Bros, M.; Haas, K.; Moll, L.; Grabbe, S. RhoA as a Key Regulator of Innate and Adaptive Immunity. Cells 2019, 8, 733. [Google Scholar] [CrossRef]
- Guo, F.; Zhang, S.; Tripathi, P.; Mattner, J.; Phelan, J.; Sproles, A.; Mo, J.; Wills-Karp, M.; Grimes, H.L.; Hildeman, D.; et al. Distinct roles of Cdc42 in thymopoiesis and effector and memory T cell differentiation. PLoS ONE 2011, 6, e18002. [Google Scholar] [CrossRef]
- Ubukawa, K.; Goto, T.; Asanuma, K.; Sasaki, Y.; Guo, Y.M.; Kobayashi, I.; Sawada, K.; Wakui, H.; Takahashi, N. Cdc42 regulates cell polarization and contractile actomyosin rings during terminal differentiation of human erythroblasts. Sci. Rep. 2020, 10, 11806. [Google Scholar] [CrossRef]
- Ji, P.; Lodish, H.F. Rac GTPases play multiple roles in erythropoiesis. Haematologica 2010, 95, 2–4. [Google Scholar] [CrossRef][Green Version]
- Liang, X.; Draghi, N.A.; Resh, M.D. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J. Neurosci. 2004, 24, 7140–7149. [Google Scholar] [CrossRef]
- Zhao, Y.; He, L.; Zhang, Y.; Zhao, J.; Liu, Z.; Xing, F.; Liu, M.; Feng, Z.; Li, W.; Zhang, J. Estrogen receptor alpha and beta regulate actin polymerization and spatial memory through an SRC-1/mTORC2-dependent pathway in the hippocampus of female mice. J. Steroid Biochem. Mol. Biol. 2017, 174, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Iksen; Pothongsrisit, S.; Pongrakhananon, V. Targeting the PI3K/AKT/mTOR Signaling Pathway in Lung Cancer: An Update Regarding Potential Drugs and Natural Products. Molecules 2021, 26, 4100. [Google Scholar] [CrossRef]
- Martin, H.L.; Smith, L.; Tomlinson, D.C. Multidrug-resistant breast cancer: Current perspectives. Breast Cancer 2014, 6, 1–13. [Google Scholar] [PubMed]
- Collins, B.C.; Arpke, R.W.; Larson, A.A.; Baumann, C.W.; Xie, N.; Cabelka, C.A.; Nash, N.L.; Juppi, H.K.; Laakkonen, E.K.; Sipila, S.; et al. Estrogen Regulates the Satellite Cell Compartment in Females. Cell Rep. 2019, 28, 368–381.e6. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xu, J.; Qi, J.; Zhang, L.; Wang, J.; Liang, J.; Qian, N.; Zhou, H.; Wei, L.; Deng, L. MicroRNA-17-92a upregulation by estrogen leads to Bim targeting and inhibition of osteoblast apoptosis. J. Cell Sci. 2013, 126 Pt 4, 978–988. [Google Scholar] [CrossRef]
- Zhou, Z.; Ribas, V.; Rajbhandari, P.; Drew, B.G.; Moore, T.M.; Fluitt, A.H.; Reddish, B.R.; Whitney, K.A.; Georgia, S.; Vergnes, L.; et al. Estrogen receptor alpha protects pancreatic beta-cells from apoptosis by preserving mitochondrial function and suppressing endoplasmic reticulum stress. J. Biol. Chem. 2018, 293, 4735–4751. [Google Scholar] [CrossRef]
- Garber, J.J.; Mallick, E.M.; Scanlon, K.M.; Turner, J.R.; Donnenberg, M.S.; Leong, J.M.; Snapper, S.B. Attaching-and-Effacing Pathogens Exploit Junction Regulatory Activities of N-WASP and SNX9 to Disrupt the Intestinal Barrier. Cell. Mol. Gastroenterol. Hepatol. 2018, 5, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, S.; Ridley, A.J.; Lutz, S. The Function of Rho-Associated Kinases ROCK1 and ROCK2 in the Pathogenesis of Cardiovascular Disease. Front. Pharmacol. 2015, 6, 276. [Google Scholar] [CrossRef]
