Exploring In Vivo Models of Musculoskeletal Frailty: A Comprehensive Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Risk of Bias Assessment
3. Results
3.1. In Vivo Models of Frailty
3.1.1. Elderly Models
3.1.2. Genetically Modified Animal Model
3.1.3. Peptide Injection Animal Model
3.1.4. Tail Suspension Animal Model
3.2. Gender Differences
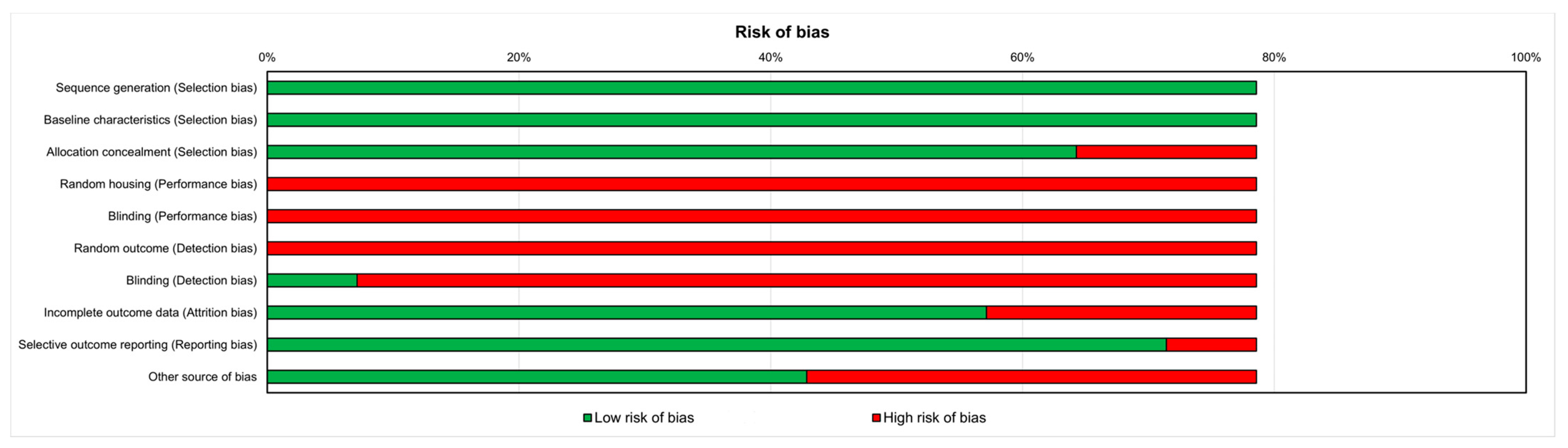
3.3. Risk of Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef]
- Rockwood, K.; Bergman, H. FRAILTY: A report from the 3rd Joint Workshop of IAGG/WHO/SFGG, Athens, January 2012. Can. Geriatr. J. 2021, 15, 31–36. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Vina, J.; Borras, C.; Gomez-Cabrera, M.C. A free radical theory of frailty. Free Radic. Biol. Med. 2018, 124, 358–363. [Google Scholar] [CrossRef]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of frailty in communitydwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef]
- McPhee, J.S.; French, D.P.; Jackson, D.; Nazroo, J.; Pendleton, N.; Degens, H. Physical activity in older age: Perspectives for healthy ageing and frailty. Biogerontology 2016, 17, 567–580. [Google Scholar] [CrossRef]
- McGuigan, F.E.; Bartosch, P.; Åkesson, K.E. Musculoskeletal health and frailty. Best Pract. Res. Clin. Rheumatol. 2017, 31, 145–159. [Google Scholar] [CrossRef]
- Milte, R.; Crotty, M. Musculoskeletal health, frailty and functional decline. Best Pract. Res. Clin. Rheumatol. 2014, 28, 395–410. [Google Scholar] [CrossRef]
- Frisoli, A.; Chaves, P.H.; Ingham, S.J.M.; Fried, L.P. Severe osteopenia and osteoporosis, sarcopenia, and frailty status in community-dwelling older women: Results from the Women’s Health and Aging Study (WHAS) II. Bone 2011, 48, 952–957. [Google Scholar] [CrossRef]
- Fede, C.; Fan, C.; Pirri, C.; Petrelli, L.; Biz, C.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. The Effects of Aging on the Intramuscular Connective Tissue. Int. J. Mol. Sci. 2022, 23, 11061. [Google Scholar] [CrossRef]
- Fan, C.; Pirri, C.; Fede, C.; Guidolin, D.; Biz, C.; Petrelli, L.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Age-Related Alterations of Hyaluronan and Collagen in Extracellular Matrix of the Muscle Spindles. J. Clin. Med. 2021, 11, 86. [Google Scholar] [CrossRef] [PubMed]
- Pavan, P.; Monti, E.; Bondí, M.; Fan, C.; Stecco, C.; Narici, M.; Reggiani, C.; Marcucci, L. Alterations of Extracellular Matrix Mechanical Properties Contribute to Age-Related Functional Impairment of Human Skeletal Muscles. Int. J. Mol. Sci. 2020, 21, 3992. [Google Scholar] [CrossRef] [PubMed]
- Buta, B.J.; Walston, J.D.; Godino, J.G.; Park, M.; Kalyani, R.R.; Xue, Q.; Bandeen-Roche, K.; Varadhan, R. Frailty assessment instruments: Systematic characterization of the uses and contexts of highlycited instruments. Ageing Res. Rev. 2016, 26, 53–61. [Google Scholar] [CrossRef]
- Searle, S.D.; Mitnitski, A.; Gahbauer, E.A.; Gill, T.M.; Rockwood, K. A standard procedure for creating a frailty index. BMC Geriatr. 2008, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, J.C.; Hildebrand, B.A.; Sun, M.; Rockwood, M.R.; Rose, R.A.; Rockwood, K.; Howlett, S.E. A clinical frailty index in aging mice: Comparisons with frailty index data in humans. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 621–632. [Google Scholar] [CrossRef]
