Modulation of Heme-Induced Inflammation Using MicroRNA-Loaded Liposomes: Implications for Hemolytic Disorders Such as Malaria and Sickle Cell Disease
Abstract
:1. Introduction
2. Results
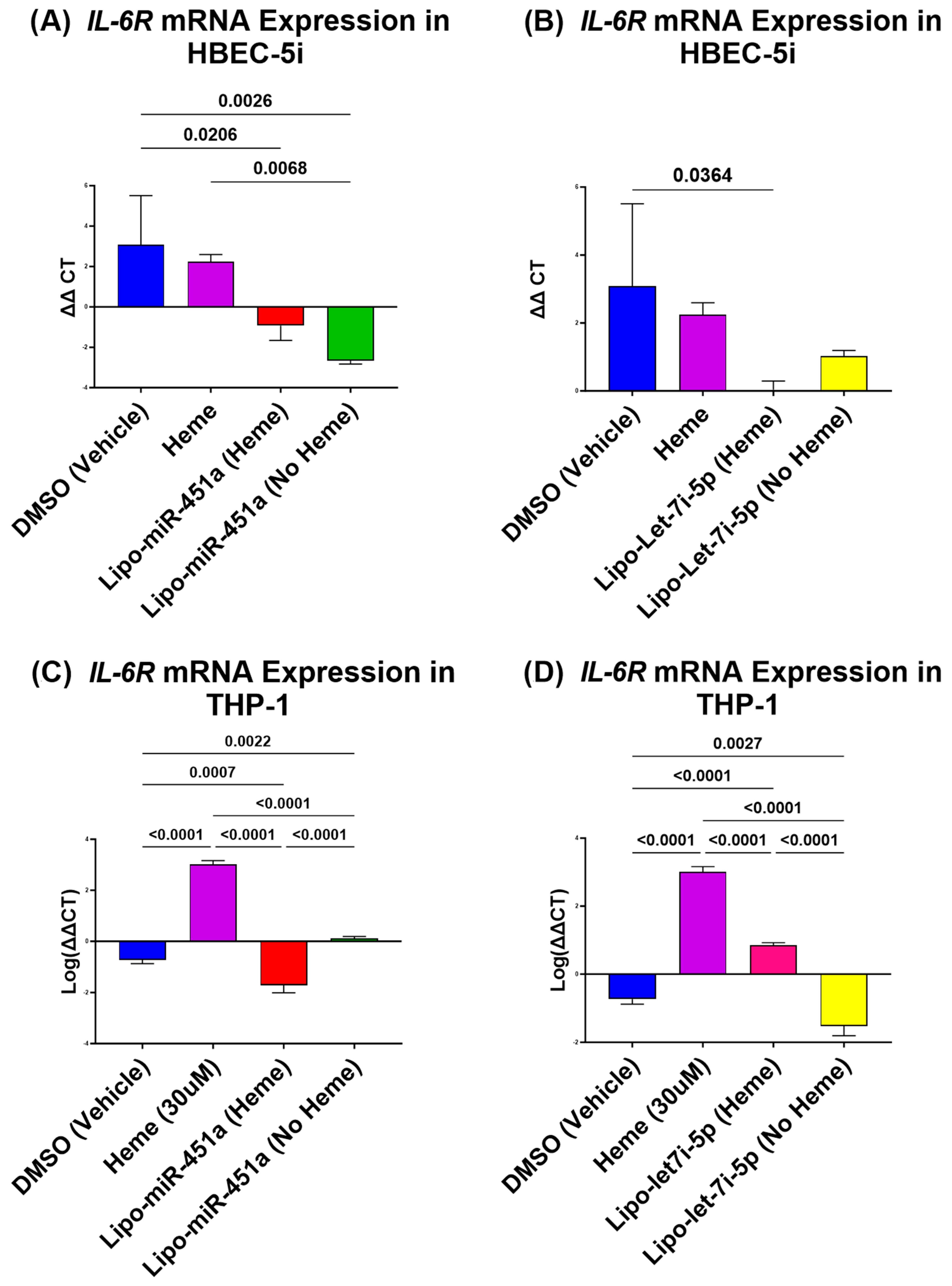
2.1. Lipo-miR-451a Downregulates IL-6R mRNA in HBEC-5i and THP-1 Cells
2.2. Lipo-let-7i-5 Downregulates TLR4 mRNA in HBEC-5i and THP-1 Cells
2.3. Lipo-miR-451a and Lipo-let-7i-5p Upregulates 14-3-3ζ in HBEC-5i Cells
2.4. Lipo-let-7i-5 and Lipo-miR-451a Downregulates p65/NFκB mRNA in HBEC-5i and THP-1 Cells
2.5. Lipo-let-7i-5p Upregulates HMOX1 in HBEC-5i Cells
2.6. Lipo-let-7i-5p Upregulates Hemopexin in HBEC-5i and THP-1 Cells
2.7. Arginase and Nitrite Concentration Is Decreased in THP-1 (Macrophages) Treated with miRNA
2.8. In Vitro Cell Death Assay
3. Discussion
4. Materials and Methods
4.1. Liposome Formulation
4.2. Cell Culture
4.3. Macrophage Conversion
4.4. Liposome Treatment
4.5. Total RNA Isolation and RT-qPCR
4.6. Nitrite Assay Analysis
4.7. Arginase Assay
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. World malaria report 2022. In World Health Organization Global Malaria Programme Report; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Garcia, L.S. Malaria. Clin. Lab. Med. 2010, 30, 93–129. [Google Scholar] [CrossRef] [PubMed]
- Gbotosho, O.T.; Kapetanaki, M.G.; Kato, G.J. The Worst Things in Life are Free: The Role of Free Heme in Sickle Cell Disease. Front. Immunol. 2020, 11, 561917. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.Y.; Ahmad, I.M.; Switzer, B.; Britigan, B.E. Induction of heme oxygenase-1 contributes to survival of Mycobacterium abscessus in human macrophages-like THP-1 cells. Redox Biol. 2015, 4, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, A.; Hanscheid, T.; Epiphanio, S.; Mota, M.M.; Vigário, A.M. Cerebral malaria and the hemolysis/methemoglobin/heme hypothesis: Shedding new light on an old disease. Int. J. Biochem. Cell Biol. 2009, 41, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, M.H. Predicting clinical severity in sickle cell anaemia. Br. J. Haematol. 2005, 129, 465–481. [Google Scholar] [CrossRef] [PubMed]
