Biomarkers for Salvage Therapy in Testicular Germ Cell Tumors
Abstract
1. Introduction
2. Prognostic Model for Patients Who Failed Cisplatin-Based First-Line Chemotherapy
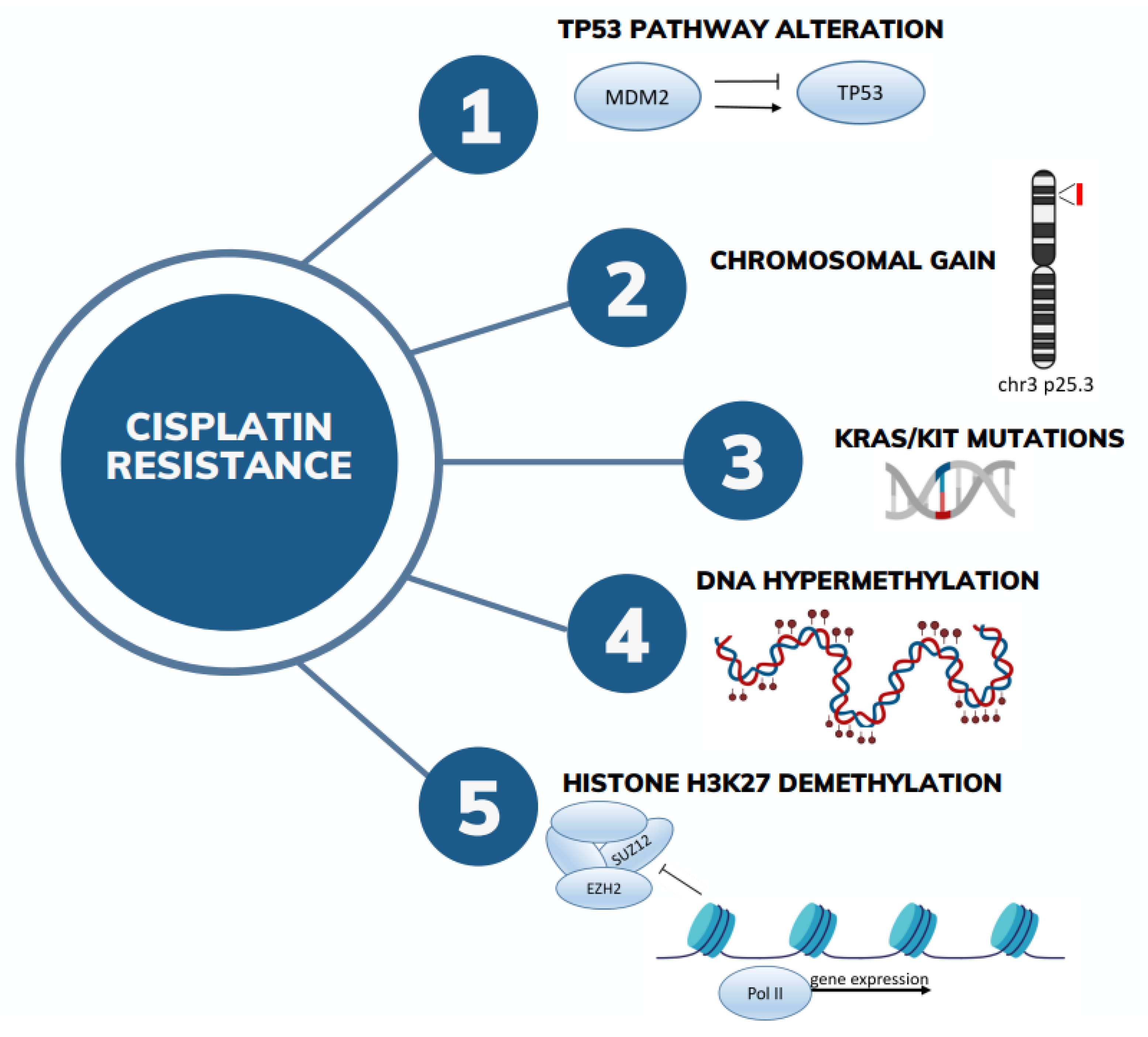
3. Genetic Determinants
4. MicroRNA Dysregulation
5. PD-1/PDL-1/TIGIT/Inflammatory Biomarkers
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oosterhuis, J.W.; Looijenga, L.H.J. Testicular Germ-Cell Tumours in a Broader Perspective. Nat. Rev. Cancer 2005, 5, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Lorch, A.; Beyer, J. How We Treat Germ Cell Cancers. Cancer 2017, 123, 2190–2192. [Google Scholar] [CrossRef] [PubMed]
- Gori, S.; Porrozzi, S.; Roila, F.; Gatta, G.; De Giorgi, U.; Marangolo, M. Germ Cell Tumours of the Testis. Crit. Rev. Oncol. Hematol. 2005, 53, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, J.; Berney, D.M.; Bokemeyer, C.; Climent, M.A.; Daugaard, G.; Gietema, J.A.; De Giorgi, U.; Haugnes, H.S.; Huddart, R.A.; Leão, R.; et al. Testicular Seminoma and Non-Seminoma: ESMO-EURACAN Clinical Practice Guideline for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2022, 33, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Oldenburg, J.; Dunant, A.; Chen, I.; Salvioni, R.; Hartmann, J.T.; De Santis, M.; Daugaard, G.; Flechon, A.; de Giorgi, U.; et al. Assessing Prognosis and Optimizing Treatment in Patients with Postchemotherapy Viable Nonseminomatous Germ-Cell Tumors (NSGCT): Results of the sCR2 International Study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2008, 19, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Feldman, D.R.; Lorch, A.; Kramar, A.; Albany, C.; Einhorn, L.H.; Giannatempo, P.; Necchi, A.; Flechon, A.; Boyle, H.; Chung, P.; et al. Brain Metastases in Patients With Germ Cell Tumors: Prognostic Factors and Treatment Options—An Analysis From the Global Germ Cell Cancer Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Gravis, G.; Flechon, A.; Geoffrois, L.; Chevreau, C.; Laguerre, B.; Delva, R.; Eymard, J.C.; Rolland, F.; Houede, N.; et al. Combining Gemcitabine, Cisplatin, and Ifosfamide (GIP) Is Active in Patients with Relapsed Metastatic Germ-Cell Tumors (GCT): A Prospective Multicenter GETUG Phase II Trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2014, 25, 987–991. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, U.; Rosti, G.; Aieta, M.; Testore, F.; Burattini, L.; Fornarini, G.; Naglieri, E.; Lo Re, G.; Zumaglini, F.; Marangolo, M. Phase II Study of Oxaliplatin and Gemcitabine Salvage Chemotherapy in Patients with Cisplatin-Refractory Nonseminomatous Germ Cell Tumor. Eur. Urol. 2006, 50, 1032–1038; discussion 1038–1039. [Google Scholar] [CrossRef]
- Kondagunta, G.V.; Bacik, J.; Donadio, A.; Bajorin, D.; Marion, S.; Sheinfeld, J.; Bosl, G.J.; Motzer, R.J. Combination of Paclitaxel, Ifosfamide, and Cisplatin Is an Effective Second-Line Therapy for Patients with Relapsed Testicular Germ Cell Tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 6549–6555. [Google Scholar] [CrossRef]
- Lorch, A.; Bascoul-Mollevi, C.; Kramar, A.; Einhorn, L.; Necchi, A.; Massard, C.; De Giorgi, U.; Fléchon, A.; Margolin, K.; Lotz, J.-P.; et al. Conventional-Dose versus High-Dose Chemotherapy as First Salvage Treatment in Male Patients with Metastatic Germ Cell Tumors: Evidence from a Large International Database. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 2178–2184. [Google Scholar] [CrossRef]
