Human Chondrocytes, Metabolism of Articular Cartilage, and Strategies for Application to Tissue Engineering
Abstract
1. Introduction
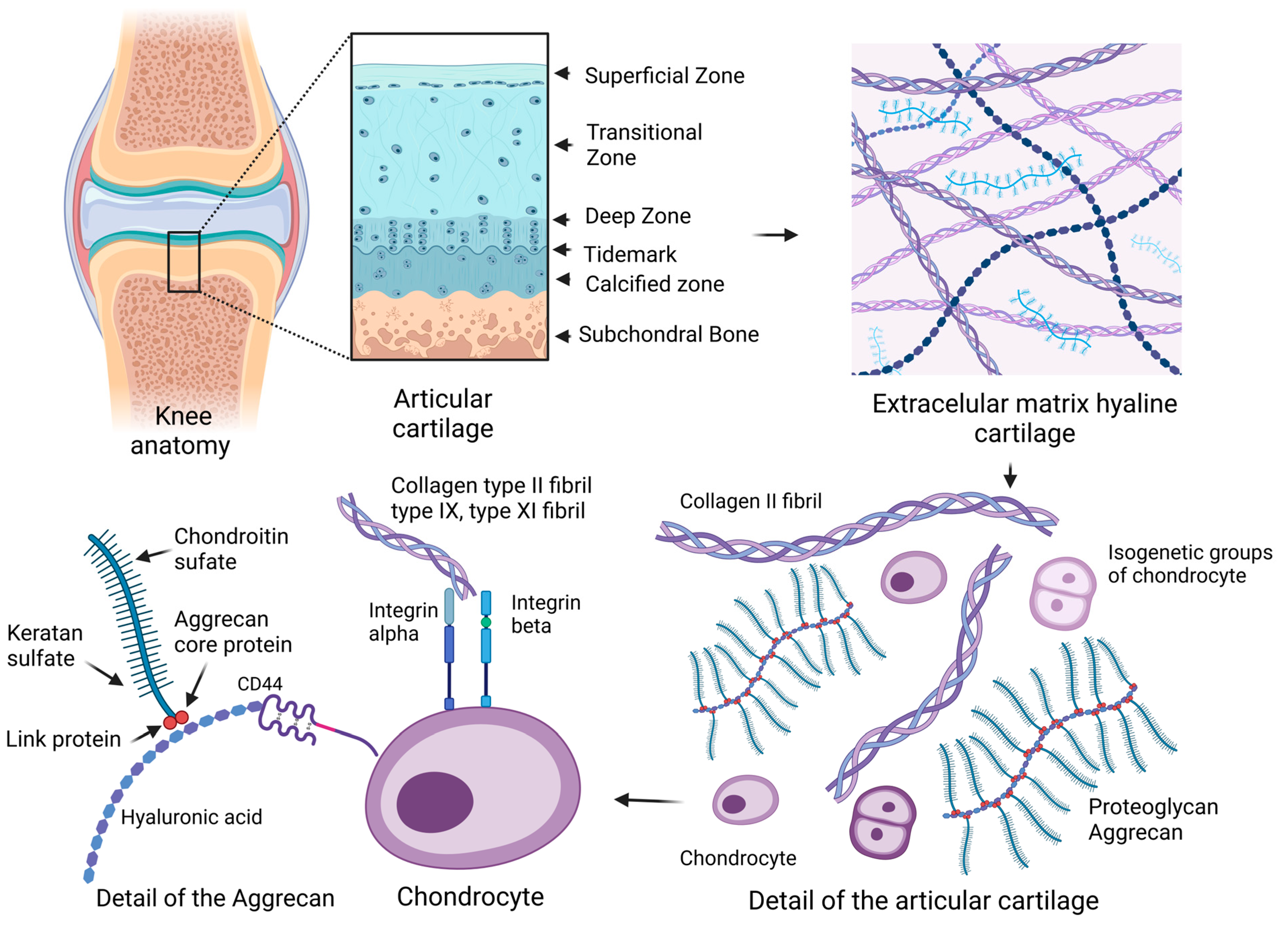
2. Articular Cartilage
2.1. Extracellular Matrix
2.2. Chondrocyte
2.2.1. Progenitor Cells of Cartilage
2.2.2. Characteristic Phenotype of Chondrocytes
2.2.3. Specific Growth Factors during the Expansion of Articular Chondrocytes
2.2.4. Biomechanical Principles of Articular Cartilage and Chondrocytes
3. Degenerative Processes Affecting the Metabolism of Cartilage
3.1. Age-Related Degeneration of Articular Cartilage
3.2. Oxidative Stress
4. Diseases of Articular Cartilage
4.1. Osteoarthritis
4.2. Rheumatoid Arthritis
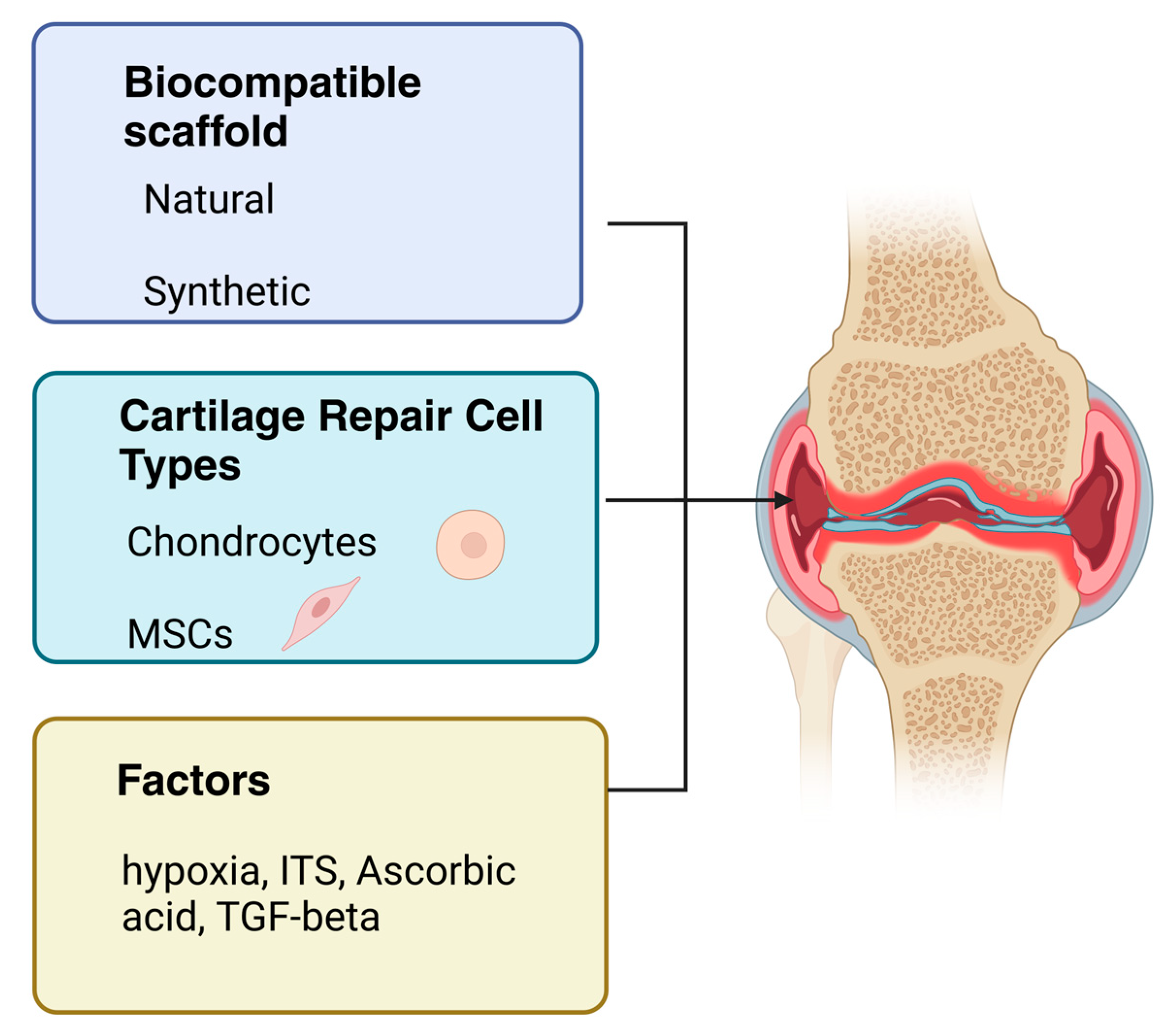
5. Cartilage Repair Cell Types
5.1. Mesenchymal Stem Cells
5.2. Articular Chondrocytes
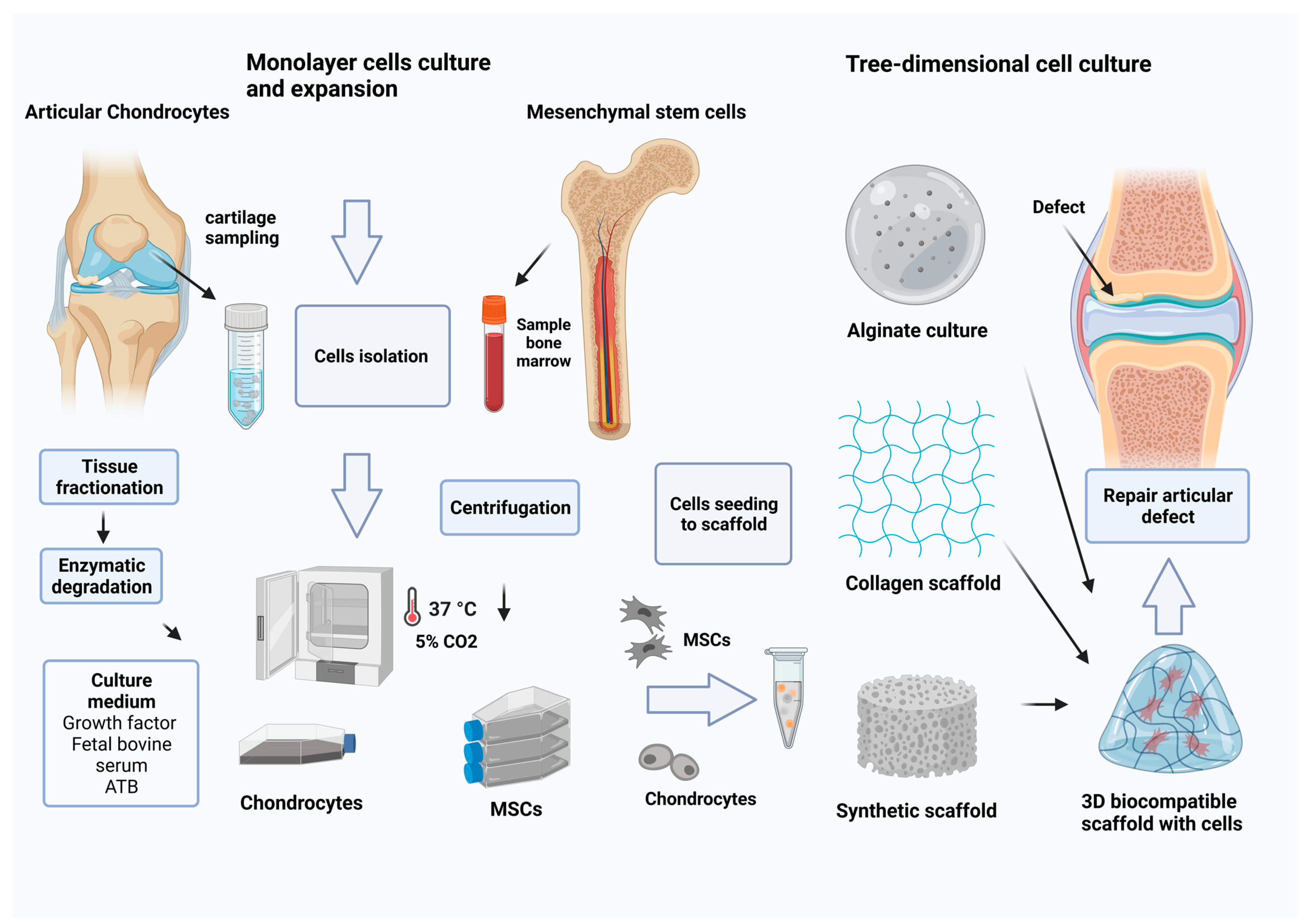
6. Cell Culture In Vitro
6.1. Chondroprogenitors and Mesenchymal Stem Cells
6.2. Monolayer Culture of Chondrocytes
Activation Factors
6.3. Three-Dimensional Culture
6.3.1. Alginate Culture
6.3.2. Collagen- and Hyaluronan-Based Scaffolds
6.3.3. Gelatin Hydrogel
6.4. Biocompatible Synthetic Scaffold
6.5. 3D Printing Technology
6.6. Cartilage Tissue Engineering
7. Clinical Approach in Advanced Cartilage Therapy
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schulze-Tanzil, G. Activation and dedifferentiation of chondrocytes: Implications in cartilage injury and repair. Ann. Anat. Anat. Anz. 2009, 191, 325–338. [Google Scholar] [CrossRef]
- Duan, L.; Liang, Y.; Ma, B.; Zhu, W.; Wang, D. Epigenetic regulation in chondrocyte phenotype maintenance for cell-based cartilage repair. Am. J. Transl. Res. 2015, 7, 2127. [Google Scholar]
- Fox, S.; Bedi, A.J.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sport. Health 2009, 1, 461–468. [Google Scholar]
- Enders, J.T.; Otto, T.J.; Peters, H.C.; Wu, J.; Hardouin, S.; Moed, B.R.; Zhang, Z. A model for studying human articular cartilage integration in vitro. J. Biomed. Mater. Res. Part A 2010, 94, 509–514. [Google Scholar] [CrossRef]
- Roughley, P.J.; Lee, E.R. Cartilage proteoglycans: Structure and potential functions. Microsc. Res. Tech. 1994, 28, 385–397. [Google Scholar] [CrossRef]
- Iwanaga, T.; Shikichi, M. Morphology and functional roles of synoviocytes in the joint. Arch. Histol. Cytol. 2000, 63, 17–31. [Google Scholar] [CrossRef]
- Hunziker, E.B.; Lippuner, K.; Keel, M.J.B.; Shintani, N. An educational review of cartilage repair: Precepts & practice—Myths &misconceptions—Progress & prospects. Osteoarth Cart. 2015, 23, 334–350. [Google Scholar]
- Kurenkova, A.D.; Romanova, I.A.; Kibirskiy, P.D.; Timashev, P. Strategies to convert cells into hyaline cartilage: Magic spells for adult stem cells. Int. J. Mol. Sci. 2022, 23, 11169. [Google Scholar] [CrossRef]
- Hall, A.C.; Horwitz, E.R.; Wilkins, R.J. The cellular physiology of articular cartilage. Exp. Physiol. Transl. Integr. 1996, 81, 535–545. [Google Scholar] [CrossRef]
- Dudhia, J. Aggrecan, aging and assembly in articular cartilage. Cell. Mol. Life Sci. 2005, 62, 2241–2256. [Google Scholar] [CrossRef]
