Examining the Potential of Vitamin C Supplementation in Tissue-Engineered Equine Superficial Digital Flexor Tendon Constructs
Abstract
:1. Introduction
2. Results
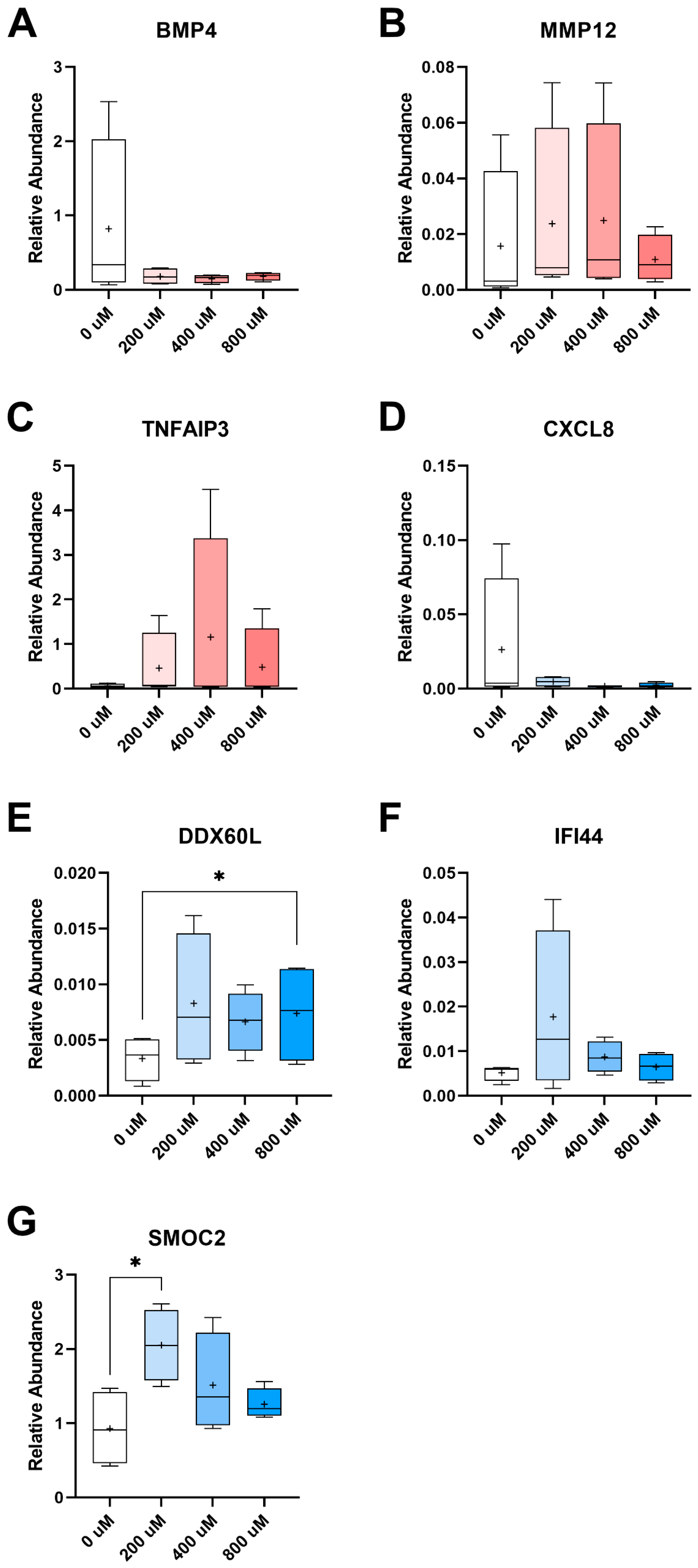
2.1. Transcriptomic Assessment of Vitamin C Supplementation
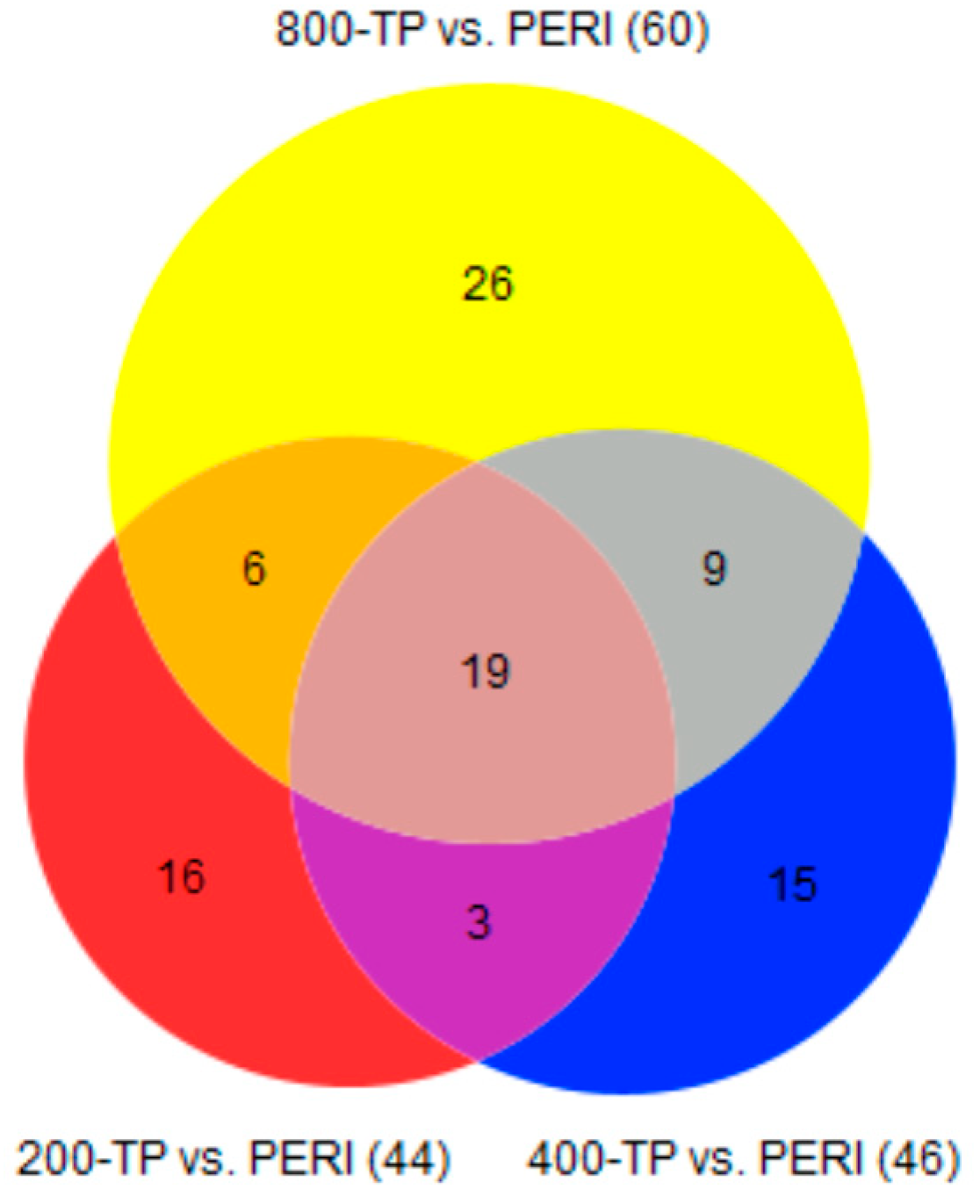
2.2. Comparisons of Transcriptomic Profiles of TP versus PERI-Cell-Derived Constructs
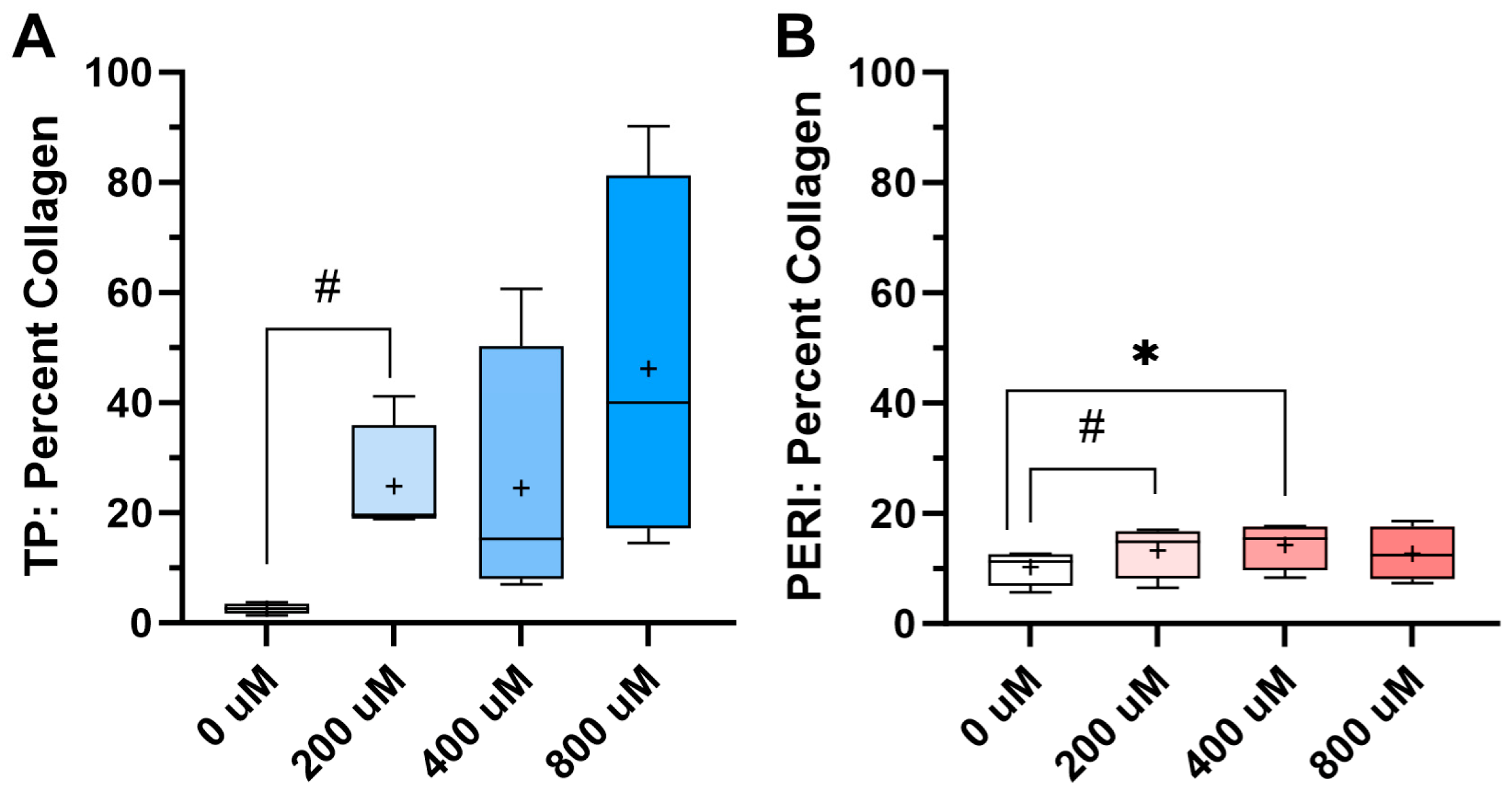
2.3. Collagen Content
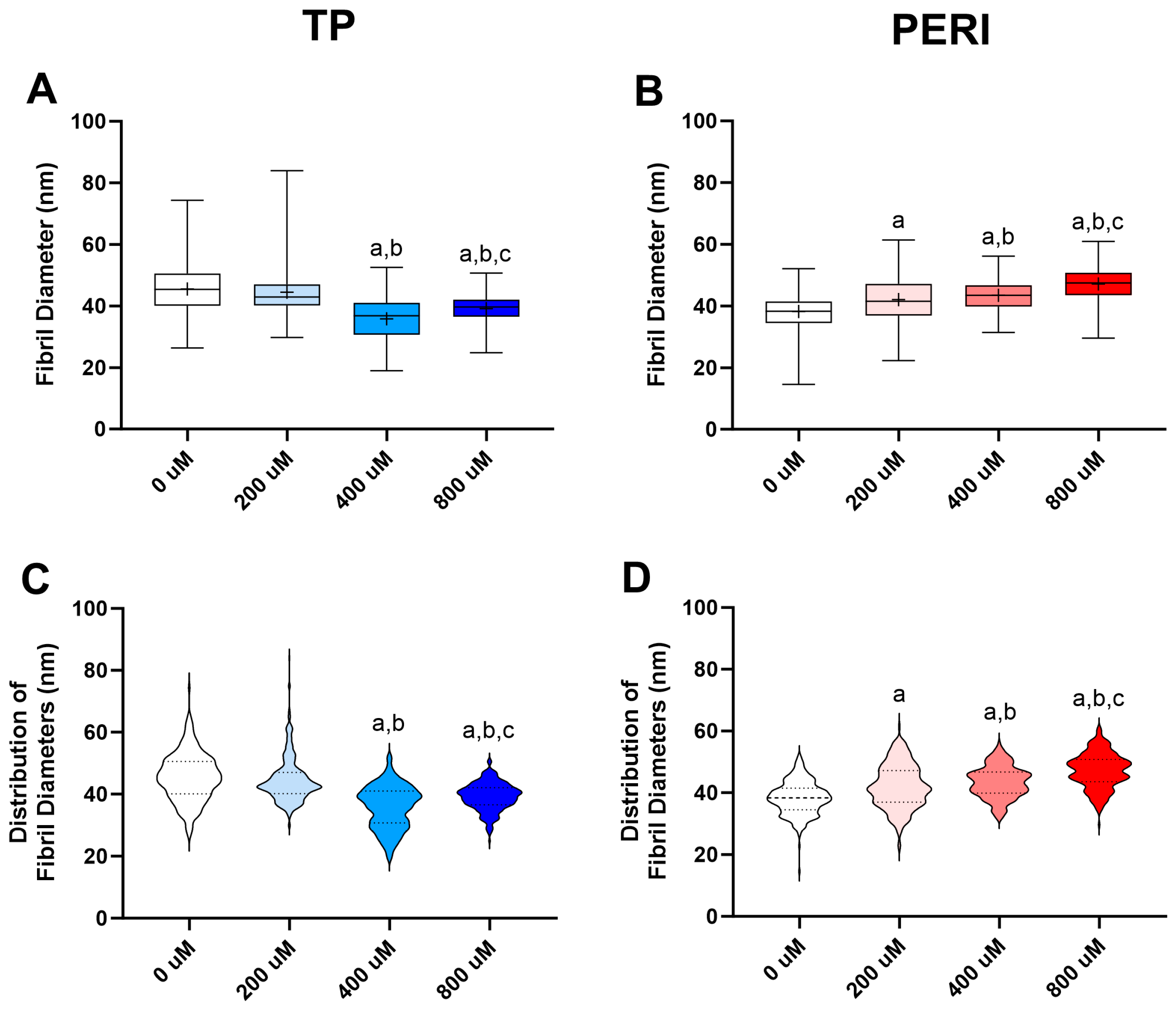
2.4. Collagen Fibril Diameters from Transmission Electron Microscopy
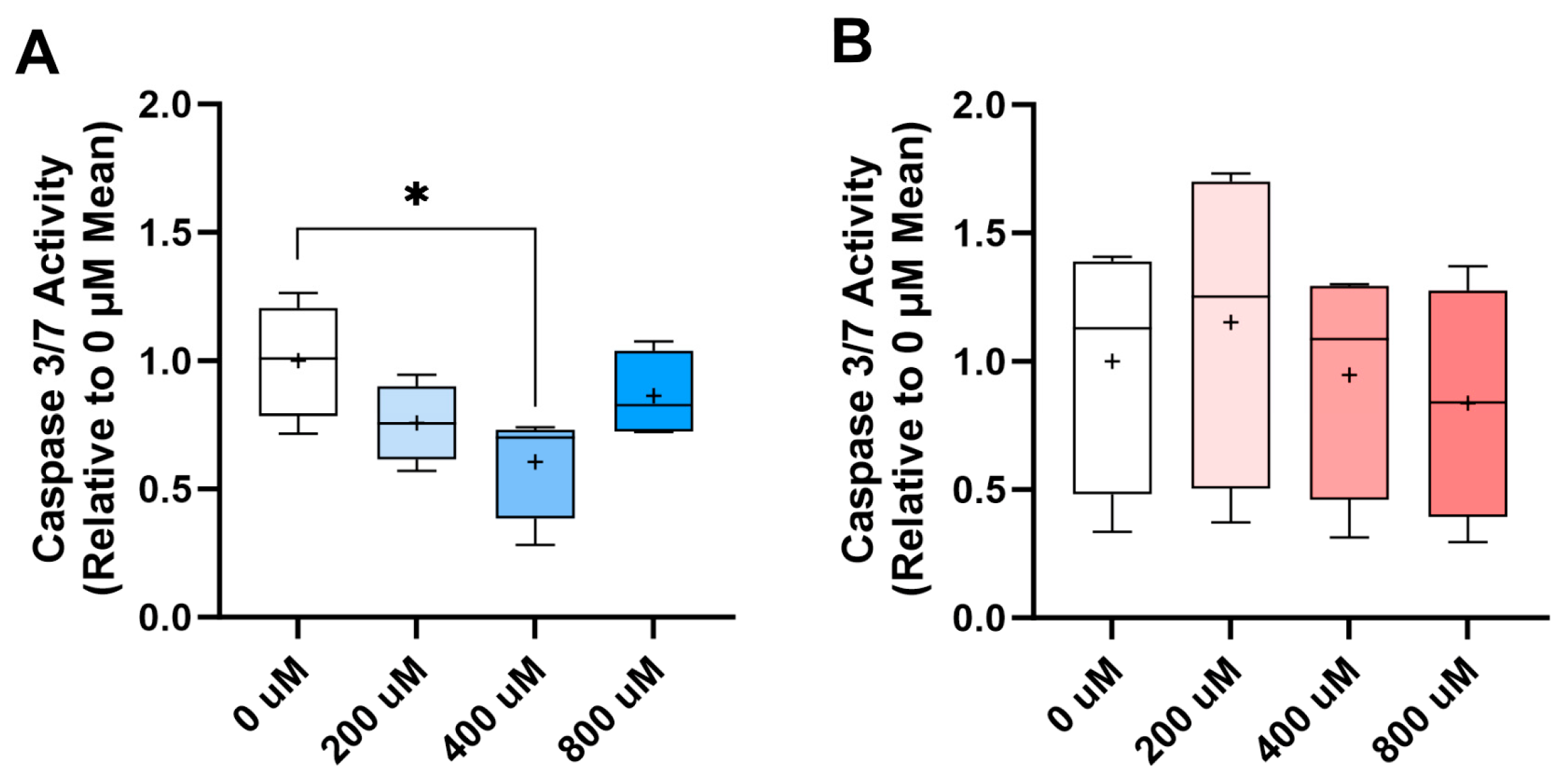
2.5. Dexamethasone Challenge
3. Discussion
4. Materials and Methods
4.1. Progenitor Cell Isolation
4.2. Constructs with Vitamin C Treatment
4.3. 3′-Tag-seq
4.4. RT-qPCR
4.5. Hydroxyproline Assay
4.6. Transmission Electron Microscopy
4.7. Dexamethasone Challenge
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thorpe, C.T.; Clegg, P.D.; Birch, H.L. A review of tendon injury: Why is the equine superficial digital flexor tendon most at risk? Equine Vet. J. 2010, 42, 174–180. [Google Scholar] [CrossRef]
- Kasashima, Y.; Takahashi, T.; Smith, R.K.; Goodship, A.E.; Kuwano, A.; Ueno, T.; Hirano, S. Prevalence of superficial digital flexor tendonitis and suspensory desmitis in Japanese Thoroughbred flat racehorses in 1999. Equine Vet. J. 2004, 36, 346–350. [Google Scholar] [CrossRef]
- Ely, E.R.; Verheyen, K.L.; Wood, J.L. Fractures and tendon injuries in National Hunt horses in training in the UK: A pilot study. Equine Vet. J. 2004, 36, 365–367. [Google Scholar] [CrossRef]
- O’Brien, C.; Marr, N.; Thorpe, C. Microdamage in the equine superficial digital flexor tendon. Equine Vet. J. 2021, 53, 417–430. [Google Scholar] [CrossRef]
- Birch, H.L.; Bailey, A.J.; Goodship, A.E. Macroscopic ‘degeneration’ of equine superficial digital flexor tendon is accompanied by a change in extracellular matrix composition. Equine Vet. J. 1998, 30, 534–539. [Google Scholar] [CrossRef]
- Beason, D.P.; Kuntz, A.F.; Hsu, J.E.; Miller, K.S.; Soslowsky, L.J. Development and evaluation of multiple tendon injury models in the mouse. J. Biomech. 2012, 45, 1550–1553. [Google Scholar] [CrossRef]
- Mienaltowski, M.J.; Adams, S.M.; Birk, D.E. Regional differences in stem cell/progenitor cell populations from the mouse achilles tendon. Tissue Eng. Part. A 2013, 19, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Mienaltowski, M.J.; Adams, S.M.; Birk, D.E. Tendon proper- and peritenon-derived progenitor cells have unique tenogenic properties. Stem Cell Res. Ther. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Mienaltowski, M.J.; Birk, D.E. Mouse models in tendon and ligament research. Adv. Exp. Med. Biol. 2014, 802, 201–230. [Google Scholar] [PubMed]
- Mienaltowski, M.J.; Gonzales, N.L.; Beall, J.M.; Pechanec, M.Y. Basic structure, physiology, and biochemistry of connective tissues and extracellular matrix collagens. Adv. Exp. Med. Biol. 2021, 1348, 5–43. [Google Scholar]