- Barone, I.; Brusco, L.; Gu, G.; Selever, J.; Beyer, A.; Covington, K.R.; Tsimelzon, A.; Wang, T.; Hilsenbeck, S.G.; Chamness, G.C.; et al. Loss of Rho GDIalpha and resistance to tamoxifen via effects on estrogen receptor alpha. J. Natl. Cancer Inst. 2011, 103, 538–552. [Google Scholar] [CrossRef]
- Zhou, K.; Sumigray, K.D.; Lechler, T. The Arp2/3 complex has essential roles in vesicle trafficking and transcytosis in the mammalian small intestine. Mol. Biol. Cell 2015, 26, 1995–2004. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Abbas, A.K.; Nelson, F.; Mitchell, R. Robbins Basic Pathology; Saunders Elsevier: Philadelphia, PA, USA, 2007; Volume 8, pp. 516–522. [Google Scholar]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: Recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ. 2009, 16, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Wohlan, K.; Goy, S.; Olling, A.; Srivaratharajan, S.; Tatge, H.; Genth, H.; Gerhard, R. Pyknotic cell death induced by Clostridium difficile TcdB: Chromatin condensation and nuclear blister are induced independently of the glucosyltransferase activity. Cell. Microbiol. 2014, 16, 1678–1692. [Google Scholar] [CrossRef] [PubMed]
- Moller, L.L.V.; Klip, A.; Sylow, L. Rho GTPases-Emerging Regulators of Glucose Homeostasis and Metabolic Health. Cells 2019, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Vig, A.T.; Foldi, I.; Szikora, S.; Migh, E.; Gombos, R.; Toth, M.A.; Huber, T.; Pinter, R.; Talian, G.C.; Mihaly, J.; et al. The activities of the C-terminal regions of the formin protein disheveled-associated activator of morphogenesis (DAAM) in actin dynamics. J. Biol. Chem. 2017, 292, 13566–13583. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.; Merino, F.; Venkova, L.; Heydenreich, L.; Kierfeld, J.; Vargas, P.; Raunser, S.; Piel, M.; Bieling, P. Profilin and formin constitute a pacemaker system for robust actin filament growth. eLife 2019, 8, e50963. [Google Scholar] [CrossRef]
- Hurst, V.; Shimada, K.; Gasser, S.M. Nuclear Actin and Actin-Binding Proteins in DNA Repair. Trends Cell Biol. 2019, 29, 462–476. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Cerione, R.A. Cdc42 is a substrate for caspases and influences Fas-induced apoptosis. J. Biol. Chem. 2001, 276, 19656–19663. [Google Scholar] [CrossRef]
- Pixley, F.J. Macrophage Migration and Its Regulation by CSF-1. Int. J. Cell Biol. 2012, 2012, 501962. [Google Scholar] [CrossRef]
- Taylor, M.P.; Koyuncu, O.O.; Enquist, L.W. Subversion of the actin cytoskeleton during viral infection. Nat. Rev. Microbiol. 2011, 9, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Juvekar, A.; Lyssiotis, C.A.; Lien, E.C.; Albeck, J.G.; Oh, D.; Varma, G.; Hung, Y.P.; Ullas, S.; Lauring, J.; et al. Phosphoinositide 3-Kinase Regulates Glycolysis through Mobilization of Aldolase from the Actin Cytoskeleton. Cell 2016, 164, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, A.; Zhang, F.; Somasekharan, S.P.; Tse, C.; Leachman, L.; Gleaye, A.; Li, B.; Asmaro, I.; Huang, T.; Kotula, L.; et al. Stress-induced tunneling nanotubes support treatment adaptation in prostate cancer. Sci. Rep. 2019, 9, 7826. