Lacticaseibacillus rhamnosus CRL1505 Peptidoglycan Modulates the Inflammation-Coagulation Response Triggered by Poly(I:C) in the Respiratory Tract
Abstract
:1. Introduction
2. Results
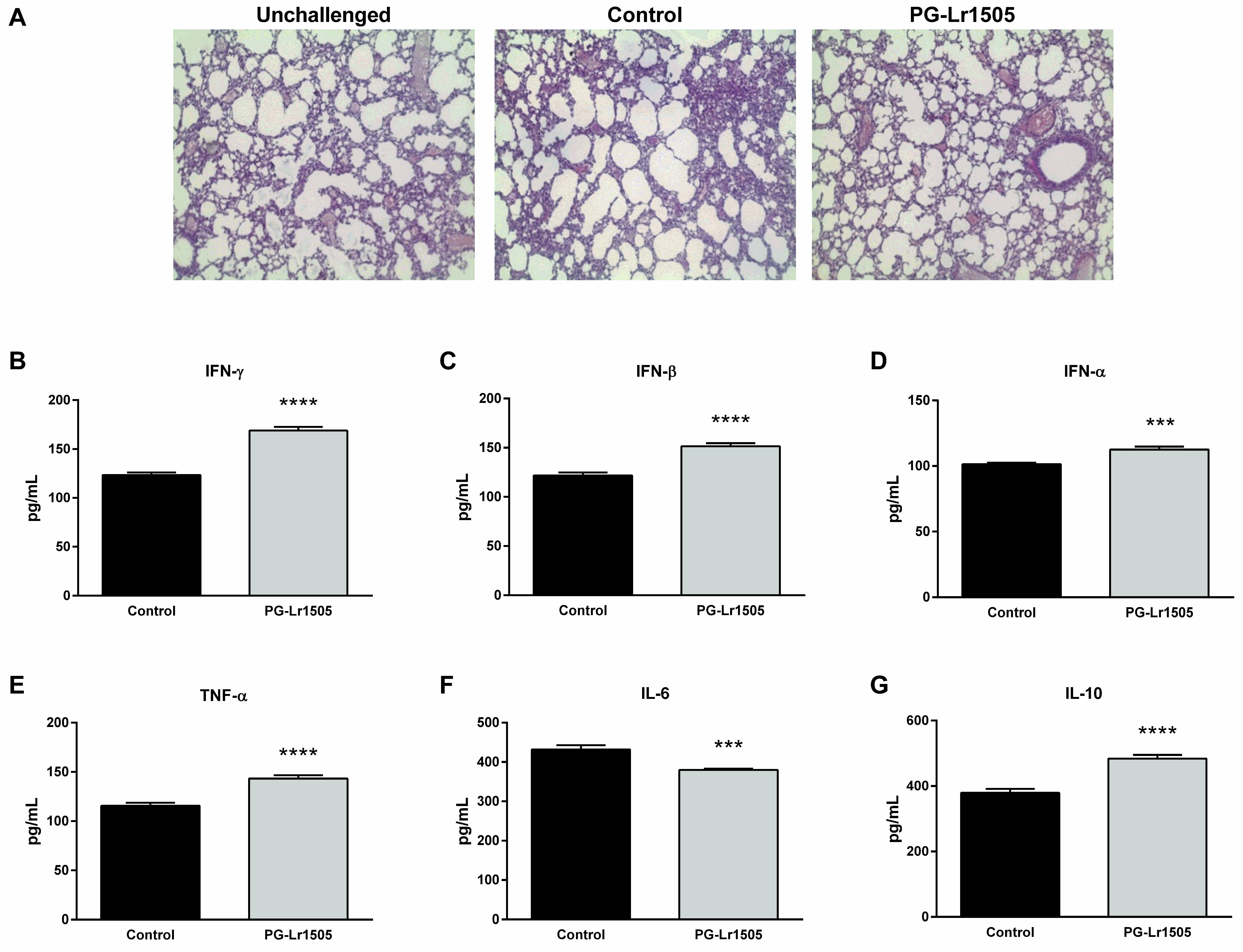
2.1. Nasally Administered PG-Lr1505 Reduces Lung Injuries in Poly(I:C)-Challenged Mice
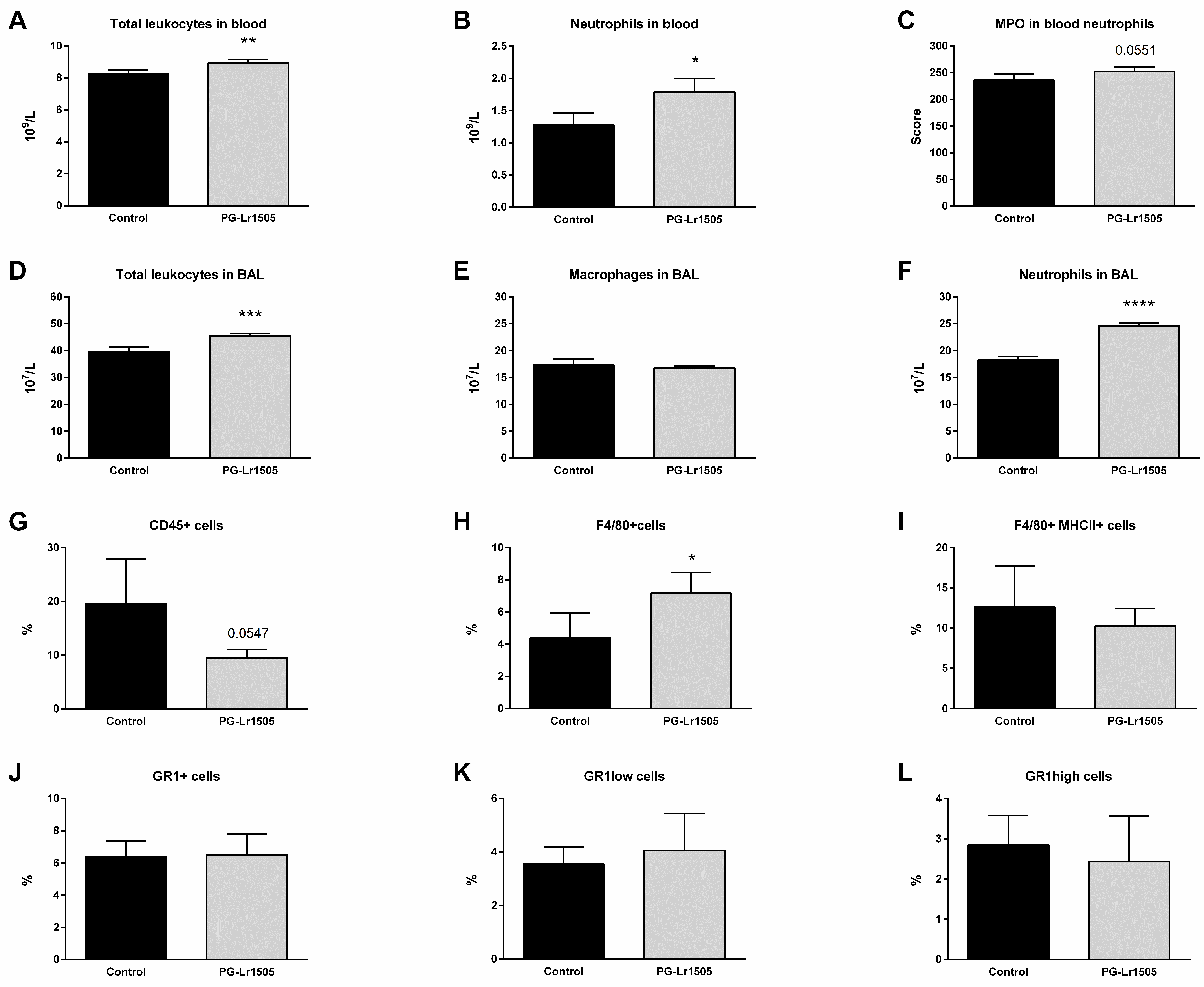
2.2. Nasally Administered PG-Lr1505 Beneficially Modulates Inflammatory Response in Poly(I:C)-Challenged Mice
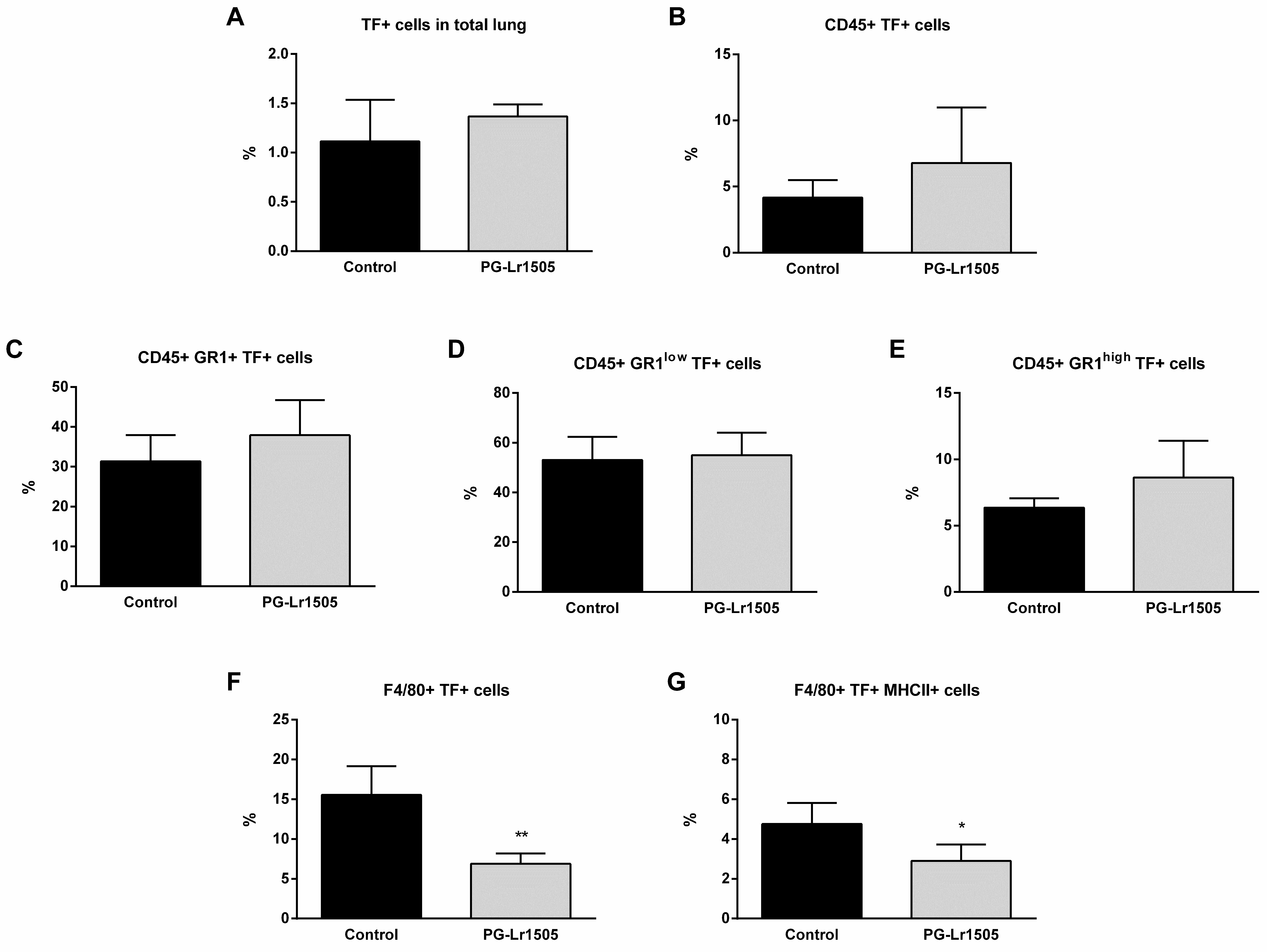
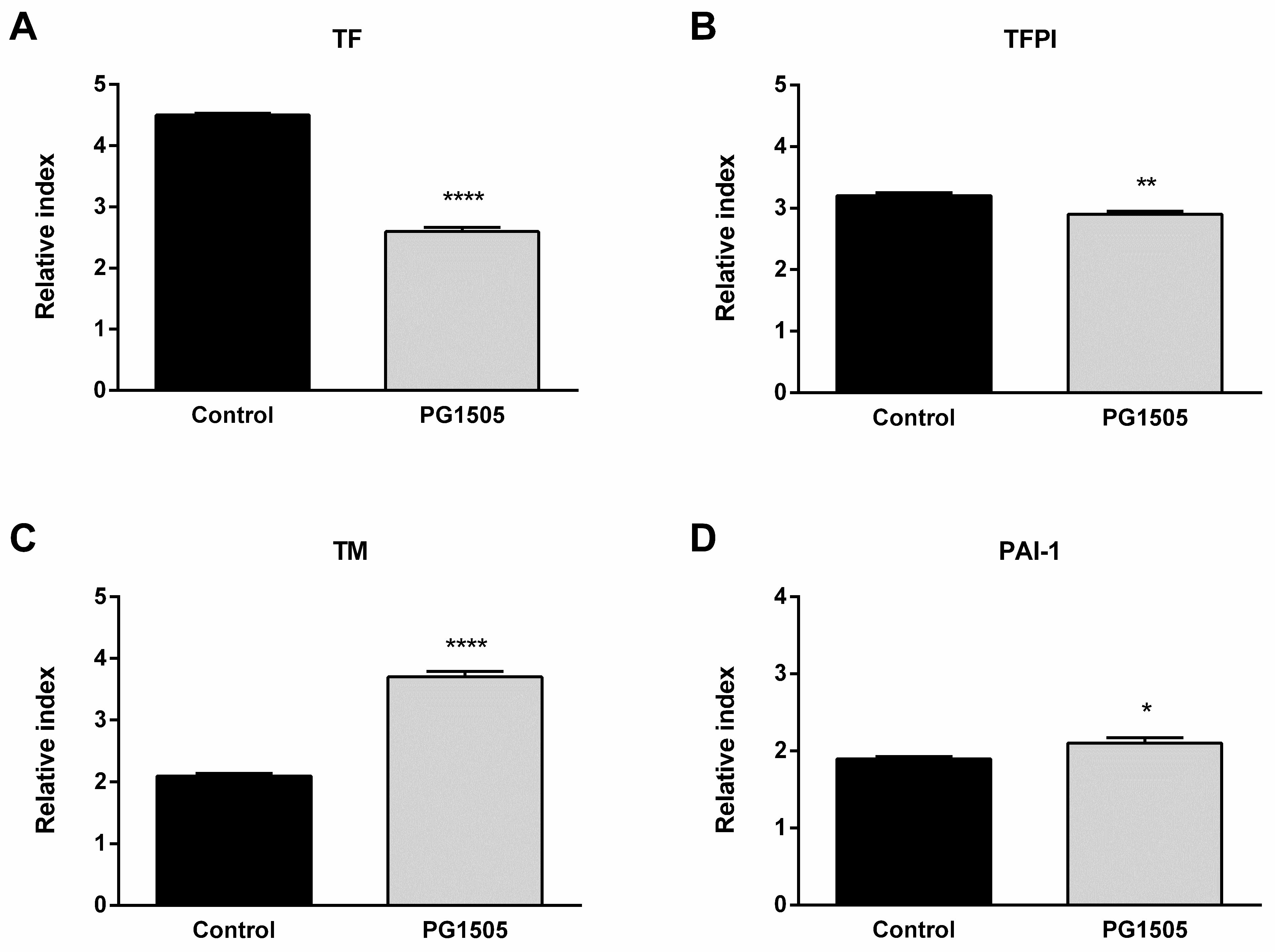
2.3. Nasally Administered PG-Lr1505 Regulates Hemostatic Parameters in Poly(I:C)-Challenged Mice
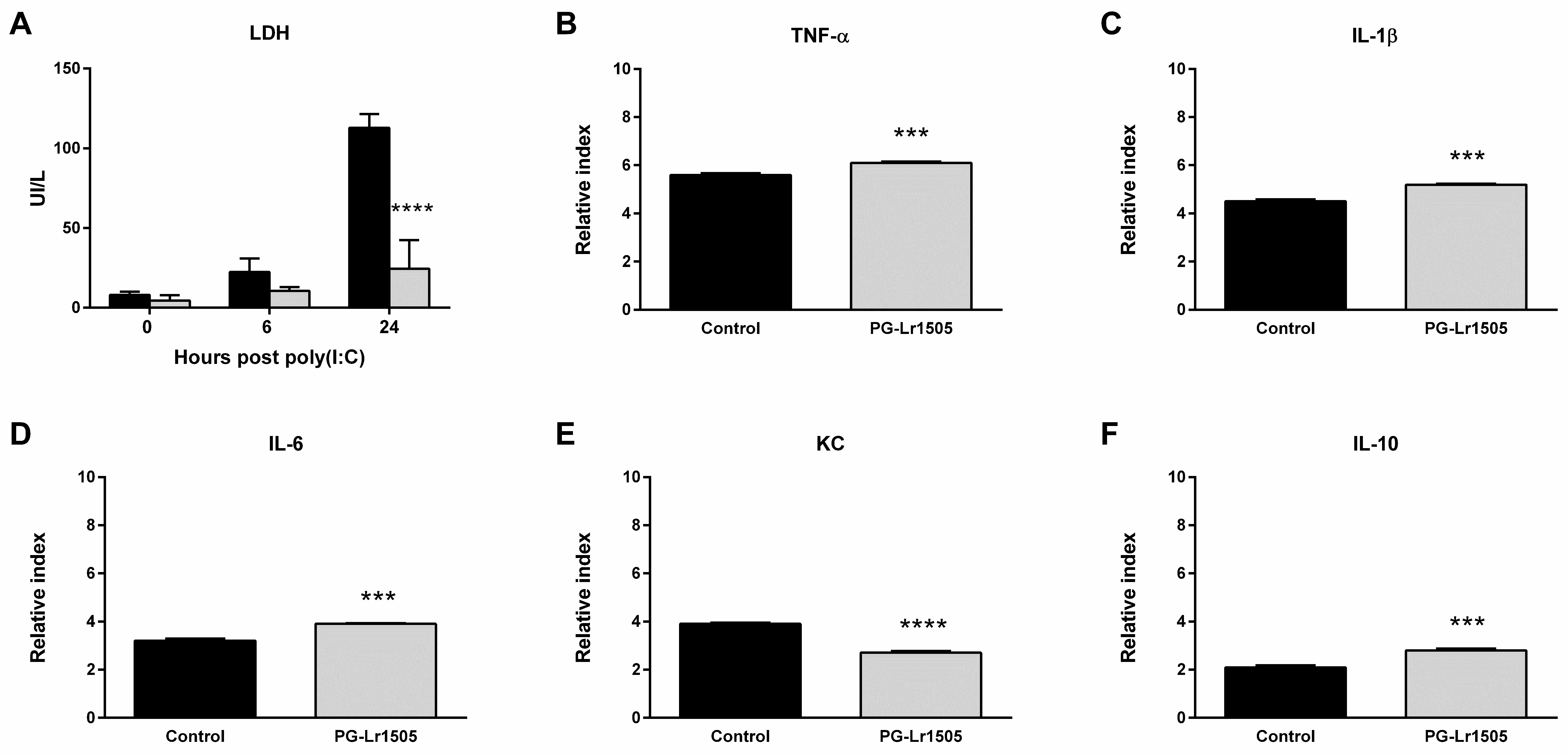
2.4. PG-Lr1505 Modulates Macrophage Response to Poly(I:C) In Vitro
2.5. The Production of Pro-Inflammatory Mediators in Poly(I:C)-Challenged Macrophages Is Regulated by PG-Lr1505
2.6. PG-Lr1505 Modulates Hemostatic Parameters in Poly(I:C)-Stimulated Macrophages
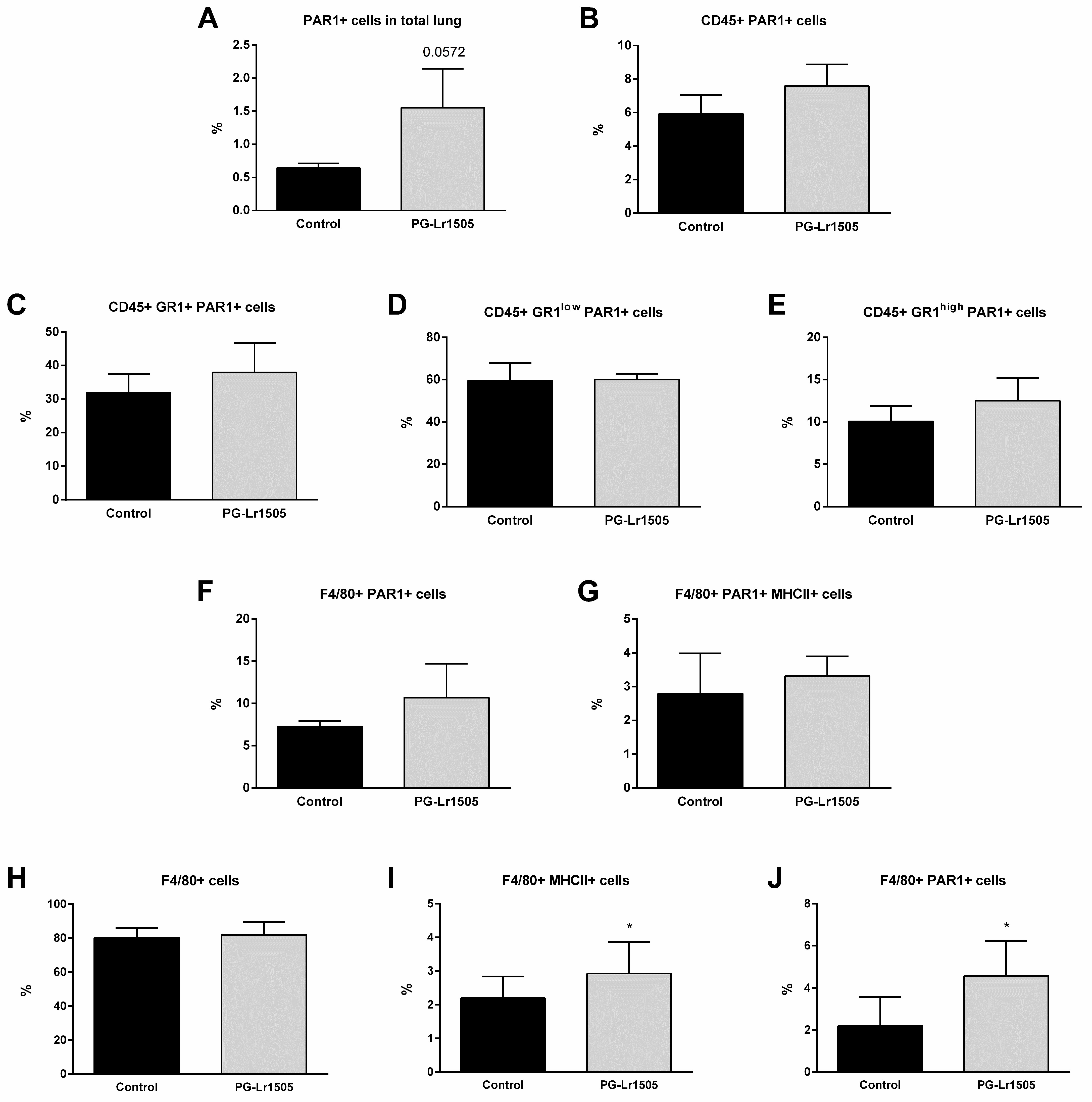
2.7. Poly(I:C)-Stimulated Macrophages Induce Protease Activated Receptor 1 (PAR1) Activation Modulated by PG-Lr1505
3. Discussion
4. Materials and Methods
4.1. Microorganism and Peptidoglycan
4.2. Experimental Murine Model
4.3. Lung Histology
4.4. Cytokine Concentrations in BAL
4.5. Total and Differential Leukocyte Counts in Blood and BAL
4.6. Activation of Blood Neutrophils
4.7. Lung Cell Preparation
4.8. Flow Cytometry Studies
4.9. Quantitative Expression Analysis by Real-Time PCR
4.10. Coagulation Tests
4.11. Platelet Counts
4.12. Determination of vWF in Plasma
4.13. Raw 264.7 Cells and Treatment with PG-Lr1505
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TF | tissue factor |
| dsRNA | double-stranded RNA |
| poly(I:C) | polyinosinic-polycytidylic acid |
| NETs | neutrophil extracellular traps |
| TLR | toll-like receptor |
| PG-Lr1505 | Lacticaseibacillus rhamnosus CRL1505 peptidoglycan |
| H-E | hematoxilin-eosin |
| LDH | lactate dehydrogenase |
| BAL | broncho-alveolar lavage |