- Kane, A.E.; Hilmer, S.N.; Mach, J.; Mitchell, S.J.; de Cabo, R.; Howlett, S.E. Animal models of frailty: Current applications in clinical research. Clin. Interv. Aging 2016, 11, 1519–1529. [Google Scholar] [CrossRef]
- von Zglinicki, T.; Varela-Nieto, I.; Brites, D.; Karagianni, N.; Ortolano, S.; Georgopoulos, S.; Cardoso, A.L.; Novella, S.; Lepperdinger, G.; Trendelenburg, A.U.; et al. Frailty in mouse ageing: A conceptual approach. Mech. Ageing Dev. 2016, 160, 34–40. [Google Scholar] [CrossRef]
- Banga, S.; Heinze-Milne, S.D.; Howlett, S.E. Rodent models of frailty and their application in preclinical research. Mech. Ageing Dev. 2019, 179, 1–10. [Google Scholar] [CrossRef]
- Asadi Shahmirzadi, A.; Edgar, D.; Liao, C.Y.; Hsu, Y.M.; Lucanic, M.; Asadi Shahmirzadi, A.; Wiley, C.D.; Gan, G.; Kim, D.E.; Kasler, H.G.; et al. Alpha-Ketoglutarate, an Endogenous Metabolite, Extends Lifespan and Compresses Morbidity in Aging Mice. Cell Metab. 2020, 32, 447–456.e6. [Google Scholar] [CrossRef]
- Graber, T.G.; Maroto, R.; Fry, C.S.; Brightwell, C.R.; Rasmussen, B.B. Measuring Exercise Capacity and Physical Function in Adult and Older Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 819–824. [Google Scholar] [CrossRef]
- Petr, M.A.; Alfaras, I.; Krawcyzk, M.; Bair, W.N.; Mitchell, S.J.; Morrell, C.H.; Studenski, S.A.; Price, N.L.; Fishbein, K.W.; Spencer, R.G.; et al. A cross-sectional study of functional and metabolic changes during aging through the lifespan in male mice. eLife 2021, 10, e62952. [Google Scholar] [CrossRef]
- Arc-Chagnaud, C.; Salvador-Pascual, A.; Garcia-Dominguez, E.; Olaso-Gonzalez, G.; Correas, A.G.; Serna, E.; Brioche, T.; Chopard, A.; Fernandez-Marcos, P.J.; Serrano, M.; et al. Glucose 6-P dehydrogenase delays the onset of frailty by protecting against muscle damage. J. Cachexia Sarcopenia Muscle 2021, 12, 1879–1896. [Google Scholar] [CrossRef]
- Sabini, E.; O’Mahony, A.; Caturegli, P. MyMD-1 Improves Health Span and Prolongs Life Span in Old Mice: A Noninferiority Study to Rapamycin. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 227–235. [Google Scholar] [CrossRef]
- Akki, A.; Yang, H.; Gupta, A.; Chacko, V.P.; Yano, T.; Leppo, M.K.; Steenbergen, C.; Walston, J.; Weiss, R.G. Skeletal muscle ATP kinetics are impaired in frail mice. Age 2014, 36, 21–30. [Google Scholar] [CrossRef]
- Scheuren, A.C.; Kuhn, G.A.; Müller, R. Effects of long-term in vivo micro-CT imaging on hallmarks of osteopenia and frailty in aging mice. PLoS ONE 2020, 15, e0239534. [Google Scholar] [CrossRef]
- Scheuren, A.C.; D’Hulst, G.; Kuhn, G.A.; Masschelein, E.; Wehrle, E.; De Bock, K.; Müller, R. Hallmarks of frailty and osteosarcopenia in prematurely aged PolgA(D257A/D257A) mice. J. Cachexia Sarcopenia Muscle 2020, 11, 1121–1140. [Google Scholar] [CrossRef]
