Transcriptional Targeting of Dendritic Cells Using an Optimized Human Fascin1 Gene Promoter
Abstract
1. Introduction
2. Results and Discussion
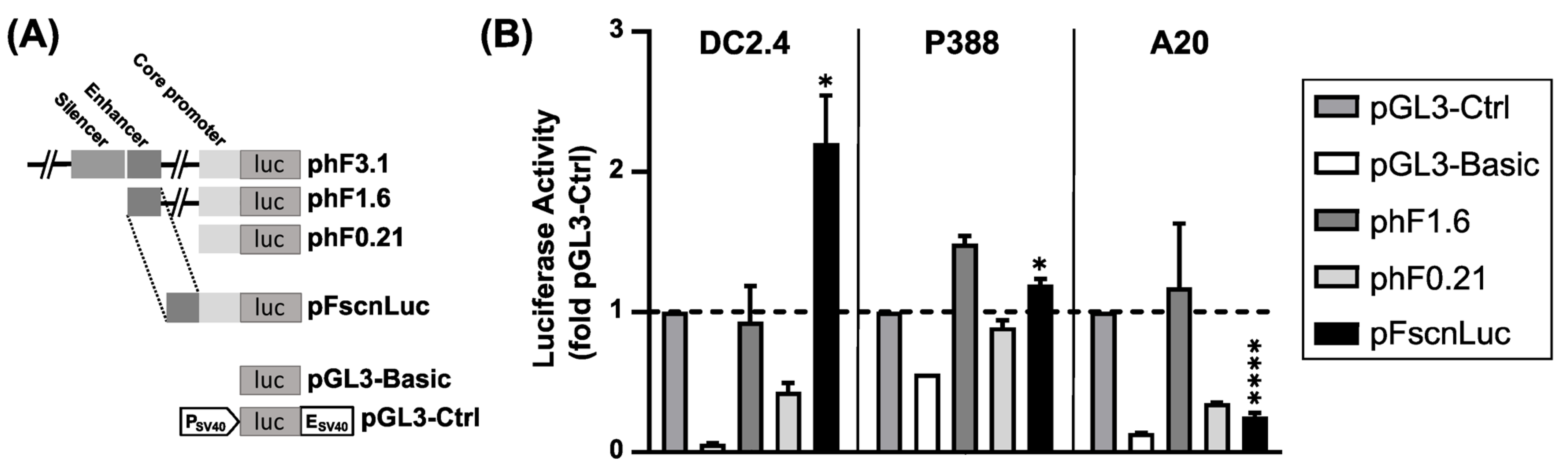
2.1. Firefly Luciferase Reporter Gene Construct under the Control of an Optimized Human Fscn1 Gene Promoter
2.2. Tumor Cell Lines Express Fscn1 at Lower Levels Than DCs
2.3. PEI Derivatives Are Suitable Carriers for pDNA Delivery
2.4. The Optimized Human Fscn1 Promoter Confers Enhanced Reporter Gene Expression in Fscn1-Expressing Cell Lines
2.5. The Optimized Human Fscn1 Promoter Mediates Preferential Reporter Expression in DCs also In Vivo
2.5.1. LPEI at Higher N/P Ratio Exerts Moderate Toxicity and Activation of DCs at the Same Time
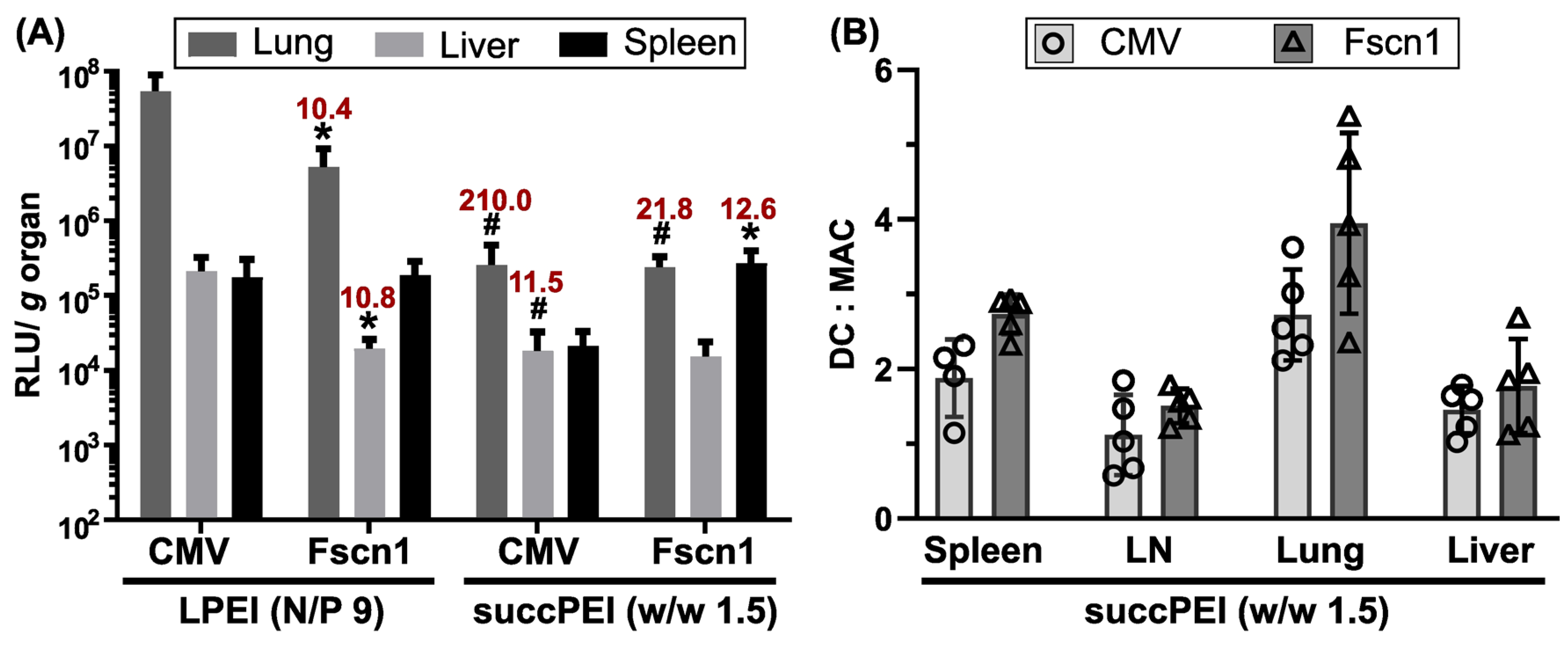
2.5.2. The Optimized Human Fscn1 Gene Promoter Evokes Reporter Gene Expression Preferably in Spleens
2.5.3. The Optimized Human Fscn1 Gene Promoter Shows DC-Focused Activity In Vivo
3. Materials and Methods
3.1. Materials
3.1.1. Plasmids
3.1.2. Chemicals
3.1.3. Transfection Agents
3.1.4. Cell Lines
3.1.5. Antibodies
3.2. pDNA Polyplex Formation
3.3. Physico-Chemical Characterization of pDNA Polyplexes
3.4. Cell Culture
3.5. Confocal Laser Scanning Microscopy (CLSM)
3.6. In Vitro Transfection and Luciferase Detection
3.7. Metabolic Activity of Transfectd Cells
3.8. In Vivo Appliction of pDNA Polyplexes
3.9. Ex vivo Luciferase Gene Expression Assay after In Vivo Transfection
3.10. Singe Cell Preparation after In Vivo Transfection
3.11. Flow Cytometry
3.12. Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hager, S.; Fittler, F.J.; Wagner, E.; Bros, M. Nucleic Acid-Based Approaches for Tumor Therapy. Cells 2020, 9, 2061. [Google Scholar] [CrossRef] [PubMed]
- Mai, D.; June, C.H.; Sheppard, N.C. In vivo gene immunotherapy for cancer. Sci. Transl. Med. 2022, 14, eabo3603. [Google Scholar] [CrossRef] [PubMed]
- Gary, E.N.; Weiner, D.B. DNA vaccines: Prime time is now. Curr. Opin. Immunol. 2020, 65, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Tombácz, I.; Weissman, D.; Pardi, N. Vaccination with Messenger RNA: A Promising Alternative to DNA Vaccination. Methods Mol. Biol. 2021, 2197, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Nabel, G.J.; Chang, A.; Nabel, E.G.; Plautz, G.; Fox, B.A.; Huang, L.; Shu, S. Immunotherapy of Malignancy by In Vivo Gene Transfer into Tumors. Hum. Gene Ther. 1992, 3, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Nabel, G.J.; Nabel, E.G.; Yang, Z.Y.; Fox, B.A.; Plautz, G.E.; Gao, X.; Huang, L.; Shu, S.; Gordon, D.; Chang, A.E. Direct gene transfer with DNA-liposome complexes in melanoma: Expression, biologic activity, and lack of toxicity in humans. Proc. Natl. Acad. Sci. USA 1993, 90, 11307. [Google Scholar] [CrossRef] [PubMed]