| IFN | interferon |
| TNF | tumor necrosis factor |
| IL | interleukin |
| MPO | myeloperoxidase |
| vWF | von Willebrand factor |
| APTT | activated partial thromboplastin time |
| TFPI | tissue factor pathway inhibitor |
| TM | thrombomodulin |
| TFPI | tissue factor pathway inhibitor |
| PAI-1 | plasminogen activator inhibitor-1 |
| PAR1 | protease activated receptor 1 |
| MRS | Man–Rogosa–Sharpe broth |
| PBS | phosphate buffer saline |
| ELISA | enzyme-linked immunosorbent assay |
| FBS | fetal bovine serum |
| PT | prothrombin time |
References
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; He, F.; Li, P.; Hardwidge, P.R.; Li, N.; Peng, Y. The Role of Innate Immunity in Pulmonary Infections. Biomed. Res. Int. 2021, 2021, 6646071. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, B.; Massberg, S. Thrombosis as an intravascular effector of innate immunity. Nat. Rev. Immunol. 2013, 13, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Colling, M.E.; Tourdot, B.E.; Kanthi, Y. Inflammation, Infection and Venous Thromboembolism. Circ. Res. 2021, 128, 2017–2036. [Google Scholar] [CrossRef] [PubMed]
- Klavina, P.A.; Leon, G.; Curtis, A.M.; Preston, R.J.S. Dysregulated haemostasis in thrombo-inflammatory disease. Clin. Sci. 2022, 136, 1809–1829. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Coagulation and sepsis. Thromb. Res. 2017, 149, 38–44. [Google Scholar] [CrossRef]
- Mauad, T.; Duarte-Neto, A.N.; da Silva, L.F.F.; de Oliveira, E.P.; de Brito, J.M.; do Nascimento, E.C.T.; de Almeida Monteiro, R.A.; Ferreira, J.C.; de Carvalho, C.R.R.; do Nascimento Saldiva, P.H.; et al. Tracking the time course of pathological patterns of lung injury in severe COVID-19. Respir. Res. 2021, 22, 32. [Google Scholar] [CrossRef] [PubMed]
- Leberzammer, J.; von Hundelshausen, P. Chemokines, molecular drivers of thromboinflammation and immunothrombosis. Front. Immunol. 2023, 14, 1276353. [Google Scholar] [CrossRef]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Sachetto, A.T.A.; Mackman, N. Monocyte Tissue Factor Expression: Lipopolysaccharide Induction and Roles in Pathological Activation of Coagulation. Thromb. Haemost. 2023, 123, 1017–1033. [Google Scholar] [CrossRef]