- De Giorgi, U.; Demirer, T.; Wandt, H.; Taverna, C.; Siegert, W.; Bornhauser, M.; Kozak, T.; Papiani, G.; Ballardini, M.; Rosti, G.; et al. Second-Line High-Dose Chemotherapy in Patients with Mediastinal and Retroperitoneal Primary Non-Seminomatous Germ Cell Tumors: The EBMT Experience. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2005, 16, 146–151. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, U.; Rosti, G.; Slavin, S.; Yaniv, I.; Harousseau, J.L.; Ladenstein, R.; Demirer, T.; Dini, G.; European Group for Blood and Marrow Transplantation Solid Tumours and Paediatric Disease Working Parties. Salvage High-Dose Chemotherapy for Children with Extragonadal Germ-Cell Tumours. Br. J. Cancer 2005, 93, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Mead, G.M. International Germ Cell Consensus Classification: A Prognostic Factor-Based Staging System for Metastatic Germ Cell Cancers. International Germ Cell Cancer Collaborative Group. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997, 15, 594–603. [Google Scholar] [CrossRef]
- Beyer, J.; Collette, L.; Sauvé, N.; Daugaard, G.; Feldman, D.R.; Tandstad, T.; Tryakin, A.; Stahl, O.; Gonzalez-Billalabeitia, E.; De Giorgi, U.; et al. Survival and New Prognosticators in Metastatic Seminoma: Results From the IGCCCG-Update Consortium. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Gillessen, S.; Sauvé, N.; Collette, L.; Daugaard, G.; de Wit, R.; Albany, C.; Tryakin, A.; Fizazi, K.; Stahl, O.; Gietema, J.A.; et al. Predicting Outcomes in Men With Metastatic Nonseminomatous Germ Cell Tumors (NSGCT): Results From the IGCCCG Update Consortium. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 1563–1574. [Google Scholar] [CrossRef]
- International Prognostic Factors Study Group; Lorch, A.; Beyer, J.; Bascoul-Mollevi, C.; Kramar, A.; Einhorn, L.H.; Necchi, A.; Massard, C.; De Giorgi, U.; Fléchon, A.; et al. Prognostic Factors in Patients with Metastatic Germ Cell Tumors Who Experienced Treatment Failure with Cisplatin-Based First-Line Chemotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2010, 28, 4906–4911. [Google Scholar] [CrossRef]
- Oosterhuis, J.W.; Looijenga, L.H.J. Human germ cell tumours from a developmental perspective. Nat. Rev. Cancer 2019, 19, 522–537. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Shih, J.; Hollern, D.P.; Wang, L.; Bowlby, R.; Tickoo, S.K.; Thorsson, V.; Mungall, A.J.; Newton, Y.; Hegde, A.M.; et al. Integrated Molecular Characterization of Testicular Germ Cell Tumors. Cell Rep. 2018, 23, 3392–3406. [Google Scholar] [CrossRef]
- Loveday, C.; Litchfield, K.; Proszek, P.Z.; Cornish, A.J.; Santo, F.; Levy, M.; Macintyre, G.; Holryod, A.; Broderick, P.; Dudakia, D.; et al. Genomic Landscape of Platinum Resistant and Sensitive Testicular Cancers. Nat. Commun. 2020, 11, 2189. [Google Scholar] [CrossRef]
- Mata, D.A.; Yang, S.-R.; Ferguson, D.C.; Liu, Y.; Sharma, R.; Benhamida, J.K.; Al-Ahmadie, H.A.; Chakravarty, D.; Solit, D.B.; Tickoo, S.K.; et al. RAS/MAPK Pathway Driver Alterations Are Significantly Associated With Oncogenic KIT Mutations in Germ-Cell Tumors. Urology 2020, 144, 111–116. [Google Scholar] [CrossRef]
- Bagrodia, A.; Lee, B.H.; Lee, W.; Cha, E.K.; Sfakianos, J.P.; Iyer, G.; Pietzak, E.J.; Gao, S.P.; Zabor, E.C.; Ostrovnaya, I.; et al. Genetic Determinants of Cisplatin Resistance in Patients With Advanced Germ Cell Tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2016, 34, 4000–4007. [Google Scholar] [CrossRef]
