Proton Compared to X-Irradiation Induces Different Protein Profiles in Oral Cancer Cells and Their Derived Extracellular Vesicles
Abstract
:1. Introduction
2. Results
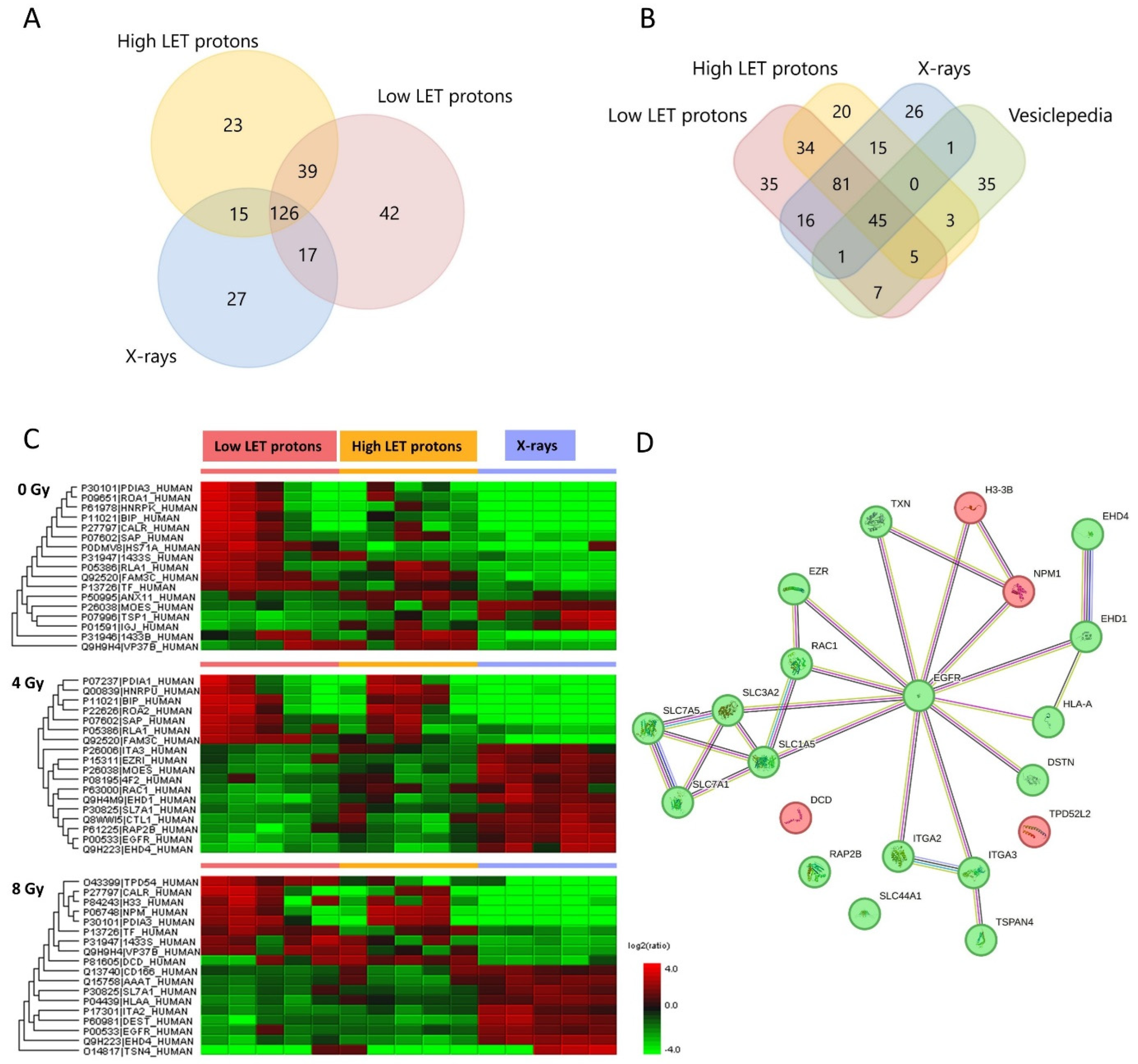
2.1. EV Characterization and Overview of Protein Content
2.2. Up- and Downregulated EV Proteins after Irradiation
2.3. Up- and Downregulated OSCC Cell Proteins after Irradiation
3. Discussion
4. Materials and Methods
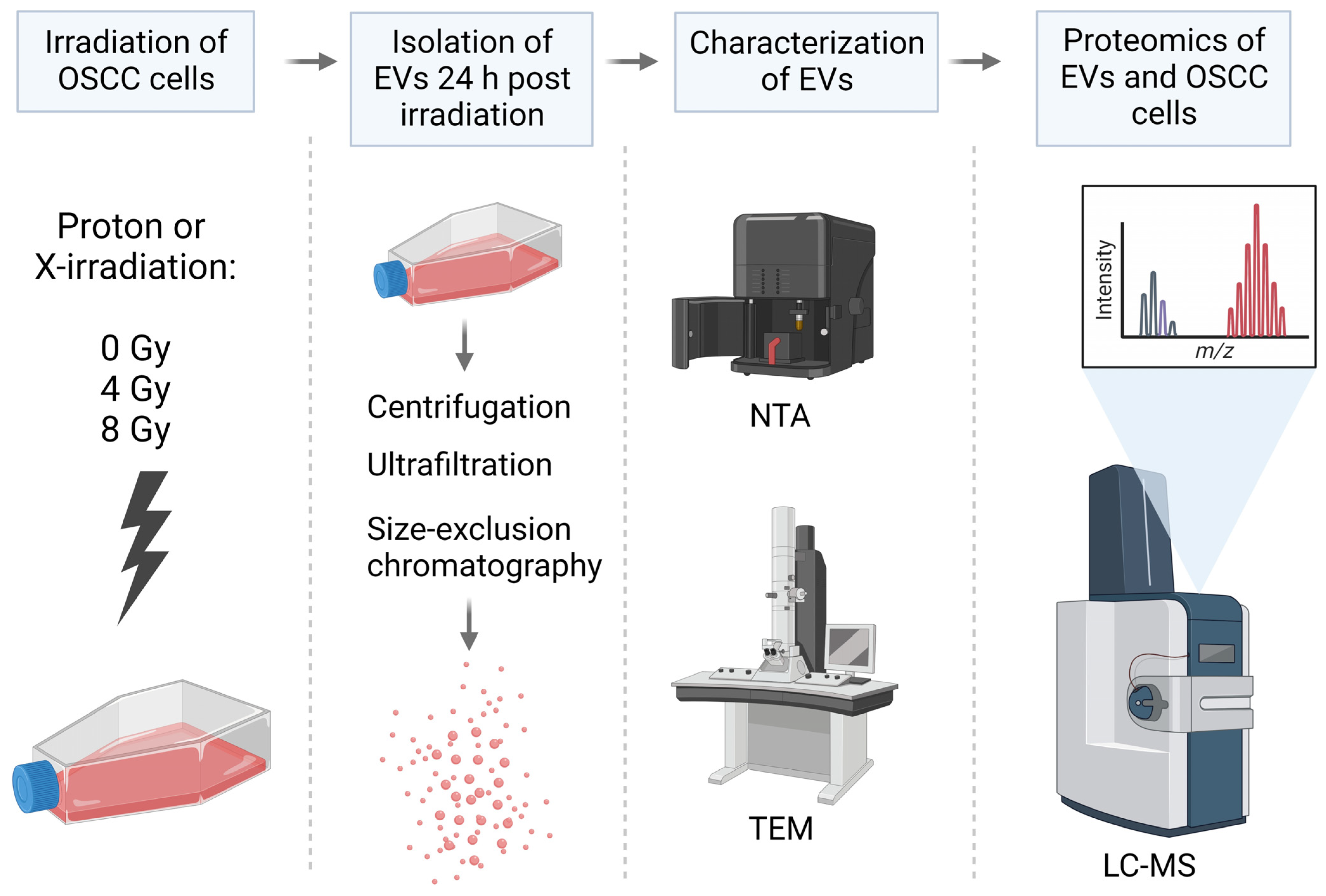
4.1. Cell Irradiation
4.2. EV Isolation
4.3. EV Characterization
4.4. Proteomic Analysis
4.4.1. In-Solution Digestion
4.4.2. LC-MS Analysis
4.4.3. Database Search
4.4.4. Label-Free Quantitation
4.5. Protein Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Bucci, J.; Malouf, D.; Knox, M.; Graham, P.; Li, Y. Exosomes in Cancer Radioresistance. Front. Oncol. 2019, 9, 869. [Google Scholar] [CrossRef]
- Szatmari, T.; Hargitai, R.; Safrany, G.; Lumniczky, K. Extracellular Vesicles in Modifying the Effects of Ionizing Radiation. Int. J. Mol. Sci. 2019, 20, 5527. [Google Scholar] [CrossRef]
- Kadhim, M.; Tuncay Cagatay, S.; Elbakrawy, E.M. Non-targeted effects of radiation: A personal perspective on the role of exosomes in an evolving paradigm. Int. J. Radiat. Biol. 2022, 98, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Le, M.; Fernandez-Palomo, C.; McNeill, F.E.; Seymour, C.B.; Rainbow, A.J.; Mothersill, C.E. Exosomes are released by bystander cells exposed to radiation-induced biophoton signals: Reconciling the mechanisms mediating the bystander effect. PLoS ONE 2017, 12, e0173685. [Google Scholar] [CrossRef] [PubMed]
- Le, M.; McNeill, F.E.; Seymour, C.; Rainbow, A.J.; Mothersill, C.E. An observed effect of ultraviolet radiation emitted from beta-irradiated HaCaT cells upon non-beta-irradiated bystander cells. Radiat. Res. 2015, 183, 279–290. [Google Scholar] [CrossRef]
- Al-Mayah, A.H.; Irons, S.L.; Pink, R.C.; Carter, D.R.; Kadhim, M.A. Possible role of exosomes containing RNA in mediating nontargeted effect of ionizing radiation. Radiat. Res. 2012, 177, 539–545. [Google Scholar] [CrossRef]
- Jella, K.K.; Rani, S.; O’Driscoll, L.; McClean, B.; Byrne, H.J.; Lyng, F.M. Exosomes are involved in mediating radiation induced bystander signaling in human keratinocyte cells. Radiat. Res. 2014, 181, 138–145. [Google Scholar] [CrossRef]
- Mothersill, C.; Rusin, A.; Seymour, C. Relevance of Non-Targeted Effects for Radiotherapy and Diagnostic Radiology; A Historical and Conceptual Analysis of Key Players. Cancers 2019, 11, 1236. [Google Scholar] [CrossRef] [PubMed]
- Mutschelknaus, L.; Azimzadeh, O.; Heider, T.; Winkler, K.; Vetter, M.; Kell, R.; Tapio, S.; Merl-Pham, J.; Huber, S.M.; Edalat, L.; et al. Radiation alters the cargo of exosomes released from squamous head and neck cancer cells to promote migration of recipient cells. Sci. Rep. 2017, 7, 12423. [Google Scholar] [CrossRef] [PubMed]
