Col1A-2 Mutation in Osteogenesis Imperfecta Mice Contributes to Long Bone Fragility by Modifying Cell-Matrix Organization
Abstract
:1. Introduction
2. Results
2.1. Clinimetry
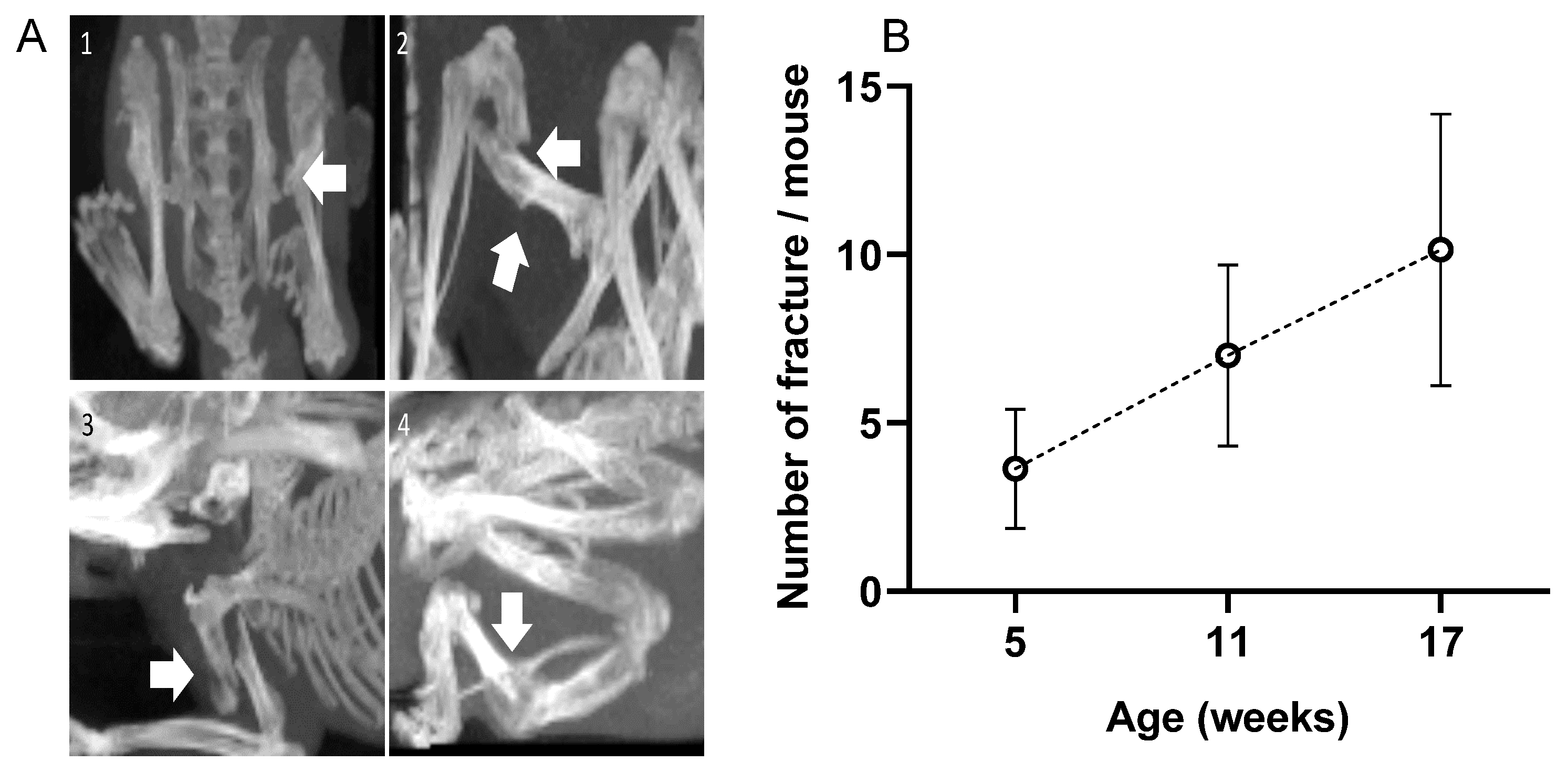
2.2. Fractures
2.3. pQCT Data
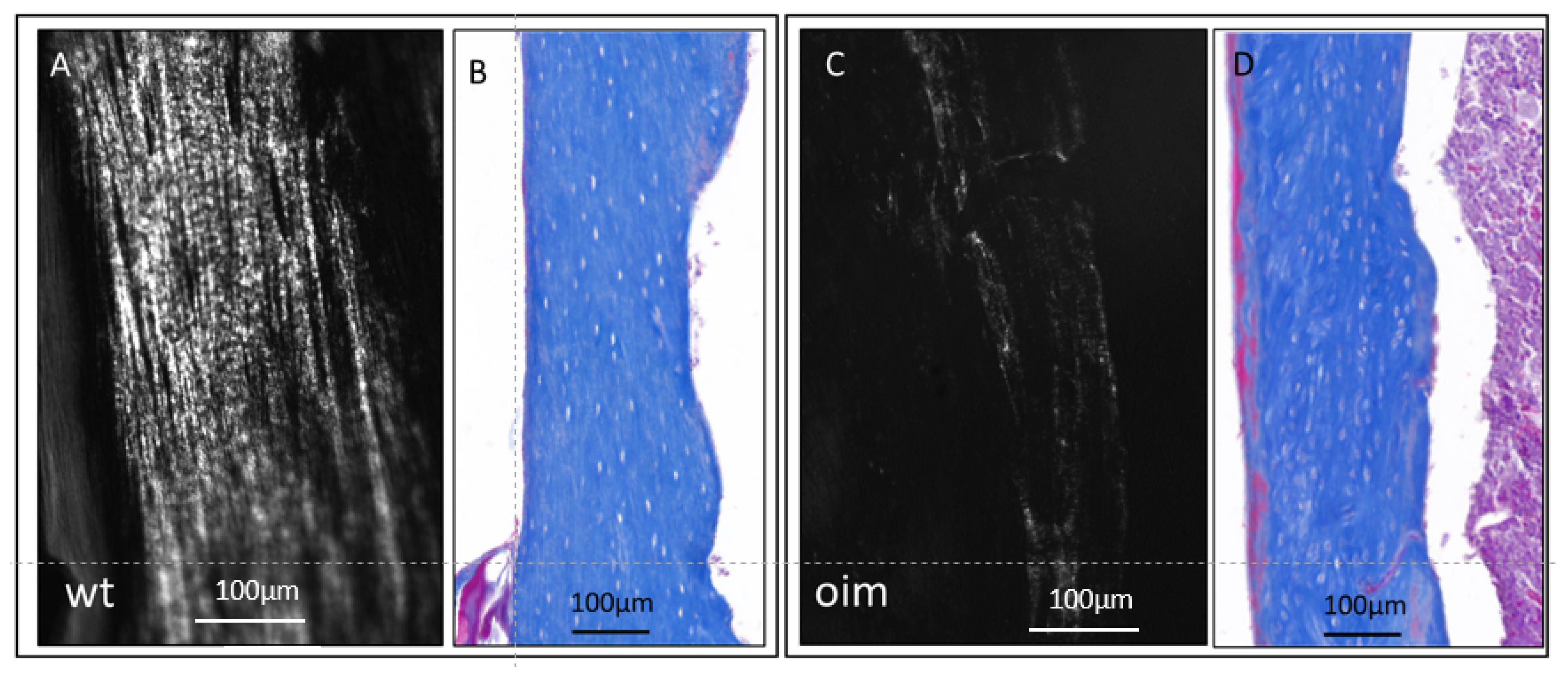
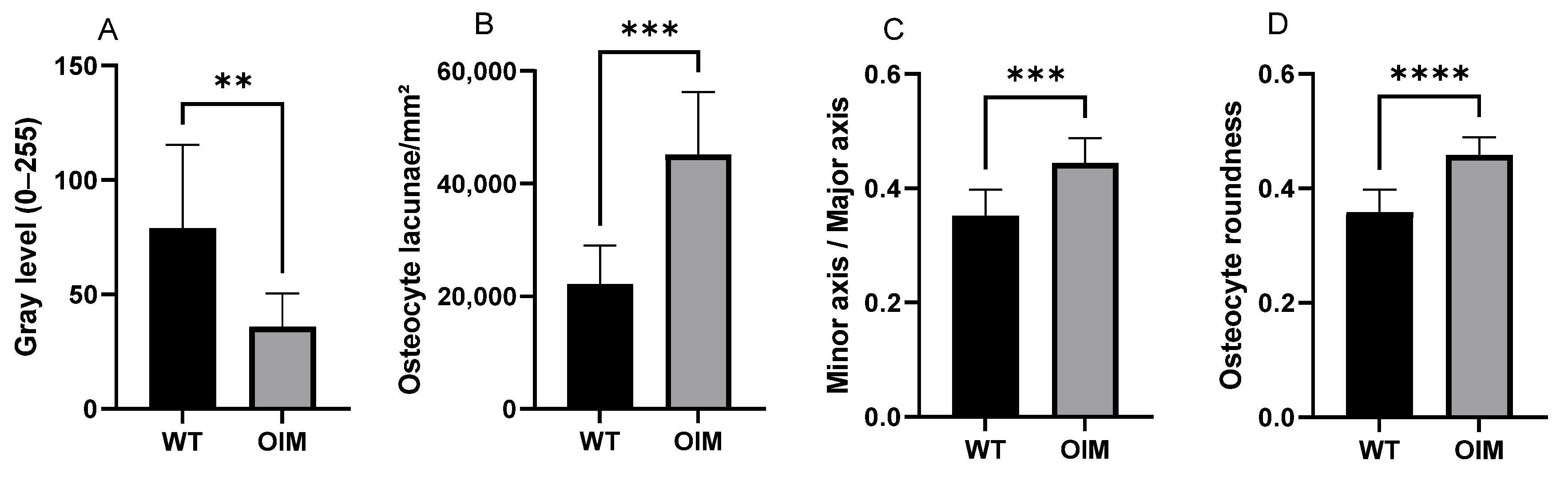