- Timmerman, D.M.; Eleveld, T.F.; Gillis, A.J.M.; Friedrichs, C.C.; Hillenius, S.; Remmers, T.L.; Sriram, S.; Looijenga, L.H.J. The Role of TP53 in Cisplatin Resistance in Mediastinal and Testicular Germ Cell Tumors. Int. J. Mol. Sci. 2021, 22, 11774. [Google Scholar] [CrossRef] [PubMed]
- Urbini, M.; Schepisi, G.; Bleve, S.; Virga, A.; Gianni, C.; Gurioli, G.; Ulivi, P.; De Giorgi, U. Primary Mediastinal and Testicular Germ Cell Tumors in Adolescents and Adults: A Comparison of Genomic Alterations and Clinical Implications. Cancers 2021, 13, 5223. [Google Scholar] [CrossRef] [PubMed]
- Necchi, A.; Bratslavsky, G.; Chung, J.; Millis, S.; Gay, L.M.; Ali, S.M.; Ross, J.S. Genomic Features for Therapeutic Insights of Chemotherapy-Resistant, Primary Mediastinal Nonseminomatous Germ Cell Tumors and Comparison with Gonadal Counterpart. Oncologist 2019, 24, e142–e145. [Google Scholar] [CrossRef] [PubMed]
- Bagrodia, A.; Sawa, Y.; Jia, L.; Krause, H.B.; Millard, F.; Farrell, A.P.; Elliott, A.; Lafin, J.T.; Jamieson, C.; Antonarakis, E.S.; et al. The Genomic and Transcriptomic Landscapes of Chemotherapy Naïve vs Post-Chemotherapy Germ Cell Tumors. J. Clin. Oncol. 2023, 41, 5007. [Google Scholar] [CrossRef]
- Timmerman, D.M.; Eleveld, T.F.; Sriram, S.; Dorssers, L.C.J.; Gillis, A.J.M.; Schmidtova, S.; Kalavska, K.; van de Werken, H.J.G.; Oing, C.; Honecker, F.; et al. Chromosome 3p25.3 Gain Is Associated With Cisplatin Resistance and Is an Independent Predictor of Poor Outcome in Male Malignant Germ Cell Tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 3077–3087. [Google Scholar] [CrossRef] [PubMed]
- Fichtner, A.; Bohnenberger, H.; Elakad, O.; Richter, A.; Lenz, C.; Oing, C.; Ströbel, P.; Kueffer, S.; Nettersheim, D.; Bremmer, F. Proteomic Profiling of Cisplatin-Resistant and Cisplatin-Sensitive Germ Cell Tumour Cell Lines Using Quantitative Mass Spectrometry. World J. Urol. 2022, 40, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Chovanec, M.; Albany, C.; Mego, M.; Montironi, R.; Cimadamore, A.; Cheng, L. Emerging Prognostic Biomarkers in Testicular Germ Cell Tumors: Looking Beyond Established Practice. Front. Oncol. 2018, 8, 571. [Google Scholar] [CrossRef]
- Lobo, J.; Constâncio, V.; Leite-Silva, P.; Guimarães, R.; Cantante, M.; Braga, I.; Maurício, J.; Looijenga, L.H.J.; Henrique, R.; Jerónimo, C. Differential Methylation EPIC Analysis Discloses Cisplatin-Resistance Related Hypermethylation and Tumor-Specific Heterogeneity within Matched Primary and Metastatic Testicular Germ Cell Tumor Patient Tissue Samples. Clin. Epigenet. 2021, 13, 70. [Google Scholar] [CrossRef]
- Fazal, Z.; Singh, R.; Fang, F.; Bikorimana, E.; Baldwin, H.; Corbet, A.; Tomlin, M.; Yerby, C.; Adra, N.; Albany, C.; et al. Hypermethylation and Global Remodelling of DNA Methylation Is Associated with Acquired Cisplatin Resistance in Testicular Germ Cell Tumours. Epigenetics 2021, 16, 1071–1084. [Google Scholar] [CrossRef]
- Singh, R.; Fazal, Z.; Corbet, A.K.; Bikorimana, E.; Rodriguez, J.C.; Khan, E.M.; Shahid, K.; Freemantle, S.J.; Spinella, M.J. Epigenetic Remodeling through Downregulation of Polycomb Repressive Complex 2 Mediates Chemotherapy Resistance in Testicular Germ Cell Tumors. Cancers 2019, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Fazal, Z.; Bikorimana, E.; Boyd, R.I.; Yerby, C.; Tomlin, M.; Baldwin, H.; Shokry, D.; Corbet, A.K.; Shahid, K.; et al. Reciprocal Epigenetic Remodeling Controls Testicular Cancer Hypersensitivity to Hypomethylating Agents and Chemotherapy. Mol. Oncol. 2022, 16, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wang, X.; Xu, Y.; Yang, L.; Qian, Q.; Ju, S.; Chen, Y.; Chen, S.; Qin, N.; Ma, Z.; et al. Comprehensive Characterization of Cancer-Testis Genes in Testicular Germ Cell Tumor. Cancer Med. 2019, 8, 3511–3519. [Google Scholar] [CrossRef] [PubMed]