- Yentrapalli, R.; Merl-Pham, J.; Azimzadeh, O.; Mutschelknaus, L.; Peters, C.; Hauck, S.M.; Atkinson, M.J.; Tapio, S.; Moertl, S. Quantitative changes in the protein and miRNA cargo of plasma exosome-like vesicles after exposure to ionizing radiation. Int. J. Radiat. Biol. 2017, 93, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Al-Mayah, A.; Bright, S.; Chapman, K.; Irons, S.; Luo, P.; Carter, D.; Goodwin, E.; Kadhim, M. The non-targeted effects of radiation are perpetuated by exosomes. Mutat. Res. 2015, 772, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Abramowicz, A.; Wojakowska, A.; Marczak, L.; Lysek-Gladysinska, M.; Smolarz, M.; Story, M.D.; Polanska, J.; Widlak, P.; Pietrowska, M. Ionizing radiation affects the composition of the proteome of extracellular vesicles released by head-and-neck cancer cells in vitro. J. Radiat. Res. 2019, 60, 289–297. [Google Scholar] [CrossRef]
- Blanchard, P.; Garden, A.S.; Gunn, G.B.; Rosenthal, D.I.; Morrison, W.H.; Hernandez, M.; Crutison, J.; Lee, J.J.; Ye, R.; Fuller, C.D.; et al. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer—A case matched analysis. Radiother. Oncol. 2016, 120, 48–55. [Google Scholar] [CrossRef]
- Tian, X.; Liu, K.; Hou, Y.; Cheng, J.; Zhang, J. The evolution of proton beam therapy: Current and future status. Mol. Clin. Oncol. 2018, 8, 15–21. [Google Scholar] [CrossRef]
- McNamara, A.L.; Schuemann, J.; Paganetti, H. A phenomenological relative biological effectiveness (RBE) model for proton therapy based on all published in vitro cell survival data. Phys. Med. Biol. 2015, 60, 8399–8416. [Google Scholar] [CrossRef]
- Paganetti, H. Mechanisms and review of clinical evidence of variations in relative biological effectiveness in proton therapy. Int. J. Radiat. Oncol. Biol. Phys. 2022, 112, 222–236. [Google Scholar] [CrossRef]
- Paganetti, H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys. Med. Biol. 2014, 59, R419–R472. [Google Scholar] [CrossRef]
- Byun, H.K.; Han, M.C.; Yang, K.; Kim, J.S.; Yoo, G.S.; Koom, W.S.; Kim, Y.B. Physical and biological characteristics of particle therapy for oncologists. Cancer Res. Treat. 2021, 53, 611–620. [Google Scholar] [CrossRef]
- Sorensen, B.S.; Bassler, N.; Nielsen, S.; Horsman, M.R.; Grzanka, L.; Spejlborg, H.; Swakon, J.; Olko, P.; Overgaard, J. Relative biological effectiveness (RBE) and distal edge effects of proton radiation on early damage in vivo. Acta Oncol. 2017, 56, 1387–1391. [Google Scholar] [CrossRef]
- Sorensen, B.S.; Pawelke, J.; Bauer, J.; Burnet, N.G.; Dasu, A.; Hoyer, M.; Karger, C.P.; Krause, M.; Schwarz, M.; Underwood, T.S.A.; et al. Does the uncertainty in relative biological effectiveness affect patient treatment in proton therapy? Radiother. Oncol. 2021, 163, 177–184. [Google Scholar] [CrossRef]