- Huebener, N.; Lange, B.; Lemmel, C.; Rammensee, H.G.; Strandsby, A.; Wenkel, J.; Jikai, J.; Zeng, Y.; Gaedicke, G.; Lode, H.N. Vaccination with minigenes encoding for novel ′self′ antigens are effective in DNA-vaccination against neuroblastoma. Cancer Lett. 2003, 197, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Pertl, U.; Wodrich, H.; Ruehlmann, J.M.; Gillies, S.D.; Lode, H.N.; Reisfeld, R.A. Immunotherapy with a posttranscriptionally modified DNA vaccine induces complete protection against metastatic neuroblastoma. Blood 2003, 101, 649–654. [Google Scholar] [CrossRef][Green Version]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C.; Meng, M.; Fritz, D.; Vascotto, F.; Hefesha, H.; et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Sahin, U.; Derhovanessian, E.; Miller, M.; Kloke, B.P.; Simon, P.; Löwer, M.; Bukur, V.; Tadmor, A.D.; Luxemburger, U.; Schrörs, B.; et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017, 547, 222–226. [Google Scholar] [CrossRef]
- Sahin, U.; Oehm, P.; Derhovanessian, E.; Jabulowsky, R.A.; Vormehr, M.; Gold, M.; Maurus, D.; Schwarck-Kokarakis, D.; Kuhn, A.N.; Omokoko, T.; et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature 2020, 585, 107–112. [Google Scholar] [CrossRef] [PubMed]
- McNeel, D.G.; Eickhoff, J.C.; Jeraj, R.; Staab, M.J.; Straus, J.; Rekoske, B.; Liu, G. DNA vaccine with pembrolizumab to elicit antitumor responses in patients with metastatic, castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. 2017, 35, 168. [Google Scholar] [CrossRef]
- McNeel, D.G.; Eickhoff, J.C.; Wargowski, E.; Zahm, C.; Staab, M.J.; Straus, J.; Liu, G. Concurrent, but not sequential, PD-1 blockade with a DNA vaccine elicits anti-tumor responses in patients with metastatic, castration-resistant prostate cancer. Oncotarget 2018, 9, 25586–25596. [Google Scholar] [CrossRef] [PubMed]
- Meleshko, A.; Piatrouskaya, N.; Vashkevich, K.; Lutskovich, D.; Wang, C.; Dormeshkin, D.; Savelyeva, N.; Katsin, M. Safety and Immunogenicity of Combined DNA-Polyethylenimine and Oral Bacterial Idiotypic Vaccine for Patients with B-Cell Non-Hodgkin Lymphoma: A Pilot Study. Cancers 2022, 14, 3298. [Google Scholar] [CrossRef] [PubMed]
- Rojas, L.A.; Sethna, Z.; Soares, K.C.; Olcese, C.; Pang, N.; Patterson, E.; Lihm, J.; Ceglia, N.; Guasp, P.; Chu, A.; et al. Personalized RNA neoantigen vaccines stimulate T cells in pancreatic cancer. Nature 2023, 618, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, R.; Malek, T.R. Fueling Cancer Vaccines to Improve T Cell-Mediated Antitumor Immunity. Front. Oncol. 2022, 12, 878377. [Google Scholar] [CrossRef] [PubMed]
- Lopes, A.; Vandermeulen, G.; Préat, V. Cancer DNA vaccines: Current preclinical and clinical developments and future perspectives. J. Exp. Clin. Cancer Res. 2019, 38, 146. [Google Scholar] [CrossRef]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine adjuvants: Putting innate immunity to work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef]
- Dubensky, T.W., Jr.; Reed, S.G. Adjuvants for cancer vaccines. Semin. Immunol. 2010, 22, 155–161. [Google Scholar] [CrossRef]
