Chemical Structure Diversity and Extensive Biological Functions of Specialized Metabolites in Rice
Abstract
:1. Introduction
2. Chemical Structure Diversity of SPMs in Rice
2.1. Chemical Structure Diversity of Terpenoids in Rice
2.1.1. Chemical Structure Diversity of Rice Monoterpenoids
2.1.2. Chemical Structure Diversity of Rice Sesquiterpenoids
2.1.3. Chemical Structure Diversity of Rice Diterpenoids
2.1.4. Chemical Structure Diversity of Triterpenoids and Steroids from Rice
2.2. Chemical Structure Diversity of Rice Phenolics
2.2.1. Simple Rice Phenolics
2.2.2. Rice Flavonoids
2.2.3. Other Rice Phenolics
2.3. Chemical Structure Diversity of Rice Alkaloids
2.4. Chemical Structure Diversity of Other Types of Compounds in Rice
3. Interactions between Rice SPMs and Herbivores
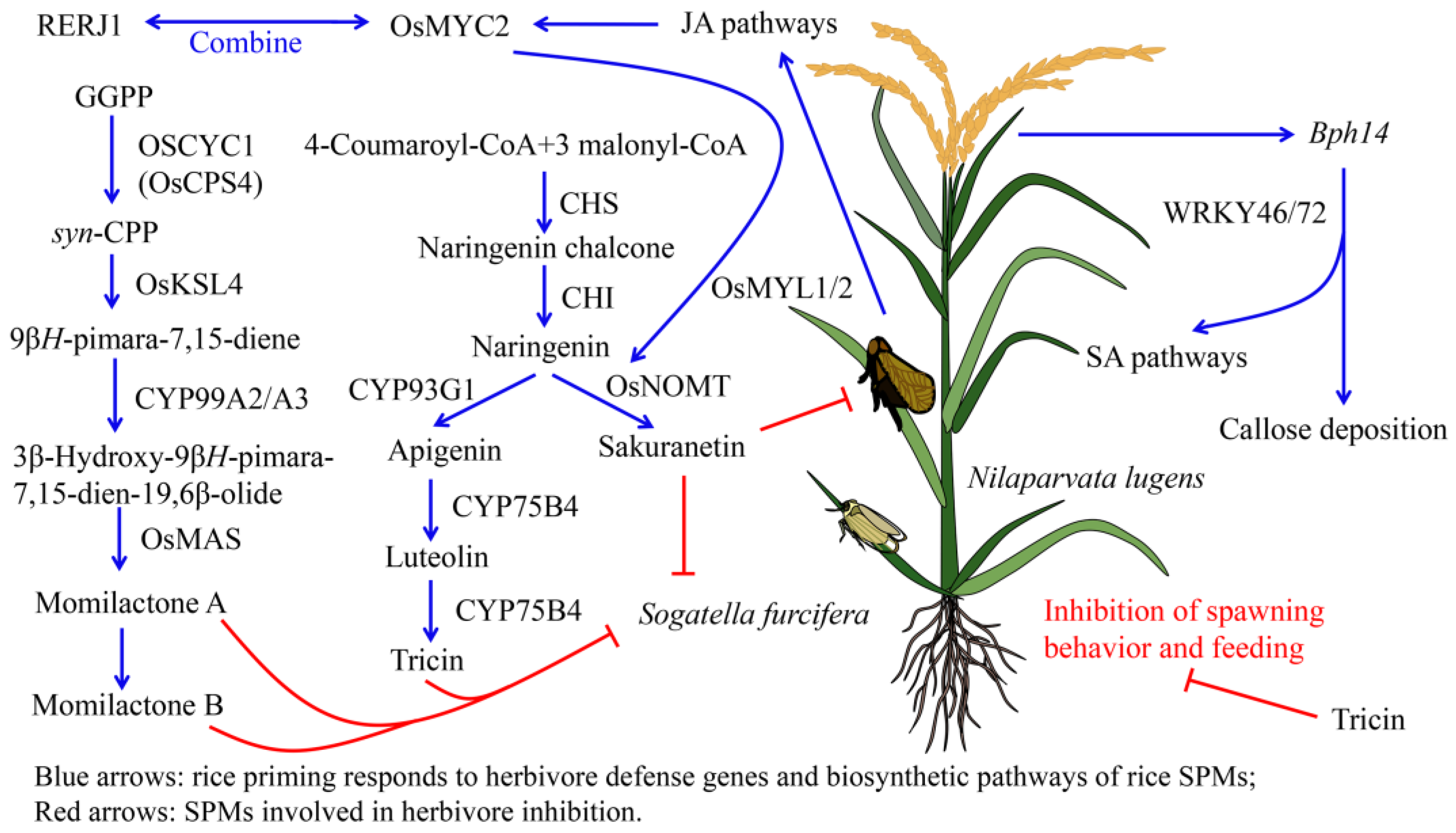
3.1. Rice SPMs Related to Herbivore Resistance
3.1.1. Anti-Insect Activity of Rice Terpenoids
3.1.2. Anti-Insect Activity of Phenolic Substances in Rice
3.1.3. Anti-Insect Activity of Alkaloids in Rice
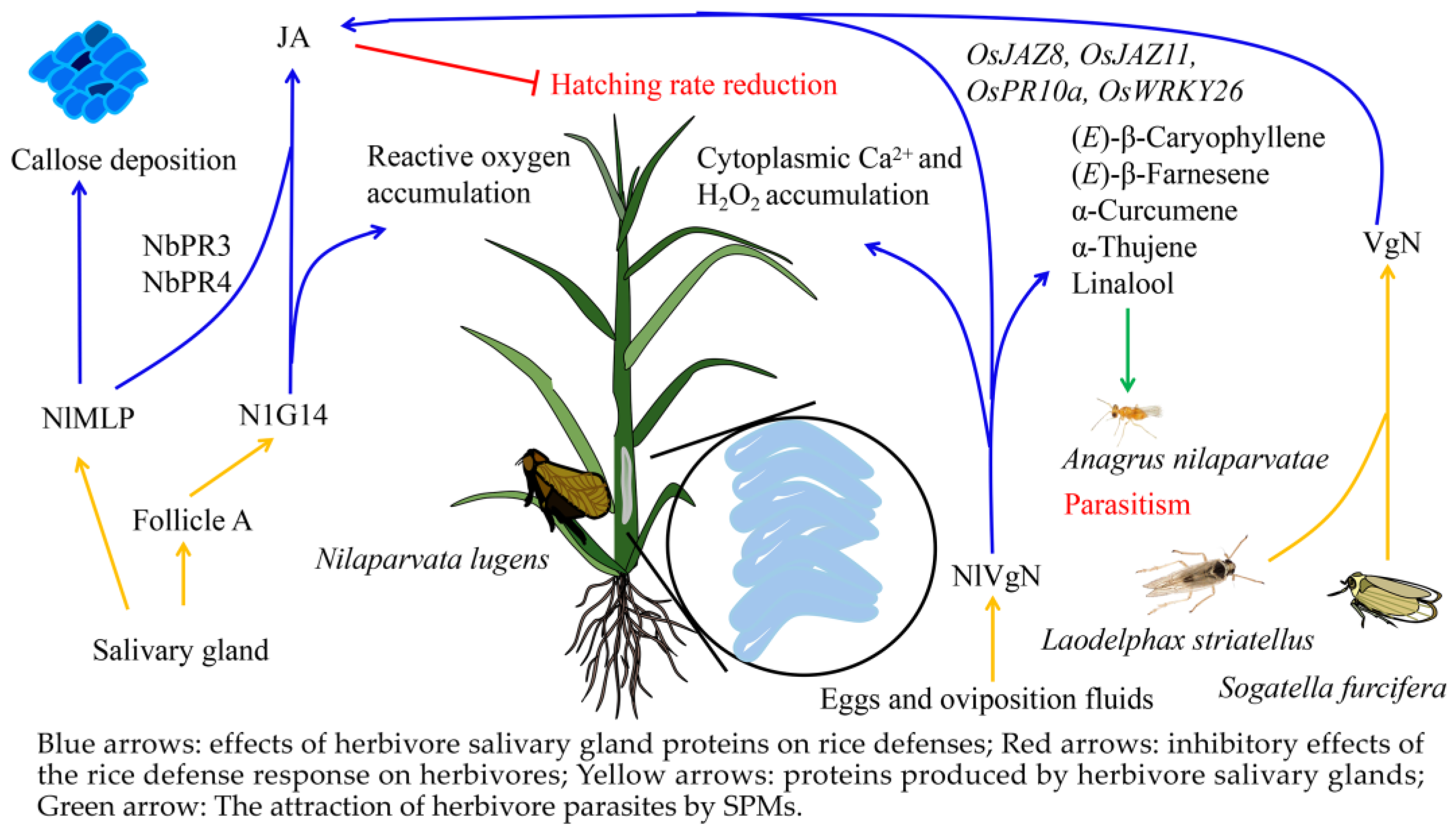
3.2. Salivary Metabolites from Herbivores Induce Defense Responses in Rice
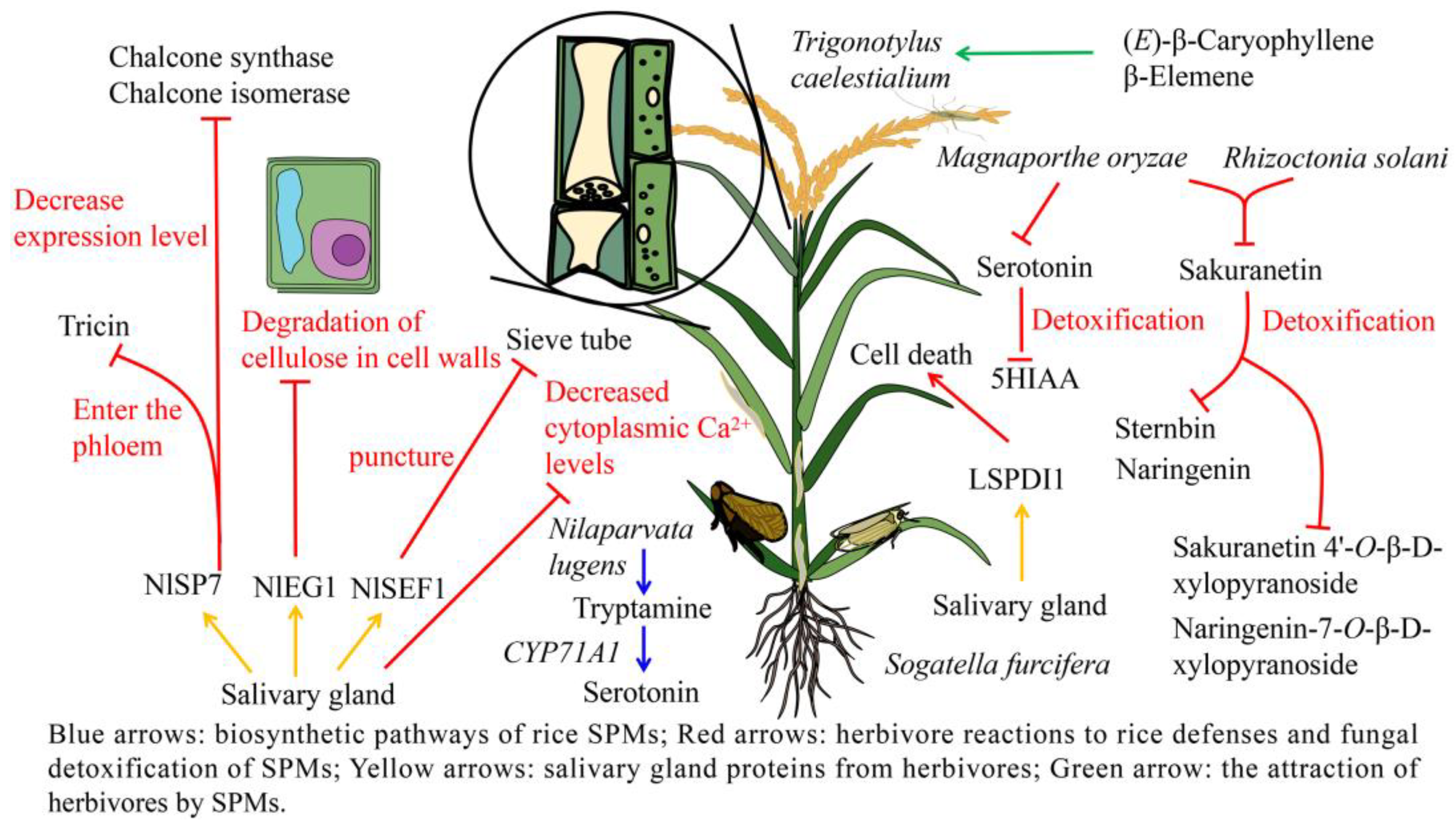
3.3. Adaptive Mechanisms of Herbivores to Rice Defense Response
4. Interactions between SPMs and Microorganisms in Rice
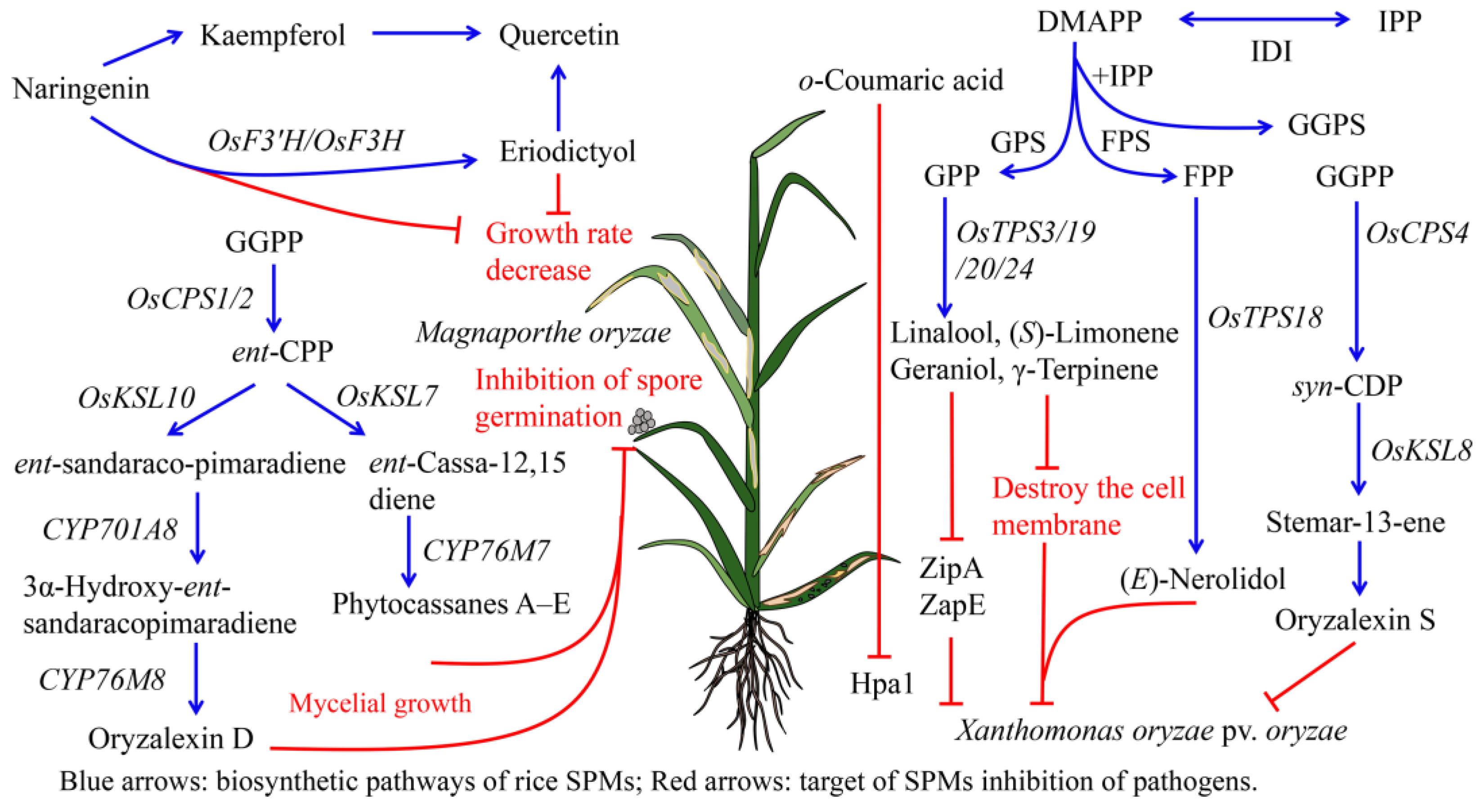
4.1. Antipathogen Activities of Rice SPMs
4.1.1. Antipathogen Activities of Rice Terpenoids
4.1.2. Antipathogen Activities of Rice Phenolic Compounds
4.1.3. Antipathogen Activities of Rice Alkaloids
4.2. Main Bacterial Targets of Rice SPMs
4.3. Adaptation of Fungus to Rice SPMs
4.4. Interactions between Rice SPMs and Rhizosphere Microbial Communities
5. Rice SPMs Regulate Plant-to-Plant Relationships
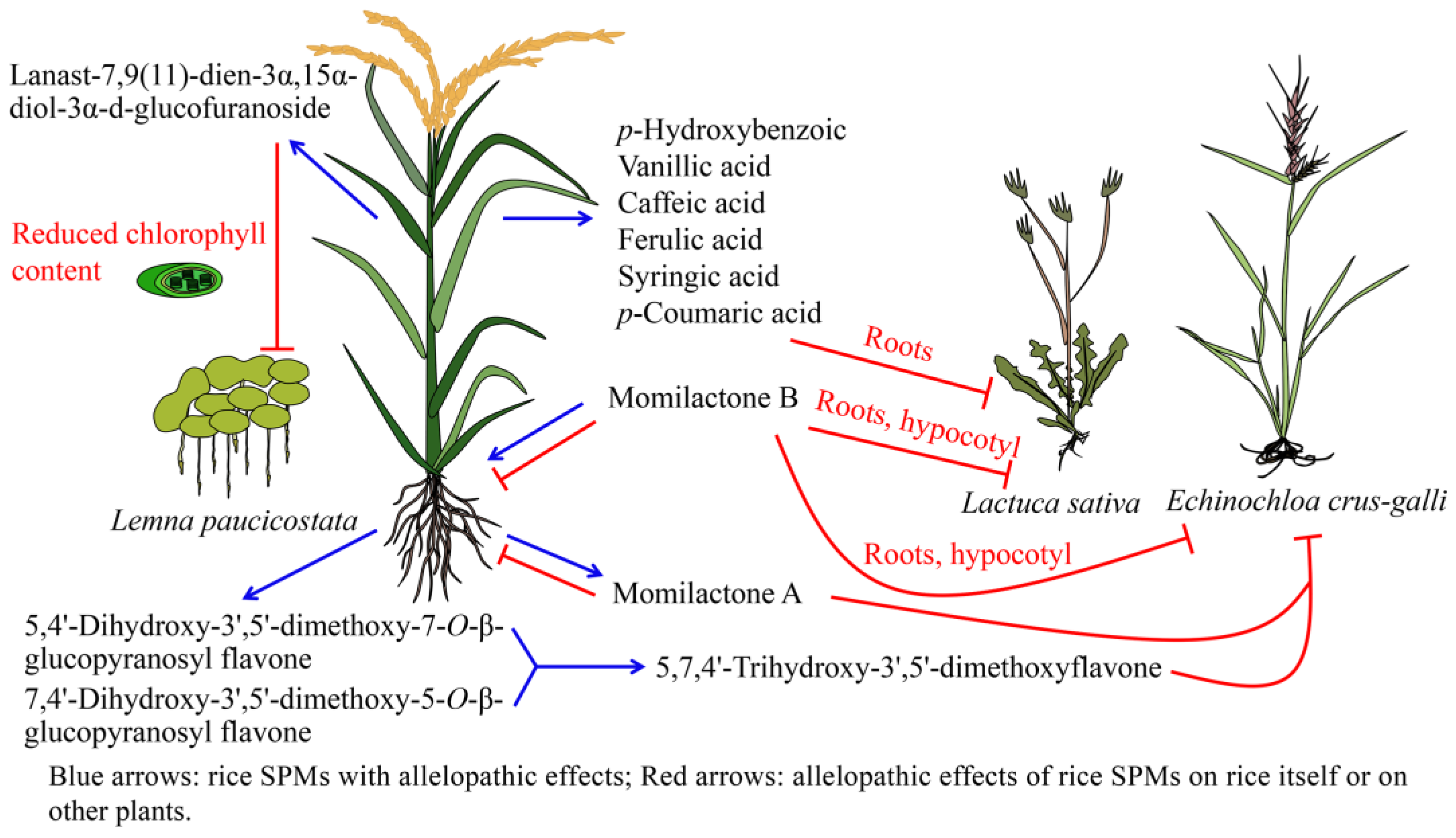
5.1. Allelopathy of Rice Terpenoids
5.2. Allelopathy of Rice Phenolic Compounds
6. Endophytic Microorganisms Promote Rice Growth
7. Prospect
8. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovach, M.J.; Sweeney, M.T.; McCouch, S.R. New insights into the history of rice domestication. Trends Genet. 2007, 23, 578–587. [Google Scholar] [CrossRef]
- Zhou, H.W.; Hua, J.; Li, H.D.; Song, X.Y.; Luo, S.H. Structurally diverse specialized metabolites of maize and their extensive biological functions. J. Cell. Physiol. 2023, 1–15. [Google Scholar] [CrossRef]
- Zhou, H.W.; Hua, J.; Zhang, J.M.; Luo, S.H. Negative interactions balance growth and defense in plants confronted with herbivores or pathogens. J. Agric. Food Chem. 2022, 70, 12723–12732. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K.-M. Plant secondary metabolite biosynthesis and transcriptional regulation in response to biotic and abiotic stress conditions. Agronomy 2021, 11, 968. [Google Scholar]
- Kim, E.-G.; Yun, S.; Park, J.-R.; Kim, K.-M. Identification of F3H, major secondary metabolite-related gene that confers resistance against whitebacked planthopper through qtl mapping in rice. Plants 2021, 10, 81. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-H.; Chung, I.-M.; Ahmad, A.; Kim, B.-H.; Hwang, S.-J. Growth inhibition of unicellular and colonial Microcystis strains (Cyanophyceae) by compounds isolated from rice (Oryza sativa) hulls. Aquat. Bot. 2009, 90, 309–314. [Google Scholar] [CrossRef]
- Pang, Y.H.; Ahmed, S.; Xu, Y.J.; Beta, T.; Zhu, Z.W.; Shao, Y.F.; Bao, J.S. Bound phenolic compounds and antioxidant properties of whole grain and bran of white, red and black rice. Food Chem. 2018, 240, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Ota, K.; Ino, T. Release of momilactone A and B from rice plants into the rhizosphere and its bioactivities. Allelopathy J. 2008, 22, 321–328. [Google Scholar]
- Wang, Q.; Hillwig, M.L.; Wu, Y.S.; Peters, R.J. CYP701A8: A rice ent-kaurene oxidase paralog diverted to more specialized diterpenoid metabolism. Plant Physiol. 2012, 158, 1418–1425. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, Q.L.; Xia, X.M.; Dong, L.M.; Luo, B.; Liu, W.B.; Tan, J.W. A new phenylpropane-pimarane heterodimer and a new ent-kaurene diterpene from the husks of Oryza sativa. Phytochem. Lett. 2018, 24, 120–124. [Google Scholar] [CrossRef]
- Umemura, K.; Ogawa, N.; Shimura, M.; Koga, J.; Usami, H.; Kono, T. Possible role of phytocassane, rice phytoalexin, in disease resistance of rice against the blast fungus Magnaporthe grisea. Biosci. Biotechnol. Biochem. 2003, 67, 899–902. [Google Scholar] [CrossRef] [PubMed]
- Mitsuaki, T.; Akihiro, O.; Nobuki, S.; Chizuko, K.; Tadahiro, K.; Yoshio, K.; Norindo, T. Momilactone-C, a minor constituent of growth inhibitors in rice husk. Chem. Lett. 1976, 5, 1157–1158. [Google Scholar]
- Wang, W.X.; Li, Y.Y.; Dang, P.Q.; Zhao, S.J.; Lai, D.W.; Zhou, L.G. Rice secondary metabolites: Structures, roles, biosynthesis, and metabolic regulation. Molecules 2018, 23, 3098. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Shimizu, T.; Okada, K. Transcriptional regulation of the biosynthesis of phytoalexin: A lesson from specialized metabolites in rice. Plant Biotechnol. 2014, 31, 377–388. [Google Scholar] [CrossRef]
- Toyomasu, T.; Usui, M.; Sugawara, C.; Otomo, K.; Hirose, Y.; Miyao, A.; Hirochika, H.; Okada, K.; Shimizu, T.; Koga, J. Reverse-genetic approach to verify physiological roles of rice phytoalexins: Characterization of a knockdown mutant of OsCPS4 phytoalexin biosynthetic gene in rice. Physiol. Plant. 2014, 150, 55–62. [Google Scholar] [CrossRef]
- Matsuda, F.; Nakabayashi, R.; Yang, Z.G.; Okazaki, Y.; Yonemaru, J.i.; Ebana, K.; Yano, M.; Saito, K. Metabolome-genome-wide association study dissects genetic architecture for generating natural variation in rice secondary metabolism. Plant J. 2015, 81, 13–23. [Google Scholar] [CrossRef]
- Horie, K.; Sakai, K.; Okugi, M.; Toshima, H.; Hasegawa, M. Ultraviolet-induced amides and casbene diterpenoids from rice leaves. Phytochem. Lett. 2016, 15, 57–62. [Google Scholar] [CrossRef]
- Koga, J.; Shimura, M.; Oshima, K.; Ogawa, N.; Yamauchi, T.; Ogasawara, N. Phytocassanes A, B, C and D, novel diterpene phytoalexins from rice, Oryza sativa L. Tetrahedron 1995, 51, 7907–7918. [Google Scholar] [CrossRef]
- Koga, J.; Ogawa, N.; Yamauchi, T.; Kikuchi, M.; Ogasawara, N.; Shimura, M. Functional moiety for the antifungal activity of phytocassane E, a diterpene phytoalexin from rice. Phytochemistry 1997, 44, 249–253. [Google Scholar] [CrossRef]
- Horie, K.; Inoue, Y.; Sakai, M.; Yao, Q.; Tanimoto, Y.; Koga, J.; Toshima, H.; Hasegawa, M. Identification of UV-induced diterpenes including a new diterpene phytoalexin, phytocassane F, from rice leaves by complementary GC/MS and LC/MS approaches. J. Agric. Food Chem. 2015, 63, 4050–4059. [Google Scholar] [CrossRef]
- Kono, Y.; Kojima, A.; Nagai, R.; Watanabe, M.; Kawashima, T.; Onizawa, T.; Teraoka, T.; Watanab, M.; Koshino, H.; Uzawa, J.; et al. Antibacterial diterpenes and their fatty acid conjugates from rice leaves. Phytochemistry 2004, 65, 1291–1298. [Google Scholar] [CrossRef]