- Jing, Y.; Zuo, Y.; Sun, L.; Yu, Z.R.; Ma, S.; Hu, H.; Zhao, Q.; Huang, D.; Zhang, W.; Izpisua Belmonte, J.C.; et al. SESN1 is a FOXO3 effector that counteracts human skeletal muscle ageing. Cell Prolif. 2023, 56, e13455. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Barros, C.; Barateiro, A.; Howlett, S.E.; Fernandes, A. Improved Assessment of Overall Health in Variably Aged Murine Models of Multiple Sclerosis With a Novel Frailty Index Tool. J. Gerontol. A Biol. Sci. Med. Sci. 2022, 77, 1–9. [Google Scholar] [CrossRef]
- Ono, T.; Denda, R.; Tsukahara, Y.; Nakamura, T.; Okamoto, K.; Takayanagi, H.; Nakashima, T. Simultaneous augmentation of muscle and bone by locomomimetism through calcium-PGC-1α signaling. Bone Res. 2022, 10, 52. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Method 2014, 14, 43. [Google Scholar] [CrossRef]
- Joseph, B.; Phelan, H.; Hassan, A.; Orouji Jokar, T.; O’Keeffe, T.; Azim, A.; Gries, L.; Kulvatunyou, N.; Latifi, R.; Rhee, P. The impact of frailty on failure-torescue in geriatric trauma patients: A prospective study. J. Trauma Acute Care Surg. 2016, 81, 1150–1155. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Han, H.S.; Jung, H.W.; Kim, K.I.; Hwang, D.W.; Kang, S.B.; Kim, C.H. Multidimensional frailty score for the prediction of postoperative mortality risk. JAMA Surg. 2014, 149, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Kositsawat, J.; Duque, G.; Kirk, B. Nutrients with anabolic/anticatabolic, antioxidant, and anti-inflammatory properties: Targeting the biological mechanisms of aging to support musculoskeletal health. Exp. Gerontol. 2021, 154, 111521. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.A.; Harper, J.M.; Galecki, A.; Burke, D.T. Big mice die young: Early life body weight predicts longevity in genetically heterogeneous mice. Aging Cell 2002, 1, 22–29. [Google Scholar] [CrossRef]
- Mitchell, S.J.; Bernier, M.; Mattison, J.A.; Aon, M.A.; Kaiser, T.A.; Anson, R.M.; Ikeno, Y.; Anderson, R.M.; Ingram, D.K.; de Cabo, R. Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab. 2019, 29, 221–228. [Google Scholar] [CrossRef]
- TeSlaa, T.; Chaikovsky, A.C.; Lipchina, I.; Escobar, S.L.; Hochedlinger, K.; Huang, J.; Graeber, T.G.; Braas, D.; Teitell, M.A. α-Ketoglutarate Accelerates the Initial Differentiation of Primed Human Pluripotent Stem Cells. Cell Metab. 2016, 24, 485–493. [Google Scholar] [CrossRef]
- Su, Y.; Wang, T.; Wu, N.; Li, D.; Fan, X.; Xu, Z.; Mishra, S.K.; Yang, M. Alpha-ketoglutarate extends Drosophila lifespan by inhibiting mTOR and activating AMPK. Aging 2019, 11, 4183–4197. [Google Scholar] [CrossRef]
- Di Dalmazi, G.; Chalan, P.; Caturegli, P. MYMD-1, a novel immunometabolic regulator, ameliorates autoimmune thyroiditis via suppression of Th1 responses and TNF-α release. J. Immunol. 2019, 202, 1350–1362. [Google Scholar] [CrossRef]
- Berg, E.L.; Polokoff, M.A.; O’Mahony, A.; Nguyen, D.; Li, X. Elucidating mechanisms of toxicity using phenotypic data from primary human cell systems―a chemical biology approach for thrombosis-related side effects. Int. J. Mol. Sci. 2015, 16, 1008–1029. [Google Scholar] [CrossRef]