- Platt, O.S.; Thorington, B.D.; Brambilla, D.J.; Milner, P.F.; Rosse, W.F.; Vichinsky, E.; Kinney, T.R. Pain in sickle cell disease: Rates and risk factors. N. Engl. J. Med. 1991, 325, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, M.H.; Sebastiani, P. Genetic modifiers of sickle cell disease. Am. J. Hematol. 2012, 87, 795–803. [Google Scholar] [CrossRef]
- Driss, A.; Hibbert, J.M.; Wilson, N.O.; Iqbal, S.A.; Adamkiewicz, T.V.; Stiles, J.K. Genetic polymorphisms linked to susceptibility to malaria. Malar. J. 2011, 10, 271. [Google Scholar] [CrossRef]
- Archer, N.M.; Petersen, N.; Clark, M.A.; Buckee, C.O.; Childs, L.M.; Duraisingh, M.T. Resistance to Plasmodium falciparum in sickle cell trait erythrocytes is driven by oxygen-dependent growth inhibition. Proc. Natl. Acad. Sci. USA 2018, 115, 7350–7355. [Google Scholar] [CrossRef]
- Driss, A.; Asare, K.; Hibbert, J.; Gee, B.; Adamkiewicz, T.; Stiles, J. Sickle Cell Disease in the Post Genomic Era: A Monogenic Disease with a Polygenic Phenotype. Genom. Insights 2009, 2, 23–48. [Google Scholar] [CrossRef]
- Harp, K.O.; Botchway, F.; Dei-Adomakoh, Y.; Wilson, M.D.; Hood, J.L.; Adjei, A.A.; Stiles, J.K.; Driss, A. Hemoglobin Genotypes Modulate Inflammatory Response to Plasmodium Infection. Front. Immunol. 2020, 11, 593546. [Google Scholar] [CrossRef] [PubMed]
- Wassmer, S.C.; Moxon, C.A.; Taylor, T.; Grau, G.E.; Molyneux, M.E.; Craig, A.G. Vascular endothelial cells cultured from patients with cerebral or uncomplicated malaria exhibit differential reactivity to TNF. Cell. Microbiol. 2010, 13, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Maitland, K.; Marsh, K. Pathophysiology of severe malaria in children. Acta Trop. 2004, 90, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Duperray, A.; Mantovani, A.; Introna, M.; Dejana, E. Endothelial cell regulation of leukocyte infiltration in inflammatory tissues. Mediat. Inflamm. 1995, 4, 322–330. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Environmental Health Sciences. Autoimmune Diseases. 2022. Available online: https://www.niehs.nih.gov/health/topics/conditions/autoimmune/index.cfm#:~:text=Some%20are%20well%20known%2C%20such,these%20diseases%20have%20no%20cure (accessed on 8 August 2023).