- Verbeke, R.; Lentacker, I.; Wayteck, L.; Breckpot, K.; Van Bockstal, M.; Descamps, B.; Vanhove, C.; De Smedt, S.C.; Dewitte, H. Co-delivery of nucleoside-modified mRNA and TLR agonists for cancer immunotherapy: Restoring the immunogenicity of immunosilent mRNA. J. Control. Release 2017, 266, 287–300. [Google Scholar] [CrossRef]
- Abbasi, S.; Uchida, S. Multifunctional Immunoadjuvants for Use in Minimalist Nucleic Acid Vaccines. Pharmaceutics 2021, 13, 644. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Halabi, E.A.; Fredrich, I.R.; Oh, J.; Peterson, H.M.; Ge, X.; Scott, E.; Kohler, R.H.; Garris, C.S.; Weissleder, R. Hybrid LNP Prime Dendritic Cells for Nucleotide Delivery. Adv. Sci. 2023, 10, 2303576. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yao, R.; Xia, X. The advances of adjuvants in mRNA vaccines. NPJ Vaccines 2023, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Felgner, P.L.; Barenholz, Y.; Behr, J.P.; Cheng, S.H.; Cullis, P.; Huang, L.; Jessee, J.A.; Seymour, L.; Szoka, F.; Thierry, A.R.; et al. Nomenclature for synthetic gene delivery systems. Hum. Gene Ther. 1997, 8, 511–512. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhao, B.; Ruiz, E.F.; Zhang, F. Advances in the polymeric delivery of nucleic acid vaccines. Theranostics 2022, 12, 4081–4109. [Google Scholar] [CrossRef]
- Abd Elwakil, M.M.; Suzuki, R.; Khalifa, A.M.; Elshami, R.M.; Isono, T.; Elewa, Y.H.A.; Sato, Y.; Nakamura, T.; Satoh, T.; Harashima, H. Harnessing Topology and Stereochemistry of Glycidylamine-Derived Lipid Nanoparticles for in Vivo mRNA Delivery to Immune Cells in Spleen and Their Application for Cancer Vaccination. Adv. Funct. Mater. 2023, 33, 2303795. [Google Scholar] [CrossRef]
- Ben-Akiva, E.; Karlsson, J.; Hemmati, S.; Yu, H.; Tzeng, S.Y.; Pardoll, D.M.; Green, J.J. Biodegradable lipophilic polymeric mRNA nanoparticles for ligand-free targeting of splenic dendritic cells for cancer vaccination. Proc. Natl. Acad. Sci. USA 2023, 120, e2301606120. [Google Scholar] [CrossRef]
- Li, Z.; Amaya, L.; Pi, R.; Wang, S.K.; Ranjan, A.; Waymouth, R.M.; Blish, C.A.; Chang, H.Y.; Wender, P.A. Charge-altering releasable transporters enhance mRNA delivery in vitro and exhibit in vivo tropism. Nat. Commun. 2023, 14, 6983. [Google Scholar] [CrossRef]
- Banchereau, J.; Steinman, R.M. Dendritic cells and the control of immunity. Nature 1998, 392, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Sudowe, S.; Beisner, J.; Ross, X.L.; Ludwig-Portugall, I.; Steitz, J.; Tuting, T.; Knop, J.; Reske-Kunz, A.B. Transcriptional targeting of dendritic cells for gene therapy using the promoter of the cytoskeletal protein fascin. Gene Ther. 2003, 10, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.J.; Jiang, Y.P.; Zhang, J.Y.; Tang, X.Q.; Lou, J.S.; Huang, X.Y. Roles of Fascin in Dendritic Cells. Cancers 2023, 15, 3691. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Taylor, M.D.; Singh, P.K.; Yang, S. How does fascin promote cancer metastasis? FEBS J. 2021, 288, 1434–1446. [Google Scholar] [CrossRef] [PubMed]
