Investigating the Cardiovascular Benefits of Dapagliflozin: Vasodilatory Effect on Isolated Rat Coronary Arteries
Abstract
1. Introduction
2. Results
2.1. Effect of Dapagliflozin on High-K+- or U-46619-Induced Contraction in Rat Coronary Arteries
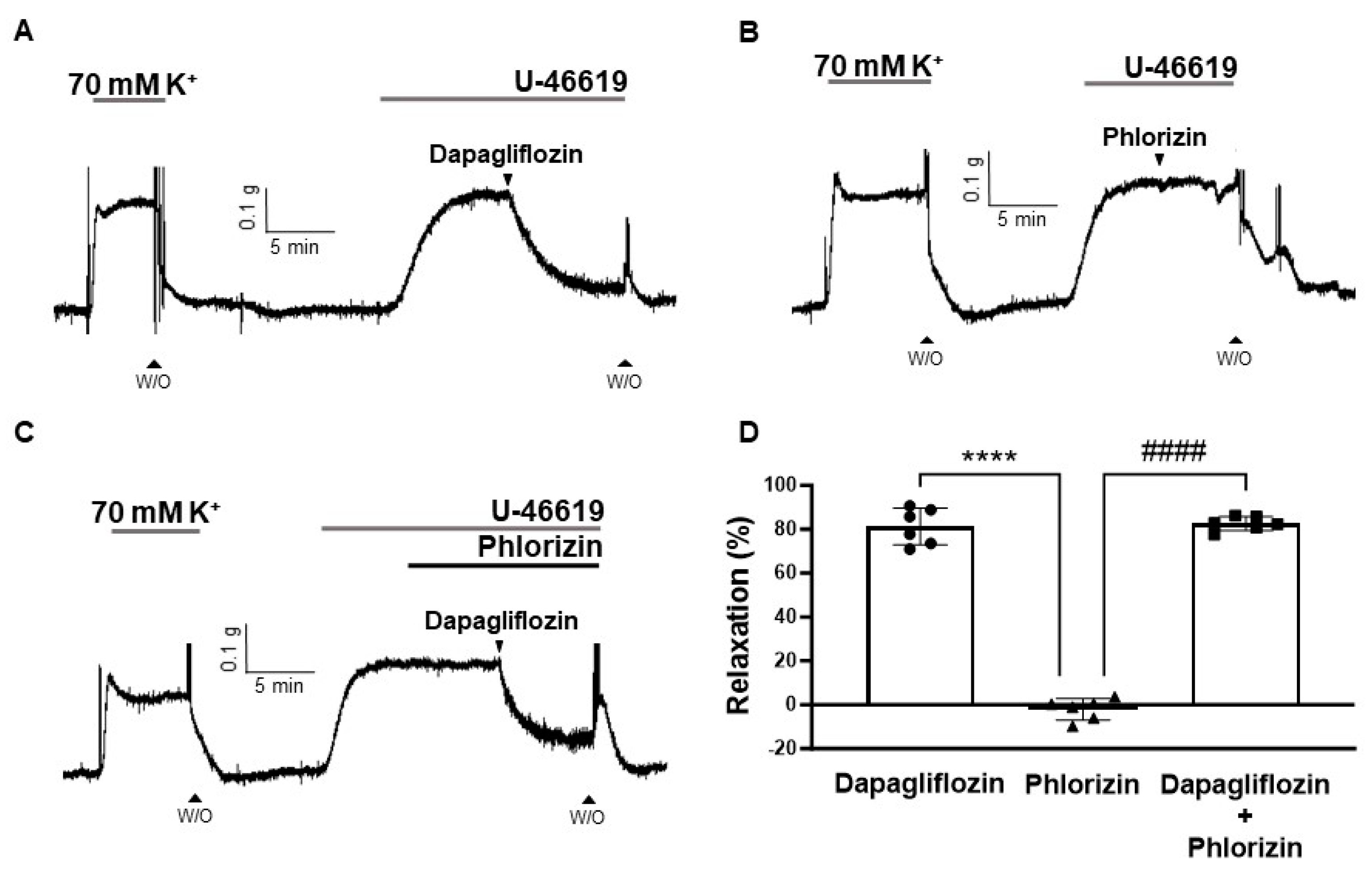
2.2. Effects of SGLT2 Inhibition on Rat Coronary Arteries
2.3. Dapagliflozin-Induced Endothelium-Independent Relaxation
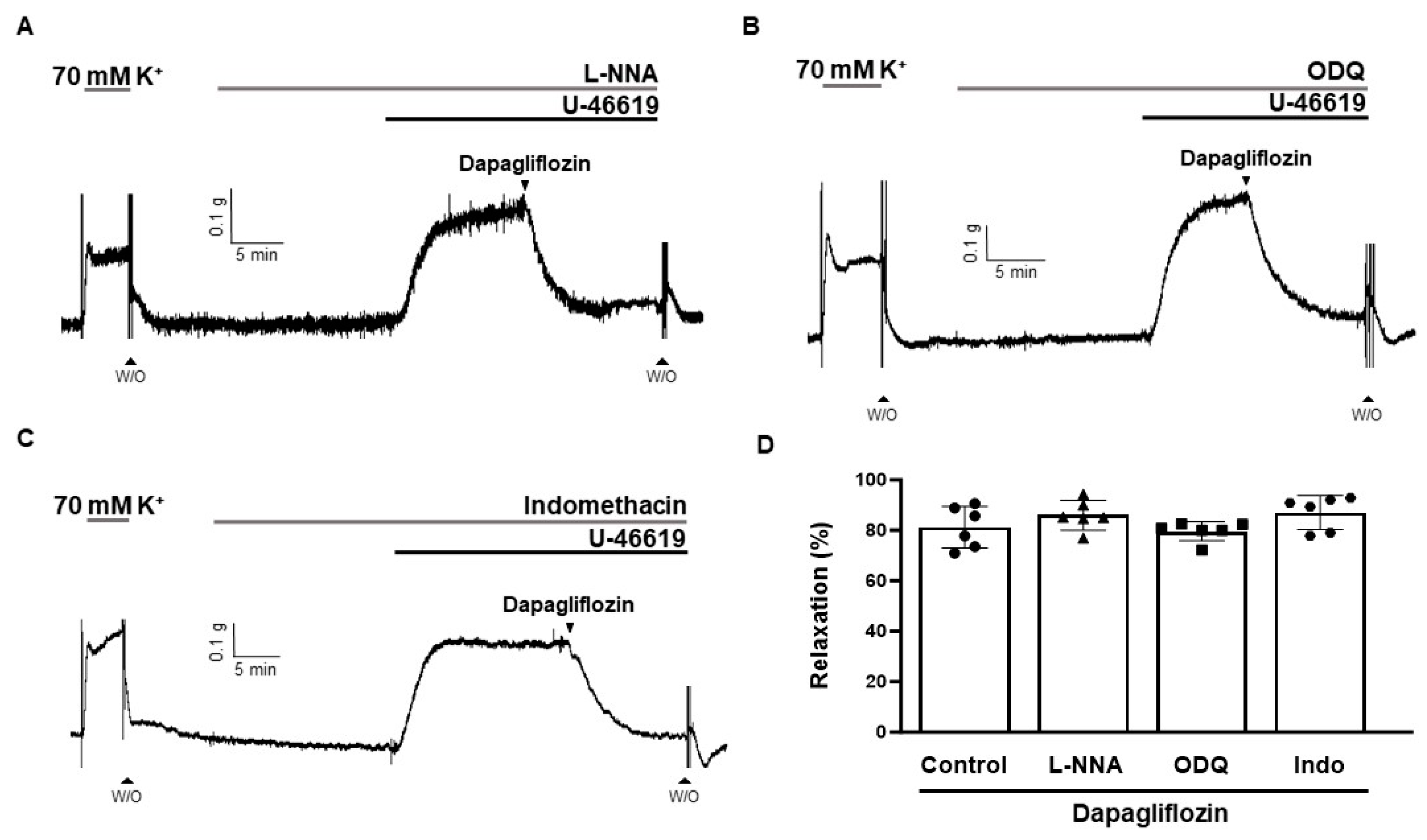
2.4. Effects of L-NNA, ODQ, and Indomethacin on Dapagliflozin-Induced Vasodilation
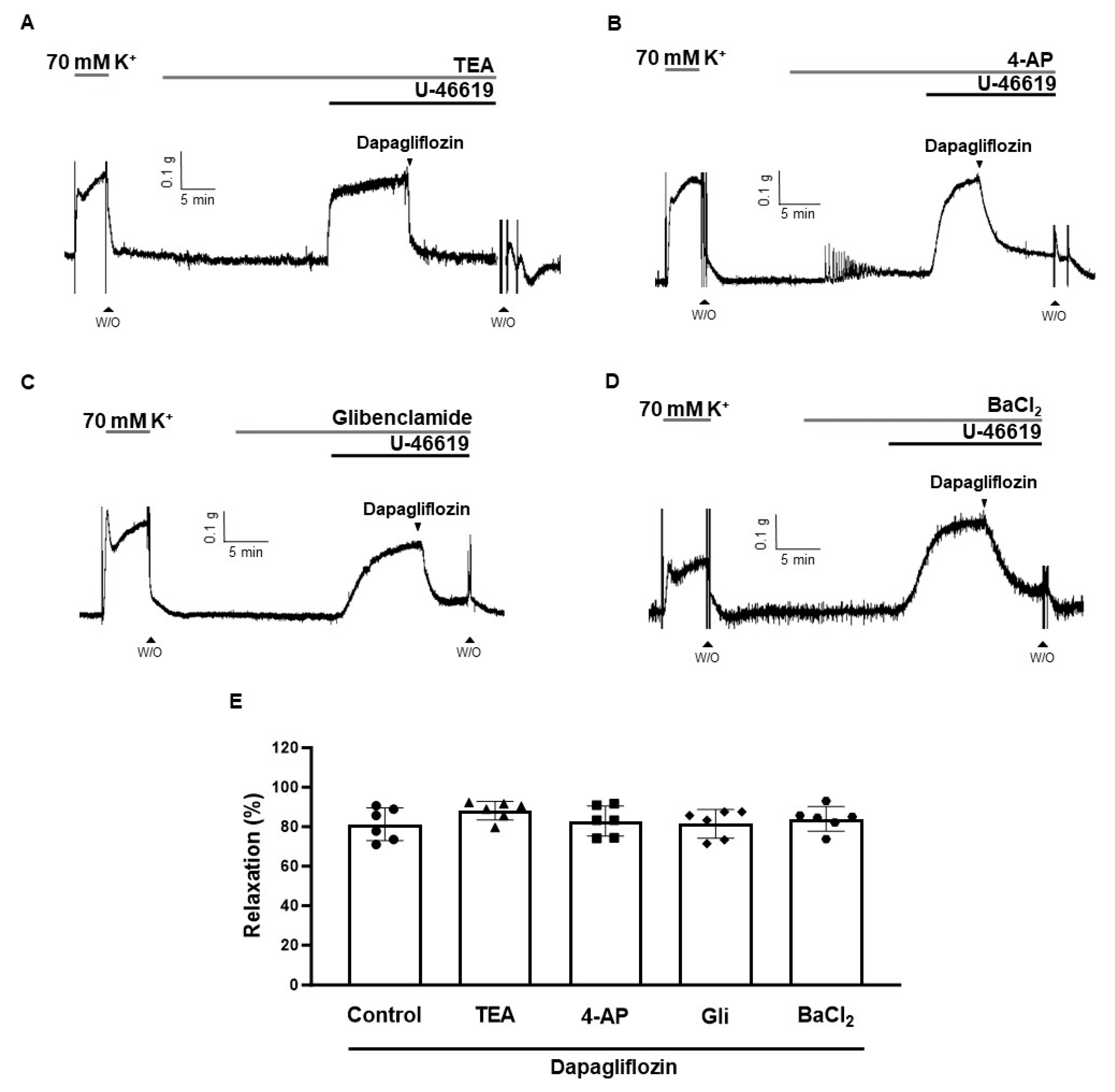
2.5. Effects of K+ Channel Blockers on Dapagliflozin-Induced Vascular Relaxation
2.6. Effect of Dapagliflozin on Extracellular-Ca2+-Induced Contraction
2.7. Decrease in Phosphorylation Level of MLC20 by Dapagliflozin in Human Aortic Smooth Muscle Cells
3. Discussion
4. Materials and Methods
4.1. Animals and Tissue Preparation
4.2. Measurement of Isometric Tension in Coronary Arteries
4.3. Cell Culture
4.4. Measurement of MLC20 Phosphorylation Level (Western Blot Analysis)
4.5. Chemicals and Reagents
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chin, K.L.; Ofori-Asenso, R.; Hopper, I.; von Lueder, T.G.; Reid, C.M.; Zoungas, S.; Wang, B.H.; Liew, D. Potential mechanisms underlying the cardiovascular benefits of sodium glucose cotransporter 2 inhibitors: A systematic review of data from preclinical studies. Cardiovasc. Res. 2019, 115, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, E.; Lim, S.; Lamptey, R.; Webb, D.R.; Davies, M.J. Type 2 diabetes. Lancet 2022, 400, 1803–1820. [Google Scholar] [CrossRef] [PubMed]
- Martin-Timon, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; Del Canizo-Gomez, F.J. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.X.; Ma, X.N.; Guan, C.H.; Li, Y.D.; Mauricio, D.; Fu, S.B. Cardiovascular disease in type 2 diabetes mellitus: Progress toward personalized management. Cardiovasc. Diabetol. 