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Jung, S. Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 2014, 14, 392–404. [Google Scholar] [CrossRef] [PubMed]
- Mills, C. M1 and M2 macrophages: Oracles of health and disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef]
- Hibbs, J.B., Jr.; Vavrin, Z.; Taintor, R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J. Immunol. 1987, 138, 550–565. [Google Scholar] [CrossRef]
- Ozarslan, N.; Robinson, J.F.; Gaw, S.L. Circulating monocytes, tissue macrophages, and malaria. J. Trop. Med. 2019, 2019, 3720838. [Google Scholar] [CrossRef]
- Su, X.-Z.; Lane, K.D.; Xia, L.; Sá, J.M.; Wellems, T.E. Plasmodium Genomics and Genetics: New Insights into Malaria Pathogenesis, Drug Resistance, Epidemiology, and Evolution. Clin. Microbiol. Rev. 2019, 32, e00019-19. [Google Scholar] [CrossRef]
- Rubio, M.; Bassat, Q.; Estivill, X.; Mayor, A. Tying malaria and microRNAs: From the biology to future diagnostic perspectives. Malar. J. 2016, 15, 167. [Google Scholar] [CrossRef]
- LaMonte, G.; Philip, N.; Reardon, J.; Lacsina, J.R.; Majoros, W.; Chapman, L.; Thornburg, C.D.; Telen, M.J.; Ohler, U.; Nicchitta, C.V.; et al. Translocation of sickle cell erythrocyte microRNAs into Plasmodium falciparum inhibits parasite translation and contributes to malaria resistance. Cell Host Microbe 2012, 12, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Harp, K.O.; Bashi, A.; Botchway, F.; Dei-Adomakoh, Y.; Iqbal, S.A.; Wilson, M.D.; Adjei, A.A.; Stiles, J.K.; Driss, A. MicroRNAs miR-451a and Let-7i-5p Profiles in Circulating Exosomes Vary among Individuals with Different Sickle Hemoglobin Genotypes and Malaria. J. Clin. Med. 2022, 11, 500. [Google Scholar] [CrossRef] [PubMed]
- Chamnanchanunt, S.; Fucharoen, S.; Umemura, T. Circulating microRNAs in malaria infection: Bench to bedside. Malar. J. 2017, 16, 334. [Google Scholar] [CrossRef] [PubMed]
- Munier, C.C.; Ottmann, C.; Perry, M.W. 14-3-3 modulation of the inflammatory response. Pharmacol. Res. 2021, 163, 105236. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Xu, Z.; Jin, X.; He, Y.; Zhang, J.; Jiang, T.; Chen, J. miR-451a suppression of IL-6R can inhibit proliferation and increase apoptosis through the JAK2/STAT3 pathway in multiple myeloma. Oncol. Lett. 2020, 20, 339. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, D.; Jiang, W.; Zhao, M.; Gan, J. Role of TLR4-Mediated PI3K/AKT/GSK-3beta Signaling Pathway in Apoptosis of Rat Hepatocytes. BioMed Res. Int. 2015, 2015, 631326. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Yang, R.; Chen, J.; Qi, E.; Zhou, S.; Wang, Y.; Fu, Q.; Chen, R.; Fang, X. Let-7i-5p Regulation of Cell Morphology and Migration Through Distinct Signaling Pathways in Normal and Pathogenic Urethral Fibroblasts. Front. Bioeng. Biotechnol. 2020, 8, 428. [Google Scholar] [CrossRef]
- Punsawad, C.; Krudsood, S.; Maneerat, Y.; Chaisri, U.; Tangpukdee, N.; Pongponratn, E.; Nantavisai, K.; Udomsangpetch, R.; Viriyavejakul, P. Activation of nuclear factor kappa B in peripheral blood mononuclear cells from malaria patients. Malar. J. 2012, 11, 191. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, L.; Xie, G.-L.; Bao, J.-F.; Yu, Q. Let-7 miRNAs Modulate the Activation of NF-kappaB by Targeting TNFAIP3 and Are Involved in the Pathogenesis of Lupus Nephritis. PLoS ONE 2015, 10, e0121256. [Google Scholar] [CrossRef]