- Akatsuka, T.; Kodama, O.; Sekido, H.; Kono, Y.; Takeuchi, S. Novel phytoalexins (oryzalexins A, B and C) isolated from rice blast leaves infected with Pyricularia oryzae. Part I: Isolation, characterization and biological activities of oryzalexins. Agric. Biol. Chem. 1985, 49, 1689–1694. [Google Scholar] [CrossRef]
- Cho, J.-G.; Cha, B.-J.; Min Lee, S.; Shrestha, S.; Jeong, R.-H.; Sung Lee, D.; Kim, Y.-C.; Lee, D.-G.; Kang, H.-C.; Kim, J.; et al. Diterpenes from the roots of Oryza sativa L. and their inhibition activity on NO production in LPS-stimulated RAW264.7 macrophages. Chem. Biodivers. 2015, 12, 1356–1364. [Google Scholar] [CrossRef]
- Cartwright, D.W.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Isolation and characterization of two phytoalexins from rice as momilactones A and B. Phytochemistry 1981, 20, 535–537. [Google Scholar] [CrossRef]
- Kato, T.; Kabuto, C.; Sasaki, N.; Tsunagawa, M.; Aizawa, H.; Fujita, K.; Kato, Y.; Kitahara, Y.; Takahashi, N. Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedron Lett. 1973, 14, 3861–3864. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, J.T.; Kim, S.-H. Evaluation of allelopathic potential and quantification of momilactone A,B from rice hull extracts and assessment of inhibitory bioactivity on paddy field weeds. J. Agric. Food Chem. 2006, 54, 2527–2536. [Google Scholar] [CrossRef]
- Ramazani, E.; Akaberi, M.; Emami, S.A.; Tayarani-Najaran, Z. Biological and pharmacological effects of γ-oryzanol: An updated review of the molecular mechanisms. Curr. Pharm. Des. 2021, 27, 2299–2316. [Google Scholar] [CrossRef] [PubMed]
- Chumpolsri, W.; Wijit, N.; Boontakham, P.; Nimmanpipug, P.; Sookwong, P.; Luangkamin, S.; Wongpornchai, S. Variation of terpenoid flavor odorants in bran of some black and white rice varieties analyzed by GC×GC-MS. J. Food Nutr. Res. 2015, 3, 114–120. [Google Scholar] [CrossRef]
- Lee, G.W.; Chung, M.-S.; Kang, M.; Chung, B.Y.; Lee, S. Direct suppression of a rice bacterial blight (Xanthomonas oryzae pv. oryzae) by monoterpene (S)-limonene. Protoplasma 2016, 253, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Obara, N.; Hasegawa, M.; Kodama, O. Induced volatiles in elicitor-treated and rice blast fungus-inoculated rice leaves. Biosci. Biotechnol. Biochem. 2002, 66, 2549–2559. [Google Scholar] [CrossRef]
- Taniguchi, S.; Hosokawa-Shinonaga, Y.; Tamaoki, D.; Yamada, S.; Akimitsu, K.; Gomi, K. Jasmonate induction of the monoterpene linalool confers resistance to rice bacterial blight and its biosynthesis is regulated by JAZ protein in rice. Plant Cell Environ. 2014, 37, 451–461. [Google Scholar] [CrossRef]
- Kiyama, H.; Matsunaga, A.; Suzuki, G.; Gomi, K. Monoterpene geraniol produced by rice terpene synthase 21 suppresses the expression of cell-division related genes in the rice bacterial pathogen, Xanthomonas oryzae pv. oryzae. Physiol. Mol. Plant Pathol. 2021, 115, 101673. [Google Scholar] [CrossRef]
- Lee, H.; Lee, G.; Kim, Y.; Ahn, H.; Lee, K.-G. Analysis of volatile compounds and antioxidant activity in rice extracts (Oryza sativa L.) extracted by various conditions. Inst. Food Sci. Technol. 2022, 57, 5289–5296. [Google Scholar] [CrossRef]
- Yoshitomi, K.; Taniguchi, S.; Tanaka, K.; Uji, Y.; Akimitsu, K.; Gomi, K. Rice terpene synthase 24 (OsTPS24) encodes a jasmonate-responsive monoterpene synthase that produces an antibacterial γ-terpinene against rice pathogen. J. Plant Physiol. 2016, 191, 120–126. [Google Scholar] [CrossRef]
- Concepcion, J.C.T.; Ouk, S.; Riedel, A.; Calingacion, M.; Zhao, D.; Ouk, M.; Garson, M.J.; Fitzgerald, M.A. Quality evaluation, fatty acid analysis and untargeted profiling of volatiles in Cambodian rice. Food Chem. 2018, 240, 1014–1021. [Google Scholar] [CrossRef]
- Kiryu, M.; Hamanaka, M.; Yoshitomi, K.; Mochizuki, S.; Akimitsu, K.; Gomi, K. Rice terpene synthase 18 (OsTPS18) encodes a sesquiterpene synthase that produces an antibacterial (E)-nerolidol against a bacterial pathogen of rice. J. Gen. Plant Pathol. 2018, 84, 221–229. [Google Scholar] [CrossRef]
- Chung, I.M.; Ali, M.; Hahn, S.J.; Siddiqui, N.A.; Lim, Y.H.; Ahmad, A. Chemical constituents from the hulls of Oryza sativa with cytotoxic activity. Chem. Nat. Compd. 2005, 41, 182–189. [Google Scholar] [CrossRef]
- Sridharan, A.; Thankappan, S.; Karthikeyan, G.; Uthandi, S. Comprehensive profiling of the VOCs of Trichoderma longibrachiatum EF5 while interacting with Sclerotium rolfsii and Macrophomina phaseolina. Microbiol. Res. 2020, 236, 126436. [Google Scholar]
- Xiao, Y.H.; Wang, Q.J.; Erb, M.; Turlings, T.C.; Ge, L.M.; Hu, L.F.; Li, J.; Han, X.; Zhang, T.; Lu, J. Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol. Lett. 2012, 15, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Sakai, M.; Yao, Q.; Tanimoto, Y.; Toshima, H.; Hasegawa, M. Identification of a novel casbane-type diterpene phytoalexin, ent-10-oxodepressin, from rice leaves. Biosci. Biotechnol. Biochem. 2013, 77, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Xu, Q.L.; He, C.M.; Zeng, L.; Wang, H.F. Two new anti-fungal diterpenoids from the husks of Oryza sativa. Phytochem. Lett. 2014, 10, 309–312. [Google Scholar] [CrossRef]
- Gu, C.Z.; Xia, X.M.; Lv, J.; Tan, J.W.; Baerson, S.R.; Pan, Z.Q.; Song, Y.Y.; Zeng, R.S. Diterpenoids with herbicidal and antifungal activities from hulls of rice (Oryza sativa). Fitoterapia 2019, 136, 104183. [Google Scholar] [CrossRef]
- Kodama, O.; Li, W.X.; Tamogami, S.; Akatsuka, T. Oryzalexin S, a novel stemarane-type diterpene rice phytoalexin. Biosci. Biotechnol. Biochem. 1992, 56, 1002–1003. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Kodama, O.; Akatsuka, T. Oryzalexin E, a diterpene phytoalexin from UV-irradiated rice leaves. Phytochemistry 1993, 33, 79–81. [Google Scholar] [CrossRef]
- Nemoto, T.; Cho, E.-M.; Okada, A.; Okada, K.; Otomo, K.; Kanno, Y.; Toyomasu, T.; Mitsuhashi, W.; Sassa, T.; Minami, E. Stemar-13-ene synthase, a diterpene cyclase involved in the biosynthesis of the phytoalexin oryzalexin S in rice. FEBS Lett. 2004, 571, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Kurogochi, S.; Murofushi, N.; Ota, Y.; Takahashi, N. Identification of gibberellins in the rice plant and quantitative changes of gibberellin A19 throughout its life cycle. Planta 1979, 146, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Shimura, K.; Okada, A.; Okada, K.; Jikumaru, Y.; Ko, K.-W.; Toyomasu, T.; Sassa, T.; Hasegawa, M.; Kodama, O.; Shibuya, N. Identification of a biosynthetic gene cluster in rice for momilactones. J. Biol. Chem. 2007, 282, 34013–34018. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.S.; Wang, Q.; Hillwig, M.L.; Peters, R.J. Picking sides: Distinct roles for CYP76M6 and CYP76M8 in rice oryzalexin biosynthesis. Biochem. J. 2013, 454, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Kodama, O.; Akatsuka, T. Oryzalexin F, a diterpene phytoalexin from UV-irradiated rice leaves. Phytochemistry 1994, 36, 299–301. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ino, T. Concentration and release level of momilactone B in the seedlings of eight rice cultivars. J. Plant Physiol. 2005, 162, 965–969. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ino, T. Possible involvement of momilactone B in rice allelopathy. J. Plant Physiol. 2005, 162, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Kono, Y.; Esumi, Y.; Teraoka, T.; Hosokawa, D.; Suzuki, Y.; Sakurai, A.; Watanabe, M. Studies on a quantitative analysis of oryzalides and oryzalic acids in rice plants by GC-SIM. Biosci. Biotechnol. Biochem. 1996, 60, 1460–1463. [Google Scholar] [CrossRef]