- Funke, K.; Einsfelder, U.; Hansen, A.; Arévalo, L.; Schneider, S.; Nettersheim, D.; Stein, V.; Schorle, H. Genome-Scale CRISPR Screen Reveals Neddylation to Contribute to Cisplatin Resistance of Testicular Germ Cell Tumours. Br. J. Cancer 2023, 128, 2270–2282. [Google Scholar] [CrossRef] [PubMed]
- Smolarz, B.; Durczyński, A.; Romanowicz, H.; Szyłło, K.; Hogendorf, P. miRNAs in Cancer (Review of Literature). Int. J. Mol. Sci. 2022, 23, 2805. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Junker, K.; Heinzelmann, J. Prognostic and Predictive miRNA Biomarkers in Bladder, Kidney and Prostate Cancer: Where Do We Stand in Biomarker Development? J. Cancer Res. Clin. Oncol. 2016, 142, 1673–1695. [Google Scholar] [CrossRef] [PubMed]
- Constâncio, V.; Tavares, N.T.; Henrique, R.; Jerónimo, C.; Lobo, J. MiRNA Biomarkers in Cancers of the Male Reproductive System: Are We Approaching Clinical Application? Andrology 2023, 11, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Halsall, D.J.; Hook, C.E.; Williams, D.M.; Nicholson, J.C.; Coleman, N. Identification of microRNAs From the miR-371~373 and miR-302 Clusters as Potential Serum Biomarkers of Malignant Germ Cell Tumors. Am. J. Clin. Pathol. 2011, 135, 119–125. [Google Scholar] [CrossRef]
- Belge, G.; Dieckmann, K.-P.; Spiekermann, M.; Balks, T.; Bullerdiek, J. Serum Levels of microRNAs miR-371-3: A Novel Class of Serum Biomarkers for Testicular Germ Cell Tumors? Eur. Urol. 2012, 61, 1068–1069. [Google Scholar] [CrossRef]
- Syring, I.; Bartels, J.; Holdenrieder, S.; Kristiansen, G.; Müller, S.C.; Ellinger, J. Circulating Serum miRNA (miR-367-3p, miR-371a-3p, miR-372-3p and miR-373-3p) as Biomarkers in Patients with Testicular Germ Cell Cancer. J. Urol. 2015, 193, 331–337. [Google Scholar] [CrossRef]
- Van Agthoven, T.; Looijenga, L.H.J. Accurate Primary Germ Cell Cancer Diagnosis Using Serum Based microRNA Detection (ampTSmiR Test). Oncotarget 2017, 8, 58037–58049. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, K.-P.; Radtke, A.; Geczi, L.; Matthies, C.; Anheuser, P.; Eckardt, U.; Sommer, J.; Zengerling, F.; Trenti, E.; Pichler, R.; et al. Serum Levels of MicroRNA-371a-3p (M371 Test) as a New Biomarker of Testicular Germ Cell Tumors: Results of a Prospective Multicentric Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.J.; Coleman, N. MicroRNA Dysregulation in Malignant Germ Cell Tumors: More Than a Biomarker? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 1432–1435. [Google Scholar] [CrossRef]
- Bezan, A.; Gerger, A.; Pichler, M. MicroRNAs in Testicular Cancer: Implications for Pathogenesis, Diagnosis, Prognosis and Therapy. Anticancer Res. 2014, 34, 2709–2713. [Google Scholar] [PubMed]