- Chung, V.; Jhaveri, K.L.; Von Hoff, D.D.; Huang, X.-Y.; Garmey, E.G.; Zhang, J.; Tsai, F.Y.-C. Phase 1A clinical trial of the first-in-class fascin inhibitor NP-G2-044 evaluating safety and anti-tumor activity in patients with advanced and metastatic solid tumors. J. Clin. Oncol. 2021, 39, 2548. [Google Scholar] [CrossRef]
- Wang, Y.; Song, M.; Liu, M.; Zhang, G.; Zhang, X.; Li, M.O.; Ma, X.; Zhang, J.J.; Huang, X.Y. Fascin inhibitor increases intratumoral dendritic cell activation and anti-cancer immunity. Cell Rep. 2021, 35, 108948. [Google Scholar] [CrossRef] [PubMed]
- Bros, M.; Ross, X.L.; Pautz, A.; Reske-Kunz, A.B.; Ross, R. The human fascin gene promoter is highly active in mature dendritic cells due to a stage-specific enhancer. J. Immunol. 2003, 171, 1825–1834. [Google Scholar] [CrossRef]
- Zou, S.M.; Erbacher, P.; Remy, J.S.; Behr, J.P. Systemic linear polyethylenimine (L-PEI)-mediated gene delivery in the mouse. J. Gene Med. 2000, 2, 128–134. [Google Scholar] [CrossRef]
- Wightman, L.; Kircheis, R.; Rössler, V.; Carotta, S.; Ruzicka, R.; Kursa, M.; Wagner, E. Different behavior of branched and linear polyethylenimine for gene delivery in vitro and in vivo. J. Gene Med. 2001, 3, 362–372. [Google Scholar] [CrossRef]
- Zintchenko, A.; Philipp, A.; Dehshahri, A.; Wagner, E. Simple modifications of branched PEI lead to highly efficient siRNA carriers with low toxicity. Bioconjug. Chem. 2008, 19, 1448–1455. [Google Scholar] [CrossRef]
- Höhn, Y.; Sudowe, S.; Reske-Kunz, A.B. Dendritic cell-specific biolistic transfection using the fascin gene promoter. Methods Mol. Biol. 2013, 940, 199–213. [Google Scholar] [CrossRef]
- Sudowe, S.; Ludwig-Portugall, I.; Montermann, E.; Ross, R.; Reske-Kunz, A.B. Prophylactic and therapeutic intervention in IgE responses by biolistic DNA vaccination primarily targeting dendritic cells. J. Allergy Clin. Immunol. 2006, 117, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Sudowe, S.; Dominitzki, S.; Montermann, E.; Bros, M.; Grabbe, S.; Reske-Kunz, A.B. Uptake and presentation of exogenous antigen and presentation of endogenously produced antigen by skin dendritic cells represent equivalent pathways for the priming of cellular immune responses following biolistic DNA immunization. Immunology 2009, 128, e193–e205. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Reznikoff, G.; Dranoff, G.; Rock, K.L. Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J. Immunol. 1997, 158, 2723–2730. [Google Scholar] [CrossRef] [PubMed]
- Mohr, C.F.; Gross, C.; Bros, M.; Reske-Kunz, A.B.; Biesinger, B.; Thoma-Kress, A.K. Regulation of the tumor marker Fascin by the viral oncoprotein Tax of human T-cell leukemia virus type 1 (HTLV-1) depends on promoter activation and on a promoter-independent mechanism. Virology 2015, 485, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Zeyn, Y.; Harms, G.; Tubbe, I.; Montermann, E.; Röhrig, N.; Hartmann, M.; Grabbe, S.; Bros, M. Inhibitors of the Actin-Bundling Protein Fascin-1 Developed for Tumor Therapy Attenuate the T-Cell Stimulatory Properties of Dendritic Cells. Cancers 2022, 14, 2738. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Kim, D.J.; Adams, J.C. The roles of fascins in health and disease. J. Pathol. 2011, 224, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.; Jonuleit, H.; Bros, M.; Ross, X.-L.; Enk, A.H.; Knop, J.; Reske-Kunz, A.B.; Yamashiro, S.; Matsumura, F. Expression of the Actin-Bundling Protein Fascin in Cultured Human Dendritic Cells Correlates with Dendritic Morphology and Cell Differentiation. J. Investig. Dermatol. 2000, 115, 658–663. [Google Scholar] [CrossRef]
- Hall, A.; Lächelt, U.; Bartek, J.; Wagner, E.; Moghimi, S.M. Polyplex Evolution: Understanding Biology, Optimizing Performance. Mol. Ther. 2017, 25, 1476–1490. [Google Scholar] [CrossRef]
- Merkel, O.M.; Urbanics, R.; Bedocs, P.; Rozsnyay, Z.; Rosivall, L.; Toth, M.; Kissel, T.; Szebeni, J. In vitro and in vivo complement activation and related anaphylactic effects associated with polyethylenimine and polyethylenimine-graft-poly(ethylene glycol) block copolymers. Biomaterials 2011, 32, 4936–4942. [Google Scholar] [CrossRef]
- Chen, H.; Li, P.; Yin, Y.; Cai, X.; Huang, Z.; Chen, J.; Dong, L.; Zhang, J. The promotion of type 1 T helper cell responses to cationic polymers in vivo via toll-like receptor-4 mediated IL-12 secretion. Biomaterials 2010, 31, 8172–8180. [Google Scholar] [CrossRef]
- Cubillos-Ruiz, J.R.; Engle, X.; Scarlett, U.K.; Martinez, D.; Barber, A.; Elgueta, R.; Wang, L.; Nesbeth, Y.; Durant, Y.; Gewirtz, A.T.; et al. Polyethylenimine-based siRNA nanocomplexes reprogram tumor-associated dendritic cells via TLR5 to elicit therapeutic antitumor immunity. J. Clin. Investig. 2009, 119, 2231–2244. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, Y.; Jiang, Y.; Shao, J.; Sun, X.; Chen, J.; Dong, L.; Zhang, J. Anti-tumor immune responses of tumor-associated macrophages via toll-like receptor 4 triggered by cationic polymers. Biomaterials 2013, 34, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Lezoualc, H.F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297. [Google Scholar] [CrossRef] [PubMed]
- Coll, J.L.; Chollet, P.; Brambilla, E.; Desplanques, D.; Behr, J.P.; Favrot, M. In vivo delivery to tumors of DNA complexed with linear polyethylenimine. Hum. Gene Ther. 1999, 10, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Chollet, P.; Favrot, M.C.; Hurbin, A.; Coll, J.L. Side-effects of a systemic injection of linear polyethylenimine-DNA complexes. J. Gene Med. 2002, 4, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Plank, C.; Zatloukal, K.; Cotten, M.; Mechtler, K.; Wagner, E. Gene transfer into hepatocytes using asialoglycoprotein receptor mediated endocytosis of DNA complexed with an artificial tetra-antennary galactose ligand. Bioconjug. Chem. 1992, 3, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Carswell, E.A.; Old, L.J.; Kassel, R.L.; Green, S.; Fiore, N.; Williamson, B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. USA 1975, 72, 3666–3670. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, K.C.; Green, D.R. How cells die: Apoptosis pathways. J. Allergy Clin. Immunol. 2001, 108, S99–S103. [Google Scholar] [CrossRef]