- Nóbrega-Pereira, S.; Fernandez-Marcos, P.J.; Brioche, T.; Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Viña, J.; Serrano, M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Fiorelli, G. Glucose-6-phosphate dehydrogenase deficiency. Lancet 2008, 371, 64–74. [Google Scholar] [CrossRef]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Trifunovic, A.; Wredenberg, A.; Falkenberg, M.; Spelbrink, J.N.; Rovio, A.T.; Bruder, C.E.; Bohlooly-Y, M.; Gidlöf, S.; Oldfors, A.; Wibom, R.; et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Walston, J.; Fedarko, N.; Yang, H.; Leng, S.; Beamer, B.; Espinoza, S.; Lipton, A.; Zheng, H.; Becker, K. The physical and biological characterization of a frail mouse model. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.; Yu, Q.; Xue, Q.L.; Yao, W.; Brayton, C.; Yang, H.; Fedarko, N.; Walston, J. Inflammation and mortality in a frail mouse model. Age 2012, 34, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.W.; Richmond, S.B.; Sharp, B.E.; Fling, B.W. Middle-age people with multiple sclerosis demonstrate similar mobility characteristics to neurotypical older adults. Mult. Scler. Relat. Disord. 2021, 51, 102924. [Google Scholar] [CrossRef]
- Kim, M.; Kowalsky, A.H.; Lee, J.H. Sestrins in physiological stress responses. Annu. Rev. Physiol. 2021, 83, 381–403. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, T.; Yu, Z.; Yu, Q.; Wang, Y.; Hu, J.; Shi, J.; Yang, G. The functions and roles of sestrins in regulating human diseases. Cell Mol. Biol. Lett. 2022, 27, 2. [Google Scholar] [CrossRef]
- Dent, E.; Kowal, P.; Hoogendijk, E.O. Frailty measurement in research and clinical practice: A review. Europ. J. Int. Med. 2016, 31, 3–10. [Google Scholar] [CrossRef]
- de Vries, N.M.; Staal, J.B.; van Ravensberg, C.D.; Hobbelen, J.S.; Olde Rikkert, M.G.; Nijhuis-van der Sanden, M.W. Outcome instruments to measure frailty: A systematic review. Ageing Res. Rev. 2011, 10, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Fink, J.; Schoenfeld, B.J.; Nakazato, K. The role of hormones in muscle hypertrophy. Phys. Sportsmed. 2018, 46, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Hart, D.A.; Enoka, M.; Nicolella, D.P.; Resnick, E.; Berkley, K.J.; Sluka, K.A.; Kwoh, C.K.; Tosi, L.L.; O’Connor, M.I.; et al. Hormonal modulation of connective tissue homeostasis and sex differences in risk for osteoarthritis of the knee. Biol. Sex Differ. 2013, 4, 3. [Google Scholar] [CrossRef] [PubMed]

| In Vivo Model of Frailty | Evaluations | Main Results | Ref. | |
|---|---|---|---|---|
| Elderly models | C57BL/6J female and male mice (18 mo old): Group 1: mice + standard-2918 diet; Group 2: mice + 2% w/w AKG supplemented on 2918 diet | FI; Survival; Indirect calorimetry (O2 consumption, CO2 production, whole-body composition); Treadmill exhaustion test (maximal speed and distance to exhaustion) | Group 2: ↑ survival and lifespan; ↓ FI, O2 consumption, CO2 production, energy expenditure | [20] |