- Saager, M.; Peschke, P.; Brons, S.; Debus, J.; Karger, C.P. Determination of the proton RBE in the rat spinal cord: Is there an increase towards the end of the spread-out Bragg peak? Radiother. Oncol. 2018, 128, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Jones, B. Why RBE must be a variable and not a constant in proton therapy. Br. J. Radiol. 2016, 89, 20160116. [Google Scholar] [CrossRef]
- Witwer, K.W.; Goberdhan, D.C.; O’Driscoll, L.; Thery, C.; Welsh, J.A.; Blenkiron, C.; Buzas, E.I.; Di Vizio, D.; Erdbrugger, U.; Falcon-Perez, J.M.; et al. Updating MISEV: Evolving the minimal requirements for studies of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12182. [Google Scholar] [CrossRef] [PubMed]
- Witwer, K.W.; Soekmadji, C.; Hill, A.F.; Wauben, M.H.; Buzás, E.I.; Di Vizio, D.; Falcon-Perez, J.M.; Gardiner, C.; Hochberg, F.; Kurochkin, I.V. Updating the MISEV minimal requirements for extracellular vesicle studies: Building bridges to reproducibility. J. Extracell. Vesicles 2017, 6, 1396823. [Google Scholar] [CrossRef] [PubMed]
- Mutschelknaus, L.; Peters, C.; Winkler, K.; Yentrapalli, R.; Heider, T.; Atkinson, M.J.; Moertl, S. Exosomes Derived from Squamous Head and Neck Cancer Promote Cell Survival after Ionizing Radiation. PLoS ONE 2016, 11, e0152213. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Targeting ferroptosis in pancreatic cancer: A double-edged sword. Trends Cancer 2021, 7, 891–901. [Google Scholar] [CrossRef] [PubMed]
- Nachef, M.; Ali, A.K.; Almutairi, S.M.; Lee, S.H. Targeting SLC1A5 and SLC3A2/SLC7A5 as a Potential Strategy to Strengthen Anti-Tumor Immunity in the Tumor Microenvironment. Front. Immunol. 2021, 12, 624324. [Google Scholar] [CrossRef]
- Liang, J.; Sun, Z. Overexpression of membranal SLC3A2 regulates the proliferation of oral squamous cancer cells and affects the prognosis of oral cancer patients. J. Oral Pathol. Med. 2021, 50, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Linge, A.; Lohaus, F.; Lock, S.; Nowak, A.; Gudziol, V.; Valentini, C.; von Neubeck, C.; Jutz, M.; Tinhofer, I.; Budach, V.; et al. HPV status, cancer stem cell marker expression, hypoxia gene signatures and tumour volume identify good prognosis subgroups in patients with HNSCC after primary radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation Oncology Group (DKTK-ROG). Radiother. Oncol. 2016, 121, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Pizzagalli, M.D.; Bensimon, A.; Superti-Furga, G. A guide to plasma membrane solute carrier proteins. FEBS J. 2021, 288, 2784–2835. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, S.M.; Ali, A.K.; He, W.; Yang, D.S.; Ghorbani, P.; Wang, L.; Fullerton, M.D.; Lee, S.H. Interleukin-18 up-regulates amino acid transporters and facilitates amino acid-induced mTORC1 activation in natural killer cells. J. Biol. Chem. 2019, 294, 4644–4655. [Google Scholar] [CrossRef] [PubMed]