- Maugeri, N.; Manfredi, A.A. Tissue Factor Expressed by Neutrophils: Another Piece in the Vascular Inflammation Puzzle. Semin. Thromb. Hemost. 2015, 41, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Hottz, E.D.; Martins-Goncalves, R.; Palhinha, L.; Azevedo-Quintanilha, I.G.; de Campos, M.M.; Sacramento, C.Q.; Temerozo, J.R.; Soares, V.C.; Dias, S.S.G.; Teixeira, L.; et al. Platelet-monocyte interaction amplifies thromboinflammation through tissue factor signaling in COVID-19. Blood Adv. 2022, 6, 5085–5099. [Google Scholar] [CrossRef] [PubMed]
- Zelaya, H.; Rothmeier, A.S.; Ruf, W. Tissue factor at the crossroad of coagulation and cell signaling. J. Thromb. Haemost. 2018, 16, 1941–1952. [Google Scholar] [CrossRef]
- Iba, T.; Levi, M.; Levy, J.H. Intracellular communication and immunothrombosis in sepsis. J. Thromb. Haemost. 2022, 20, 2475–2484. [Google Scholar] [CrossRef]
- Wilhelm, G.; Mertowska, P.; Mertowski, S.; Przysucha, A.; Struzyna, J.; Grywalska, E.; Torres, K. The Crossroads of the Coagulation System and the Immune System: Interactions and Connections. Int. J. Mol. Sci. 2023, 24, 12563. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Zuliani, G.; Roncon, L. The risk of thrombosis after acute-COVID-19 infection. QJM Int. J. Med. 2021, 114, 619–620. [Google Scholar] [CrossRef] [PubMed]
- Son, K.N.; Liang, Z.; Lipton, H.L. Double-Stranded RNA Is Detected by Immunofluorescence Analysis in RNA and DNA Virus Infections, Including Those by Negative-Stranded RNA Viruses. J. Virol. 2015, 89, 9383–9392. [Google Scholar] [CrossRef]
- Jarrahi, A.; Khodadadi, H.; Moore, N.S.; Lu, Y.; Awad, M.E.; Salles, E.L.; Vaibhav, K.; Baban, B.; Dhandapani, K.M. Recombinant human DNase-I improves acute respiratory distress syndrome via neutrophil extracellular trap degradation. J. Thromb. Haemost. 2023, 21, 2473–2484. [Google Scholar] [CrossRef]