| C57BL/6J male mice: Group 1: mice (6 mo old); Group 2: mice (24 mo old); Group 3: mice (>28 mo old) | Functional test (endurance capacity, forelimb strenght, four limb strenght/endurance, balance, ccordination, gait speed, power generation, voluntary wheel running and activity rate, CFAB score); Body composition and muscle mass; Muscle contractile physiology of dorsiflexor torque | Groups 2, 3: ↓ grip, CFAB score, muscle contractile physiology; ↑ muscle mass than group 1. Group 3: ↓ inverted cling than group 1. Group 3: ↓ voluntary wheel running than groups 1, 2 Group 2: ↓ voluntary wheel running; ↑ body mass than group 1 | [21] | |
| C57BL/6N male mice: Group 1: mice (3–8 mo old); Group 2: mice (13–23 mo old); Group 3: mice (27–36 mo old) | FI; Body composition (body fat, free body fluid, lean tissue content); Functional test (gait speed, tail height, forelimb strength); Micro-CT (Cr.Th, Tr.BMD, % fat around tibia, % lean around tibia); Metabolic assessment (natural walking gait speed); Indirect calorimetry (O2 consumption) | Group 3: ↑ FI, RER, % lean tissue around tibia; ↓ gait speed, forelimb muscle strength, locomotor activity, Cr.Th and Tr. BMD, body weight, % fat around tibia than groups 1, 2 | [22] | |
| Group 1: C57BL/6 female mice (18–26 mo old); Group 2: G6PD-Tg female mice (18–26 mo old); Group 3: C57BL/6 female mice (34 mo old); Group 4: G6PD-Tg female mice (34 mo old) | Frailty score; Histology of gastrocnemius and tibialis anterior muscles (H&E staining); IHC of gastrocnemius and tibialis anterior muscles (eMHC); Metabolic assessment (EE, locomotory activity, RER); Body composition (BMD, lean mass, fat mass, fat in tissue) | Group 2: ↓ frailty score, RER; ↑ locomotory activity than group 1. Group 4: ↑ muscle fiber size than group 3 | [23] | |
| C57BL/6J female and male mice (19 mo old): Group 1: mice + MyMD-1; Group 2: mice + high-dose (126 ppm) rapamycin; Group 3: mice + low-dose (14 ppm) rapamycin + metformin | FI; Health span assessment (body weight, grip strength, locomotor activity, motor coordination and endurance, learning and memory); Lifespan assessment | Groups 1, 2, 3: ↓ body weight, muscle strength; ↑ FI with age Group 1: ↑ health span characteristics, muscle strength; ↓ body weight loss, progression to frailty than group 3 Group 1: ↑ survival, lifespan than groups 2, 3 | [24] | |
| Genetically modified models | Group 1: C57BL/6J male mice (92 wks old); Group 2: IL10tm/tm male mice (92 wks old) | 31P MRS (PCr, Pi, ADP, rate of ATP synthesis via CK (PCr → ATP), rate of ATP synthesis from Pi (Pi → ATP), free energy released from ATP hydrolysis (ΔG∼ATP)) in hind limb skeletal muscle | Group 2: ↓ PCr, ATP flux via CK, ATP synthesis from Pi, free energy released from ATP hydrolysis; ↑ Pi than group 1 | [25] |
| Group 1: PolgA(D257A/D257A) female mice (34 wks old); Group 2: PolgA(+/+) female mice (34 wks old); Group 3: PolgA(D257A/D257A) female mice (40 wks old); Group 4: PolgA(+/+) female mice (40 wks old); Group 5: PolgA(D257A/D257A) female mice (46 wks old); Group 6: PolgA(+/+) female mice (46 wks old) | FI; Functional test (forelimb grip strength); Micro-CT of the right femur (BV/TV, Tb.Th, Tb.N, Tb.Sp, Ct.Ar/Tt.Ar, Ct.BV, Ct.MV, Ct.Ar, Tt.Ar, Ct.Th, Ps.Pm, Ec.Pm, AVD, length, BFR, BRR, MAR, MRR, MS, ES) | Groups 1, 3, 5: ↑ health deficits; ↓ bone mass, BVF, Tb.Th, Cr.Th, remodeling activities than groups 2, 4, 6 Groups 3, 5: ↑ FI; ↓ grip strength and concentric muscle forces than groups 4, 6 | [26] | |