- Mienaltowski, M.J.; Canovas, A.; Fates, V.A.; Hampton, A.R.; Pechanec, M.Y.; Islas-Trejo, A.; Medrano, J.F. Transcriptome profiles of isolated murine Achilles tendon proper- and peritenon-derived progenitor cells. J. Orthop. Res. 2019, 37, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- De Micheli, A.J.; Swanson, J.B.; Disser, N.P.; Martinez, L.M.; Walker, N.R.; Oliver, D.J.; Cosgrove, B.D.; Mendias, C.L. Single-cell transcriptomic analysis identifies extensive heterogeneity in the cellular composition of mouse Achilles tendons. Am. J. Physiol. Cell Physiol. 2020, 319, C885–C894. [Google Scholar] [CrossRef]
- Dyment, N.A.; Hagiwara, Y.; Matthews, B.G.; Li, Y.; Kalajzic, I.; Rowe, D.W. Lineage tracing of resident tendon progenitor cells during growth and natural healing. PLoS ONE 2014, 9, e96113. [Google Scholar] [CrossRef] [PubMed]
- Dyment, N.A.; Liu, C.F.; Kazemi, N.; Aschbacher-Smith, L.E.; Kenter, K.; Breidenbach, A.P.; Shearn, J.T.; Wylie, C.; Rowe, D.W.; Butler, D.L. The Paratenon Contributes to Scleraxis-Expressing Cells during Patellar Tendon Healing. PLoS ONE 2013, 8, e59944. [Google Scholar] [CrossRef]
- Howell, K.; Chien, C.; Bell, R.; Laudier, D.; Tufa, S.F.; Keene, D.R.; Andarawis-Puri, N.; Huang, A.H. Novel Model of Tendon Regeneration Reveals Distinct Cell Mechanisms Underlying Regenerative and Fibrotic Tendon Healing. Sci. Rep. 2017, 7, 45238. [Google Scholar] [CrossRef] [PubMed]
- Dyment, N.A.; Galloway, J.L. Regenerative biology of tendon: Mechanisms for renewal and repair. Curr. Mol. Biol. Rep. 2015, 1, 124–131. [Google Scholar] [CrossRef]
- Schneider, M.; Angele, P.; Järvinen, T.A.H.; Docheva, D. Rescue plan for Achilles: Therapeutics steering the fate and functions of stem cells in tendon wound healing. Adv. Drug Deliv. Rev. 2018, 129, 352–375. [Google Scholar] [CrossRef]
- Huang, Z.; Yin, Z.; Xu, J.; Fei, Y.; Heng, B.C.; Jiang, X.; Chen, W.; Shen, W. Tendon stem/progenitor cell subpopulations and their implications in tendon biology. Front. Cell Dev. Biol. 2021, 9, 63127. [Google Scholar] [CrossRef]
- Shoulders, M.D.; Raines, R.T. Collagen structure and stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef]
- Hung, L.K.; Fu, S.C.; Lee, Y.W.; Mok, T.Y.; Chan, K.M. Local vitamin-C injection reduced tendon adhesion in a chicken model of flexor digitorum profundus tendon injury. J. Bone Joint Surg. Am. 2013, 95, e41. [Google Scholar] [CrossRef]
- Dincel, Y.M.; Adanir, O.; Arikan, Y.; Caglar, A.K.; Dogru, S.C.; Arslan, Y.Z. Effects of High-Dose Vitamin C and Hyaluronic Acid on Tendon Healing. Acta Ortop. Bras. 2018, 26, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Omeroglu, S.; Peker, T.; Turkozkan, N.; Omeroglu, H. High-dose vitamin C supplementation accelerates the Achilles tendon healing in healthy rats. Arch. Orthop. Trauma. Surg. 2009, 129, 281–286. [Google Scholar] [CrossRef] [PubMed]
- di Giacomo, V.; Berardocco, M.; Gallorini, M.; Oliva, F.; Colosimo, A.; Cataldi, A.; Maffulli, N.; Berardi, A.C. Combined supplementation of ascorbic acid and thyroid hormone T3 affects tenocyte proliferation. The effect of ascorbic acid in the production of nitric oxide. Muscles Ligaments Tendons J. 2017, 7, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, O.; Poulson, R.; Thakkar, D.; Yapp, C.; Carr, A. Ascorbic acid is essential for significant collagen deposition by human tenocytes in vitro. Oxid. Antioxid. Med. Sci. 2014, 3, 119–127. [Google Scholar] [CrossRef]