- Hodgkinson, T.; Kelly, D.C.; Curtin, C.M. Mechanosignalling in cartilage: An emerging target for the treatment of osteoarthritis. Nat. Rev. Rheumatol. 2022, 18, 67–84. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, J.; Athanasiou, K.A. The role of tissue engineering in articular cartilage repair and regeneration. Crit. Rev. Biomed. Eng. 2009, 37, 1–57. [Google Scholar] [CrossRef]
- Kheir, E.; Shaw, D. Hyaline articular cartilage. Orthop. Trauma 2009, 23, 450–455. [Google Scholar] [CrossRef]
- Hayes, A.J.; Melrose, J. Glycosaminoglycan and proteoglycan biotherapeutics in articular cartilage protection and repair strategies: Novel approaches to visco-supplementation in orthobiologics. Adv. Ther. 2019, 2, 1900034. [Google Scholar] [CrossRef]
- Pomin, V.H. Keratan sulfate: An up-to-date review. Int. J. Biol. Macromol. 2015, 72, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.R.; Trindade, M.C.D.; Ikenoue, T. Effects of shear stress on articular chondrocyte metabolism. Biorheology 2000, 37, 95–107. [Google Scholar]
- Lin, Z.; Willers, C.; Xu, J.; Zheng, M.H. The chondrocyte: Biology and clinical application. Tissue Eng. 2006, 12, 1971–1984. [Google Scholar] [CrossRef]
- Cancedda, R.; Cancedda, F.D.; Castagnola, P. Chondrocyte differentiation. Int. Rev. Cytol. 1995, 159, 265–358. [Google Scholar]
- Responte, D.J.; Lee, J.K.; Hu, J.C.; Athanasiou, K.A. Biomechanics-driven chondrogenesis: From embryo to adult. FASEB J. 2012, 26, 3614. [Google Scholar] [CrossRef]
- Zhao, Q.; Eberspaecher, H.; Lefebvre, V.; de Crombrugghe, B. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1997, 209, 377–386. [Google Scholar]
- Loeser, R.F. Integrins and chondrocyte–matrix interactions in articular cartilage. Matrix Biol. 2014, 39, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Heinegård, D. Fell-Muir Lecture: Proteoglycans and more–from molecules to biology. Int. J. Exp. Pathol. 2009, 90, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.C.; Chen, Y.J.; Chang, W.A.; Tsai, W.C. Dual role of chondrocytes in rheumatoid arthritis: The chicken and the egg. Int. J. Mol. Sci. 2020, 21, 1071. [Google Scholar] [CrossRef]
- Ecke, A.; Lutter, A.H.; Scholka, J.; Hansch, A. Tissue specific differentiation of human chondrocytes depends on cell microenvironment and serum selection. Cells 2019, 8, 934. [Google Scholar] [CrossRef]
- Camper, L.; Hellman, U.; Lundgren-Åkerlund, E. Isolation, cloning, and sequence analysis of the integrin subunit α10, a β1-associated collagen binding integrin expressed on chondrocytes. J. Biol. Chem. 1998, 273, 20383–20389. [Google Scholar] [CrossRef] [PubMed]
- Lundgren-Åkerlund, E.; Aszòdi, A. Integrin α10β1: A collagen receptor critical in skeletal development. Adv. Exp. Med. Biol. 2014, 819, 61–71. [Google Scholar]
- Bengtsson, T.; Aszodi, A.; Nicolae, C.; Hunziker, E.B.; Lundgren-Åkerlund, E. Loss of α10β1 integrin expression leads to moderate dysfunction of growth plate chondrocytes. J. Cell Sci. 2005, 118, 929–936. [Google Scholar] [CrossRef]