2022, 21, 74. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Kang, B.; Zhou, J. The current role of sodium-glucose cotransporter 2 inhibitors in type 2 diabetes mellitus management. Cardiovasc. Diabetol. 2022, 21, 83. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Ma, Y.; Fu, Y.; Xun, X.; Cui, Y. Development of UPLC-MS/MS Method to Study the Pharmacokinetic Interaction between Sorafenib and Dapagliflozin in Rats. Molecules 2022, 27, 6190. [Google Scholar] [CrossRef]
- da Silva, P.N.; da Conceicao, R.A.; do Couto Maia, R.; de Castro Barbosa, M.L. Sodium-glucose cotransporter 2 (sglt-2) inhibitors: A new antidiabetic drug class. Medchemcomm 2018, 9, 1273–1281. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Solini, A.; Giannini, L.; Seghieri, M.; Vitolo, E.; Taddei, S.; Ghiadoni, L.; Bruno, R.M. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: A pilot study. Cardiovasc. Diabetol. 2017, 16, 138. [Google Scholar] [CrossRef]
- Saleem, F. Dapagliflozin: Cardiovascular safety and benefits in type 2 diabetes mellitus. Cureus 2017, 9, e1751. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Cruz, C.; Gonzalez-Ortiz, M.; Rosales-Rivera, L.Y.; Patino-Laguna, A.J.; Ramirez-Rodriguez, Z.G.; Diaz-Cruz, K.; Martinez-Abundis, E. Effects of dapagliflozin on blood pressure variability in patients with prediabetes and prehypertension without pharmacological treatment: A randomized trial. Blood Press. Monit. 2020, 25, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Batra, M.; Green, K.; Hejna, J.; Abuaysheh, S.; Makdissi, A.; Chaudhuri, A.; Dandona, P. Dapagliflozin reduces systolic blood pressure and modulates vasoactive factors. Diabetes Obes. Metab. 2021, 23, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.A.; Norton, L.; DeFronzo, R.A. Renal sodium-glucose cotransporter inhibition in the management of type 2 diabetes mellitus. Am. J. Physiol. Renal. Physiol. 2015, 309, F889–F900. [Google Scholar] [CrossRef]