- Verardo, V.; Gómez-Caravaca, A.M.; Marconi, E.; Segura-Carretero, A.; Garrido-Frenich, A.; Fernández-Gutiérrez, A. Determination of lipophilic and hydrophilic bioactive compounds in raw and parboiled rice bran. RSC Adv. 2016, 6, 50786–50796. [Google Scholar] [CrossRef]
- Akihisa, T.; Yasukawa, K.; Yamaura, M.; Ukiya, M.; Kimura, Y.; Shimizu, N.; Arai, K. Triterpene alcohol and sterol ferulates from rice bran and their anti-inflammatory effects. J. Agric. Food Chem. 2000, 48, 2313–2319. [Google Scholar] [CrossRef]
- Suttiarporn, P.; Chumpolsri, W.; Mahatheeranont, S.; Luangkamin, S.; Teepsawang, S.; Leardkamolkarn, V. Structures of phytosterols and triterpenoids with potential anti-cancer activity in bran of black non-glutinous rice. Nutrients 2015, 7, 1672–1687. [Google Scholar] [CrossRef]
- Fang, N.B.; Yu, S.G.; Badger, T.M. Characterization of triterpene alcohol and sterol ferulates in rice bran using LC-MS/MS. J. Agric. Food Chem. 2003, 51, 3260–3267. [Google Scholar] [CrossRef]
- Oka, T.; Fujimoto, M.; Nagasaka, R.; Ushio, H.; Hori, M.; Ozaki, H. Cycloartenyl ferulate, a component of rice bran oil-derived γ-oryzanol, attenuates mast cell degranulation. Phytomedicine 2010, 17, 152–156. [Google Scholar] [CrossRef]
- Chung, I.M.; Ali, M.; Ahmad, A.; Lim, J.D.; Yu, C.Y.; Kim, J.S. Chemical constituents of rice (Oryza sativa) hulls and their herbicidal activity against duckweed (Lemna paucicostata Hegelm 381). Phytochem. Analysis 2006, 17, 36–45. [Google Scholar] [CrossRef]
- Sabir, A.; Rafi, M.; Darusman, L.K. Discrimination of red and white rice bran from Indonesia using HPLC fingerprint analysis combined with chemometrics. Food Chem. 2017, 221, 1717–1722. [Google Scholar] [CrossRef]
- Liu, C.; Xi, X.J.; Liu, Y.Y.; Lu, Y.Z.; Che, F.F.; Gu, Y.X.; Yu, Y.C.; Li, H.; Liu, J.G.; Wei, Y. Isolation of four major compounds of γ-oryzanol from rice bran oil by ionic liquids modified high-speed countercurrent chromatography and antimicrobial activity and neuroprotective effect of cycloartenyl ferulate in vitro. Chromatographia 2021, 84, 635–644. [Google Scholar] [CrossRef]
- Luo, H.F.; Li, Q.L.; Yu, S.G.; Badger, T.M.; Fang, N.B. Cytotoxic hydroxylated triterpene alcohol ferulates from rice bran. J. Nat. Prod. 2005, 68, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Zhong, H.M.; Che, C.T. Cycloartanes from the red alga Galaxaura sp. J. Asian Nat. Prod. Res. 2005, 7, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.L.; Frank, T.; Shu, Q.Y.; Engel, K.-H. Metabolite profiling of germinating rice seeds. J. Agric. Food Chem. 2008, 56, 11612–11620. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Kwon, C.; An, Y.; Ali, M.; Lee, H.; Lim, J.-D.; Kim, S.; Yang, Y.J.; Kim, S.-H.; Ahmad, A. Characterization of new polyphenolic glycosidic constituents and evaluation of cytotoxicity on a macrophage cell line and allelopathic activities of Oryza sativa. Molecules 2018, 23, 1933. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Ali, M.; Ahmad, A. A and B from rice hulls of Oryza sativa. Indian J. Chem. 2007, 3, 516–522. [Google Scholar]
- Norvienyeku, J.; Lin, L.L.; Waheed, A.; Chen, X.M.; Bao, J.D.; Aliyu, S.R.; Lin, L.Y.; Shabbir, A.; Batool, W.; Zhong, Z.H.; et al. Bayogenin 3-O-cellobioside confers non-cultivar-specific defence against the rice blast fungus Pyricularia oryzae. Plant Biotechnol. J. 2021, 19, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Okahara, F.; Suzuki, J.; Hashizume, K.; Osaki, N.; Shimotoyodome, A. Triterpene alcohols and sterols from rice bran reduce postprandial hyperglycemia in rodents and humans. Mol. Nutr. Food Res. 2016, 60, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Chakraborty, R.; Kalita, P. Rice-not just a staple food: A comprehensive review on its phytochemicals and therapeutic potential. Trends Food Sci. Technol. 2020, 97, 265–285. [Google Scholar] [CrossRef]
- Ali, M.; Ahmad, A.; Sultana, S.; Mir, S.R. Chemical constituents from the seed husks of Oryza sativa L. Nat. Prod. Res. 2022, 36, 5530–5538. [Google Scholar] [CrossRef]
- Jung, Y.J.; Park, J.-H.; Shrestha, S.; Song, M.-C.; Cho, S.; Lee, C.-H.; Han, D.; Baek, N.-I. Phytosterols from the rice (Oryza sativa) bran. J. Appl. Biol. Chem. 2014, 57, 175–178. [Google Scholar] [CrossRef]
- Ohnishi, M.; Fujino, Y. Novel glycolipids; cellobiosylsterol and cellotriosylsterol in rice bran. Agric. Biol. Chem. 1978, 42, 2423–2425. [Google Scholar]
- Ohnishi, M.; Fujino, Y. Structural study on new sterylglycosides in rice bran: Cellotetraosylsitosterol and cellopentaosylsitosterol. Agric. Biol. Chem. 1980, 44, 333–338. [Google Scholar]
- Chung, I.M.; Ali, M.; Ahmad, A.; Chun, S.-C.; Kim, J.-T.; Sultana, S.; Kim, J.-S.; Min, S.-K.; Seo, B.-R. Steroidal constituents of rice (Oryza sativa) hulls with algicidal and herbicidal activity against blue–green algae and duckweed. Phytochem. Anal. 2007, 18, 133–145. [Google Scholar] [CrossRef]
- Luang-In, V.; Yotchaisarn, M.; Somboonwatthanakul, I.; Deeseenthum, S. Bioactivities of organic riceberry broken rice and crude riceberry rice oil. J. Pharm. Anal. 2018, 42. [Google Scholar]
- Xu, Z.M.; Godber, J.S. Purification and identification of components of γ-oryzanol in rice bran oil. J. Agric. Food Chem. 1999, 47, 2724–2728. [Google Scholar] [CrossRef]
- Kumar, M.S.; Ali, K.; Dahuja, A.; Tyagi, A. Role of phytosterols in drought stress tolerance in rice. Plant Physiol. Biochem. 2015, 96, 83–89. [Google Scholar] [CrossRef]
- Ding, C.; Liu, Q.; Li, P.; Pei, Y.S.; Tao, T.T.; Wang, Y.; Yan, W.; Yang, G.F.; Shao, X.L. Distribution and quantitative analysis of phenolic compounds in fractions of Japonica and Indica rice. Food Chem. 2019, 274, 384–391. [Google Scholar] [CrossRef]
- Wang, W.; Guo, J.; Zhang, J.N.; Peng, J.; Liu, T.X.; Xin, Z.H. Isolation, identification and antioxidant activity of bound phenolic compounds present in rice bran. Food Chem. 2015, 171, 40–49. [Google Scholar] [CrossRef]
- Seal, A.N.; Haig, T.; Pratley, J.E. Evaluation of putative allelochemicals in rice root exudates for their role in the suppression of arrowhead root growth. J. Chem. Ecol. 2004, 30, 1663–1678. [Google Scholar] [CrossRef]
- Ti, H.H.; Li, Q.; Zhang, R.F.; Zhang, M.W.; Deng, Y.Y.; Wei, Z.C.; Chi, J.W.; Zhang, Y. Free and bound phenolic profiles and antioxidant activity of milled fractions of different indica rice varieties cultivated in southern China. Food Chem. 2014, 159, 166–174. [Google Scholar] [CrossRef]