- Terren, I.; Orrantia, A.; Vitalle, J.; Zenarruzabeitia, O.; Borrego, F. NK Cell Metabolism and Tumor Microenvironment. Front. Immunol. 2019, 10, 2278. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.J.; Zhang, W.; Lin, S.H.; Yang, W.H.; Wang, J.Z.; Shen, J.; Zhang, Y.; Lu, Y.; Wang, H.; Yu, J.; et al. Systems biology approach reveals a link between mTORC1 and G2/M DNA damage checkpoint recovery. Nat. Commun. 2018, 9, 3982. [Google Scholar] [CrossRef]
- Ma, Y.; Vassetzky, Y.; Dokudovskaya, S. mTORC1 pathway in DNA damage response. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2018, 1865, 1293–1311. [Google Scholar] [CrossRef]
- Ribeiro, F.A.; Noguti, J.; Oshima, C.T.; Ribeiro, D.A. Effective targeting of the epidermal growth factor receptor (EGFR) for treating oral cancer: A promising approach. Anticancer Res. 2014, 34, 1547–1552. [Google Scholar]
- Mohanapure, N.S.; Khandeparkar, S.G.S.; Saragade, P.B.; Gogate, B.P.; Joshi, A.R.; Mehta, S.R. Immunohistochemical study of epidermal growth factor receptor, human epidermal growth factor receptor 2/neu, p53, and Ki67 in oral squamous cell carcinoma. J. Oral Maxillofac. Pathol. 2022, 26, 127–128. [Google Scholar] [CrossRef]
- Shahsavari, F.; Miri, R.; Ghorbanpour, M. Expression of epidermal growth factor receptor in oral and esophageal squamous-cell carcinoma. Dent. Res. J. 2020, 17, 85–91. [Google Scholar]
- Gaudet, P.; Livstone, M.S.; Lewis, S.E.; Thomas, P.D. Phylogenetic-based propagation of functional annotations within the Gene Ontology consortium. Brief. Bioinform. 2011, 12, 449–462. [Google Scholar] [CrossRef]
- Temkin, V.; Huang, Q.; Liu, H.; Osada, H.; Pope, R.M. Inhibition of ADP/ATP exchange in receptor-interacting protein-mediated necrosis. Mol. Cell. Biol. 2006, 26, 2215–2225. [Google Scholar] [CrossRef] [PubMed]
- Chandra, D.; Choy, G.; Tang, D.G. Cytosolic accumulation of HSP60 during apoptosis with or without apparent mitochondrial release: Evidence that its pro-apoptotic or pro-survival functions involve differential interactions with caspase-3. J. Biol. Chem. 2007, 282, 31289–31301. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, J.C.; Dohi, T.; Kang, B.H.; Altieri, D.C. Hsp60 regulation of tumor cell apoptosis. J. Biol. Chem. 2008, 283, 5188–5194. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Asano, Y.; Shintani, Y.; Aoyama, H.; Kioka, H.; Tsukamoto, O.; Hikita, M.; Shinzawa-Itoh, K.; Takafuji, K.; Higo, S. Higd1a is a positive regulator of cytochrome c oxidase. Proc. Natl. Acad. Sci. USA 2015, 112, 1553–1558. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Chen, M.; Mu, W.J.; Luo, H.Y.; Guo, L. The functional role of Higd1a in mitochondrial homeostasis and in multiple disease processes. Genes Dis. 2023, 10, 1833–1845. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Liang, H.; Fu, X.; Wu, P.; Wang, C.; Chen, H.; Zheng, B.; Zhang, J.; Hu, S.; Zeng, R.; et al. SLC25A22 promotes proliferation and metastasis by activating MAPK/ERK pathway in gallbladder cancer. Cancer Cell Int. 2019, 19, 33. [Google Scholar] [CrossRef]