- Lee, Y.W.; Fu, S.C.; Yeung, M.Y.; Lau, C.M.L.; Chan, K.M.; Hung, L.K. Effects of redox modulation on cell proliferation, viability, and migration in cultured rat and human tendon progenitor cells. Oxid. Med. Cell Longev. 2017, 2017, 8785042. [Google Scholar] [CrossRef] [PubMed]
- Evrova, O.; Kellenberger, D.; Calcagni, M.; Vogel, V.; Buschmann, J. Supporting cell-based tendon therapy: Effect of PDGF-BB and ascorbic acid on rabbit Achilles Tenocytes in vitro. Int. J. Mol. Sci. 2020, 21, 458. [Google Scholar] [CrossRef] [PubMed]
- Pechanec, M.Y.; Lee-Barthel, A.; Baar, K.; Mienaltowski, M.J. Evaluation and optimization of a three-dimensional construct model for equine superficial digital flexor tendon. J. Equine Vet. Sci. 2018, 71, 90–97. [Google Scholar] [CrossRef]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef]
- Gutierrez-Nibeyro, S.D.; White, N.A., II; Werpy, N.M. Outcome of medical treatment for horses with foot pain: 56 cases. Equine Vet. J. 2010, 42, 680–685. [Google Scholar] [CrossRef]
- Wright, I.M.; McMahon, P.J. Tenosynovitis associated with longitudinal tears of the digital flexor tendons in horses: A report of 20 cases. Equine Vet. J. 1999, 31, 12–18. [Google Scholar] [CrossRef]
- Pechanec, M.Y.; Boyd, T.N.; Baar, K.; Mienaltowski, M.J. Adding exogenous biglycan or decorin improves tendon formation for equine peritenon and tendon proper cells in vitro. BMC Musculoskelet. Disord. 2020, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- Löscher, W.; Jaeschke, G.; Keller, H. Pharmacokinetics of ascorbic acid in horses. Equine Vet. J. 1984, 16, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, R.L.; Carroll, M.D.; Ford, E.S.; Lacher, D.A. Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003–2004 National Health and Nutrition Examination Survey (NHANES). Am. J. Clin. Nutr. 2009, 90, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Storey, J.D.; Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 2003, 100, 9440–9445. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.F.; Breidenbach, A.; Aschbacher-Smith, L.; Butler, D.; Wylie, C. A role for hedgehog signaling in the differentiation of the insertion site of the patellar tendon in the mouse. PLoS ONE 2013, 8, e65411. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, Y.; Yang, Z.; Lan, H.; Liu, G.; Zhang, Y.; Xia, H.; Wang, X.; Han, F.; Tu, X.; et al. TNFAIP3 ameliorates the degeneration of inflammatory human nucleus pulposus cells by inhibiting mTOR signaling and promoting autophagy. Aging 2020, 12, 24242–24254. [Google Scholar] [CrossRef]
- Liu, S.L.; Bae, Y.H.; Yu, C.; Monslow, J.; Hawthorne, E.A.; Castagnino, P.; Branchetti, E.; Ferrari, G.; Damrauer, S.M.; Puré, E.; et al. Matrix metalloproteinase-12 is an essential mediator of acute and chronic arterial stiffening. Sci. Rep. 2015, 5, 17189. [Google Scholar] [CrossRef]