- Webb, R.C. Smooth muscle contraction and relaxation. Adv. Physiol. Educ. 2003, 27, 201–206. [Google Scholar] [CrossRef]
- Pi, X.; Xie, L.; Patterson, C. Emerging roles of vascular endothelium in metabolic homeostasis. Circ. Res. 2018, 123, 477–494. [Google Scholar] [CrossRef]
- Kruger-Genge, A.; Blocki, A.; Franke, R.P.; Jung, F. Vascular endothelial cell biology: An update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef]
- Furchgott, R.F.; Vanhoutte, P.M. Endothelium-derived relaxing and contracting factors. FASEB J. 1989, 3, 2007–2018. [Google Scholar] [CrossRef]
- Feletou, M.; Vanhoutte, P.M. Endothelium-dependent hyperpolarization: No longer an f-word! J. Cardiovasc. Pharmacol. 2013, 61, 91–92. [Google Scholar] [CrossRef]
- Nagao, T.; Illiano, S.; Vanhoutte, P.M. Heterogeneous distribution of endothelium-dependent relaxations resistant to ng-nitro-l-arginine in rats. Am. J. Physiol. 1992, 263, H1090–H1094. [Google Scholar] [CrossRef]
- Nagao, T.; Vanhoutte, P.M. Hyperpolarization contributes to endothelium-dependent relaxations to acetylcholine in femoral veins of rats. Am. J. Physiol. 1991, 261, H1034–H1037. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Closs, E.I.; Pollock, J.S.; Nakane, M.; Schwarz, P.; Gath, I.; Kleinert, H. Nitric oxide synthase isozymes. Characterization, purification, molecular cloning, and functions. Hypertension 1994, 23, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Menon, S.N.; Zerin, F.; Hasan, R. Dapagliflozin induces vasodilation in resistance-size mesenteric arteries by stimulating smooth muscle cell k(v)7 ion channels. Heliyon 2022, 8, e09503. [Google Scholar] [CrossRef] [PubMed]