- Syn, N.L.; Wang, L.; Chow, E.K.-H.; Lim, C.T.; Goh, B.-C. Exosomes in Cancer Nanomedicine and Immunotherapy: Prospects and Challenges. Trends Biotechnol. 2017, 35, 665–676. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Harp, K.O.; Bashi, A.; Botchway, F.; Addo-Gyan, D.; Tetteh-Tsifoanya, M.; Lamptey, A.; Djameh, G.; Iqbal, S.A.; Lekpor, C.; Banerjee, S.; et al. Sickle Cell Hemoglobin Genotypes Affect Malaria Parasite Growth and Correlate with Exosomal miR-451a and let-7i-5p Levels. Int. J. Mol. Sci. 2023, 24, 7546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Krappmann, D.; Wegener, E.; Sunami, Y.; Esen, M.; Thiel, A.; Mordmuller, B.; Scheidereit, C. The IkappaB kinase complex and NF-kappaB act as master regulators of lipopolysaccharide-induced gene expression and control subordinate activation of AP-1. Mol. Cell. Biol. 2004, 24, 6488–6500. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Basu, A. miR-451a Regulates Neuronal Apoptosis by Modulating 14-3-3zeta-JNK Axis upon Flaviviral Infection. mSphere 2022, 7, e0020822. [Google Scholar] [CrossRef]
- Wang, X.; Hong, Y.; Wu, L.; Duan, X.; Hu, Y.; Sun, Y.; Wei, Y.; Dong, Z.; Wu, C.; Yu, D.; et al. Deletion of MicroRNA-144/451 Cluster Aggravated Brain Injury in Intracerebral Hemorrhage Mice by Targeting 14-3-3zeta. Front. Neurol. 2020, 11, 551411. [Google Scholar] [CrossRef]
- Bohuslav, J.; Kravchenko, V.V.; Parry, G.C.; Erlich, J.H.; Gerondakis, S.; Mackman, N.; Ulevitch, R.J. Regulation of an essential innate immune response by the p50 subunit of NF-kappaB. J. Clin. Investig. 1998, 102, 1645–1652. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Sha, W.; Shulaev, V.; Stins, M.F.; Sullivan, D.J., Jr. Plasmodium falciparum–infected erythrocytes induce NF-kappaB regulated inflammatory pathways in human cerebral endothelium. Blood 2009, 114, 4243–4252. [Google Scholar] [CrossRef]
- Newton, C.R.J.C.; Hien, T.T.; White, N. Cerebral malaria. J. Neurol. Neurosurg. Psychiatry 2000, 69, 433–441. [Google Scholar] [CrossRef]
- Nagel, R.; Roth, E.J., Jr. Malaria and red cell genetic defects. Blood 1989, 74, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Hood, J.L.; San, R.S.; Wickline, S.A. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011, 71, 3792–3801. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Dickinson-Copeland, C.; Hassana, S.; Stiles, J.K. Plasmodium-infected erythrocytes (pRBC) induce endothelial cell apoptosis via a heme-mediated signaling pathway. Drug Des. Dev. Ther. 2016, 10, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Young, H.; Hurlstone, A.; Wellbrock, C. Differentiation of THP1 Cells into Macrophages for Transwell Co-culture Assay with Melanoma Cells. Bio-Protocol 2015, 5, e1638. [Google Scholar] [CrossRef] [PubMed]
- Dickinson-Copeland, C.M.; Wilson, N.O.; Liu, M.; Driss, A.; Salifu, H.; Adjei, A.A.; Wilson, M.; Gyan, B.; Oduro, D.; Badu, K.; et al. Heme-Mediated Induction of CXCL10 and Depletion of CD34+ Progenitor Cells Is Toll-Like Receptor 4 Dependent. PLoS ONE 2015, 10, e0142328. [Google Scholar] [CrossRef]