- Magra, M.; Maffulli, N. Matrix metalloproteases: A role in overuse tendinopathies. Br. J. Sports Med. 2005, 39, 789–791. [Google Scholar] [CrossRef]
- Karlsen, A.; Yeung, C.C.; Schjerling, P.; Denz, L.; Hoegsbjerg, C.; Jakobsen, J.R.; Krogsgaard, M.R.; Koch, M.; Schiaffino, S.; Kjaer, M.; et al. Distinct myofibre domains of the human myotendinous junction revealed by single-nucleus RNA sequencing. J. Cell Sci. 2023, 136, jcs260913. [Google Scholar] [CrossRef]
- Derler, R.; Gesslbauer, B.; Weber, C.; Strutzmann, E.; Miller, I.; Kungl, A. Glycosaminoglycan-Mediated Downstream Signaling of CXCL8 Binding to Endothelial Cells. Int. J. Mol. Sci. 2017, 18, 2605. [Google Scholar] [CrossRef]
- Orchard, K.J.A.; Akbar, M.; Crowe, L.A.N.; Cole, J.; Millar, N.L.; Raleigh, S.M. Characterization of Histone Modifications in Late-Stage Rotator Cuff Tendinopathy. Genes 2023, 14, 496. [Google Scholar] [CrossRef]
- Harman, R.M.; Patel, R.S.; Fan, J.C.; Park, J.E.; Rosenberg, B.R.; Van de Walle, G.R. Single-cell RNA sequencing of equine mesenchymal stromal cells from primary donor-matched tissue sources reveals functional heterogeneity in immune modulation and cell motility. Stem Cell Res. Ther. 2020, 11, 524. [Google Scholar] [CrossRef]
- Wu, Y.; He, X.; Huang, N.; Yu, J.; Shao, B. A20: A master regulator of arthritis. Arthritis Res. Ther. 2020, 22, 220. [Google Scholar] [CrossRef]
- Kendal, A.R.; Layton, T.; Al-Mossawi, H.; Appleton, L.; Dakin, S.; Brown, R.; Loizou, C.; Rogers, M.; Sharp, R.; Carr, A. Multi-omic single cell analysis resolves novel stromal cell populations in healthy and diseased human tendon. Sci. Rep. 2020, 10, 13939. [Google Scholar] [CrossRef]
- Mendez-Enriquez, E.; Garcia-Zepeda, E.A. The multiple faces of CCL13 in immunity and inflammation. Inflammopharmacology 2013, 21, 397–406. [Google Scholar] [CrossRef]
- Fung, K.Y.; Louis, C.; Metcalfe, R.D.; Kosasih, C.C.; Wicks, I.P.; Griffin, M.D.W.; Putoczki, T.L. Emerging roles for IL-11 in inflammatory diseases. Cytokine 2022, 149, 155750. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.W.; Corden, B.; Ng, B.; Vanezis, K.; D’Agostino, G.; Widjaja, A.A.; Song, W.H.; Xie, C.; Su, L.; Kwek, X.Y.; et al. Interleukin-11 is important for vascular smooth muscle phenotypic switching and aortic inflammation, fibrosis and remodeling in mouse models. Sci. Rep. 2020, 10, 17853. [Google Scholar] [CrossRef] [PubMed]
- De Buck, M.; Gouwy, M.; Berghmans, N.; Opdenakker, G.; Proost, P.; Struyf, S.; Van Damme, J. COOH-terminal SAA1 peptides fail to induce chemokines but synergize with CXCL8 and CCL3 to recruit leukocytes via FPR2. Blood 2018, 131, 439–449. [Google Scholar] [CrossRef]
- Fu, W.; Yang, R.; Li, J. Single-cell and spatial transcriptomics reveal changes in cell heterogeneity during progression of human tendinopathy. BMC Biol. 2023, 21, 132. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Abraham, T.; Sampaio, A.; Cowan, M.; Underhill, M.; Scott, A. Sodium cromolyn reduces expression of CTGF, ADAMTS1, and TIMP3 and modulates post-injury patellar tendon morphology. J. Orthop. Res. 2011, 29, 678–683. [Google Scholar] [CrossRef]