- Stowell, N.C.; Seideman, J.; Raymond, H.A.; Smalley, K.A.; Lamb, R.J.; Egenolf, D.D.; Bugelski, P.J.; Murray, L.A.; Marsters, P.A.; Bunting, R.A.; et al. Long-term activation of TLR3 by poly(I:C) induces inflammation and impairs lung function in mice. Respir. Res. 2009, 10, 43. [Google Scholar] [CrossRef]
- Mei, X.; Lu, R.; Cui, L.; Tian, Y.; Zhao, P.; Li, J. Poly I:C Exacerbates Airway Inflammation and Remodeling in Cigarette Smoke-Exposed Mice. Lung 2022, 200, 677–686. [Google Scholar] [CrossRef]
- Zelaya, H.; Tada, A.; Vizoso-Pinto, M.G.; Salva, S.; Kanmani, P.; Aguero, G.; Alvarez, S.; Kitazawa, H.; Villena, J. Nasal priming with immunobiotic Lactobacillus rhamnosus modulates inflammation-coagulation interactions and reduces influenza virus-associated pulmonary damage. Inflamm. Res. 2015, 64, 589–602. [Google Scholar] [CrossRef] [PubMed]
- Zelaya, H.; Tsukida, K.; Chiba, E.; Marranzino, G.; Alvarez, S.; Kitazawa, H.; Aguero, G.; Villena, J. Immunobiotic lactobacilli reduce viral-associated pulmonary damage through the modulation of inflammation-coagulation interactions. Int. Immunopharmacol. 2014, 19, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Salva, S.; Tiscornia, I.; Gutierrez, F.; Alvarez, S.; Bollati-Fogolin, M. Lactobacillus rhamnosus postbiotic-induced immunomodulation as safer alternative to the use of live bacteria. Cytokine 2021, 146, 155631. [Google Scholar] [CrossRef] [PubMed]
- Clua, P.; Kanmani, P.; Zelaya, H.; Tada, A.; Kober, A.; Salva, S.; Alvarez, S.; Kitazawa, H.; Villena, J. Peptidoglycan from Immunobiotic Lactobacillus rhamnosus Improves Resistance of Infant Mice to Respiratory Syncytial Viral Infection and Secondary Pneumococcal Pneumonia. Front. Immunol. 2017, 8, 948. [Google Scholar] [CrossRef] [PubMed]