- Baretella, O.; Chung, S.K.; Barton, M.; Xu, A.; Vanhoutte, P.M. Obesity and heterozygous endothelial overexpression of prepro-endothelin-1 modulate responsiveness of mouse main and segmental renal arteries to vasoconstrictor agents. Life Sci. 2014, 118, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Gluais, P.; Lonchampt, M.; Morrow, J.D.; Vanhoutte, P.M.; Feletou, M. Acetylcholine-induced endothelium-dependent contractions in the shr aorta: The janus face of prostacyclin. Br. J. Pharmacol. 2005, 146, 834–845. [Google Scholar] [CrossRef]
- Billington, C.K.; Penn, R.B. Signaling and regulation of g protein-coupled receptors in airway smooth muscle. Respir. Res. 2003, 4, 2. [Google Scholar] [CrossRef]
- Stitham, J.; Stojanovic, A.; Ross, L.A.; Blount, A.C., Jr.; Hwa, J. Clusters of transmembrane residues are critical for human prostacyclin receptor activation. Biochemistry 2004, 43, 8974–8986. [Google Scholar] [CrossRef]
- Gao, Y.D.; Zou, J.J.; Zheng, J.W.; Shang, M.; Chen, X.; Geng, S.; Yang, J. Promoting effects of il-13 on ca2+ release and store-operated ca2+ entry in airway smooth muscle cells. Pulm. Pharmacol. Ther. 2010, 23, 182–189. [Google Scholar] [CrossRef]
- Gilani, A.H.; Khan, A.U.; Jabeen, Q.; Subhan, F.; Ghafar, R. Antispasmodic and blood pressure lowering effects of valeriana wallichii are mediated through k+ channel activation. J. Ethnopharmacol. 2005, 100, 347–352. [Google Scholar] [CrossRef]
- Tykocki, N.R.; Boerman, E.M.; Jackson, W.F. Smooth muscle ion channels and regulation of vascular tone in resistance arteries and arterioles. Compr. Physiol. 2017, 7, 485–581. [Google Scholar]
- Jackson, W.F. K(v) channels and the regulation of vascular smooth muscle tone. Microcirculation 2018, 25, e12421. [Google Scholar] [CrossRef]
- Jackson, W.F. Potassium channels in regulation of vascular smooth muscle contraction and growth. Adv. Pharmacol. 2017, 78, 89–144. [Google Scholar]
- Arow, M.; Waldman, M.; Yadin, D.; Nudelman, V.; Shainberg, A.; Abraham, N.G.; Freimark, D.; Kornowski, R.; Aravot, D.; Hochhauser, E.; et al. Sodium-glucose cotransporter 2 inhibitor dapagliflozin attenuates diabetic cardiomyopathy. Cardiovasc. Diabetol. 2020, 19, 7. [Google Scholar] [CrossRef]