- Wunderli, S.L.; Blache, U.; Beretta Piccoli, A.; Niederöst, B.; Holenstein, C.N.; Passini, F.S.; Silván, U.; Bundgaard, L.; Auf dem Keller, U.; Snedeker, J.G. Tendon response to matrix unloading is determined by the patho-physiological niche. Matrix Biol. 2020, 89, 11–26. [Google Scholar] [CrossRef]
- Gumucio, J.P.; Schonk, M.M.; Kharaz, Y.A.; Comerford, E.; Mendias, C.L. Scleraxis is required for the growth of adult tendons in response to mechanical loading. JCI Insight 2020, 5, e138295. [Google Scholar] [CrossRef]
- Ruan, D.; Fei, Y.; Qian, S.; Huang, Z.; Chen, W.; Tang, C.; Xiang, X.; Xu, J.; Yin, Z.; Chen, X.; et al. Early-Stage Primary Anti-inflammatory Therapy Enhances the Regenerative Efficacy of Platelet-Rich Plasma in a Rabbit Achilles Tendinopathy Model. Am. J. Sports Med. 2021, 49, 3357–3371. [Google Scholar] [CrossRef]
- Davis, M.E.; Gumucio, J.P.; Sugg, K.B.; Bedi, A.; Mendias, C.L. MMP inhibition as a potential method to augment the healing of skeletal muscle and tendon extracellular matrix. J. Appl. Physiol. 2013, 115, 884–891. [Google Scholar] [CrossRef] [PubMed]
- Blitz, E.; Viukov, S.; Sharir, A.; Shwartz, Y.; Galloway, J.L.; Pryce, B.A.; Johnson, R.L.; Tabin, C.J.; Schweitzer, R.; Zelzer, E. Bone ridge patterning during musculoskeletal assembly is mediated through SCX regulation of Bmp4 at the tendon-skeleton junction. Dev. Cell. 2009, 17, 861–873. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.A.; Ray, D.W.; Zeef, L.A.; O’Neill, T.; Bruce, I.N.; Alexander, M.Y. The effect of type 1 IFN on human aortic endothelial cell function in vitro: Relevance to systemic lupus erythematosus. J. Interferon Cytokine Res. 2014, 34, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Wright, V.; Peng, H.; Usas, A.; Young, B.; Gearhart, B.; Cummins, J.; Huard, J. BMP4-expressing muscle-derived stem cells differentiate into osteogenic lineage and improve bone healing in immunocompetent mice. Mol. Ther. 2002, 6, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Bochon, K.; Zielniok, K.; Gawlak, M.; Zawada, K.; Zarychta-Wiśniewska, W.; Siennicka, K.; Struzik, S.; Pączek, L.; Burdzińska, A. The Effect of L-Ascorbic Acid and Serum Reduction on Tenogenic Differentiation of Human Mesenchymal Stromal Cells. Int. J. Stem Cells 2021, 14, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.; Moraes, S.A.S.; de Paula, D.R.; Maciel, A.A.; Batista, E.J.O.; Silva, D.G.F.; Bahia, C.P.; Oliveira, K.R.H.M.; Herculano, A.M. Local treatment with ascorbic acid accelerates recovery of post-sutured Achilles tendon in male Wistar rats. Braz. J. Med. Biol. Res. 2019, 52, e8290. [Google Scholar] [CrossRef]