- Ryan, T.A.J.; O’Neill, L.A.J. Innate immune signaling and immunothrombosis: New insights and therapeutic opportunities. Eur. J. Immunol. 2022, 52, 1024–1034. [Google Scholar] [CrossRef]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Yiang, G.T.; Wu, Y.K.; Tsai, K.W.; Tzeng, I.S.; Hu, W.C.; Liao, M.T.; Lu, K.C.; Chung, H.W.; Chao, Y.C.; Su, W.L. Immunothrombosis biomarkers as potential predictive factors of acute respiratory distress syndrome in moderate-to-critical COVID-19: A single-center, retrospective cohort study. Immunol. Lett. 2023, 254, 30–38. [Google Scholar] [CrossRef]
- Conway, E.M.; Mackman, N.; Warren, R.Q.; Wolberg, A.S.; Mosnier, L.O.; Campbell, R.A.; Gralinski, L.E.; Rondina, M.T.; van de Veerdonk, F.L.; Hoffmeister, K.M.; et al. Understanding COVID-19-associated coagulopathy. Nat. Rev. Immunol. 2022, 22, 639–649. [Google Scholar] [CrossRef]
- Pannone, G.; Caponio, V.C.A.; De Stefano, I.S.; Ramunno, M.A.; Meccariello, M.; Agostinone, A.; Pedicillo, M.C.; Troiano, G.; Zhurakivska, K.; Cassano, T.; et al. Lung histopathological findings in COVID-19 disease—A systematic review. Infect. Agent. Cancer 2021, 16, 34. [Google Scholar] [CrossRef]
- Kumar, Y.; Liang, C.; Limmon, G.V.; Liang, L.; Engelward, B.P.; Ooi, E.E.; Chen, J.; Tannenbaum, S.R. Molecular analysis of serum and bronchoalveolar lavage in a mouse model of influenza reveals markers of disease severity that can be clinically useful in humans. PLoS ONE 2014, 9, e86912. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Sacramento, C.Q.; Pereira-Dutra, F.S.; Fintelman-Rodrigues, N.; Silva, P.P.; Mattos, M.; de Freitas, C.S.; Marttorelli, A.; de Melo, G.R.; Campos, M.M.; et al. Severe influenza infection is associated with inflammatory programmed cell death in infected macrophages. Front. Cell Infect. Microbiol. 2023, 13, 1067285. [Google Scholar] [CrossRef] [PubMed]
- Tosi, M.F. Innate immune responses to infection. J. Allergy Clin. Immunol. 2005, 116, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, F.T.; Traber, K.E. Innate immune responses in pneumonia. Pneumonia 2023, 15, 4. [Google Scholar] [CrossRef]