| Group 1: PolgA(D257A/D257A) female mice (20–40 wks old); Group 2: PolgA(+/+) female mice (20–40 wks old); Group 3: PolgA(D257A/D257A) female mice (26–34 wks old); Group 4: PolgA(+/+) female mice (26–34 wks old); Group 5: PolgA(D257A/D257A) female mice (32–40 wks old); Group 6: PolgA(+/+) female mice (32–40 wks old); Group 7: PolgA(D257A/D257A) female mice (40–46 wks old); Group 8: PolgA(+/+) female mice (40–46 wks old) | FI; Micro-CT of the 6th caudal vertebrae (BFR, BRR, MAR, MRR, MS, ES, BV/TV, Tb.Th, Tb.N., Ct.Ar/Tt.Ar, Ct.Th, Tb.Sp) | Groups 1, 3, 5, 7: ↓ BV/TV, Tb.Th, Tb.N, Ct.Ar/Tt.Ar, Ct.Th, BFR, BRR, MAR, MRR, MS; ↑ Tb.Sp, FI than groups 2, 4, 6, 8 Groups 7, 8: ↓ BV/TV, Tb.Th than groups 1, 2 Group 8: ↑ Tb.N, Ct.Ar/Tt.Ar; ↓ Tb.Sp, BRR, MRR than group 2 | [27] | |
| Peptide injection model | C57BL/6J male mice (16 mo old): Group 1: mice sham; Group 2: mice + CTX + PBS into tibialis anterior muscle/ quadriceps muscle; Group 3: mice + CTX + PBS + rSESN1 protein into tibialis anterior muscle/quadriceps muscle | Functional test (grip strength of forelimb and hind limb, motor coordination, maximal speed, time and distance to exhaustion) | Group 2: ↓ grip strength, physical endurance, maximal running time and running distance than groups 1, 3 | [28] |
| C57BL/6 female mice: Group 1: mice (3 mo old); Group 2: mice (3 mo old) + MOG peptide emulsified in CFA supplemented with heat-inactivated Mycobacterium tuberculosis; Group 3: mice (6 mo old) + MOG peptide emulsified in CFA supplemented with heat-inactivated Mycobacterium tuberculosis; Group 4: mice (12 mo old) + MOG peptide emulsified in CFA supplemented with heat-inactivated Mycobacterium tuberculosis | FI; Traditional 5-point clinical paralysis scale | Group 2: ↓ body weight; ↑ FI than group 1 Group 4: ↑ FI than groups 2, 3 | [29] | |
| Tail-suspension model | C57BL/6 J male mice (6 wks old): Group 1: TS model mice; Group 2: TS model mice + LAMZ (10 mg·kg−1 once a day for 14 days) | Micro-CT of femur (BV/TV, Tb.Sp, Tb.N, Tb.Th, BMC/TV, Cr.Th); Histology of proximal tibia (TRAP, toluidine blu/calcein staining); Histology of soleus muscle (H&E staining); Histomorphometry (Ob.Surf., Osteoid surf., BFR, Oc.N., Eroded surf., muscle fiber width); Functional test (fatigue-like behavior, travel distance, adjusted maximum muscle strength/g) | Group 1: ↓ muscle fiber width, travel distance, maximal muscle strength, BV/TV, Tb.N., Tb.Th., BMC/TV, Cr.Th., BFR, Ob.Surf., bone mass, osteoid surf.; ↑ episodes of fatigue-like behavior, Oc.N., eroded surf. than group 2 | [30] |
| Frailty Index | Items | Sub-Items | Refs. |
|---|---|---|---|
| Mouse frailty assessment of Whitehead et al. | Integument | Alopecia; Loss of fur colour; Dermatitis; Loss of whiskers; Coat condition | [20,22,26,27] |
| Physical/musculoskeletal | Tumors; Distended abdomen; Kyphosis; Tail stiffening; Gait disorders; Tremor; Forelimb grip strength; Body condition score | ||