- Emmert, D.A.; Fee, J.A.; Goeckeler, Z.M.; Grojean, J.M.; Wakatsuki, T.; Elson, E.L.; Herring, B.P.; Gallagher, P.J.; Wysolmerski, R.B. Rho-kinase-mediated Ca2+-independent contraction in rat embryo fibroblasts. Am. J. Physiol. Cell Physiol. 2004, 286, C8–C21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ikebe, M.; Hartshorne, D.J.; Elzinga, M. Phosphorylation of the 20,000-dalton light chain of smooth muscle myosin by the calcium-activated, phospholipid-dependent protein kinase. Phosphorylation sites and effects of phosphorylation. J. Biol. Chem. 1987, 262, 9569–9573. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Verma, S. Mechanisms of cardiovascular benefits of sodium glucose co-transporter 2 (sglt2) inhibitors: A state-of-the-art review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.A.; Mansfield, T.A.; Cain, V.A.; Iqbal, N.; Parikh, S.; Ptaszynska, A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: A randomised, double-blind, placebo-controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016, 4, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Sayour, A.A.; Korkmaz-Icoz, S.; Loganathan, S.; Ruppert, M.; Sayour, V.N.; Olah, A.; Benke, K.; Brune, M.; Benko, R.; Horvath, E.M.; et al. Acute canagliflozin treatment protects against in vivo myocardial ischemia-reperfusion injury in non-diabetic male rats and enhances endothelium-dependent vasorelaxation. J. Transl. Med. 2019, 17, 127. [Google Scholar] [CrossRef]
- Makino, A.; Kamata, K. Elevated plasma endothelin-1 level in streptozotocin-induced diabetic rats and responsiveness of the mesenteric arterial bed to endothelin-1. Br. J. Pharmacol. 1998, 123, 1065–1072. [Google Scholar] [CrossRef]
- Choi, S.K.; Ahn, D.S.; Lee, Y.H. Comparison of contractile mechanisms of sphingosylphosphorylcholine and sphingosine-1-phosphate in rabbit coronary artery. Cardiovasc. Res. 2009, 82, 324–332. [Google Scholar] [CrossRef][Green Version]