- Shaw, G.; Lee-Barthel, A.; Ross, M.L.; Wang, B.; Baar, K. Vitamin C-enriched gelatin supplementation before intermittent activity augments collagen synthesis. Am. J. Clin. Nutr. 2017, 105, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, I.; Gueltekin, S.; Keith, M.F.; Minichmair, K.; Peham, C.; Jenner, F.; Egerbacher, M. Age-related changes of tendon fibril micro-morphology and gene expression. J. Anat. 2020, 236, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Ali, S.; Tahir, H.M.; Kazmi, S.A.R.; Shakir, H.A.; Mughal, T.A.; Mumtaz, S.; Summer, M.; Farooq, M.A. Aging and its treatment with vitamin C: A comprehensive mechanistic review. Mol. Biol. Rep. 2021, 48, 8141–8153. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.J.; Katz, A.; Wang, Y.; Eck, P.; Kwon, O.; Lee, J.H.; Chen, S.; Corpe, C.; Dutta, A.; Dutta, S.K.; et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 2003, 22, 18–35. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, D.; Nojiri, H.; Itoigawa, Y.; Ozawa, Y.; Kaneko, K.; Shimizu, T. Antioxidant treatment with vitamin C attenuated rotator cuff degeneration caused by oxidative stress in Sod1-deficient mice. JSES Open Access 2018, 2, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Itoigawa, Y.; Morikawa, D.; Koga, A.; Tsurukami, H.; Maruyama, Y.; Ishijima, M. The Effect of Vitamin C and N-Acetylcysteine on Tendon-to-Bone Healing in a Rodent Model of Rotator Cuff Repair. Am. J. Sports Med. 2023, 51, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.H.; Chen, P.; Chen, A.C.; Chan, Y.S.; Hsu, K.Y.; Rei, H.; Lei, K.F. Real-Time Monitoring of Ascorbic Acid-Mediated Reduction of Cytotoxic Effects of Analgesics and NSAIDs on Tenocytes Proliferation. Dose Response 2019, 17, 1559325819832143. [Google Scholar] [CrossRef]
- May, J.M.; Harrison, F.E. Role of vitamin C in the function of the vascular endothelium. Antioxid. Redox Signal. 2013, 19, 2068–2083. [Google Scholar] [CrossRef]
- Pechanec, M.Y.; Beall, J.M.; Katzman, S.; Maga, E.A.; Mienaltowski, M.J. Examining the Effects of In Vitro Co-Culture of Equine Adipose-Derived Mesenchymal Stem Cells With Tendon Proper and Peritenon Cells. J. Equine Vet. Sci. 2023, 126, 104262. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.J.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Lui, P.P. Markers for the identification of tendon-derived stem cells in vitro and tendon stem cells in situ—Update and future development. Stem Cell Res. Ther. 2015, 6, 106. [Google Scholar] [CrossRef]
- Bundgaard, L.; Stensballe, A.; Elbæk, K.J.; Berg, L.C. Mapping of equine mesenchymal stromal cell surface proteomes for identification of specific markers using proteomics and gene expression analysis: An in vitro cross-sectional study. Stem Cell Res. Ther. 2018, 9, 288. [Google Scholar] [CrossRef]
- Meyer, E.; Aglyamova, G.V.; Matz, M.V. Profiling gene expression responses of coral larvae (Acropora millepora) to elevated temperature and settlement inducers using a novel RNA-Seq procedure. Mol. Ecol. 2011, 20, 3599–3616. [Google Scholar] [CrossRef] [PubMed]