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, A.; Rao, P.; Neuzil, J.; Pountney, D.L.; Nath, S. Oxidative stress and Rho GTPases in the biogenesis of tunnelling nanotubes: Implications in disease and therapy. Cell. Mol. Life Sci. 2022, 79, 36. [Google Scholar] [CrossRef]
- Stow, J.L. and R.Z. Murray, Intracellular trafficking and secretion of inflammatory cytokines. Cytokine Growth Factor Rev. 2013, 24, 227–239. [Google Scholar] [CrossRef]
- Andrews, C.; McLean, M.H.; Durum, S.K. Cytokine Tuning of Intestinal Epithelial Function. Front. Immunol. 2018, 9, 1270. [Google Scholar] [CrossRef]
- Uehara, I.; Tanaka, N. Role of p53 in the Regulation of the Inflammatory Tumor Microenvironment and Tumor Suppression. Cancers 2018, 10, 219. [Google Scholar] [CrossRef]
- Lauer, J.C.; Selig, M.; Hart, M.L.; Kurz, B.; Rolauffs, B. Articular Chondrocyte Phenotype Regulation through the Cytoskeleton and the Signaling Processes That Originate from or Converge on the Cytoskeleton: Towards a Novel Understanding of the Intersection between Actin Dynamics and Chondrogenic Function. Int. J. Mol. Sci. 2021, 22, 3279. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, X.; Wang, J.; Li, Z.; Kong, X.; Qian, J.; Hu, Y.; Fang, J.Y. RhoGAPs attenuate cell proliferation by direct interaction with p53 tetramerization domain. Cell Rep. 2013, 3, 1526–1538. [Google Scholar] [CrossRef]
- Campos, S.B.; Ashworth, S.L.; Wean, S.; Hosford, M.; Sandoval, R.M.; Hallett, M.A.; Atkinson, S.J.; Molitoris, B.A. Cytokine-induced F-actin reorganization in endothelial cells involves RhoA activation. Am. J. Physiol. Ren. Physiol. 2009, 296, F487–F495. [Google Scholar] [CrossRef][Green Version]
- Ku, N.O.; Zhou, X.; Toivola, D.M.; Omary, M.B. The cytoskeleton of digestive epithelia in health and disease. Am. J. Physiol. 1999, 277, G1108–G1137. [Google Scholar] [CrossRef]
- Kayalar, C.; Ord, T.; Testa, M.P.; Zhong, L.T.; Bredesen, D.E. Cleavage of actin by interleukin 1 beta-converting enzyme to reverse DNase I inhibition. Proc. Natl. Acad. Sci. USA 1996, 93, 2234–2238. [Google Scholar] [CrossRef] [PubMed]
- Leverrier, Y.; Ridley, A.J. Apoptosis: Caspases orchestrate the ROCK ‘n’ bleb. Nat. Cell Biol. 2001, 3, E91–E93. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Lai, X.N.; Jiao, X.Q.; Xiong, J.P.; Xiong, L.X. Focus on Cdc42 in Breast Cancer: New Insights, Target Therapy Development and Non-Coding RNAs. Cells 2019, 8, 146. [Google Scholar] [CrossRef]
- Adan, H.; Guy, S.; Arulanandam, R.; Geletu, M.; Daniel, J.; Raptis, L. Activated Src requires Cadherin-11, Rac, and gp130 for Stat3 activation and survival of mouse Balb/c3T3 fibroblasts. Cancer Gene Ther. 2022, 29, 1502–1513. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.C.; Zheng, Y.S.; Li, X.H.; Takahashi, H.; Hara, T.; Masuda, S.; Yang, X.H.; Guan, Y.F.; Takano, Y. Arp2/3 overexpression contributed to pathogenesis, growth and invasion of gastric carcinoma. Anticancer Res. 2008, 28, 2225–2232. [Google Scholar]