- Szymonowicz, K.; Krysztofiak, A.; Linden, J.V.; Kern, A.; Deycmar, S.; Oeck, S.; Squire, A.; Koska, B.; Hlouschek, J.; Vullings, M.; et al. Proton Irradiation Increases the Necessity for Homologous Recombination Repair Along with the Indispensability of Non-Homologous End Joining. Cells 2020, 9, 889. [Google Scholar] [CrossRef]
- Carter, R.J.; Nickson, C.M.; Thompson, J.M.; Kacperek, A.; Hill, M.A.; Parsons, J.L. Complex DNA damage induced by high linear energy transfer alpha-particles and protons triggers a specific cellular DNA damage response. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 776–784. [Google Scholar] [CrossRef]
- Chaudhary, P.; Marshall, T.I.; Currell, F.J.; Kacperek, A.; Schettino, G.; Prise, K.M. Variations in the processing of DNA double-strand breaks along 60-MeV therapeutic proton beams. Int. J. Radiat. Oncol. Biol. Phys. 2016, 95, 86–94. [Google Scholar] [CrossRef]
- Miszczyk, J.; Rawojc, K.; Panek, A.; Borkowska, A.; Prasanna, P.G.S.; Ahmed, M.M.; Swakon, J.; Galas, A. Do protons and X-rays induce cell-killing in human peripheral blood lymphocytes by different mechanisms? Clin. Transl. Radiat. Oncol. 2018, 9, 23–29. [Google Scholar] [CrossRef]
- Lei, G.; Zhang, Y.; Koppula, P.; Liu, X.; Zhang, J.; Lin, S.H.; Ajani, J.A.; Xiao, Q.; Liao, Z.; Wang, H.; et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 2020, 30, 146–162. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Carter, B.Z.; Andreeff, M.; Ishizawa, J. Molecular Mechanisms of Ferroptosis and Updates of Ferroptosis Studies in Cancers and Leukemia. Cells 2023, 12, 1128. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Pan, J.; Gan, L.; Xue, J. Ferroptosis, necroptosis, and pyroptosis in cancer: Crucial cell death types in radiotherapy and post-radiotherapy immune activation. Radiother. Oncol. 2023, 184, 109689. [Google Scholar] [CrossRef] [PubMed]
- Qu, K.; Chen, K.; Wang, H.; Li, X.; Chen, Z. Structure of the NuA4 acetyltransferase complex bound to the nucleosome. Nature 2022, 610, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, K.; Fradet-Turcotte, A.; Avvakumov, N.; Lambert, J.-P.; Roques, C.; Pandita, R.K.; Paquet, E.; Herst, P.; Gingras, A.-C.; Pandita, T.K. The TIP60 complex regulates bivalent chromatin recognition by 53BP1 through direct H4K20me binding and H2AK15 acetylation. Mol. Cell 2016, 62, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Grosse, N.; Fontana, A.O.; Hug, E.B.; Lomax, A.; Coray, A.; Augsburger, M.; Paganetti, H.; Sartori, A.A.; Pruschy, M. Deficiency in homologous recombination renders Mammalian cells more sensitive to proton versus photon irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 175–181. [Google Scholar] [CrossRef]