- Rosado, M.J.; Rencoret, J.; Marques, G.; Gutiérrez, A.; Del Río, J.C. Structural characteristics of the guaiacyl-rich lignins from rice (Oryza sativa L.) husks and straw. Front. Plant Sci. 2021, 12, 640475. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.G.; Nakabayashi, R.; Okazaki, Y.; Mori, T.; Takamatsu, S.; Kitanaka, S.; Kikuchi, J.; Saito, K. Toward better annotation in plant metabolomics: Isolation and structure elucidation of 36 specialized metabolites from Oryza sativa (rice) by using MS/MS and NMR analyses. Metabolomics 2014, 10, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.C.; Hu, X.T.; McClements, D.J.; Luo, S.J.; Liu, C.M.; Gong, E.; Huang, K. Hydrothermal stability of phenolic extracts of brown rice. Food Chem. 2019, 271, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Zaupa, M.; Calani, L.; Del Rio, D.; Brighenti, F.; Pellegrini, N. Characterization of total antioxidant capacity and (poly) phenolic compounds of differently pigmented rice varieties and their changes during domestic cooking. Food Chem. 2015, 187, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Ryu, S.N.; Kang, S.S. A new 2-arylbenzofuran with antioxidant activity from the black colored rice (Oryza sativa L.) bran. Chem. Pharm. Bull. 2004, 52, 1365–1366. [Google Scholar] [CrossRef]
- Himeno, N.; Saburi, W.; Wakuta, S.; Takeda, R.; Matsuura, H.; Nabeta, K.; Sansenya, S.; Cairns, J.R.K.; Mori, H.; Imai, R. Identification of rice β-glucosidase with high hydrolytic activity towards salicylic acid β-D-glucoside. Biosci. Biotechnol. Biochem. 2013, 77, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Nakanishi, T.; Shimoda, H.; Nakamura, S.; Tsuruma, K.; Shimazawa, M.; Matsuda, H.; Yoshikawa, M.; Hara, H. Purple rice extract and its constituents suppress endoplasmic reticulum stress-induced retinal damage in vitro and in vivo. Life Sci. 2013, 92, 17–25. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Zhang, H.C.; Shao, Y.F.; Bao, J.S.; Beta, T. Phenolic compounds and antioxidant properties of breeding lines between the white and black rice. Food Chem. 2015, 172, 630–639. [Google Scholar] [CrossRef]
- Deng, G.F.; Xu, X.R.; Zhang, Y.; Li, D.; Gan, R.Y.; Li, H.B. Phenolic compounds and bioactivities of pigmented rice. Crit. Rev. Food Sci. 2013, 53, 296–306. [Google Scholar] [CrossRef]
- Samyor, D.; Das, A.B.; Deka, S.C. Pigmented rice a potential source of bioactive compounds: A review. Int. J. Food Sci. Technol. 2017, 52, 1073–1081. [Google Scholar] [CrossRef]
- Zhang, F.; Yang, L.M.; Huang, W.X.; Luo, X.D.; Xie, J.K.; Hu, B.L.; Chen, Y.L. Flavonoid metabolic profiles and gene mapping of rice (Oryza sativa L.) purple gradient grain hulls. Rice 2022, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Irakli, M.N.; Samanidou, V.F.; Biliaderis, C.G.; Papadoyannis, I.N. Simultaneous determination of phenolic acids and flavonoids in rice using solid-phase extraction and RP-HPLC with photodiode array detection. J. Sep. Sci. 2012, 35, 1603–1611. [Google Scholar] [CrossRef]
- Yu, X.T.; Yang, T.; Qi, Q.Q.; Du, Y.M.; Shi, J.; Liu, X.M.; Liu, Y.H.; Zhang, H.B.; Zhang, Z.F.; Yan, N. Comparison of the contents of phenolic compounds including flavonoids and antioxidant activity of rice (Oryza sativa) and Chinese wild rice (Zizania latifolia). Food Chem. 2021, 344, 128600. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Lin, F.Q.; Hasegawa, M.; Okada, K.; Nojiri, H.; Yamane, H. Purification and identification of naringenin 7-O-methyltransferase, a key enzyme in biosynthesis of flavonoid phytoalexin sakuranetin in rice. J. Biol. Chem. 2012, 287, 19315–19325. [Google Scholar] [CrossRef]
- Chung, I.M.; Park, S.-K.; Ali, M.; Prabakaran, M.; Oh, Y.-T.; Kim, S.-H.; Siddiqui, N.A.; Ahmad, A. Flavonoid glycosides from leaves and straw of Oryza sativa and their effects of cytotoxicity on a macrophage cell line and allelopathic on weed germination. Saudi Pharm. J. 2018, 26, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Kodama, O.; Miyakawa, J.; Akatsuka, T.; Kiyosawa, S. Sakuranetin, a flavanone phytoalexin from ultraviolet-irradiated rice leaves. Phytochemistry 1992, 31, 3807–3809. [Google Scholar] [CrossRef]
- Katsumata, S.; Hamana, K.; Horie, K.; Toshima, H.; Hasegawa, M. Identification of sternbin and naringenin as detoxified metabolites from the rice flavanone phytoalexin sakuranetin by Pyricularia oryzae. Chem. Biodivers. 2017, 14, e1600240. [Google Scholar] [CrossRef]
- Katsumata, S.; Toshima, H.; Hasegawa, M. Xylosylated detoxification of the rice flavonoid phytoalexin sakuranetin by the rice sheath blight fungus Rhizoctonia solani. Molecules 2018, 23, 276. [Google Scholar] [CrossRef]
- Jan, R.; Khan, M.; Asaf, S.; Lubna; Asif, S.; Kim, K.-M. Bioactivity and therapeutic potential of kaempferol and quercetin: New insights for plant and human health. Plants 2022, 11, 2623. [Google Scholar] [CrossRef]
- Kim, B.; Woo, S.; Kim, M.-J.; Kwon, S.-W.; Lee, J.; Sung, S.H.; Koh, H.-J. Identification and quantification of flavonoids in yellow grain mutant of rice (Oryza sativa L.). Food Chem. 2018, 241, 154–162. [Google Scholar] [CrossRef]
- Jeong, R.-H.; Lee, D.-Y.; Cho, J.-G.; Lee, S.-M.; Kang, H.-C.; Seo, W.-D.; Kang, H.-W.; Kim, J.-Y.; Baek, N.-I. A new flavonolignan from the aerial parts of Oryza sativa L. inhibits nitric oxide production in RAW 264.7 macrophage cells. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 865–870. [Google Scholar] [CrossRef]
- Kim, C.; Kikuchi, S.; Kim, Y.; Park, S.; Yoon, U.; Lee, G.; Choi, J.; Kim, Y.; Park, S. Computational identification of seed-specific transcription factors involved in anthocyanin production in black rice. Biochip J. 2010, 4, 247–255. [Google Scholar] [CrossRef]
- Hou, Z.H.; Qin, P.Y.; Zhang, Y.; Cui, S.H.; Ren, G.X. Identification of anthocyanins isolated from black rice (Oryza sativa L.) and their degradation kinetics. Food Res. Int. 2013, 50, 691–697. [Google Scholar] [CrossRef]
- Chen, X.Q.; Nagao, N.; Itani, T.; Irifune, K. Anti-oxidative analysis, and identification and quantification of anthocyanin pigments in different coloured rice. Food Chem. 2012, 135, 2783–2788. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Zhu, H.; Zhang, Z.Q.; Yang, S.L.; Li, H.R. Identification of anthocyanins in black rice (Oryza sativa L.) by UPLC/Q-TOF-MS and their in vitro and in vivo antioxidant activities. J. Cereal Sci. 2015, 64, 92–99. [Google Scholar] [CrossRef]
- Tamura, S.; Yan, K.; Shimoda, H.; Murakami, N. Anthocyanins from Oryza sativa L. subsp. indica. Biochem. Syst. Ecol. 2010, 38, 438–440. [Google Scholar] [CrossRef]
- Besson, E.; Dellamonica, G.; Chopin, J.; Markham, K.R.; Kim, M.; Koh, H.-S.; Fukami, H. C-glycosylflavones from Oryza sativa. Phytochemistry 1985, 24, 1061–1064. [Google Scholar] [CrossRef]
- Cho, J.-G.; Song, N.Y.; Nam, T.-G.; Shrestha, S.; Park, H.-J.; Lyu, H.-N.; Kim, D.-O.; Lee, G.; Woo, Y.-M.; Jeong, T.-S.; et al. Flavonoids from the grains of C1/R-S transgenic rice, the transgenic Oryza sativa spp. japonica, and their radical scavenging activities. J. Agric. Food Chem. 2013, 61, 10354–10359. [Google Scholar] [CrossRef]