- Zhao, Y.; Schoeps, B.; Yao, D.; Zhang, Z.; Schuck, K.; Tissen, V.; Jager, C.; Schlitter, A.M.; van der Kammen, R.; Ludwig, C.; et al. mTORC1 and mTORC2 Converge on the Arp2/3 Complex to Promote Kras(G12D)-Induced Acinar-to-Ductal Metaplasia and Early Pancreatic Carcinogenesis. Gastroenterology 2021, 160, 1755–1770.e17. [Google Scholar] [CrossRef]
- Yasuda, K.; Takahashi, M.; Mori, N. Mdm20 Modulates Actin Remodeling through the mTORC2 Pathway via Its Effect on Rictor Expression. PLoS ONE 2015, 10, e0142943. [Google Scholar] [CrossRef]
- Pfeilschifter, J.; Koditz, R.; Pfohl, M.; Schatz, H. Changes in proinflammatory cytokine activity after menopause. Endocr. Rev. 2002, 23, 90–119. [Google Scholar] [CrossRef]
- Miller, K.E.; Kang, P.J.; Park, H.O. Regulation of Cdc42 for polarized growth in budding yeast. Microb. Cell 2020, 7, 175–189. [Google Scholar] [CrossRef]
- Gomez-Cavazos, J.S.; Lee, K.Y.; Lara-Gonzalez, P.; Li, Y.; Desai, A.; Shiau, A.K.; Oegema, K. A Non-canonical BRCT-Phosphopeptide Recognition Mechanism Underlies RhoA Activation in Cytokinesis. Curr. Biol. CB 2020, 30, 3101–3115.e11. [Google Scholar] [CrossRef]
- Maldonado, M.D.M.; Medina, J.I.; Velazquez, L.; Dharmawardhane, S. Targeting Rac and Cdc42 GEFs in Metastatic Cancer. Front. Cell Dev. Biol. 2020, 8, 201. [Google Scholar] [CrossRef] [PubMed]
- Desouza, M.; Gunning, P.W.; Stehn, J.R. The actin cytoskeleton as a sensor and mediator of apoptosis. Bioarchitecture 2012, 2, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Barko, S.; Szatmari, D.; Bodis, E.; Turmer, K.; Ujfalusi, Z.; Popp, D.; Robinson, R.C.; Nyitrai, M. Large-scale purification and in vitro characterization of the assembly of MreB from Leptospira interrogans. Biochim. Biophys. Acta 2016, 1860, 1942–1952. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halász, H.; Szatmári, Z.; Kovács, K.; Koppán, M.; Papp, S.; Szabó-Meleg, E.; Szatmári, D. Changes of Ex Vivo Cervical Epithelial Cells Due to Electroporation with JMY. Int. J. Mol. Sci. 2023, 24, 16863. https://doi.org/10.3390/ijms242316863
Halász H, Szatmári Z, Kovács K, Koppán M, Papp S, Szabó-Meleg E, Szatmári D. Changes of Ex Vivo Cervical Epithelial Cells Due to Electroporation with JMY. International Journal of Molecular Sciences. 2023; 24(23):16863. https://doi.org/10.3390/ijms242316863
Chicago/Turabian StyleHalász, Henriett, Zoltán Szatmári, Krisztina Kovács, Miklós Koppán, Szilárd Papp, Edina Szabó-Meleg, and Dávid Szatmári. 2023. "Changes of Ex Vivo Cervical Epithelial Cells Due to Electroporation with JMY" International Journal of Molecular Sciences 24, no. 23: 16863. https://doi.org/10.3390/ijms242316863
APA StyleHalász, H., Szatmári, Z., Kovács, K., Koppán, M., Papp, S., Szabó-Meleg, E., & Szatmári, D. (2023). Changes of Ex Vivo Cervical Epithelial Cells Due to Electroporation with JMY. International Journal of Molecular Sciences, 24(23), 16863. https://doi.org/10.3390/ijms242316863