- Diaz Arguello, O.A.; Haisma, H.J. Apoptosis-Inducing TNF Superfamily Ligands for Cancer Therapy. Cancers 2021, 13, 1543. [Google Scholar] [CrossRef]
- Josephs, S.F.; Ichim, T.E.; Prince, S.M.; Kesari, S.; Marincola, F.M.; Escobedo, A.R.; Jafri, A. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. J. Transl. Med. 2018, 16, 242. [Google Scholar] [CrossRef] [PubMed]
- Rothoeft, T.; Balkow, S.; Krummen, M.; Beissert, S.; Varga, G.; Loser, K.; Oberbanscheidt, P.; van den Boom, F.; Grabbe, S. Structure and duration of contact between dendritic cells and T cells are controlled by T cell activation state. Eur. J. Immunol. 2006, 36, 3105–3117. [Google Scholar] [CrossRef] [PubMed]
- Cronin, S.J.; Penninger, J.M. From T-cell activation signals to signaling control of anti-cancer immunity. Immunol. Rev. 2007, 220, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.S.; Anandasabapathy, N. The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues. Semin. Immunopathol. 2017, 39, 137–152. [Google Scholar] [CrossRef]
- Paudel, Y.N.; Angelopoulou, E.; Piperi, C.; Balasubramaniam, V.; Othman, I.; Shaikh, M.F. Enlightening the role of high mobility group box 1 (HMGB1) in inflammation: Updates on receptor signalling. Eur. J. Pharmacol. 2019, 858, 172487. [Google Scholar] [CrossRef] [PubMed]
- Rödl, W.; Taschauer, A.; Schaffert, D.; Wagner, E.; Ogris, M. Synthesis of Polyethylenimine-Based Nanocarriers for Systemic Tumor Targeting of Nucleic Acids. In Nanotechnology for Nucleic Acid Delivery: Methods and Protocols; Ogris, M., Sami, H., Eds.; Springer: New York, NY, USA, 2019; pp. 83–99. [Google Scholar]
- Medina-Montano, C.; Cacicedo, M.L.; Svensson, M.; Limeres, M.J.; Zeyn, Y.; Chaves-Giraldo, J.E.; Röhrig, N.; Grabbe, S.; Gehring, S.; Bros, M. Enrichment Methods for Murine Liver Non-Parenchymal Cells Differentially Affect Their Immunophenotype and Responsiveness towards Stimulation. Int. J. Mol. Sci. 2022, 23, 6543. [Google Scholar] [CrossRef] [PubMed]
- Seya, T.; Shingai, M.; Kawakita, T.; Matsumoto, M. Two Modes of Th1 Polarization Induced by Dendritic-Cell-Priming Adjuvant in Vaccination. Cells 2023, 12, 1504. [Google Scholar] [CrossRef]
- Trevejo, J.M.; Marino, M.W.; Philpott, N.; Josien, R.; Richards, E.C.; Elkon, K.B.; Falck-Pedersen, E. TNF-alpha -dependent maturation of local dendritic cells is critical for activating the adaptive immune response to virus infection. Proc. Natl. Acad. Sci. USA 2001, 98, 12162–12167. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Z.; Li, F.; Kamencic, H.; Juurlink, B.; Gordon, J.R.; Xiang, J. Tumour necrosis factor-alpha (TNF-alpha) transgene-expressing dendritic cells (DCs) undergo augmented cellular maturation and induce more robust T-cell activation and anti-tumour immunity than DCs generated in recombinant TNF-alpha. Immunology 2003, 108, 177–188. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, C.; Yi, H.; Li, P.; Pan, H.; Liu, L.; Cai, L.; Ma, Y. Nanovaccine loaded with poly I:C and STAT3 siRNA robustly elicits anti-tumor immune responses through modulating tumor-associated dendritic cells in vivo. Biomaterials 2015, 38, 50–60. [Google Scholar] [CrossRef]