- Knudson, W.; Casey, B.; Nishida, Y.; Eger, W. Hyaluronan oligosaccharides perturb cartilage matrix homeostasis and induce chondrocytic chondrolysis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2000, 43, 1165–1174. [Google Scholar] [CrossRef]
- Salter, D.M.; Hughes, D.E.; Simpson, R. Integrin expression by human articular chondrocytes. Rheumatology 1992, 31, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, M.; Leboy, P.S.; Menko, A.S. β1 integrins mediate chondrocyte interaction with type I collagen, type II collagen, and fibronectin. Exp. Cell Res. 1993, 205, 276–285. [Google Scholar] [CrossRef]
- Aguiar, D.J.; Knudson, W.; Knudson, C.B. Internalization of the hyaluronan receptor CD44 by chondrocytes. Exp. Cell Res. 1999, 252, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Ishida, O.; Tanaka, Y.; Morimoto, I.; Takigawa, M. Chondrocytes are regulated by cellular adhesion through CD44 and hyaluronic acid pathway. J. Bone Miner. Res. 1997, 12, 1657–1663. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, W.; Li, X.; Zhong, D.; Li, Y.; Li, J. Strategies to modulate the redifferentiation of chondrocytes. Front. Bioeng. Biotechnol. 2021, 9, 764193. [Google Scholar] [CrossRef] [PubMed]
- Jakob, M.; Demarteau, O.; Schäfer, D.; Hintermann, B.; Dick, W. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J. Cell. Biochem. 2001, 81, 368–377. [Google Scholar] [CrossRef]
- Benya, P.D.; Shaffer, J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell 1982, 30, 215–224. [Google Scholar] [CrossRef]
- Burt, D.W.; Law, A.S. Evolution of the transforming growth factor-beta superfamily. Prog. Growth Factor Res. 1994, 5, 99–118. [Google Scholar] [CrossRef]
- De Caestecker, M. The transforming growth factor-β superfamily of receptors. Cytokine Growth Factor Rev. 2004, 15, 1–11. [Google Scholar] [CrossRef]
- Yang, D.; Dai, F.; Yuan, M.; Zheng, Y. Role of transforming growth factor-β1 in regulating fetal-maternal immune tolerance in normal and pathological pregnancy. Front. Immunol. 2021, 12, 689181. [Google Scholar] [CrossRef] [PubMed]
- Mantel, P.Y.; Schmidt-Weber, C.B. Transforming growth factor-beta: Recent advances on its role in immune tolerance. Methods Mol. Biol. 2011, 677, 303–338. [Google Scholar] [PubMed]
- Van Osch, G.J.; Van Der Veen, S.W.; Buma, P.; Verwoerd-Verhoef, H.L. Effect of transforming growth factor-β on proteoglycan synthesis by chondrocytes in relation to differentiation stage and the presence of pericellular matrix. Matrix Biol. 1998, 17, 413–424. [Google Scholar] [CrossRef]
- Luo, K. Signaling cross talk between TGF-β/Smad and other signaling pathways. Cold Spring Harb. Perspect. Biol. 2017, 9, a022137. [Google Scholar] [CrossRef]
- Duan, D.; Derynck, R. Transforming growth factor–β (TGF-β)–induced up-regulation of TGF-β receptors at the cell surface amplifies the TGF-β response. J. Biol. Chem. 2019, 294, 8490–8504. [Google Scholar] [CrossRef]
- Battegay, E.J.; Raines, E.W.; Seifert, R.A.; Bowen-Pope, D.F. TGF-β induces bimodal proliferation of connective tissue cells via complex control of an autocrine PDGF loop. Cell 1990, 63, 515–524. [Google Scholar] [CrossRef]