2.4. Histology
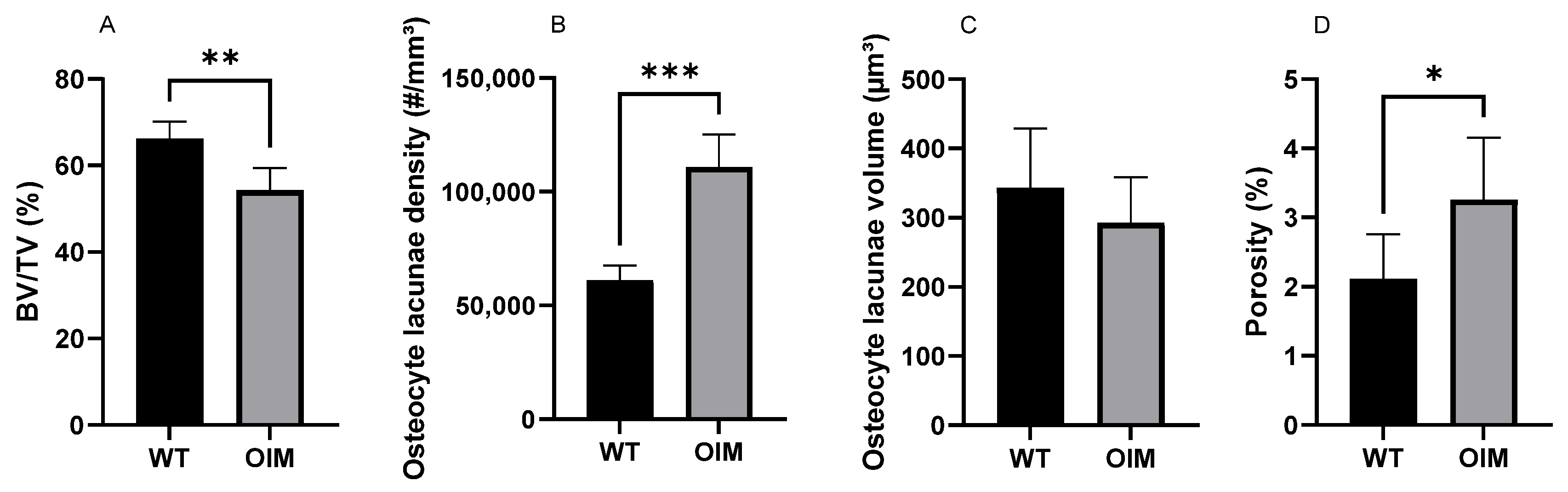
2.5. Nano-CT
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Schedule of Procedure
4.3. Fracture Count
4.4. Peripheral Quantitative Computed Tomography (pQCT)
4.5. Histology
4.6. Nano-CT
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marini, J.C.; Dang Do, A.N. Osteogenesis Imperfecta. In Endotext; Feingold, K.R., Anawalt, B., Blackman, M.R., Boyce, A., Chrousos, G., Corpas, E., de Herder, W.W., Dhatariya, K., Dungan, K., Hofland, J., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK279109/ (accessed on 3 August 2023).