- Hasan, R.; Leo, M.D.; Muralidharan, P.; Mata-Daboin, A.; Yin, W.; Bulley, S.; Fernandez-Pena, C.; MacKay, C.E.; Jaggar, J.H. Sumo1 modification of pkd2 channels regulates arterial contractility. Proc. Natl. Acad. Sci. USA 2019, 116, 27095–27104. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Lasker, S.; Hasan, A.; Zerin, F.; Zamila, M.; Chowdhury, F.I.; Nayan, S.I.; Rahman, M.M.; Khan, F.; Subhan, N.; et al. Canagliflozin attenuates isoprenaline-induced cardiac oxidative stress by stimulating multiple antioxidant and anti-inflammatory signaling pathways. Sci. Rep. 2020, 10, 14459. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Haam, C.E.; Byeon, S.; Oh, E.Y.; Choi, S.-K.; Lee, Y.-H. Investigating the Cardiovascular Benefits of Dapagliflozin: Vasodilatory Effect on Isolated Rat Coronary Arteries. Int. J. Mol. Sci. 2023, 24, 16873. https://doi.org/10.3390/ijms242316873
Choi S, Haam CE, Byeon S, Oh EY, Choi S-K, Lee Y-H. Investigating the Cardiovascular Benefits of Dapagliflozin: Vasodilatory Effect on Isolated Rat Coronary Arteries. International Journal of Molecular Sciences. 2023; 24(23):16873. https://doi.org/10.3390/ijms242316873
Chicago/Turabian StyleChoi, Sooyeon, Chae Eun Haam, Seonhee Byeon, Eun Yi Oh, Soo-Kyoung Choi, and Young-Ho Lee. 2023. "Investigating the Cardiovascular Benefits of Dapagliflozin: Vasodilatory Effect on Isolated Rat Coronary Arteries" International Journal of Molecular Sciences 24, no. 23: 16873. https://doi.org/10.3390/ijms242316873
APA StyleChoi, S., Haam, C. E., Byeon, S., Oh, E. Y., Choi, S.-K., & Lee, Y.-H. (2023). Investigating the Cardiovascular Benefits of Dapagliflozin: Vasodilatory Effect on Isolated Rat Coronary Arteries. International Journal of Molecular Sciences, 24(23), 16873. https://doi.org/10.3390/ijms242316873