| Vestibulocochlear/auditory | Vestibular disturbance; Hearing loss | ||
| Ocular/nasal | Cataracts; Corneal opacity; Eye discharge/swelling; Microphthalmia; Vision loss; Menace reflex; Nasal discharge | ||
| Digestive/urogenital | Malocclusions; Rectal prolapse; Vaginal/uterine/penile prolapse; Diarrhea | ||
| Respiratory | Breathing rate/depth | ||
| Discomfort | Mouse grimace scale; Piloerection | ||
| Other | Temperature; Weight | ||
| Frailty score | Running time (endurance) | Derived from four paw hang and rotarod measures (seconds) | [23,24] |
| Running speed (slowness) | Rotarod-training protocol (maximum speed) | ||
| Motor coordination | Voluntary wheel running (daily running distance) | ||
| Body weight | Low body weight | ||
| Grip strength | Four paw inverted hang (seconds to fall) | ||
| Mouse frailty assessment adapted from Whitehead et al. | Integument | Alopecia; Dermatitis; Loss of whiskers | [29] |
| Physical condition | Kyphosis; Tail condition; Gait; Body condition score; Distended abdomen | ||
| Neuromuscoskeletal system/ sensorimotor reflexes | Tremor; Hindlimb reflexology—foot “pinch”; Menace reflex | ||
| Paralysis and Weakness | Forelimb paralysis; Body posture; Nose down | ||
| Strength | Forelimb grip strength | ||
| Ataxia/coordination | Grid walk; Righting test; Splayed hind legs; Belly drag | ||
| Self-care and grooming | Coat condition | ||
| Vestibulocochlear system | Vestibular disturbance/head tilt | ||
| Auditory system | Hearing loss | ||
| Ocular system | Vision loss; Microphthalmia; Discharge/swollen/squinting | ||
| Nasal system | Nasal discharge | ||
| Digestive system | Diarrhoea | ||
| Urogenital system | Rectal prolapse; Vaginal/uterine prolapse | ||
| Respiratory | Breathing rate/depth | ||
| Discomfort | Mouse grimace scale; Piloerection; Temperature; Body weight |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contartese, D.; Di Sarno, L.; Salamanna, F.; Martini, L.; Fini, M.; Giavaresi, G.; Veronesi, F. Exploring In Vivo Models of Musculoskeletal Frailty: A Comprehensive Systematic Review. Int. J. Mol. Sci. 2023, 24, 16948. https://doi.org/10.3390/ijms242316948
Contartese D, Di Sarno L, Salamanna F, Martini L, Fini M, Giavaresi G, Veronesi F. Exploring In Vivo Models of Musculoskeletal Frailty: A Comprehensive Systematic Review. International Journal of Molecular Sciences. 2023; 24(23):16948. https://doi.org/10.3390/ijms242316948
Chicago/Turabian StyleContartese, Deyanira, Laura Di Sarno, Francesca Salamanna, Lucia Martini, Milena Fini, Gianluca Giavaresi, and Francesca Veronesi. 2023. "Exploring In Vivo Models of Musculoskeletal Frailty: A Comprehensive Systematic Review" International Journal of Molecular Sciences 24, no. 23: 16948. https://doi.org/10.3390/ijms242316948
APA StyleContartese, D., Di Sarno, L., Salamanna, F., Martini, L., Fini, M., Giavaresi, G., & Veronesi, F. (2023). Exploring In Vivo Models of Musculoskeletal Frailty: A Comprehensive Systematic Review. International Journal of Molecular Sciences, 24(23), 16948. https://doi.org/10.3390/ijms242316948