- Yang, Z.G.; Nakabayashi, R.; Mori, T.; Takamatsu, S.; Kitanaka, S.; Saito, K. Metabolome analysis of Oryza sativa (rice) using liquid chromatography-mass spectrometry for characterizing organ specificity of flavonoids with anti-inflammatory and anti-oxidant activity. Chem. Pharm. Bull. 2016, 64, 952–956. [Google Scholar] [CrossRef]
- Ajitha, M.J.; Mohanlal, S.; Suresh, C.H.; Jayalekshmy, A. DPPH radical scavenging activity of tricin and its conjugates isolated from “njavara” rice bran: A density functional theory study. J. Agric. Food Chem. 2012, 60, 3693–3699. [Google Scholar] [CrossRef]
- Mohanlal, S.; Parvathy, R.; Shalini, V.; Helen, A.; Jayalekshmy, A. Isolation, characterization and quantification of tricin and flavonolignans in the medicinal rice njavara (Oryza sativa L.), as compared to staple varieties. Plant Food Hum. Nutr. 2011, 66, 91–96. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Mo, H.; Packer, L.; Peterson, D.M. Isolation and identification of novel tocotrienols from rice bran with hypocholesterolemic, antioxidant, and antitumor properties. J. Agric. Food Chem. 2000, 48, 3130–3140. [Google Scholar] [CrossRef]
- Sookwong, P.; Murata, K.; Nakagawa, K.; Shibata, A.; Kimura, T.; Yamaguchi, M.; Kojima, Y.; Miyazawa, T. Cross-fertilization for enhancing tocotrienol biosynthesis in rice plants and QTL analysis of their F2 progenies. J. Agric. Food Chem. 2009, 57, 4620–4625. [Google Scholar] [CrossRef] [PubMed]
- Jeong, I.-M.; Lim, Y.-H.; Ali, M.; Sultana, S.; Ahmad, A. Novel anthracene derivatives isolated from rice hulls of Oryza sativa and their growth inhibitory activity of radish seed. Bull. Korean Chem. Soc. 2006, 27, 995–1000. [Google Scholar]
- Chung, I.M.; Park, H.Y.; Chun, S.C.; Kim, J.J.; Ahmad, A. New glycosidic and other constituents from hulls of Oryza sativa. Chem. Nat. Compd. 2007, 43, 417–421. [Google Scholar] [CrossRef]
- Chen, M.-H.; McClung, A.M.; Bergman, C.J. Phenolic content, anthocyanins and antiradical capacity of diverse purple bran rice genotypes as compared to other bran colors. J. Cereal Sci. 2017, 77, 110–119. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.-Å. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef] [PubMed]
- Aboshi, T.; Iitsuka, C.; Galis, I.; Teraishi, M.; Kamo, M.; Nishimura, A.; Ishihara, A.; Mori, N.; Murayama, T. Isopentylamine is a novel defence compound induced by insect feeding in rice. Plant Cell Environ. 2021, 44, 247–256. [Google Scholar] [CrossRef]
- Morimoto, N.; Ueno, K.; Teraishi, M.; Okumoto, Y.; Mori, N.; Ishihara, A. Induced phenylamide accumulation in response to pathogen infection and hormone treatment in rice (Oryza sativa). Biosci. Biotechnol. Biochem. 2018, 82, 407–416. [Google Scholar] [CrossRef]
- Park, H.L.; Yoo, Y.; Hahn, T.-R.; Bhoo, S.H.; Lee, S.-W.; Cho, M.H. Antimicrobial activity of UV-induced phenylamides from rice leaves. Molecules 2014, 19, 18139–18151. [Google Scholar] [CrossRef]
- Wang, W.W.; Yu, Z.X.; Meng, J.P.; Zhou, P.Y.; Luo, T.; Zhang, J.; Wu, J.; Lou, Y.G. Rice phenolamindes reduce the survival of female adults of the white-backed planthopper Sogatella furcifera. Sci. Rep. 2020, 10, 5778. [Google Scholar] [CrossRef] [PubMed]
- Alamgir, K.M.; Hojo, Y.; Christeller, J.T.; Fukumoto, K.; Isshiki, R.; Shinya, T.; Baldwin, I.T.; Galis, I. Systematic analysis of rice (Oryza sativa) metabolic responses to herbivory. Plant Cell Environ. 2016, 39, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Buttery, R.G.; Ling, L.C.; Juliano, B.O.; Turnbaugh, J.G. Cooked rice aroma and 2-acetyl-1-pyrroline. J. Agric. Food Chem. 1983, 31, 823–826. [Google Scholar] [CrossRef]
- Kinashi, H.; Suzuki, Y.; Takeuchi, S.; Kawarada, A. Possible metabolic intermediates from IAA to β-acid in rice bran. Agric. Biol. Chem. 1976, 40, 2465–2470. [Google Scholar]
- Suzuki, Y.; Kinashi, H.; Takeuchi, S.; Kawarada, A. (+)-5-Hydroxy-dioxindole-3-acetic acid, a synergist from rice bran of auxin-induced ethylene production in plant tissue. Phytochemistry 1977, 16, 635–637. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kamiya, N.; Morinaka, Y.; Matsuoka, M.; Sazuka, T. Auxin biosynthesis by the YUCCA genes in rice. Plant Physiol. 2007, 143, 1362–1371. [Google Scholar] [CrossRef]
- Ishihara, A.; Hashimoto, Y.; Miyagawa, H.; Wakasa, K. Induction of serotonin accumulation by feeding of rice striped stem borer in rice leaves. Plant Signal. Behav. 2008, 3, 714–716. [Google Scholar] [CrossRef]
- Nakano, H.; Ono, H.; Kaji, R.; Sakai, M.; Doi, S.; Kosemura, S. Oryzadiamines A and B, alkaloids from Oryza sativa with yellow grain. Tetrahedron Lett. 2020, 61, 151519. [Google Scholar] [CrossRef]
- Tadera, K.; Orite, K. Isolation and structure of a new vitamin B6 conjugate in rice bran. J. Food Sci. 1991, 56, 268–269. [Google Scholar] [CrossRef]
- Nakano, H.; Kosemura, S.; Suzuki, T.; Hirose, K.; Kaji, R.; Sakai, M. Oryzamutaic acid A, a novel yellow pigment from an Oryza sativa mutant with yellow endosperm. Tetrahedron Lett. 2009, 50, 2003–2005. [Google Scholar] [CrossRef]
- Nakano, H.; Kosemura, S.; Yoshida, M.; Suzuki, T.; Iwaura, R.; Kaji, R.; Sakai, M.; Hirose, K. Oryzamutaic acids B–G, new alkaloids from an Oryza sativa mutant with yellow endosperm. Tetrahedron Lett. 2010, 51, 49–53. [Google Scholar] [CrossRef]
- Nakano, H.; Kosemura, S.; Yoshida, M.; Iwaura, R.; Suzuki, T.; Kaji, R.; Sakai, M. Oryzamutaic acids H–J, new alkaloids from an Oryza sativa mutant with yellow endosperm. Tetrahedron Lett. 2010, 51, 4953–4956. [Google Scholar] [CrossRef]
- Kaikavoosi, K.; Kad, T.D.; Zanan, R.L.; Nadaf, A.B. 2-Acetyl-1-pyrroline augmentation in scented indica rice (Oryza sativa L.) varieties through Δ1-pyrroline-5-carboxylate synthetase (P5CS) gene transformation. Appl. Biochem. Biotechnol. 2015, 177, 1466–1479. [Google Scholar] [CrossRef]
- Tanaka, K.; Taniguchi, S.; Tamaoki, D.; Yoshitomi, K.; Akimitsu, K.; Gomi, K. Multiple roles of plant volatiles in jasmonate-induced defense response in rice. Plant Signal. Behav. 2014, 9, e29247. [Google Scholar] [CrossRef]
- Fujino, Y.; Ohnishi, M. Constituents of ceramide and ceramide monohexoside in rice bran. Chem. Phys. Lipids 1976, 17, 275–289. [Google Scholar] [CrossRef]
- Chung, I.M.; Ali, M.; Chun, S.-C.; Jin, C.W.; Cho, D.H.; Hong, S.-B.; Ahmad, A. New aliphatic alcohol and ester constituents from rice hulls of Oryza sativa. Chin. J. Chem. 2007, 25, 843–848. [Google Scholar] [CrossRef]
- Ahmad, A.; Yoon, J.; Chung, I. Chemical constituents from the rice straw of Oryza sativa. Asian J. Chem. 2013, 25, 9872–9874. [Google Scholar] [CrossRef]
- Chung, I.M.; Ali, M.; Chun, S.-C.; Lee, O.-K.; Ahmad, A. Sativalanosteronyl glycoside and oryzatriacontolide constituents from the Hulls of Oryza sativa. Asian J. Chem. 2007, 19, 1535. [Google Scholar]
- Miyazawa, M.; Nagai, S.; Oshima, T. Volatile components of the straw of Oryza sativa L. J. Oleo Sci. 2008, 57, 139–143. [Google Scholar] [CrossRef]