- Van Agthoven, T.; Eijkenboom, W.M.H.; Looijenga, L.H.J. microRNA-371a-3p as Informative Biomarker for the Follow-up of Testicular Germ Cell Cancer Patients. Cell. Oncol. Dordr. 2017, 40, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Port, M.; Glaesener, S.; Ruf, C.; Riecke, A.; Bokemeyer, C.; Meineke, V.; Honecker, F.; Abend, M. Micro-RNA expression in cisplatin resistant germ cell tumor cell lines. Mol. Cancer 2011, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Roška, J.; Lobo, J.; Ivovič, D.; Wachsmannová, L.; Mueller, T.; Henrique, R.; Jerónimo, C.; Chovanec, M.; Jurkovičová, D. Integrated Microarray-Based Data Analysis of miRNA Expression Profiles: Identification of Novel Biomarkers of Cisplatin-Resistance in Testicular Germ Cell Tumours. Int. J. Mol. Sci. 2023, 24, 2495. [Google Scholar] [CrossRef]
- Liu, L.; Lian, J.; Zhang, H.; Tian, H.; Liang, M.; Yin, M.; Sun, F. MicroRNA-302a sensitizes testicular embryonal carcinoma cells to cisplatin-induced cell death. J. Cell Physiol. 2013, 228, 2294–2304. [Google Scholar] [CrossRef]
- Huang, H.; Tian, H.; Duan, Z.; Cao, Y.; Zhang, X.-S.; Sun, F. MicroRNA-383 impairs phosphorylation of H2AX by targeting PNUTS and inducing cell cycle arrest in testicular embryonal carcinoma cells. Cell. Signal. 2014, 26, 903–911. [Google Scholar] [CrossRef]
- Wei, J.; Gan, Y.; Peng, D.; Jiang, X.; Kitazawa, R.; Xiang, Y.; Dai, Y.; Tang, Y.; Yang, J. Long non-coding RNA H19 promotes TDRG1 expression and cisplatin resistance by sequestering miRNA-106b-5p in seminoma. Cancer Med. 2018, 7, 6247–6257. [Google Scholar] [CrossRef]
- Pan, G.; Liu, Y.; Shang, L.; Zhou, F.; Yang, S. EMT-Associated microRNAs and Their Roles in Cancer Stemness and Drug Resistance. Cancer Commun. Lond. Engl. 2021, 41, 199–217. [Google Scholar] [CrossRef] [PubMed]
- Pavlíková, L.; Šereš, M.; Breier, A.; Sulová, Z. The Roles of microRNAs in Cancer Multidrug Resistance. Cancers 2022, 14, 1090. [Google Scholar] [CrossRef] [PubMed]
- Bleve, S.; Cursano, M.C.; Casadei, C.; Schepisi, G.; Menna, C.; Urbini, M.; Gianni, C.; De Padova, S.; Filograna, A.; Gallà, V.; et al. Inflammatory Biomarkers for Outcome Prediction in Patients With Metastatic Testicular Cancer. Front. Oncol. 2022, 12, 910087. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.D.; Curioni-Fontecedro, A.; Allmann, V.; Beyer, J.; Tischler, V.; Sulser, T.; Moch, H.; Bode, P.K. Frequent PD-L1 Expression in Testicular Germ Cell Tumors. Br. J. Cancer 2015, 113, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Ward, J.E.; Bao, R.; Hall, C.R.; Brockstein, B.E.; Luke, J.J. Clinical Response of a Patient to Anti-PD-1 Immunotherapy and the Immune Landscape of Testicular Germ Cell Tumors. Cancer Immunol. Res. 2016, 4, 903–909. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lobo, J.; Rodrigues, Â.; Guimarães, R.; Cantante, M.; Lopes, P.; Maurício, J.; Oliveira, J.; Jerónimo, C.; Henrique, R. Detailed Characterization of Immune Cell Infiltrate and Expression of Immune Checkpoint Molecules PD-L1/CTLA-4 and MMR Proteins in Testicular Germ Cell Tumors Disclose Novel Disease Biomarkers. Cancers 2019, 11, 1535. [Google Scholar] [CrossRef] [PubMed]
- Cierna, Z.; Mego, M.; Miskovska, V.; Machalekova, K.; Chovanec, M.; Svetlovska, D.; Hainova, K.; Rejlekova, K.; Macak, D.; Spanik, S.; et al. Prognostic Value of Programmed-Death-1 Receptor (PD-1) and Its Ligand 1 (PD-L1) in Testicular Germ Cell Tumors. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 300–305. [Google Scholar] [CrossRef]
- Cheng, X.; Dai, H.; Wan, N.; Moore, Y.; Vankayalapati, R.; Dai, Z. Interaction of Programmed Death-1 and Programmed Death-1 Ligand-1 Contributes to Testicular Immune Privilege. Transplantation 2009, 87, 1778–1786. [Google Scholar] [CrossRef]