- Zhang, Y.; Alexander, P.B.; Wang, X.F. TGF-β family signaling in the control of cell proliferation and survival. Cold Spring Harb. Perspect. Biol. 2017, 9, a022145. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.N.; Nguyen, J.; Yee, C.S.; Dole, N.S.; Dang, A. Mechanosensitive control of articular cartilage and subchondral bone homeostasis in mice requires osteocytic transforming growth factor β signaling. Arthritis Rheumatol. 2021, 73, 414–425. [Google Scholar] [CrossRef]
- Li, T.F.; O’Keefe, R.J.; Chen, D. TGF-β signaling in chondrocytes. Front. Biosci. 2005, 10, 681. [Google Scholar] [CrossRef] [PubMed]
- Puolakkainen, P.A.; Twardzik, D.R.; Ranchalis, J.E. The enhancement in wound healing by transforming growth factor-β1 (TGF-β1) depends on the topical delivery system. J. Surg. Res. 1995, 58, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Critchlow, M.A.; Bland, Y.S.; Ashhurst, D.E. The effect of exogenous transforming growth factor-β2 on healing fractures in the rabbit. Bone 1995, 16, 521–527. [Google Scholar] [CrossRef]
- Wells, R.G. TGF-β signaling pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, 845–850. [Google Scholar] [CrossRef]
- Duan, M.; Wang, Q.; Liu, Y.; Xie, J. The role of TGF-β2 in cartilage development and diseases. Bone Jt. Res. 2021, 10, 474–487. [Google Scholar] [CrossRef]
- Das, R.; Timur, U.T.; Edip, S.; Haak, E.; Wruck, C.; Weinans, H. TGF-β2 is involved in the preservation of the chondrocyte phenotype under hypoxic conditions. Ann. Anat. Anat. Anz. 2015, 198, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Ushida, T.; Akimoto, T. Control of cell differentiation by mechanical stress. J. Phys. Fit. Sport. Med. 2013, 2, 49–62. [Google Scholar] [CrossRef][Green Version]
- Guilak, F. The deformation behavior and viscoelastic properties of chondrocytes in articular cartilage. Biorheology 2000, 37, 27–44. [Google Scholar]
- Carter, D.R.; Beaupré, G.S.; Wong, M.; Smith, R.L. The mechanobiology of articular cartilage development and degeneration. Clin. Orthop. Relat. Res. 2004, 427, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Franzen, A.; Inerot, S.; Hejderup, S. Variations in the composition of bovine hip articular cartilage with distance from the articular surface. Biochem. J. 1981, 195, 535–543. [Google Scholar] [CrossRef]
- Eschweiler, J.; Horn, N.; Rath, B.; Betsch, M.; Baroncini, A. The biomechanics of cartilage—An overview. Life 2021, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.L.; Mow, V.C. Biomechanics of articular cartilage and determination of material properties. Med. Sci. Sport. Exerc. 2008, 40, 193–199. [Google Scholar] [CrossRef]
- Martin, J.A.; Buckwalter, J.A. Telomere erosion and senescence in human articular cartilage chondrocytes. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, 172–179. [Google Scholar] [CrossRef]
- Ellis, D.L.; Yannas, I.V. Recent advances in tissue synthesis in vivo by use of collagen-glycosaminoglycan copolymers. Biomaterials 1996, 17, 291–299. [Google Scholar] [CrossRef]