- Steffen, D.; Mienaltowski, M.J.; Baar, K. Scleraxis and collagen I expression increase following pilot isometric loading experiments in a rodent model of patellar tendinopathy. Matrix Biol. 2022, 109, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Mienaltowski, M.J.; Huang, L.; Frisbie, D.D.; McIlwraith, C.W.; Stromberg, A.J.; Bathke, A.C.; Macleod, J.N. Transcriptional profiling differences for articular cartilage and repair tissue in equine joint surface lesions. BMC Med. Genom. 2009, 2, 60. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, C.; Ruijter, J.M.; Deprez, R.H.; Moorman, A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003, 339, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Schefe, J.H.; Lehmann, K.E.; Buschmann, I.R.; Unger, T.; Funke-Kaise, H. Quantitative real-time RT-PCR data analysis: Current concepts and the novel “gene expression’s CT difference” formula. J. Mol. Med. 2006, 84, 901–910. [Google Scholar] [CrossRef]
- Lee-Barthel, A.; Baar, K.; West, D.W.D. Treatment of Ligament Constructs with Exercise-conditioned Serum: A Translational Tissue Engineering Model. J. Vis. Exp. 2017, 124, 55339. [Google Scholar]
- Birk, D.E.; Trelstad, R.L. Extracellular compartments in tendon morphogenesis: Collagen fibril, bundle, and macroaggregate formation. J. Cell Biol. 1986, 103, 231–240. [Google Scholar] [CrossRef]
- Mienaltowski, M.J.; Dunkman, A.A.; Buckley, M.R.; Beason, D.P.; Adams, S.M.; Birk, D.E.; Soslowsky, L.J. Injury response of geriatric mouse patellar tendons. J. Orthop. Res. 2016, 34, 1256–1263. [Google Scholar] [CrossRef] [PubMed]


| Horse ID | TP Constructs Mean Reads | TP Constructs Mean % Mapped | PERI Constructs Mean Reads | PERI Constructs Mean% Mapped |
|---|---|---|---|---|
| 5 | 5.21 million | 94.4% | 5.07 million | 94.0% |
| 6 | 5.25 million | 94.2% | 4.91 million | 94.7% |
| 7 | 5.06 million | 94.1% | 5.07 million | 91.7% |
| 10 | 4.86 million | 94.5% | 5.10 million | 95.1% |
| Compared to 0 μM | TP p < 0.05 | TP q < 0.20 | PERI p < 0.05 | PERI q < 0.20 |
|---|---|---|---|---|
| 200 μM vs. 0 μM | 210 | 3 | 328 | 8 |
| 400 μM vs. 0 μM | 182 | 4 | 235 | 0 |
| 800 μM vs. 0 μM | 226 | 1 | 255 | 2 |
| Gene | TP/ PERI | [Vit C] | Fold Change | p-Value | q-Value | Annotation |
|---|---|---|---|---|---|---|
| ADAMTS1 | PERI | 200 vs. 0 | −4.20 | 1.28 × 10−4 | 0.12 | Encodes a proteoglycanase |
| BMP4 | PERI | 200 vs. 0 | −6.50 | 1.01 × 10−5 | 0.03 | Promotes calcification and ossification |
| CCL13 | PERI | 200 vs. 0 | −7.71 | 1.34 × 10−5 | 0.03 | Pro-inflammatory protein |
| GPC3 | PERI | 200 vs. 0 | 4.72 | 2.02 × 10−5 | 0.03 | Expressed at tendon insertion |
| IL11 | PERI | 200 vs. 0 | −3.27 | 5.89 × 10−5 | 0.08 | Fibrotic tissue marker |
| IRF8 | PERI | 200 vs. 0 | −21.95 | 1.96 × 10−4 | 0.17 | Pro-inflammatory protein |
| MMP12 | PERI | 800 vs. 0 | −6.50 | 7.92 × 10−6 | 0.05 | Encodes degradative protein |
| MYH7 | PERI | 200 vs. 0 | −45.62 | 8.31 × 10−6 | 0.03 | Encodes cardiac and skeletal myosin protein |
| PERI | 800 vs. 0 | −50.46 | 4.72 × 10−6 | 0.05 | ||
| TNFAIP3 | PERI | 200 vs. 0 | 32.01 | 8.71 × 10−5 | 0.10 | Anti-inflammatory protein |
| CXCL6 | TP | 400 vs. 0 | −6.80 | 1.42 × 10−4 | 0.14 | Pro-inflammatory marker |
| CXCL8 | TP | 200 vs. 0 | −15.50 | 2.58 × 10−5 | 0.11 | GAG-binding pro-inflammatory marker |
| TP | 400 vs. 0 | −14.37 | 1.19 × 10−4 | 0.14 | ||
| DDX60L | TP | 200 vs. 0 | 7.46 | 6.41 × 10−6 | 0.04 | DExD/H-box helicase protein |