- Sadigh, S.; Farahani, S.J.; Shah, A.; Vaughn, D.; Lal, P. Differences in PD-L1-Expressing Macrophages and Immune Microenvironment in Testicular Germ Cell Tumors. Am. J. Clin. Pathol. 2020, 153, 387–395. [Google Scholar] [CrossRef]
- Siska, P.J.; Johnpulle, R.A.N.; Zhou, A.; Bordeaux, J.; Kim, J.Y.; Dabbas, B.; Dakappagari, N.; Rathmell, J.C.; Rathmell, W.K.; Morgans, A.K.; et al. Deep Exploration of the Immune Infiltrate and Outcome Prediction in Testicular Cancer by Quantitative Multiplexed Immunohistochemistry and Gene Expression Profiling. Oncoimmunology 2017, 6, e1305535. [Google Scholar] [CrossRef]
- Bols, B.; Jensen, L.; Jensen, A.; Braendstrup, O. Immunopathology of in Situ Seminoma. Int. J. Exp. Pathol. 2000, 81, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Boldrini, R.; De Pasquale, M.D.; Melaiu, O.; Chierici, M.; Jurman, G.; Benedetti, M.C.; Salfi, N.C.; Castellano, A.; Collini, P.; Furlanello, C.; et al. Tumor-Infiltrating T Cells and PD-L1 Expression in Childhood Malignant Extracranial Germ-Cell Tumors. Oncoimmunology 2019, 8, e1542245. [Google Scholar] [CrossRef] [PubMed]
- Chovanec, M.; Cierna, Z.; Miskovska, V.; Machalekova, K.; Svetlovska, D.; Kalavska, K.; Rejlekova, K.; Spanik, S.; Kajo, K.; Babal, P.; et al. Prognostic Role of Programmed-Death Ligand 1 (PD-L1) Expressing Tumor Infiltrating Lymphocytes in Testicular Germ Cell Tumors. Oncotarget 2017, 8, 21794–21805. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, H.; Hai, J.; Socinski, M.A.; Lim, E.; Chen, H.; Stebbing, J. Impact of PD-L1 Expression, Driver Mutations and Clinical Characteristics on Survival after Anti-PD-1/PD-L1 Immunotherapy versus Chemotherapy in Non-Small-Cell Lung Cancer: A Meta-Analysis of Randomized Trials. Oncoimmunology 2018, 7, e1396403. [Google Scholar] [CrossRef] [PubMed]
- Pęksa, R.; Kunc, M.; Popęda, M.; Piątek, M.; Bieńkowski, M.; Żok, J.; Starzyńska, A.; Perdyan, A.; Sowa, M.; Duchnowska, R.; et al. Combined Assessment of Immune Checkpoint Regulator VISTA on Tumor-Associated Immune Cells and Platelet-to-Lymphocyte Ratio Identifies Advanced Germ Cell Tumors with Higher Risk of Unfavorable Outcomes. Cancers 2021, 13, 1750. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, C.D.; Sander, S.; Roth, L.; Gross, O.; Eberli, D.; Sulser, T.; Seifert, B.; Beyer, J.; Hermanns, T. Systemic Inflammatory Markers Have Independent Prognostic Value in Patients with Metastatic Testicular Germ Cell Tumours Undergoing First-Line Chemotherapy. Br. J. Cancer 2018, 118, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Chovanec, M.; Cierna, Z.; Miskovska, V.; Machalekova, K.; Kalavska, K.; Rejlekova, K.; Svetlovska, D.; Macak, D.; Spanik, S.; Kajo, K.; et al. Systemic Immune-Inflammation Index in Germ-Cell Tumours. Br. J. Cancer 2018, 118, 831–838. [Google Scholar] [CrossRef]
- Ribnikar, D.; Stukalin, I.; Bedard, P.L.; Hamilton, R.J.; Jewett, M.; Warde, P.; Chung, P.; Anson-Cartwright, L.; Templeton, A.J.; Amir, E.; et al. The Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Metastatic Testicular Cancer. Curr. Oncol. 2020, 28, 107–114. [Google Scholar] [CrossRef]
- Cursano, M.C.; Kopf, B.; Scarpi, E.; Menna, C.; Casadei, C.; Schepisi, G.; Lolli, C.; Altavilla, A.; Gallà, V.; Santini, D.; et al. Prognostic Role of Systemic Inflammatory Indexes in Germ Cell Tumors Treated With High-Dose Chemotherapy. Front. Oncol. 2020, 10, 1325. [Google Scholar] [CrossRef]