- Aurich, M.; Poole, A.R.; Reiner, A. Matrix homeostasis in aging normal human ankle cartilage. Arthritis. Rheum. 2002, 46, 2903–2910. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.P.; Neame, P.J.; Sasse, J. Length variation in the keratan sulfate domain of mammalian aggrecan. Matrix. Biol. 1994, 14, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Szychlinska, M.A.; Mobasheri, A. Age-related degeneration of articular cartilage in the pathogenesis of osteoarthritis. Histol. Histopathol. 2015, 30, 1–12. [Google Scholar]
- Baynes, J.W. Role of oxidative stress in development of complications in diabetes. Diabetes 1991, 40, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, A.; Yabu, A.; Nakamura, H. Advanced glycation end products in musculoskeletal system and disorders. Methods 2022, 203, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, S.R.; Baynes, J.W. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids 2003, 25, 275–281. [Google Scholar] [CrossRef]
- Ames, J.M. The maillard reaction. In Biochemistry of Food Proteins; Springer: Boston, MA, USA, 1992; pp. 99–153. [Google Scholar]
- Tessier, F.J. The Maillard reaction in the human body. The main discoveries and factors that affect glycation. Pathol. Biol. 2010, 58, 214–219. [Google Scholar] [CrossRef]
- Ozawa, J.; Kaneguchi, A.; Minamimoto, K.; Tanaka, R.; Kito, N. Accumulation of advanced-glycation end products (AGEs) accelerates arthrogenic joint contracture in immobilized rat knee. J. Orthop. Res. 2018, 36, 854–863. [Google Scholar] [CrossRef]
- Singh, P.; Marcu, K.B.; Goldring, M.B.; Otero, M. Phenotypic instability of chondrocytes in osteoarthritis: On a path to hypertrophy. Ann. New York Acad. Sci. 2019, 1442, 17–34. [Google Scholar] [CrossRef]
- Vos, P.A.; DeGroot, J.; Barten-van Rijbroek, A.D.; Zuurmond, A.M.; Bijlsma, J.W. Elevation of cartilage AGEs does not accelerate initiation of canine experimental osteoarthritis upon mild surgical damage. J. Orthop. Res. 2012, 30, 1398–1404. [Google Scholar] [CrossRef]
- Wiskich, J.T. Control of the Krebs cycle. In Metabolism and Respiration; Academic Press: Cambridge, MA, USA, 1980; pp. 243–278. [Google Scholar]
- Patten, D.A.; Lafleur, V.N.; Robitaille, G.A. Hypoxia-inducible factor-1 activation in nonhypoxic conditions: The essential role of mitochondrial-derived reactive oxygen species. Mol. Biol. Cell 2010, 21, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Buechter, D.D. Free radicals and oxygen toxicity. Pharm. Res. 1988, 5, 253–260. [Google Scholar] [CrossRef]
- Saxena, R.; Barta, J. Arthritis as a disease of aging and changes in antioxidant status. In Aging; Academic Press: Cambridge, MA, USA, 2020; pp. 83–94. [Google Scholar]
- Gutowski, M.; Kowalczyk, S. A study of free radical chemistry: Their role and pathophysiological significance. Acta Biochim. Pol. 2013, 60, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Pastukh, V.; Leonard, J.; Turrens, J. Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am. J. Physiol. Cell Physiol. 2008, 294, 413–422. [Google Scholar] [CrossRef]
- Zhang, P.; Omaye, S.T. β-Carotene and protein oxidation: Effects of ascorbic acid and α-tocopherol. Toxicology 2000, 146, 37–47. [Google Scholar] [CrossRef]