| IFI44 | TP | 200 vs. 0 | 6.42 | 3.77 × 10−7 | 4.78 × 10−3 | Inhibits calcification |
| JAM2 | TP | 800 vs. 0 | 6.86 | 1.68 × 10−5 | 0.10 | Proliferation/migration marker |
| SAA1 | TP | 400 vs. 0 | −4.93 | 2.17 × 10−5 | 0.09 | Active inflammation marker |
| SMOC2 | TP | 400 vs. 0 | 2.34 | 4.97 × 10−5 | 0.10 | Promotes matrix assembly |
| Comparing TP vs. PERI at Different Vitamin C Concentrations | TP vs. PERI q ≤ 0.05 | DAVID Functional Annotation Enriched Categories (FDR < 0.05) |
|---|---|---|
| 0 μM | 18 | TP: n/a |
| 8 | PERI: extracellular space (CC) | |
| 200 μM | 31 | TP: extracellular space (CC), calcium ion binding (MF), extracellular region (CC) |
| 13 | PERI: CXCR chemokine receptor binding (MF), chemokine-mediated signaling pathway (BP), chemokine activity (MF), antimicrobial humoral immune response mediated by antimicrobial peptides (BPs), neutrophil chemotaxis (BP), heparin binding (MF) | |
| 400 μM | 21 | TP: n/a |
| 25 | PERI: extracellular space (CC), chemokine activity (MF), CXCR chemokine receptor binding (MF), chemokine-mediated signaling pathway (BP), heparin binding (MF), growth factor activity (MF), cellular response to interleukin-1 (BP), neutrophil chemotaxis (BP), positive regulation of peptidyl-serine phosphorylation (BP), cellular response to tumor necrosis factor (BP), inflammatory response (BP), cytokine activity (MF), positive regulation of vascular smooth muscle cell proliferation (BP), cellular response to lipopolysaccharide (BP), positive regulation of cell proliferation (BP), extracellular region (CC) | |
| 800 μM | 39 | TP: extracellular region (CC) |
| 21 | PERI: extracellular space (CC), extracellular region (CC), CXCR chemokine receptor binding (MF), heparin binding (MF), growth factor activity (MF), chemoattractant activity (MF) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mienaltowski, M.J.; Callahan, M.; Gonzales, N.L.; Wong, A. Examining the Potential of Vitamin C Supplementation in Tissue-Engineered Equine Superficial Digital Flexor Tendon Constructs. Int. J. Mol. Sci. 2023, 24, 17098. https://doi.org/10.3390/ijms242317098
Mienaltowski MJ, Callahan M, Gonzales NL, Wong A. Examining the Potential of Vitamin C Supplementation in Tissue-Engineered Equine Superficial Digital Flexor Tendon Constructs. International Journal of Molecular Sciences. 2023; 24(23):17098. https://doi.org/10.3390/ijms242317098
Chicago/Turabian StyleMienaltowski, Michael J., Mitchell Callahan, Nicole L. Gonzales, and Angelique Wong. 2023. "Examining the Potential of Vitamin C Supplementation in Tissue-Engineered Equine Superficial Digital Flexor Tendon Constructs" International Journal of Molecular Sciences 24, no. 23: 17098. https://doi.org/10.3390/ijms242317098
APA StyleMienaltowski, M. J., Callahan, M., Gonzales, N. L., & Wong, A. (2023). Examining the Potential of Vitamin C Supplementation in Tissue-Engineered Equine Superficial Digital Flexor Tendon Constructs. International Journal of Molecular Sciences, 24(23), 17098. https://doi.org/10.3390/ijms242317098