- Chung, I.M.; Ali, M.; Ahmad, A. Dicyclohexanyl orizane constituent from the hulls of Oryza sativa and its inhibitory activity. Asian J. Chem. 2005, 17, 2616. [Google Scholar]
- Guo, H.M.; Li, H.C.; Zhou, S.R.; Xue, H.W.; Miao, X.X. cis-12-Oxo-phytodienoic acid stimulates rice defense response to a piercing-sucking insect. Mol. Plant 2014, 7, 1683–1692. [Google Scholar] [CrossRef]
- Lee, T.K.; Lee, D.; Yu, J.S.; Jo, M.S.; Baek, S.C.; Shin, M.-S.; Ko, Y.-J.; Kang, K.S.; Kim, K.H. Biological evaluation of a new lignan from the roots of rice (Oryza sativa). Chem. Biodivers. 2018, 15, e1800333. [Google Scholar] [CrossRef]
- Wang, Q.; Xin, Z.J.; Li, J.C.; Hu, L.F.; Lou, Y.G.; Lu, J. (E)-β-Caryophyllene functions as a host location signal for the rice white-backed planthopper Sogatella furcifera. Physiol. Mol. Plant Pathol. 2015, 91, 106–112. [Google Scholar] [CrossRef]
- Yuan, J.S.; Köllner, T.G.; Wiggins, G.; Grant, J.; Degenhardt, J.; Chen, F. Molecular and genomic basis of volatile-mediated indirect defense against insects in rice. Plant J. 2008, 55, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.H.; Matsuo, A.; Oku, Y.; Tebayashi, S.-i.; Kim, C.-S. Studies on the probing stimulants for the white-backed planthopper, Sogatella furcifera (Homoptera: Delphacidae) in rice plant (Oryza sativa L.). Biosci. Biotechnol. Biochem. 2016, 80, 2285–2290. [Google Scholar] [CrossRef] [PubMed]
- Kanno, H.; Hasegawa, M.; Kodama, O. Accumulation of salicylic acid, jasmonic acid and phytoalexins in rice, Oryza sativa, infested by the white-backed planthopper, Sogatella furcifera (Hemiptera: Delphacidae). Appl. Entomol. Zool. 2012, 47, 27–34. [Google Scholar] [CrossRef]
- Li, C.Y.; Luo, C.; Zhou, Z.H.; Wang, R.; Ling, F.; Xiao, L.T.; Lin, Y.J.; Chen, H. Gene expression and plant hormone levels in two contrasting rice genotypes responding to brown planthopper infestation. BMC Plant Biol. 2017, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Zhang, W.L.; Liu, B.F.; Hu, J.; Wei, Z.; Shi, Z.Y.; He, R.F.; Zhu, L.L.; Chen, R.Z.; Han, B. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc. Natl. Acad. Sci. USA 2009, 106, 22163–22168. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Wu, Y.; Wu, D.; Rao, W.W.; Guo, J.P.; Ma, Y.H.; Wang, Z.Z.; Shangguan, X.X.; Wang, H.Y.; Xu, C.X. The coiled-coil and nucleotide binding domains of BROWN PLANTHOPPER RESISTANCE14 function in signaling and resistance against planthopper in rice. Plant Cell 2017, 29, 3157–3185. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Jan, R.; Asaf, S.; Khan, A.L.; Bilal, S.; Kim, K.-M.; Al-Harrasi, A. Genome and transcriptome-wide analysis of OsWRKY and OsNAC gene families in Oryza sativa and their response to white-backed planthopper infestation. Int. J. Mol. Sci. 2022, 23, 15396. [Google Scholar] [CrossRef]
- Valea, I.; Motegi, A.; Kawamura, N.; Kawamoto, K.; Miyao, A.; Ozawa, R.; Takabayashi, J.; Gomi, K.; Nemoto, K.; Nozawa, A. The rice wound-inducible transcription factor RERJ1 sharing same signal transduction pathway with OsMYC2 is necessary for defense response to herbivory and bacterial blight. Plant Mol. Biol. 2022, 109, 651–666. [Google Scholar] [CrossRef]
- Park, S.; Choi, M.J.; Lee, J.Y.; Kim, J.K.; Ha, S.-H.; Lim, S.-H. Molecular and biochemical analysis of two rice flavonoid 3′-hydroxylase to evaluate their roles in flavonoid biosynthesis in rice grain. Int. J. Mol. Sci. 2016, 17, 1549. [Google Scholar] [CrossRef] [PubMed]
- Lam, P.Y.; Liu, H.J.; Lo, C. Completion of tricin biosynthesis pathway in rice: Cytochrome P450 75B4 is a unique chrysoeriol 5′-hydroxylase. Plant Physiol. 2015, 168, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Yamane, H. Biosynthesis of phytoalexins and regulatory mechanisms of it in rice. Biosci. Biotechnol. Biochem. 2013, 77, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Miyamoto, K.; Nemoto, K.; Sawasaki, T.; Yamane, H.; Nojiri, H.; Okada, K. OsMYC2, an essential factor for JA-inductive sakuranetin production in rice, interacts with MYC2-like proteins that enhance its transactivation ability. Sci. Rep. 2017, 7, 40175. [Google Scholar] [CrossRef] [PubMed]
- Pazini, J.D.; Martins, J.F.D.; Dorneles, K.D.; Crizel, R.L.; Da Silva, F.F.; Chaves, F.C.; Fernando, J.A.; Dallagnol, L.J.; Seidel, E.J.; Stout, M.J. Morphoanatomical and biochemical factors associated with rice resistance to the South American rice water weevil, Oryzophagus oryzae (Coleoptera: Curculionidae). Sci. Rep. 2022, 12, 22480. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Asaf, S.; Lubna; Asif, S.; Kim, E.-G.; Jang, Y.-H.; Kim, N.; Al-Harrasi, A.; Lee, G.-S.; Kim, K.-M. Enhancing the expression of the OsF3H gene in Oryza sativa leads to the regulation of multiple biosynthetic pathways and transcriptomic changes that influence insect resistance. Int. J. Mol. Sci. 2022, 23, 15308. [Google Scholar] [CrossRef] [PubMed]
- Jan, R.; Khan, M.A.; Asaf, S.; Lee, I.J.; Kim, K.M. Overexpression of OsF3H modulates WBPH stress by alteration of phenylpropanoid pathway at a transcriptomic and metabolomic level in Oryza sativa. Sci. Rep. 2020, 10, 14685. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, B.; Yan, S.; Li, Y.; Xiao, H.; Li, Y.; Zhang, Y. Evaluation of tricin, a stylet probing stimulant of brown planthopper, in infested and non-infested rice plants. J. Appl. Entomol. 2017, 141, 393–401. [Google Scholar] [CrossRef]
- Chen, S.; Sun, B.; Shi, Z.Y.; Miao, X.X.; Li, H.C. Identification of the rice genes and metabolites involved in dual resistance against brown planthopper and rice blast fungus. Plant Cell Environ. 2022, 45, 1914–1929. [Google Scholar] [CrossRef]
- Bing, L.; Xia, D.H.; Xin, Z.M.; Di, X.; Shu, W.J. Potential resistance of tricin in rice against brown planthopper Nilaparvata lugens (Stål). Acta Ecol. Sin. 2007, 27, 1300–1306. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Cui, B.Y.; Zhang, Y. Electrical penetration graphs indicate that tricin is a key secondary metabolite of rice, inhibiting phloem feeding of brown planthopper, Nilaparvata lugens. Entomol. Exp. Appl. 2015, 156, 14–27. [Google Scholar] [CrossRef]
- Adjei-Afriyie, F.; Kim, C.-S.; Takemura, M.; Ishikawa, M.; Tebayashi, S.-i.; Horiike, M. Probing stimulants from the rice plant towards the smaller brown planthopper, Laodelphax striatellus (Fallen)(Homoptera: Delphacidae). Z. Naturforsch. C 2000, 55, 1038–1044. [Google Scholar] [CrossRef]
- Thomas, J.C.; Adams, D.G.; Nessler, C.L.; Brown, J.K.; Bohnert, H.J. Tryptophan decarboxylase, tryptamine, and reproduction of the whitefly. Plant Physiol. 1995, 109, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.I.; Ellis, B.E.; Isman, M.B. Tryptamine-induced resistance in tryptophan decarboxylase transgenic poplar and tobacco plants against their specific herbivores. J. Chem. Ecol. 2003, 29, 779–793. [Google Scholar] [CrossRef]
- Gao, H.L.; Zou, J.Z.; Lin, X.M.; Zhang, H.H.; Yu, N.; Liu, Z.W. Nilaparvata lugens salivary protein NlG14 triggers defense response in plants. J. Exp. Bot. 2022, 73, 7477–7487. [Google Scholar] [CrossRef] [PubMed]