- Thomas, J.J.; Harp, K.O.; Bashi, A.; Hood, J.L.; Botchway, F.; Wilson, M.D.; Thompson, W.E.; Stiles, J.K.; Driss, A. MiR-451a and let-7i-5p loaded extracellular vesicles attenuate heme-induced inflammation in hiPSC-derived endothelial cells. Front. Immunol. 2022, 13, 1082414. [Google Scholar] [CrossRef]
- Liu, D.; Liu, C.; Wang, X.; Ingvarsson, S.; Chen, H. MicroRNA-451 suppresses tumor cell growth by down-regulating IL6R gene expression. Cancer Epidemiol. 2014, 38, 85–92. [Google Scholar] [CrossRef]
- Huang, H.; Du, T.; Huang, J.; Lin, T.; Zhang, C.; Dong, W.; Yin, X.; Guo, Z.; Xu, K.; Jiang, C. Research of the p65 gene function in the prostate cancer cell by the obtaining of shRNA sequences blocking the expression of nuclear factor kappa-B (p65) stably and construction of lentivirus vector. Chin. J. Urol. 2010, 386–390. [Google Scholar]
- Thakar, S.; Katakia, Y.T.; Ramakrishnan, S.K.; Pandya Thakkar, N.; Majumder, S. Intermittent High Glucose Elevates Nuclear Localization of EZH2 to Cause H3K27me3-Dependent Repression of KLF2 Leading to Endothelial Inflammation. Cells 2021, 10, 2548. [Google Scholar] [CrossRef]
- Brennan, G.P.; Jimenez-Mateos, E.M.; McKiernan, R.C.; Engel, T.; Tzivion, G.; Henshall, D.C. Transgenic overexpression of 14-3-3 zeta protects hippocampus against endoplasmic reticulum stress and status epilepticus in vivo. PLoS ONE 2013, 8, e54491. [Google Scholar] [CrossRef]
- Gueron, G.; De Siervi, A.; Ferrando, M.; Salierno, M.; De Luca, P.; Elguero, B.; Meiss, R.; Navone, N.; Vazquez, E.S. Critical role of endogenous heme oxygenase 1 as a tuner of the invasive potential of prostate cancer cells. Mol. Cancer Res. 2009, 7, 1745–1755. [Google Scholar] [CrossRef] [PubMed]
- Lawson, H.A.; Zayed, M.; Wayhart, J.P.; Fabbrini, E.; Love-Gregory, L.; Klein, S.; Semenkovich, C.F. Physiologic and genetic evidence links hemopexin to triglycerides in mice and humans. Int. J. Obes 2017, 41, 631–638. [Google Scholar] [CrossRef] [PubMed]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bashi, A.; Lekpor, C.; Hood, J.L.; Thompson, W.E.; Stiles, J.K.; Driss, A. Modulation of Heme-Induced Inflammation Using MicroRNA-Loaded Liposomes: Implications for Hemolytic Disorders Such as Malaria and Sickle Cell Disease. Int. J. Mol. Sci. 2023, 24, 16934. https://doi.org/10.3390/ijms242316934
Bashi A, Lekpor C, Hood JL, Thompson WE, Stiles JK, Driss A. Modulation of Heme-Induced Inflammation Using MicroRNA-Loaded Liposomes: Implications for Hemolytic Disorders Such as Malaria and Sickle Cell Disease. International Journal of Molecular Sciences. 2023; 24(23):16934. https://doi.org/10.3390/ijms242316934
Chicago/Turabian StyleBashi, Alaijah, Cecilia Lekpor, Joshua L. Hood, Winston E. Thompson, Jonathan K. Stiles, and Adel Driss. 2023. "Modulation of Heme-Induced Inflammation Using MicroRNA-Loaded Liposomes: Implications for Hemolytic Disorders Such as Malaria and Sickle Cell Disease" International Journal of Molecular Sciences 24, no. 23: 16934. https://doi.org/10.3390/ijms242316934