- Hinsch, A.; Blessin, N.C.; Simon, R.; Kluth, M.; Fischer, K.; Hube-Magg, C.; Li, W.; Makrypidi-Fraune, G.; Wellge, B.; Mandelkow, T.; et al. Expression of the Immune Checkpoint Receptor TIGIT in Seminoma. Oncol. Lett. 2019, 18, 1497–1502. [Google Scholar] [CrossRef]
- Das, M.; Zhu, C.; Kuchroo, V.K. Tim-3 and Its Role in Regulating Anti-Tumor Immunity. Immunol. Rev. 2017, 276, 97–111. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Cao, J.; Zhao, C.; Li, X.; Zhou, C.; Hirsch, F.R. TIM-3, a Promising Target for Cancer Immunotherapy. OncoTargets Ther. 2018, 11, 7005–7009. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Zhang, X.; Chen, F.; Pan, Q.; Phiphatwatchara, P.; Zeng, Y.; Chen, H. The Promising Immune Checkpoint LAG-3: From Tumor Microenvironment to Cancer Immunotherapy. Genes Cancer 2018, 9, 176–189. [Google Scholar] [CrossRef] [PubMed]
- Tu, L.; Guan, R.; Yang, H.; Zhou, Y.; Hong, W.; Ma, L.; Zhao, G.; Yu, M. Assessment of the Expression of the Immune Checkpoint Molecules PD-1, CTLA4, TIM-3 and LAG-3 across Different Cancers in Relation to Treatment Response, Tumor-Infiltrating Immune Cells and Survival. Int. J. Cancer 2020, 147, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Honecker, F.; Wermann, H.; Mayer, F.; Gillis, A.J.M.; Stoop, H.; van Gurp, R.J.L.M.; Oechsle, K.; Steyerberg, E.; Hartmann, J.T.; Dinjens, W.N.M.; et al. Microsatellite Instability, Mismatch Repair Deficiency, and BRAF Mutation in Treatment-Resistant Germ Cell Tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 2129–2136. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, C.; Melau, C.; Nielsen, J.E.; Vile Jensen, K.; Liu, D.; Pena-Diaz, J.; Rajpert-De Meyts, E.; Rasmussen, L.J.; Jørgensen, A. Involvement of the DNA Mismatch Repair System in Cisplatin Sensitivity of Testicular Germ Cell Tumours. Cell. Oncol. Dordr. 2017, 40, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Batool, A.; Wang, Y.-Q.; Hao, X.-X.; Chen, S.-R.; Liu, Y.-X. A miR-125b/CSF1-CX3CL1/Tumor-Associated Macrophage Recruitment Axis Controls Testicular Germ Cell Tumor Growth. Cell Death Dis. 2018, 9, 962. [Google Scholar] [CrossRef]
- Zhai, J.; Shen, J.; Xie, G.; Wu, J.; He, M.; Gao, L.; Zhang, Y.; Yao, X.; Shen, L. Cancer-Associated Fibroblasts-Derived IL-8 Mediates Resistance to Cisplatin in Human Gastric Cancer. Cancer Lett. 2019, 454, 37–43. [Google Scholar] [CrossRef]
- Skowron, M.A.; Eul, K.; Stephan, A.; Ludwig, G.F.; Wakileh, G.A.; Bister, A.; Söhngen, C.; Raba, K.; Petzsch, P.; Poschmann, G.; et al. Profiling the 3D Interaction between Germ Cell Tumors and Microenvironmental Cells at the Transcriptome and Secretome Level. Mol. Oncol. 2022, 16, 3107–3127. [Google Scholar] [CrossRef]
- Svetlovska, D.; Miskovska, V.; Cholujova, D.; Gronesova, P.; Cingelova, S.; Chovanec, M.; Sycova-Mila, Z.; Obertova, J.; Palacka, P.; Rajec, J.; et al. Plasma Cytokines Correlated With Disease Characteristics, Progression-Free Survival, and Overall Survival in Testicular Germ-Cell Tumor Patients. Clin. Genitourin. Cancer 2017, 15, 411–416. [Google Scholar] [CrossRef]
- Jakobsen, M.K.; Gjerstorff, M.F. CAR T-Cell Cancer Therapy Targeting Surface Cancer/Testis Antigens. Front. Immunol. 2020, 11, 1568. [Google Scholar] [CrossRef] [PubMed]
- Nilius, V.; Killer, M.C.; Timmesfeld, N.; Schmitt, M.; Moll, R.; Lorch, A.; Beyer, J.; Mack, E.; Lohoff, M.; Burchert, A.; et al. High β-1,4-Galactosyltransferase-I Expression in Peripheral T-Lymphocytes Is Associated with a Low Risk of Relapse in Germ-Cell Cancer Patients Receiving High-Dose Chemotherapy with Autologous Stem Cell Reinfusion. Oncoimmunology 2018, 7, e1423169. [Google Scholar] [CrossRef] [PubMed]