- Shih, P.C. Revisiting the development of small molecular inhibitors that directly target the signal transducer and activator of transcription 3 (STAT3) domains. Life Sci. 2020, 242, 117241. [Google Scholar] [CrossRef]
- Midoux, P.; Pichon, C. Lipid-based mRNA vaccine delivery systems. Expert Rev. Vaccines 2015, 14, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Macri, C.; Jenika, D.; Ouslinis, C.; Mintern, J.D. Targeting dendritic cells to advance cross-presentation and vaccination outcomes. Semin. Immunol. 2023, 68, 101762. [Google Scholar] [CrossRef] [PubMed]
- Vetter, V.C.; Wagner, E. Targeting nucleic acid-based therapeutics to tumors: Challenges and strategies for polyplexes. J. Control. Release 2022, 346, 110–135. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Krauthäuser, S.; Fischer, K.; Hobernik, D.; Abassi, Y.; Dzionek, A.; Nikolaev, A.; Voltz, N.; Diken, M.; Krummen, M.; et al. Vaccination with trifunctional nanoparticles that address CD8(+) dendritic cells inhibits growth of established melanoma. Nanomedicine 2016, 11, 2647–2662. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.O.; Almeida, B.S.; Sales, N.S.; Diniz, M.O.; Aps, L.; Rodrigues, K.B.; Silva, J.R.; Moreno, A.C.R.; Porchia, B.; Sulczewski, F.B.; et al. Antigen Delivery to DEC205(+) Dendritic Cells Induces Immunological Memory and Protective Therapeutic Effects against HPV-Associated Tumors at Different Anatomical Sites. Int. J. Biol. Sci. 2021, 17, 2944–2956. [Google Scholar] [CrossRef] [PubMed]
- Gül, A.; Döşkaya, M.; Can, H.; Karakavuk, M.; Anıl-İnevi, M.; Sağlam-Metiner, P.; Atalay-Şahar, E.; Değirmenci-Döşkaya, A.; Zekioğlu, O.; Gürüz, A.Y.; et al. Immunogenicity of a xenogeneic multi-epitope HER2(+) breast cancer DNA vaccine targeting the dendritic cell restricted antigen-uptake receptor DEC205. Vaccine 2022, 40, 2409–2419. [Google Scholar] [CrossRef]
- White, K.L.; Rades, T.; Furneaux, R.H.; Tyler, P.C.; Hook, S. Mannosylated liposomes as antigen delivery vehicles for targeting to dendritic cells. J. Pharm. Pharmacol. 2006, 58, 729–737. [Google Scholar] [CrossRef]
- Perche, F.; Gosset, D.; Mével, M.; Miramon, M.-L.; Yaouanc, J.-J.; Pichon, C.; Benvegnu, T.; Jaffrès, P.-A.; Midoux, P. Selective gene delivery in dendritic cells with mannosylated and histidylated lipopolyplexes. J. Drug Targeting 2011, 19, 315–325. [Google Scholar] [CrossRef]
- Gao, H.; Goncalves, C.; Gallego, T.; Francois-Heude, M.; Malard, V.; Mateo, V.; Lemoine, F.; Cendret, V.; Djedaini-Pilard, F.; Moreau, V.; et al. Comparative binding and uptake of liposomes decorated with mannose oligosaccharides by cells expressing the mannose receptor or DC-SIGN. Carbohydr. Res. 2020, 487, 107877. [Google Scholar] [CrossRef]