- Shangguan, X.X.; Zhang, J.; Liu, B.F.; Zhao, Y.; Wang, H.Y.; Wang, Z.Z.; Guo, J.P.; Rao, W.W.; Jing, S.L.; Guan, W.; et al. A mucin-like protein of planthopper is required for feeding and induces immunity response in plants. Plant Physiol. 2018, 176, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.M.; Ye, W.F.; Hu, W.H.; Jin, X.C.; Kuai, P.; Xiao, W.H.; Jian, Y.K.; Turlings, T.C.; Lou, Y.G. The N-terminal subunit of vitellogenin in planthopper eggs and saliva acts as a reliable elicitor that induces defenses in rice. New Phytol. 2022, 238, 1230–1244. [Google Scholar] [CrossRef]
- Takeda, Y.; Koshiba, T.; Tobimatsu, Y.; Suzuki, S.; Murakami, S.; Yamamura, M.; Rahman, M.M.; Takano, T.; Hattori, T.; Sakamoto, M. Regulation of CONIFERALDEHYDE 5-HYDROXYLASE expression to modulate cell wall lignin structure in rice. Planta 2017, 246, 337–349. [Google Scholar] [CrossRef]
- Lu, H.P.; Luo, T.; Fu, H.W.; Wang, L.; Tan, Y.Y.; Huang, J.Z.; Wang, Q.; Ye, G.Y.; Gatehouse, A.M.; Lou, Y.G. Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nat. Plants 2018, 4, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Hori, M.; Enya, S. Attractiveness of synthetic volatile blends of flowering rice panicles to Trigonotylus caelestialium (Kirkaldy)(Heteroptera: Miridae). J. Appl. Entomol. 2013, 137, 97–103. [Google Scholar] [CrossRef]
- Ye, W.F.; Yu, H.X.; Jian, Y.K.; Zeng, J.M.; Ji, R.; Chen, H.D.; Lou, Y.G. A salivary EF-hand calcium-binding protein of the brown planthopper Nilaparvata lugens functions as an effector for defense responses in rice. Sci. Rep. 2017, 7, 40498. [Google Scholar] [CrossRef]
- Ji, R.; Ye, W.F.; Chen, H.D.; Zeng, J.M.; Li, H.; Yu, H.X.; Li, J.C.; Lou, Y.G. A salivary endo-β-1, 4-glucanase acts as an effector that enables the brown planthopper to feed on rice. Plant Physiol. 2017, 173, 1920–1932. [Google Scholar] [CrossRef] [PubMed]
- Gong, G.; Yuan, L.Y.; Li, Y.F.; Xiao, H.X.; Li, Y.F.; Zhang, Y.; Wu, W.J.; Zhang, Z.F. Salivary protein 7 of the brown planthopper functions as an effector for mediating tricin metabolism in rice plants. Sci. Rep. 2022, 12, 3205. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.M.; Shi, Y.; Wang, L.; Zhang, H.; Li, J.; Fang, J.C.; Ji, R. Planthopper-secreted salivary disulfide isomerase activates immune responses in plants. Front. Plant Sci. 2021, 11, 622513. [Google Scholar] [CrossRef] [PubMed]
- Bussaban, B.; Lumyong, S.; Lumyong, P.; Seelanan, T.; Park, D.; McKenzie, E.; Hyde, K. Molecular and morphological characterization of Pyricularia and allied genera. Mycologia 2005, 97, 1002–1011. [Google Scholar] [CrossRef]
- Watanabe, M.; Kono, Y.; Watanabe, M.; Uzawa, J.; Teraoka, T.; Hosokawa, D.; Suzuki, Y.; Sakurai, A.; Teraguchi, M. Structures of oryzalic acid B and three related compounds, a group of novel antibacterial diterpenes, isolated from leaves of a bacterial leaf blight-resistant cultivar of rice. Biosci. Biotechnol. Biochem. 1992, 56, 113–117. [Google Scholar] [CrossRef]
- Watanabe, M.; Sakai, Y.; Teraoka, T.; Abe, H.; Kono, Y.; Uzawa, J.; Kobayashi, K.; Suzuki, Y.; Sakurai, A. Novel C19-kaurane type of diterpene (oryzalide A), a new antimicrobial compound isolated from healthy leaves of a bacterial leaf blight-resistant cultivar of rice plant. Agric. Biol. Chem. 1990, 54, 1103–1105. [Google Scholar] [CrossRef]
- Kono, Y.; Uzawa, J.; Kobayashi, K.; Suzuki, Y.; Uramoto, M.; Sakurai, A.; Watanabe, M.; Teraoka, T.; Hosokawa, D.; Watanabe, M.; et al. Structures of oryzalides A and B, and oryzalic acid A, a group of novel antimicrobial diterpenes, isolated from healthy leaves of a bacterial leaf blight-resistant cultivar of rice plant. Agric. Biol. Chem. 1991, 55, 803–811. [Google Scholar] [CrossRef]
- Lerma-García, M.; Herrero-Martínez, J.; Simó-Alfonso, E.; Mendonça, C.R.; Ramis-Ramos, G. Composition, industrial processing and applications of rice bran γ-oryzanol. Food Chem. 2009, 115, 389–404. [Google Scholar] [CrossRef]
- Wang, Q.; Quan, S.; Xiao, H. Towards efficient terpenoid biosynthesis: Manipulating IPP and DMAPP supply. Bioresour. Bioprocess. 2019, 6, 6. [Google Scholar] [CrossRef]
- Swaminathan, S.; Morrone, D.; Wang, Q.; Fulton, D.B.; Peters, R. CYP76M7 is an ent-cassadiene C11α-hydroxylase defining a second multifunctional diterpenoid biosynthetic gene cluster in rice. Plant Cell 2009, 21, 3315–3325. [Google Scholar] [CrossRef]
- Kono, Y.; Takeuchi, S.; Kodama, O.; Sekido, H.; Akatsuka, T. Novel phytoalexins (oryzalexins A, B and C) isolated from rice blast leaves infected with Pyricularia oryzae. Part II: Structural studies of oryzalexins. Agric. Biol. Chem. 1985, 49, 1695–1701. [Google Scholar] [CrossRef]
- Sekido, H.; Endo, T.; Suga, T.; Kodama, O.; Akatsuka, T.; Kono, Y.; Takeuchi, S. Oryzalexin D (3, 7-dihydroxy-(+)-sandaracopimaradiene), a new phytoalexin isolated from blast-infected rice leaves. J. Pestic. Sci. 1986, 11, 369–372. [Google Scholar] [CrossRef]
- Sekido, H.; Akatsuka, T. Mode of action of oryzalexin D against Pyricularia oryzae. Agric. Biol. Chem. 1987, 51, 1967–1971. [Google Scholar] [CrossRef]
- Chen, X.J.; Chen, H.; Yuan, J.S.; Köllner, T.G.; Chen, Y.Y.; Guo, Y.F.; Zhuang, X.F.; Chen, X.L.; Zhang, Y.J.; Fu, J.Y. The rice terpene synthase gene OsTPS19 functions as an (S)-limonene synthase in planta, and its overexpression leads to enhanced resistance to the blast fungus Magnaporthe oryzae. Plant Biotechnol. J. 2018, 16, 1778–1787. [Google Scholar] [CrossRef]
- Cartwright, D.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Chemical activation of host defence mechanisms as a basis for crop protection. Nature 1977, 267, 511–513. [Google Scholar] [CrossRef]
- Rocha, M.F.G.; Sales, J.A.; da Rocha, M.G.; Galdino, L.M.; de Aguiar, L.; Pereira-Neto, W.D.; Cordeiro, R.D.; Castelo-Branco, D.; Sidrim, J.J.C.; Brilhante, R.S.N. Antifungal effects of the flavonoids kaempferol and quercetin: A possible alternative for the control of fungal biofilms. Biofouling 2019, 35, 320–328. [Google Scholar] [CrossRef]
- Kong, C.H.; Xu, X.H.; Zhou, B.; Hu, F.; Zhang, C.X.; Zhang, M.X. Two compounds from allelopathic rice accession and their inhibitory activity on weeds and fungal pathogens. Phytochemistry 2004, 65, 1123–1128. [Google Scholar] [CrossRef]
- Hasegawa, M.; Mitsuhara, I.; Seo, S.; Okada, K.; Yamane, H.; Iwai, T.; Ohashi, Y. Analysis on blast fungus-responsive characters of a flavonoid phytoalexin sakuranetin; accumulation in infected rice leaves, antifungal activity and detoxification by fungus. Molecules 2014, 19, 11404–11418. [Google Scholar] [CrossRef]
- Ishihara, A.; Fukami, A.; Matsuda, Y.; Nakajima, H.; Miyagawa, H. Accumulation of indole-3-acetic acid in rice sl mutant leaves infected with Bipolaris oryzae. J. Phytopathol. 2016, 164, 509–519. [Google Scholar] [CrossRef]
- Ishihara, A.; Hashimoto, Y.; Tanaka, C.; Dubouzet, J.G.; Nakao, T.; Matsuda, F.; Nishioka, T.; Miyagawa, H.; Wakasa, K. The tryptophan pathway is involved in the defense responses of rice against pathogenic infection via serotonin production. Plant J. 2008, 54, 481–495. [Google Scholar] [CrossRef]