- Browning, R.J.; Reardon, P.J.T.; Parhizkar, M.; Pedley, R.B.; Edirisinghe, M.; Knowles, J.C.; Stride, E. Drug Delivery Strategies for Platinum-Based Chemotherapy. ACS Nano 2017, 11, 8560–8578. [Google Scholar] [CrossRef]
- Conti, M.; Tazzari, V.; Baccini, C.; Pertici, G.; Serino, L.P.; De Giorgi, U. Anticancer Drug Delivery with Nanoparticles. Vivo Athens Greece 2006, 20, 697–701. [Google Scholar]
- Schepisi, G.; Gianni, C.; Cursano, M.C.; Gallà, V.; Menna, C.; Casadei, C.; Bleve, S.; Lolli, C.; Martinelli, G.; Rosti, G.; et al. Immune Checkpoint Inhibitors and Chimeric Antigen Receptor (CAR)-T Cell Therapy: Potential Treatment Options against Testicular Germ Cell Tumors. Front. Immunol. 2023, 14, 1118610. [Google Scholar] [CrossRef] [PubMed]
- Pantuck, M.; Palaskas, N.; Drakaki, A. Next Generation T-Cell Therapy for Genitourinary Malignancies, Part A: Introduction and Current State of the Art. Cancer Treat. Res. Commun. 2018, 17, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, J.A.; Reidy, A.; Mirandola, L.; Trotter, K.; Suvorava, N.; Figueroa, A.; Konala, V.; Aulakh, A.; Littlefield, L.; Grizzi, F.; et al. Chimeric Antigen Receptor Engineering: A Right Step in the Evolution of Adoptive Cellular Immunotherapy. Int. Rev. Immunol. 2015, 34, 154–187. [Google Scholar] [CrossRef] [PubMed]
- Katari, U.L.; Keirnan, J.M.; Worth, A.C.; Hodges, S.E.; Leen, A.M.; Fisher, W.E.; Vera, J.F. Engineered T Cells for Pancreatic Cancer Treatment. HPB 2011, 13, 643–650. [Google Scholar] [CrossRef]
- Martino, M.; Naso, V.; Loteta, B.; Canale, F.A.; Pugliese, M.; Alati, C.; Musuraca, G.; Nappi, D.; Gaimari, A.; Nicolini, F.; et al. Chimeric Antigen Receptor T-Cell Therapy: What We Expect Soon. Int. J. Mol. Sci. 2022, 23, 13332. [Google Scholar] [CrossRef]
- Mackensen, A.; Haanen, J.B.a.G.; Koenecke, C.; Alsdorf, W.; Wagner-Drouet, E.; Heudobler, D.; Borchmann, P.; Bokemeyer, C.; Klobuch, S.; Smit, E.; et al. LBA38 BNT211-01: A Phase I Trial to Evaluate Safety and Efficacy of CLDN6 CAR T Cells and CLDN6-Encoding mRNA Vaccine-Mediated in Vivo Expansion in Patients with CLDN6-Positive Advanced Solid Tumours. Ann. Oncol. 2022, 33, S1404–S1405. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbini, M.; Bleve, S.; Schepisi, G.; Menna, C.; Gurioli, G.; Gianni, C.; De Giorgi, U. Biomarkers for Salvage Therapy in Testicular Germ Cell Tumors. Int. J. Mol. Sci. 2023, 24, 16872. https://doi.org/10.3390/ijms242316872
Urbini M, Bleve S, Schepisi G, Menna C, Gurioli G, Gianni C, De Giorgi U. Biomarkers for Salvage Therapy in Testicular Germ Cell Tumors. International Journal of Molecular Sciences. 2023; 24(23):16872. https://doi.org/10.3390/ijms242316872
Chicago/Turabian StyleUrbini, Milena, Sara Bleve, Giuseppe Schepisi, Cecilia Menna, Giorgia Gurioli, Caterina Gianni, and Ugo De Giorgi. 2023. "Biomarkers for Salvage Therapy in Testicular Germ Cell Tumors" International Journal of Molecular Sciences 24, no. 23: 16872. https://doi.org/10.3390/ijms242316872
APA StyleUrbini, M., Bleve, S., Schepisi, G., Menna, C., Gurioli, G., Gianni, C., & De Giorgi, U. (2023). Biomarkers for Salvage Therapy in Testicular Germ Cell Tumors. International Journal of Molecular Sciences, 24(23), 16872. https://doi.org/10.3390/ijms242316872