- Kang, H.; Smriti Aryal, A.C.; Marini, J.C. Osteogenesis imperfecta: New genes reveal novel mechanisms in bone dysplasia. Transl. Res. 2017, 181, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Sillence, D.O.; Senn, A.; Danks, D.M. Genetic heterogeneity in osteogenesis imperfecta. J. Med. Genet. 1979, 16, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Forlino, A.; Marini, J.C. Osteogenesis imperfecta. Lancet 2016, 387, 1657–1671. [Google Scholar] [CrossRef] [PubMed]
- Chien, A.L.; Mu, E.W.; Kang, S. Skin in Osteogenesis Imperfecta. In Osteogenesis Imperfecta: A Translational Approach to Brittle Bone Disease, 1st ed.; Shapiro, J., Kassim, J., Sponseller, P., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2013; pp. 283–288. [Google Scholar] [CrossRef]
- Cardinal, M.; Tys, J.; Roels, T.; Lafont, S.; Ominsky, M.S.; Devogelaer, J.-P.; Chappard, D.; Mabilleau, G.; Ammann, P.; Nyssen-Behets, C.; et al. Sclerostin antibody reduces long bone fractures in the oim/oim model of osteogenesis imperfecta. Bone 2019, 124, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Tiede-Lewis, L.M.; Dallas, S.L. Changes in the osteocyte lacunocanalicular network with aging. Bone 2019, 122, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Butler, J.P.; Ingber, D.E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 1993, 260, 1124–1127. [Google Scholar] [CrossRef]
- Holmbeck, K.; Bianco, P.; Pidoux, I.; Inoue, S.; Billinghurst, R.C.; Wu, W.; Chrysovergis, K.; Yamada, S.; Birkedal-Hansen, H.; Poole, A.R. The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J. Cell Sci. 2005, 118 Pt 1, 147–156. [Google Scholar] [CrossRef]
- Zhang, K.; Barragan-Adjemian, C.; Ye, L.; Kotha, S.; Dallas, M.; Lu, Y.; Zhao, S.; Harris, M.; Harris, S.E.; Feng, J.Q.; et al. E11/gp38 Selective Expression in Osteocytes: Regulation by Mechanical Strain and Role in Dendrite Elongation. Mol. Cell. Biol. 2006, 26, 4539–4552. [Google Scholar] [CrossRef]
- Creecy, A.; Damrath, J.G.; Wallace, J.M. Control of Bone Matrix Properties by Osteocytes. Front. Endocrinol. 2021, 11, 578477. [Google Scholar] [CrossRef]
- Chen, H.; Senda, T.; Kubo, K.-Y. The osteocyte plays multiple roles in bone remodeling and mineral homeostasis. Med. Mol. Morphol. 2015, 48, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bacabac, R.G.; Mizuno, D.; Schmidt, C.F.; MacKintosh, F.C.; Van Loon, J.J.; Klein-Nulend, J.; Smit, T.H. Round versus flat: Bone cell morphology, elasticity, and mechanosensing. J. Biomech. 2008, 41, 1590–1598. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.A.; Riedel, C.; Schmidt, F.N.; Stockhausen, K.E.; Chushkin, Y.; Schaible, E.; Gludovatz, B.; Vettorazzi, E.; Zontone, F.; Püschel, K.; et al. Mechanical Competence and Bone Quality Develop During Skeletal Growth. J. Bone Miner. Res. 2019, 34, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Meier, M.; Wepf, R.; Müller, R. Towards quantitative 3D imaging of the osteocyte lacuno-canalicular network. Bone 2010, 47, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Chipman, S.D.; Sweet, H.O.; McBride, D.J.; Davisson, M.T.; Marks, S.C.; Shuldiner, A.R.; Wenstrup, R.J.; Rowe, D.W.; Shapiro, J.R. Defective pro alpha 2(I) collagen synthesis in a recessive mutation in mice: A model of human osteogenesis imperfecta. Proc. Natl. Acad. Sci. USA 1993, 90, 1701–1705. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, M.; Dessain, A.; Roels, T.; Lafont, S.; Ominsky, M.S.; Devogelaer, J.-P.; Chappard, D.; Mabilleau, G.; Ammann, P.; Nyssen-Behets, C.; et al. Sclerostin-Antibody Treatment Decreases Fracture Rates in Axial Skeleton and Improves the Skeletal Phenotype in Growing oim/oim Mice. Calcif. Tissue Int. 2020, 106, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Sun, B.; Ma, C.; Wu, H.; Chen, R.; He, H.; Zhang, Y. Comparable Effects of Strontium Ranelate and Alendronate Treatment on Fracture Reduction in a Mouse Model of Osteogenesis Imperfecta. BioMed Res. Int. 2021, 2021, 4243105. [Google Scholar] [CrossRef]