- Fontana, A.O.; Augsburger, M.A.; Grosse, N.; Guckenberger, M.; Lomax, A.J.; Sartori, A.A.; Pruschy, M.N. Differential DNA repair pathway choice in cancer cells after proton- and photon-irradiation. Radiother. Oncol. 2015, 116, 374–380. [Google Scholar] [CrossRef]
- Vitti, E.T.; Kacperek, A.; Parsons, J.L. Targeting DNA Double-Strand Break Repair Enhances Radiosensitivity of HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinoma to Photons and Protons. Cancers 2020, 12, 1490. [Google Scholar] [CrossRef]
- Guerreiro, E.M.; Vestad, B.; Steffensen, L.A.; Aass, H.C.D.; Saeed, M.; Ovstebo, R.; Costea, D.E.; Galtung, H.K.; Soland, T.M. Efficient extracellular vesicle isolation by combining cell media modifications, ultrafiltration, and size-exclusion chromatography. PLoS ONE 2018, 13, e0204276. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, E.M.; Ovstebo, R.; Thiede, B.; Costea, D.E.; Soland, T.M.; Kanli Galtung, H. Cancer cell line-specific protein profiles in extracellular vesicles identified by proteomics. PLoS ONE 2020, 15, e0238591. [Google Scholar] [CrossRef] [PubMed]
- Brusevold, I.J.; Søland, T.M.; Khuu, C.; Christoffersen, T.; Bryne, M. Nuclear and cytoplasmic expression of Met in oral squamous cell carcinoma and in an organotypic oral cancer model. Eur. J. Oral Sci. 2010, 118, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Brusevold, I.J.; Husvik, C.; Schreurs, O.; Schenck, K.; Bryne, M.; Søland, T.M. Induction of invasion in an organotypic oral cancer model by CoCl2, a hypoxia mimetic. Eur. J. Oral Sci. 2010, 118, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Howard, M.; Beltran, C.; Sarkaria, J.; Herman, M.G. Characterization of relative biological effectiveness for conventional radiation therapy: A comparison of clinical 6 MV X-rays and 137Cs. J. Radiat. Res. 2017, 58, 608–613. [Google Scholar] [CrossRef]
- Jin, X.-d.; Gong, L.; Guo, C.-l.; Hao, J.-f.; Wei, W.; Dai, Z.-y.; Li, Q. Survivin expressions in human hepatoma HepG2 cells exposed to ionizing radiation of different LET. Radiat. Environ. Biophys. 2008, 47, 399–404. [Google Scholar] [CrossRef]
- Murshed, H. Fundamentals of Radiation Oncology: Physical, Biological, and Clinical Aspects; Academic Press: New York, NY, USA, 2019. [Google Scholar]
- Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, O.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; Bertier, L.; et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef]

| Downregulated after 4 Gy | Downregulated after 8 Gy | Upregulated after 8 Gy | |||
|---|---|---|---|---|---|
| Gene Name | Function | Gene Name | Function | Gene name | Function |
| ITGA3 | Cell adhesion and migration, regulator of TGF- and Wnt signalling | ITGA2 | Cell adhesion and migration, inflammatory response | TPD52L2 | Carbohydrate metabolic processes, cell proliferation |
| SLC7A1 | Amino acid transport, T-cell proliferation | SLC7A1 | Amino acid transport, T-cell proliferation | H3-3B | Nucleosome assembly, cell growth regulation |