- Furutani, A.; Tsuge, S.; Oku, T.; Tsuno, K.; Inoue, Y.; Ochiai, H.; Kaku, H.; Kubo, Y. Hpa1 secretion via type III secretion system in Xanthomonas oryzae pv. oryzae. J. Gen. Plant Pathol. 2003, 69, 271–275. [Google Scholar] [CrossRef]
- Fan, S.S.; Tian, F.; Li, J.Y.; Hutchins, W.; Chen, H.M.; Yang, F.H.; Yuan, X.C.; Cui, Z.N.; Yang, C.H.; He, C.Y. Identification of phenolic compounds that suppress the virulence of Xanthomonas oryzae on rice via the type III secretion system. Mol. Plant Pathol. 2017, 18, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Ming, D.; Wang, D.C.; Cao, F.J.; Xiang, H.; Mu, D.; Cao, J.J.; Li, B.B.; Zhong, L.; Dong, X.Y.; Zhong, X.B.; et al. Kaempferol inhibits the primary attachment phase of biofilm formation in Staphylococcus aureus. Front. Microbiol. 2017, 8, 2263. [Google Scholar] [CrossRef]
- Nasir, F.; Shi, S.H.; Tian, L.; Chang, C.L.; Ma, L.; Li, X.J.; Gao, Y.Z.; Tian, C.J. Strigolactones shape the rhizomicrobiome in rice (Oryza sativa). Plant Sci. 2019, 286, 118–133. [Google Scholar] [CrossRef]
- Li, Y.Z.; Jian, X.; Li, Y.; Zeng, X.M.; Xu, L.N.; Khan, M.U.; Lin, W.X. OsPAL2-1 mediates allelopathic interactions between rice and specific microorganisms in the rhizosphere ecosystem. Front. Microbiol. 2020, 11, 1411. [Google Scholar] [CrossRef]
- Fang, C.X.; Zhuang, Y.E.; Xu, T.C.; Li, Y.Z.; Li, Y.; Lin, W.X. Changes in rice allelopathy and rhizosphere microflora by inhibiting rice phenylalanine ammonia-lyase gene expression. J. Chem. Ecol. 2013, 39, 204–212. [Google Scholar] [CrossRef]
- Chamam, A.; Sanguin, H.; Bellvert, F.; Meiffren, G.; Comte, G.; Wisniewski-Dyé, F.; Bertrand, C.; Prigent-Combaret, C. Plant secondary metabolite profiling evidences strain-dependent effect in the Azospirillum–Oryza sativa association. Phytochemistry 2013, 87, 65–77. [Google Scholar] [CrossRef]
- Mishra, R.P.N.; Singh, R.K.; Jaiswal, H.K.; Kumar, V.; Maurya, S. Rhizobium-mediated induction of phenolics and plant growth promotion in rice (Oryza sativa L.). Curr. Microbiol. 2006, 52, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Ino, T. Rice seedlings release momilactone B into the environment. Phytochemistry 2003, 63, 551–554. [Google Scholar] [CrossRef]
- Xu, M.M.; Galhano, R.; Wiemann, P.; Bueno, E.; Tiernan, M.; Wu, W.; Chung, I.M.; Gershenzon, J.; Tudzynski, B.; Sesma, A. Genetic evidence for natural product-mediated plant-plant allelopathy in rice (Oryza sativa). New Phytol. 2012, 193, 570–575. [Google Scholar] [CrossRef]
- Thi, H.L.; Lin, C.H.; Smeda, R.J.; Leigh, N.D.; Wycoff, W.G.; Fritschi, F.B. Isolation and identification of an allelopathic phenylethylamine in rice. Phytochemistry 2014, 108, 109–121. [Google Scholar] [CrossRef]
- Kong, C.H.; Li, H.B.; Hu, F.; Xu, X.H.; Wang, P. Allelochemicals released by rice roots and residues in soil. Plant Soil 2006, 288, 47–56. [Google Scholar] [CrossRef]
- Rimando, A.M.; Olofsdotter, M.; Dayan, F.E.; Duke, S.O. Searching for rice allelochemicals: An example of bioassay-guided isolation. Agron. J. 2001, 93, 16–20. [Google Scholar] [CrossRef]
- Kong, C.H.; Zhao, H.; Xu, X.H.; Wang, P.; Gu, Y. Activity and allelopathy of soil of flavone O-glycosides from rice. J. Agric. Food Chem. 2007, 55, 6007–6012. [Google Scholar] [CrossRef]
- Zhu, Q.; Tang, M.J.; Yang, Y.; Sun, K.; Tian, L.S.; Lu, F.; Hao, A.Y.; Dai, C.C. Endophytic fungus Phomopsis liquidambaris B3 induces rice resistance to RSRD caused by Fusarium proliferatum and promotes plant growth. J. Sci. Food Agric. 2021, 101, 4059–4075. [Google Scholar] [CrossRef]
- Saravanakumar, D.; Lavanya, N.; Muthumeena, K.; Raguchander, T.; Samiyappan, R. Fluorescent pseudomonad mixtures mediate disease resistance in rice plants against sheath rot (Sarocladium oryzae) disease. Biocontrol 2009, 54, 273–286. [Google Scholar] [CrossRef]
- Hata, E.M.; Yusof, M.T.; Zulperi, D. Induction of systemic resistance against bacterial leaf streak disease and growth promotion in rice plant by Streptomyces shenzhenesis TKSC3 and Streptomyces sp. SS8. Plant Pathol. 2021, 37, 173. [Google Scholar] [CrossRef]
- Khanh, T.; Chung, M.; Xuan, T.; Tawata, S. The exploitation of crop allelopathy in sustainable agricultural production. J. Agron. Crop Sci. 2005, 191, 172–184. [Google Scholar] [CrossRef]
- Kong, C.H.; Liang, W.J.; Xu, X.H.; Hu, F.; Wang, P.; Jiang, Y. Release and activity of allelochemicals from allelopathic rice seedlings. J. Agric. Food Chem. 2004, 52, 2861–2865. [Google Scholar] [CrossRef] [PubMed]
| Category | No. | Family | Name | Site of Infection/Herbivory | Degree of Harm * |
|---|---|---|---|---|---|
| Herbivores | 1 | Delphacidae | Nilaparvata lugens | Xylem, phloem tissues | +++ |
| 2 | Sogatella furcifera | Xylem, phloem tissues | +++ | ||
| 3 | Laodelphax striatellus | Leaves, stem | + | ||
| 4 | Miridae | Trigonotylus caelestialium | Shoots | ++ | |
| 5 | Pyralidae | Chilo suppressalis | Leaves | ++ | |
| 6 | Aleyrodidae | Bemisia tabaci | Leaves | ++ | |
| 7 | Lasiocampidae | Malacosoma disstria | Leaves | + | |
| 8 | Sphingidae | Manduca sexta | Leaves, stem | + | |
| 9 | Mythimna loreyi | Leaves | ++ | ||
| 10 | Curculionidae | Oryzophagus oryzae | Leaves, stem | ++ | |
| Pathogens | 1 | Agonomycetaceae | Rhizoctonia solani | Culms, sheath | +++ |
| 2 | Pyriculariaceae | Magnaporthe grisea/Pyricularia grisea | Leaves, nodes, stems, panicles, roots | +++ | |
| 3 | Magnaporthe oryzae/Pyricularia oryzae | Leaves, nodes, stems, panicles, roots | +++ | ||
| 4 | Pleosporaceae | Cochliobolus miyabeanus/Bipolaris oryzae | Leaves | +++ | |
| 5 | Dematiaceae | Helminthosporium oryzae | Grain | +++ | |
| 6 | Agonomycetaceae | Sclerotium rolfsii | Stem | + | |
| 7 | Tuberculariaceae | Fusarium proliferatum | Spikelet | +++ | |
| 8 | Hypocreales | Sarocladium oryzae | Leaf sheath | ++ | |
| 9 | Botryosphaeriaceae | Macrophomina phaseolina | Stem | ++ | |
| 10 | Burkholderiaceae | Burkholderia glumae | Husk | ++ | |
| 11 | Pseudomonadaceae | Xanthomonas oryzae pv. oryzae (Xoo) | Leaves | +++ | |
| 12 | X. oryzae pv. oryzicola (Xoc) | Leaves | ++ | ||
| 13 | X. campestris pv. oryzae | Leaves | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, H.; Zhang, J.; Bai, L.; Liu, J.; Li, H.; Hua, J.; Luo, S. Chemical Structure Diversity and Extensive Biological Functions of Specialized Metabolites in Rice. Int. J. Mol. Sci. 2023, 24, 17053. https://doi.org/10.3390/ijms242317053
Zhou H, Zhang J, Bai L, Liu J, Li H, Hua J, Luo S. Chemical Structure Diversity and Extensive Biological Functions of Specialized Metabolites in Rice. International Journal of Molecular Sciences. 2023; 24(23):17053. https://doi.org/10.3390/ijms242317053
Chicago/Turabian StyleZhou, Huiwen, Jinjin Zhang, Liping Bai, Jiayi Liu, Hongdi Li, Juan Hua, and Shihong Luo. 2023. "Chemical Structure Diversity and Extensive Biological Functions of Specialized Metabolites in Rice" International Journal of Molecular Sciences 24, no. 23: 17053. https://doi.org/10.3390/ijms242317053