- Meidaninikjeh, S.; Sabouni, N.; Marzouni, H.Z.; Bengar, S.; Khalili, A.; Jafari, R. Monocytes and macrophages in COVID-19: Friends and foes. Life Sci. 2021, 269, 119010. [Google Scholar] [CrossRef]
- Phillipson, M.; Kubes, P. The neutrophil in vascular inflammation. Nat. Med. 2011, 17, 1381–1390. [Google Scholar] [CrossRef]
- Wei, X.; Narasimhan, H.; Zhu, B.; Sun, J. Host Recovery from Respiratory Viral Infection. Annu. Rev. Immunol. 2023, 41, 277–300. [Google Scholar] [CrossRef]
- Goeijenbier, M.; van Wissen, M.; van de Weg, C.; Jong, E.; Gerdes, V.E.; Meijers, J.C.; Brandjes, D.P.; van Gorp, E.C. Review: Viral infections and mechanisms of thrombosis and bleeding. J. Med. Virol. 2012, 84, 1680–1696. [Google Scholar] [CrossRef] [PubMed]
- Pujhari, S.; Paul, S.; Ahluwalia, J.; Rasgon, J.L. Clotting disorder in severe acute respiratory syndrome coronavirus 2. Rev. Med. Virol. 2021, 31, e2177. [Google Scholar] [CrossRef]
- Ryan, T.A.J.; O’Neill, L.A.J. An Emerging Role for Type I Interferons as Critical Regulators of Blood Coagulation. Cells 2023, 12, 778. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, X.; Tang, Y.; Qiu, X.; Wang, Z.; Fu, G.; Wu, J.; Kang, H.; Wang, J.; Wang, H.; et al. The role of type 1 interferons in coagulation induced by gram-negative bacteria. Blood 2020, 135, 1087–1100. [Google Scholar] [CrossRef]
- Grover, S.P.; Mackman, N. Tissue Factor: An Essential Mediator of Hemostasis and Trigger of Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Girard, T.J.; Antunes, L.; Zhang, N.; Amrute, J.M.; Subramanian, R.; Eldem, I.; Remy, K.E.; Mazer, M.; Erlich, E.C.; Cruchaga, C.; et al. Peripheral blood mononuclear cell tissue factor (F3 gene) transcript levels and circulating extracellular vesicles are elevated in severe coronavirus 2019 (COVID-19) disease. J. Thromb. Haemost. 2023, 21, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, P.P.; Samarasinghe, A.E. The Role of Innate Leukocytes during Influenza Virus Infection. J. Immunol. Res. 2019, 2019, 8028725. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Kanneganti, T.D. Innate immunity: The first line of defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Weiss, K.A.; Christiaansen, A.F.; Fulton, R.B.; Meyerholz, D.K.; Varga, S.M. Multiple CD4+ T cell subsets produce immunomodulatory IL-10 during respiratory syncytial virus infection. J. Immunol. 2011, 187, 3145–3154. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.E.; Varga, S.M. Cytokines and CD8 T cell immunity during respiratory syncytial virus infection. Cytokine 2020, 133, 154481. [Google Scholar] [CrossRef]
- Christiaansen, A.F.; Knudson, C.J.; Weiss, K.A.; Varga, S.M. The CD4 T cell response to respiratory syncytial virus infection. Immunol. Res. 2014, 59, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Elean, M.; Albarracin, L.; Fukuyama, K.; Zhou, B.; Tomokiyo, M.; Kitahara, S.; Araki, S.; Suda, Y.; Saavedra, L.; Villena, J.; et al. Lactobacillus delbrueckii CRL 581 Differentially Modulates TLR3-Triggered Antiviral Innate Immune Response in Intestinal Epithelial Cells and Macrophages. Microorganisms 2021, 9, 2449. [Google Scholar] [CrossRef]
- Shibamiya, A.; Hersemeyer, K.; Schmidt Woll, T.; Sedding, D.; Daniel, J.M.; Bauer, S.; Koyama, T.; Preissner, K.T.; Kanse, S.M. A key role for Toll-like receptor-3 in disrupting the hemostasis balance on endothelial cells. Blood 2009, 113, 714–722. [Google Scholar] [CrossRef]
- Antoniak, S.; Tatsumi, K.; Bode, M.; Vanja, S.; Williams, J.C.; Mackman, N. Protease-Activated Receptor 1 Enhances Poly I:C Induction of the Antiviral Response in Macrophages and Mice. J. Innate Immun. 2017, 9, 181–192. [Google Scholar] [CrossRef]
- Rovai, E.S.; Alves, T.; Holzhausen, M. Protease-activated receptor 1 as a potential therapeutic target for COVID-19. Exp. Biol. Med. 2021, 246, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Dacie, J.; Lewis, S. Dacie y Lewis. Hematología Práctica, 10th ed.; Elsevier España: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Kaplow, L.S. Simplified