| SLC7A5 * | Immune system processes, programmed cell death, mTOR pathway | SLC7A5 * | Immune system processes, programmed cell death, mTOR pathway | NPM1 | Programmed cell death, cytoskeleton organization |
| SLC3A2 | RNA and protein binding, ferroptosis regulation, mTOR pathway | SLC1A5 | Amino acid transport, ferroptosis regulation, mTOR pathway | DCD | Immune system processes, found in sweat |
| SLC44A1 | Transmembrane transport, choline transport | HLA-A | Adaptive immune response, T-cell mediated cytotoxicity | ||
| TXN * | Response to radiation, negative regulation of cell death, | TXN * | Response to radiation, negative regulation of cell death | ||
| EGFR | Mitotic cell cycle, DNA repair, programmed cell death | EGFR | Mitotic cell cycle, DNA repair, programmed cell death | ||
| EHD4 | Endocytosis, endosomal transport, growth factor response | EHD4 | Endocytosis, endosomal transport, growth factor response | ||
| EHD1 | Endocytosis, intracellular protein transport | DSTN | Cell motility, actin binding | ||
| EZR | Immune system process, cytoskeleton organization | TSPAN4 | Integral component of plasma membrane, focal adhesion | ||
| RAC1 ** | Inflammatory response, MAPK pathway, migration and proliferation | ||||
| RAP2B | Negative regulation of cell migration | ||||
| Low LET Protons | High LET Protons | X-rays | |||
|---|---|---|---|---|---|
| Gene Name | Function | Gene Name | Function | Gene Name | Function |
| ALG5 | Protein glycosylation | ATP6V0C | Autophagy, Wnt pathway | ARL1 | Vesicle-mediated transport |
| CORO1C | Cell migration, endosomal transport | CD44 | Inflammatory response, regulation of DNA damage response and apoptosis (p53) | CD99 | Cell–cell adhesion |
| EEF1A1 | Translation, EGF response | DHX15 | RNA splicing, regulation of Ikb/NF-κB signalling | DDX39A | mRNA splicing and transport |
| EEF1D | Translation, cell death, cellular response to radiation | HIGD1A | Regulation of apoptotic process (hypoxia-induced protein), stress response | TOP2A | Makes ds DNA breaks, essential during mitosis and meiosis |
| HADH | Lipid metabolism | SEC61A1 | Integral component of ER-membrane | RAC2 | Regulation of apoptosis, augments the production of ROS |
| PPIB | RNA binding, positive regulation of organism growth | FECH | Detection of and response to UV light, heme biosynthesis, ferrous iron binding | ||
| PFN1 | Cell migration | HSPD1 | Immune response, apoptosis | ||
| PSMC3 | DNA replication, transcription | HNRNPA3 | mRNA splicing and transport | ||
| RPS17 | Translation | SLC25A22 | Mitochondrial glutamate/H+ transporter | ||
| RPSA | Translation, cell adhesion | SLC25A4 | Regulation of mitochondrial membrane permeability (apoptosis) | ||
| STIP1 | Response to IL-7, HSP90 protein binding | SLC25A6 | Regulation of mitochondrial membrane permeability (apoptosis) | ||
| TST | Epithelial cell differentiation | ||||