- Bargman, R.; Huang, A.; Boskey, A.L.; Raggio, C.; Pleshko, N. RANKL inhibition improves bone properties in a mouse model of osteogenesis imperfecta. Connect. Tissue Res. 2010, 51, 123–131. [Google Scholar] [CrossRef]
- Lund, A.M.; Müller, J.; Skovby, F. Anthropometry of patients with osteogenesis imperfecta. Arch. Dis. Child. 1999, 80, 524–528. [Google Scholar] [CrossRef]
- Chretien, A.; Couchot, M.; Mabilleau, G.; Behets, C. Biomechanical, Microstructural and Material Properties of Tendon and Bone in the Young Oim Mice Model of Osteogenesis Imperfecta. Int. J. Mol. Sci. 2022, 23, 9928. [Google Scholar] [CrossRef]
- Martin, R.B.; Lau, S.T.; Mathews, P.V.; Gibson, V.A.; Stovert, S.M. Collagen Fiber organization is related to mechanical properties and remodeling in equine bone. A comparsion of two methods. J. Biomech. 1996, 29, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, F.; Maguire, K.; Swami, S.; Zhu, H.; Flynn, E.; Wang, J.; Wu, J.Y. Histopathology of osteogenesis imperfecta bone. Supramolecular assessment of cells and matrices in the context of woven and lamellar bone formation using light, polarization and ultrastructural microscopy. Bone Rep. 2021, 14, 100734. [Google Scholar] [CrossRef] [PubMed]
- Carriero, A.; A Zimmermann, E.; Paluszny, A.; Tang, S.Y.; Bale, H.; Busse, B.; Alliston, T.; Kazakia, G.; O Ritchie, R.; Shefelbine, S.J. How Tough Is Brittle Bone? Investigating Osteogenesis Imperfecta in Mouse Bone. J. Bone Miner. Res. 2014, 29, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Vanleene, M.; Porter, A.; Guillot, P.-V.; Boyde, A.; Oyen, M.; Shefelbine, S. Ultra-structural defects cause low bone matrix stiffness despite high mineralization in osteogenesis imperfecta mice. Bone 2012, 50, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Kerschnitzki, M.; Wagermaier, W.; Roschger, P.; Seto, J.; Shahar, R.; Duda, G.N.; Mundlos, S.; Fratzl, P. The organization of the osteocyte network mirrors the extracellular matrix orientation in bone. J. Struct. Biol. 2011, 173, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Blouin, S.; Fratzl-Zelman, N.; Glorieux, F.H.; Roschger, P.; Klaushofer, K.; Marini, J.C.; Rauch, F. Hypermineralization and high osteocyte lacunar density in osteogenesis imperfecta type V bone indicate exuberant primary bone formation. J. Bone Miner. Res. 2017, 32, 1884–1892. [Google Scholar] [CrossRef]
- Schemenz, V.; Gjardy, A.; Chamasemani, F.F.; Roschger, A.; Roschger, P.; Zaslansky, P.; Helfen, L.; Burghammer, M.; Fratzl, P.; Weinkamer, R.; et al. Heterogeneity of the osteocyte lacuno-canalicular network architecture and material characteristics across different tissue types in healing bone. J. Struct. Biol. 2020, 212, 107616. [Google Scholar] [CrossRef]
- Carriero, A.; Doube, M.; Vogt, M.; Busse, B.; Zustin, J.; Levchuk, A.; Schneider, P.; Müller, R.; Shefelbine, S. Altered lacunar and vascular porosity in osteogenesis imperfecta mouse bone as revealed by synchrotron tomography contributes to bone fragility. Bone 2014, 61, 116–124. [Google Scholar] [CrossRef]
- Sang, W.; Ural, A. Influence of Osteocyte Lacunar-Canalicular Morphology and Network Architecture on Osteocyte Mechanosensitivity. Curr. Osteoporos. Rep. 2023, 21, 401–413. [Google Scholar] [CrossRef]
- Maes, A. Contrast-Team/Histogram-Windowing: Initial Release. Zenodo. 30 August 2022. Available online: https://zenodo.org/records/7034265 (accessed on 6 October 2023).