Myeloperoxidase Stain Using Benzidine Dihydrochloride. Blood 1965, 26, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Clua, P.; Tomokiyo, M.; Raya Tonetti, F.; Islam, M.A.; Garcia Castillo, V.; Marcial, G.; Salva, S.; Alvarez, S.; Takahashi, H.; Kurata, S.; et al. The Role of Alveolar Macrophages in the Improved Protection against Respiratory Syncytial Virus and Pneumococcal Superinfection Induced by the Peptidoglycan of Lactobacillus rhamnosus CRL1505. Cells 2020, 9, 1653. [Google Scholar] [CrossRef] [PubMed]
- Kordich, L. Fundamentos Para el Manejo Práctico en el Laboratorio de Hemostasia. Grupo CAHT; Federación Bioquímica de la Provincia de Buenos Aires: La Plata, Argentina, 2013. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zelaya, H.; Arellano-Arriagada, L.; Fukuyama, K.; Matsumoto, K.; Marranzino, G.; Namai, F.; Salva, S.; Alvarez, S.; Agüero, G.; Kitazawa, H.; et al. Lacticaseibacillus rhamnosus CRL1505 Peptidoglycan Modulates the Inflammation-Coagulation Response Triggered by Poly(I:C) in the Respiratory Tract. Int. J. Mol. Sci. 2023, 24, 16907. https://doi.org/10.3390/ijms242316907
Zelaya H, Arellano-Arriagada L, Fukuyama K, Matsumoto K, Marranzino G, Namai F, Salva S, Alvarez S, Agüero G, Kitazawa H, et al. Lacticaseibacillus rhamnosus CRL1505 Peptidoglycan Modulates the Inflammation-Coagulation Response Triggered by Poly(I:C) in the Respiratory Tract. International Journal of Molecular Sciences. 2023; 24(23):16907. https://doi.org/10.3390/ijms242316907
Chicago/Turabian StyleZelaya, Hortensia, Luciano Arellano-Arriagada, Kohtaro Fukuyama, Kaho Matsumoto, Gabriela Marranzino, Fu Namai, Susana Salva, Susana Alvarez, Graciela Agüero, Haruki Kitazawa, and et al. 2023. "Lacticaseibacillus rhamnosus CRL1505 Peptidoglycan Modulates the Inflammation-Coagulation Response Triggered by Poly(I:C) in the Respiratory Tract" International Journal of Molecular Sciences 24, no. 23: 16907. https://doi.org/10.3390/ijms242316907