- Van der Jeught, K.; De Koker, S.; Bialkowski, L.; Heirman, C.; Tjok Joe, P.; Perche, F.; Maenhout, S.; Bevers, S.; Broos, K.; Deswarte, K.; et al. Dendritic Cell Targeting mRNA Lipopolyplexes Combine Strong Antitumor T-Cell Immunity with Improved Inflammatory Safety. ACS Nano 2018, 12, 9815–9829. [Google Scholar] [CrossRef]
- Le Moignic, A.; Malard, V.; Benvegnu, T.; Lemiègre, L.; Berchel, M.; Jaffrès, P.A.; Baillou, C.; Delost, M.; Macedo, R.; Rochefort, J.; et al. Preclinical evaluation of mRNA trimannosylated lipopolyplexes as therapeutic cancer vaccines targeting dendritic cells. J. Control. Release 2018, 278, 110–121. [Google Scholar] [CrossRef]
- Wagener, K.; Bros, M.; Krumb, M.; Langhanki, J.; Pektor, S.; Worm, M.; Schinnerer, M.; Montermann, E.; Miederer, M.; Frey, H.; et al. Targeting of Immune Cells with Trimannosylated Liposomes. Adv. Ther. 2020, 3, 1900185. [Google Scholar] [CrossRef]
- Sun, B.; Zhao, X.; Wu, Y.; Cao, P.; Movahedi, F.; Liu, J.; Wang, J.; Xu, Z.P.; Gu, W. Mannose-Functionalized Biodegradable Nanoparticles Efficiently Deliver DNA Vaccine and Promote Anti-tumor Immunity. ACS Appl. Mater. Interfaces 2021, 13, 14015–14027. [Google Scholar] [CrossRef]
- Lv, S.; Song, K.; Yen, A.; Peeler, D.J.; Nguyen, D.C.; Olshefsky, A.; Sylvestre, M.; Srinivasan, S.; Stayton, P.S.; Pun, S.H. Well-Defined Mannosylated Polymer for Peptide Vaccine Delivery with Enhanced Antitumor Immunity. Adv. Healthc. Mater. 2022, 11, e2101651. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeyn, Y.; Hobernik, D.; Wilk, U.; Pöhmerer, J.; Hieber, C.; Medina-Montano, C.; Röhrig, N.; Strähle, C.F.; Thoma-Kress, A.K.; Wagner, E.; et al. Transcriptional Targeting of Dendritic Cells Using an Optimized Human Fascin1 Gene Promoter. Int. J. Mol. Sci. 2023, 24, 16938. https://doi.org/10.3390/ijms242316938
Zeyn Y, Hobernik D, Wilk U, Pöhmerer J, Hieber C, Medina-Montano C, Röhrig N, Strähle CF, Thoma-Kress AK, Wagner E, et al. Transcriptional Targeting of Dendritic Cells Using an Optimized Human Fascin1 Gene Promoter. International Journal of Molecular Sciences. 2023; 24(23):16938. https://doi.org/10.3390/ijms242316938
Chicago/Turabian StyleZeyn, Yanira, Dominika Hobernik, Ulrich Wilk, Jana Pöhmerer, Christoph Hieber, Carolina Medina-Montano, Nadine Röhrig, Caroline F. Strähle, Andrea K. Thoma-Kress, Ernst Wagner, and et al. 2023. "Transcriptional Targeting of Dendritic Cells Using an Optimized Human Fascin1 Gene Promoter" International Journal of Molecular Sciences 24, no. 23: 16938. https://doi.org/10.3390/ijms242316938
APA StyleZeyn, Y., Hobernik, D., Wilk, U., Pöhmerer, J., Hieber, C., Medina-Montano, C., Röhrig, N., Strähle, C. F., Thoma-Kress, A. K., Wagner, E., Bros, M., & Berger, S. (2023). Transcriptional Targeting of Dendritic Cells Using an Optimized Human Fascin1 Gene Promoter. International Journal of Molecular Sciences, 24(23), 16938. https://doi.org/10.3390/ijms242316938