| Low LET Protons | High LET Protons | X-rays | |||
|---|---|---|---|---|---|
| Gene Name | Function | Gene Name | Function | Gene Name | Function |
| FAM3C | Promotes epithelial to mesenchymal transition | GRSF1 | RNA splicing and processing | ATP6V0C | Autophagy, Wnt pathway |
| DCXR | Regulation of ROS metabolic process | TARDBP | RNA splicing, apoptosis, cell cycle | ACTB | Cell cycle, DNA repair (HR), apoptosis |
| MRPL12 | Mitochondrial translation, regulation of transcription | ACTN1 | Apoptosis, transcription, cytoskeletal organization | ACTG1 | Angiogenesis, gene expression, migration, response to INF-y |
| QSOX2 | Protein folding, regulates sensitization of cells for INF-γ induced apoptosis | AP2A1 | Endocytosis, intracellular protein transport | ACTN4 | Migration, apoptosis, response to hypoxia |
| SPTLC1 | Lipid metabolism, programmed cell death, inflammatory response | CARS2 | Protein translation | ACSL3 | Antiferroptotic, lipid metabolism |
| YWHAH | Regulation of apoptosis, transcription | GARS1 | Protein translation | ||
| LAMB1 | Cell adhesion, migration and proliferation | ||||
| NUCB1 | Small GTPase-mediated signal transduction | ||||
| PSMC2 | Cell differentiation, protein degradation | ||||
| PPP1R14B | Innate immune response | ||||
| SDHAF3 | Mitochondrion organization | ||||
| TOR1AIP1 | Membrane organization | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juvkam, I.S.; Zlygosteva, O.; Sitarz, M.; Thiede, B.; Sørensen, B.S.; Malinen, E.; Edin, N.J.; Søland, T.M.; Galtung, H.K. Proton Compared to X-Irradiation Induces Different Protein Profiles in Oral Cancer Cells and Their Derived Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 16983. https://doi.org/10.3390/ijms242316983
Juvkam IS, Zlygosteva O, Sitarz M, Thiede B, Sørensen BS, Malinen E, Edin NJ, Søland TM, Galtung HK. Proton Compared to X-Irradiation Induces Different Protein Profiles in Oral Cancer Cells and Their Derived Extracellular Vesicles. International Journal of Molecular Sciences. 2023; 24(23):16983. https://doi.org/10.3390/ijms242316983
Chicago/Turabian StyleJuvkam, Inga Solgård, Olga Zlygosteva, Mateusz Sitarz, Bernd Thiede, Brita Singers Sørensen, Eirik Malinen, Nina Jeppesen Edin, Tine Merete Søland, and Hilde Kanli Galtung. 2023. "Proton Compared to X-Irradiation Induces Different Protein Profiles in Oral Cancer Cells and Their Derived Extracellular Vesicles" International Journal of Molecular Sciences 24, no. 23: 16983. https://doi.org/10.3390/ijms242316983
APA StyleJuvkam, I. S., Zlygosteva, O., Sitarz, M., Thiede, B., Sørensen, B. S., Malinen, E., Edin, N. J., Søland, T. M., & Galtung, H. K. (2023). Proton Compared to X-Irradiation Induces Different Protein Profiles in Oral Cancer Cells and Their Derived Extracellular Vesicles. International Journal of Molecular Sciences, 24(23), 16983. https://doi.org/10.3390/ijms242316983