- Goutas, A.; Syrrou, C.; Papathanasiou, I.; Tsezou, A. The autophagic response to oxidative stress in osteoarthritic chondrocytes is deregulated. Free. Radic. Biol. Med. 2018, 126, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Henrotin, Y.E.; Bruckner, P. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthr. Cartil. 2003, 11, 747–755. [Google Scholar] [CrossRef]
- Chang, Z.; Huo, L.; Li, P.; Wu, Y. Ascorbic acid provides protection for human chondrocytes against oxidative stress. Mol. Med. Rep. 2015, 12, 7086–7092. [Google Scholar] [CrossRef]
- Bhosale, A.M.; Richardson, J.B. Articular cartilage: Structure, injuries and review of management. Br. Med. Bull. 2008, 87, 77–95. [Google Scholar] [CrossRef]
- Mankin, H.J. The response of articular cartilage to mechanical injury. J. Bone Joint. Surg. Am. 1982, 64, 460–466. [Google Scholar] [CrossRef]
- de Windt, T.S.; Vonk, L.A.; Brittberg, M. Treatment and prevention of (early) osteoarthritis using articular cartilage repair—Fact or fiction? A systematic review. Cartilage 2013, 4, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Carlson, C.S.; McGee, M.P. Expression of β1 integrins by cultured articular chondrocytes and in osteoarthritic cartilage. Exp. Cell Res. 1995, 217, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Lapadula, G.; Iannone, F.; Zuccaro, C. Integrin expression on chondrocytes: Correlations with the degree of cartilage damage in human osteoarthritis. Clin. Exp. Rheumatol. 1997, 15, 247–254. [Google Scholar]
- Ostergaard, K.; Salter, D.M.; Petersen, J.; Bendtzen, K.; Hvolris, J. Expression of alpha and beta subunits of the integrin superfamily in articular cartilage from macroscopically normal and osteoarthritic human femoral heads. Ann. Rheum. Dis. 1998, 57, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Schmid, T.M.; Bonen, D.K.; Luchene, L.; Linsenmayer, T.F. Late events in chondrocyte differentiation: Hypertrophy, type X collagen synthesis and matrix calcification. In Vivo 1991, 5, 533–540. [Google Scholar]
- Rapp, A.E.; Zaucke, F. Cartilage extracellular matrix-derived matrikines in osteoarthritis. Am. J. Physiol. Cell Physiol. 2023, 324, 377–394. [Google Scholar] [CrossRef]
- Halliday, N.L.; Tomasek, J.J. Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in vitro. Exp. Cell Res. 1995, 217, 109–117. [Google Scholar] [CrossRef]
- Arnold, M.A.; Kim, Y.; Czubryt, M.P.; Phan, D.; McAnally, J.; Qi, X. MEF2C transcription factor controls chondrocyte hypertrophy and bone development. Dev. Cell 2007, 12, 377–389. [Google Scholar] [CrossRef]
- Li, J.; Narayanan, K.; Zhang, Y.; Hill, R.C.; He, F. Role of lineage-specific matrix in stem cell chondrogenesis. Biomaterials 2020, 231, 119681. [Google Scholar] [CrossRef]
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect. Tissue Res. 2017, 58, 2–14. [Google Scholar] [CrossRef]
- Couceiro Follente, J.; Carpintero Arias, P.M.T. Cultivo de condrocitos. Rev. Ortop. Traumatol. 2002, 5, 436–443. [Google Scholar]