- Maes, A.; Pestiaux, C.; Marino, A.; Balcaen, T.; Leyssens, L.; Vangrunderbeeck, S.; Pyka, G.; De Borggraeve, W.M.; Bertrand, L.; Beauloye, C.; et al. Cryogenic contrast-enhanced microCT enables nondestructive 3D quantitative histopathology of soft biological tissues. Nat. Commun. 2022, 13, 6207. [Google Scholar] [CrossRef]


| Humerus | Femur | Tibia | ||||
|---|---|---|---|---|---|---|
| WT (n = 15) | OIM (n = 8) | WT (n = 14) | OIM (n = 10) | WT (n = 15) | OIM (n = 13) | |
| BMD (mg/cm3) | 813.8 ± 27.8 | 699.1 ± 54.6 **** | 785.6 ± 67.0 | 730.7 ± 59.9 | 868.0 ± 48.3 | 881.2 ± 59.4 |
| CSA (mm2) | 1.19 ± 0.10 | 1.14 ± 0.20 | 2.05 ± 0.30 | 1.64 ± 0.10 ** | 1.36 ± 0.30 | 0.94 ± 0.20 *** |
| Cort. area (mm2) | 0.76 ± 0.10 | 0.65 ± 0.10 * | 1.18 ± 0.10 | 0.91 ± 0.10 **** | 0.88 ± 0.10 | 0.64 ± 0.10 **** |
| Cort. area/CSA (%) | 64.69 ± 2.40 | 57.61 ± 4.10 **** | 58.36 ± 4.80 | 55.20 ± 3.60 | 65.32 ± 5.30 | 68.59 ± 4.50 |
| SSI (mm3) | 0.20 ± 0.10 | 0.19 ± 0.10 | 0.55 ± 0.10 | 0.36 ± 0.10 **** | 0.31 ± 0.10 | 0.18 ± 0.10 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
André, G.; Chretien, A.; Demoulin, A.; Beersaerts, M.; Docquier, P.-L.; Behets, C. Col1A-2 Mutation in Osteogenesis Imperfecta Mice Contributes to Long Bone Fragility by Modifying Cell-Matrix Organization. Int. J. Mol. Sci. 2023, 24, 17010. https://doi.org/10.3390/ijms242317010
André G, Chretien A, Demoulin A, Beersaerts M, Docquier P-L, Behets C. Col1A-2 Mutation in Osteogenesis Imperfecta Mice Contributes to Long Bone Fragility by Modifying Cell-Matrix Organization. International Journal of Molecular Sciences. 2023; 24(23):17010. https://doi.org/10.3390/ijms242317010
Chicago/Turabian StyleAndré, Grégoire, Antoine Chretien, Antoine Demoulin, Mélanie Beersaerts, Pierre-Louis Docquier, and Catherine Behets. 2023. "Col1A-2 Mutation in Osteogenesis Imperfecta Mice Contributes to Long Bone Fragility by Modifying Cell-Matrix Organization" International Journal of Molecular Sciences 24, no. 23: 17010. https://doi.org/10.3390/ijms242317010
APA StyleAndré, G., Chretien, A., Demoulin, A., Beersaerts, M., Docquier, P.-L., & Behets, C. (2023). Col1A-2 Mutation in Osteogenesis Imperfecta Mice Contributes to Long Bone Fragility by Modifying Cell-Matrix Organization. International Journal of Molecular Sciences, 24(23), 17010. https://doi.org/10.3390/ijms242317010