- Lin, Z.; Fitzgerald, J.B.; Xu, J.; Willers, C.; Wood, D.; Grodzinsky, A.J. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J. Orthop. Res. 2008, 26, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhou, Y.; Polson, S.W.; Wan, L.Q.; Wang, M. Identification of chondrocyte genes and signaling pathways in response to acute joint inflammation. Sci. Rep. 2019, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Dickhut, A.; Rickert, M. Human articular chondrocytes secrete parathyroid hormone–related protein and inhibit hypertrophy of mesenchymal stem cells in coculture during chondrogenesis. Arthritis Rheum. 2010, 62, 2696–2706. [Google Scholar] [CrossRef]
- Rim, Y.A.; Ju, J.H. The role of fibrosis in osteoarthritis progression. Life 2020, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Favero, M.; Dragomir, C.; Hachem, K.E. Human chondrocyte cultures as models of cartilage-specific gene regulation. Methods Mol. Biol. 2012, 806, 301–336. [Google Scholar]
- Mhanna, R.; Kashyap, A.; Palazzolo, G.; Vallmajo-Martin, Q. Chondrocyte culture in three dimensional alginate sulfate hydrogels promotes proliferation while maintaining expression of chondrogenic markers. Tissue Eng. Part A 2014, 20, 1454–1464. [Google Scholar] [CrossRef]
- Bačenková, D.; Trebuňová, M.; Morochovič, R.; Dosedla, E.; Findrik Balogová, A.; Gašparová, P.; Živčák, J. Interaction between Mesenchymal Stem Cells and the Immune System in Rheumatoid Arthritis. Pharmaceuticals 2022, 15, 941. [Google Scholar] [CrossRef]
- Gotoh, H.; Kawaguchi, Y.; Harigai, M.; Hara, M.; Saito, S.; Yamaguchi, T. Increased CD40 expression on articular chondrocytes from patients with rheumatoid arthritis: Contribution to production of cytokines and matrix metalloproteinases. J. Rheumatol. 2004, 31, 1506–1512. [Google Scholar]
- Faust, H.J.; Guo, Q.; Elisseeff, J.H. Cartilage tissue engineering. Princ. Regen. Med. 2019, 1, 937–952. [Google Scholar]
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Kangari, P.; Talaei-Khozani, T.; Razeghian-Jahromi, I.; Razmkhah, M. Mesenchymal stem cells: Amazing remedies for bone and cartilage defects. Stem Cell Res. Ther. 2020, 11, 492. [Google Scholar] [CrossRef] [PubMed]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Prolif. 1970, 3, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.L.B.K.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Bühring, H.J. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef] [PubMed]
- de Girolamo, L.; Lucarelli, E.; Alessandri, G. Mesenchymal stem/stromal cells: A new “cells as drugs” paradigm. Efficacy and critical aspects in cell therapy. Curr. Pharm. Des. 2013, 19, 2459–2473. [Google Scholar] [PubMed]
- Lu, H.H.; Kofron, M.D.; El-Amin, S.F.; Attawia, M.A. In vitro bone formation using muscle-derived cells: A new paradigm for bone tissue engineering using polymer–bone morphogenetic protein matrices. Biochem. Biophys. Res. Commun. 2003, 305, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liao, L.; Wang, Q.; Ma, L.; Ma, G.; Jiang, X. Isolation and identification of mesenchymal stem cells from human fetal pancreas. J. Lab. Clin. Med. 2003, 141, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Bačenková, D.; Rosocha, J.; Tóthová, T.; Rosocha, L.; Šarisský, M. Isolation and basic characterization of human term amnion and chorion mesenchymal stromal cells. Cytotherapy 2011, 13, 1047–1056. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Ringdén, O. Immunobiology of human mesenchymal stem cells and future use in hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. 2005, 11, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Bačenková, D.; Trebuňová, M.; Zachar, L.; Hudák, R.; Ižaríková, G.; Šurínová, K.; Živčák, J. Analysis of same selected immunomodulatory properties of chorionic mesenchymal stem cells. Appl. Sci. 2020, 10, 9040. [Google Scholar] [CrossRef]
- von Bomhard, A.; Faust, J.; Elsaesser, A.F.; Schwarz, S. Impact of expansion and redifferentiation under hypothermia on chondrogenic capacity of cultured human septal chondrocytes. J. Tissue Eng. 2017, 8, 2041731417732655. [Google Scholar] [CrossRef] [PubMed]
- Correia-Melo, C.; Hewitt, G. Telomeres, oxidative stress and inflammatory factors: Partners in cellular senescence? Longev. Heal. 2014, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Urlić, I.; Ivković, A. Cell Sources for Cartilage Repair—Biological and Clinical Perspective. Cells 2021, 10, 154–196. [Google Scholar] [CrossRef] [PubMed]
- Freshney, R.I. Culture of Animal Cells: A Manual of Basic Technique and Specialized Applications, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Oegema, T.R.; Thompson, R.C. Characterization of a hyaluronic acid-dermatan sulfate proteoglycan complex from dedifferentiated human chondrocyte cultures. J. Biol. Chem. 1981, 256, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Madry, H.; Cucchiarini, M. Application of alginate hydrogels for next-generation articular cartilage regeneration. Int. J. Mol. Sci. 2022, 23, 1147. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Brown, W.E.; Lee, C.A.; Wang, D.; Paschos, N. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019, 15, 550–570. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.H.; Aminuddin, B.S.; Fuzina, N.H.; Ruszymah, B.H. Insulin-transferrin-selenium prevent human chondrocyte dedifferentiation and promote the formation of high quality tissue engineered human hyaline cartilage. Eur. Cell Mater. 2005, 9, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Bonaventure, J.; Kadhom, N.; Cohen-Solal, L.; Ng, K.H.; Bourguignon, J. Reexpression of cartilage-specific genes by dedifferentiated human articular chondrocytes cultured in alginate beads. Exp. Cell Res. 1994, 212, 97–104. [Google Scholar] [CrossRef]
- Bačenková, D.; Rosocha, J.; Švihla, R.; Vaško, G.; Bodnar, J. Repair of chondral defects of the knee using a combination of autologous chondrocytes and osteochondral allograft—An animal model. Part I: In vitro culture of autologous chondrocytes. Acta Chir. Orthop. Traumatol. Cechoslov. 2001, 68, 363–368. [Google Scholar]
- Jeyakumar, V.; Niculescu-Morzsa, E.; Bauer, C.; Lacza, Z. Redifferentiation of articular chondrocytes by hyperacute serum and platelet rich plasma in collagen type I hydrogels. Int. J. Mol. Sci. 2019, 20, 316. [Google Scholar] [CrossRef] [PubMed]
- Cobzac, V.; Verestiuc, L.; Jian, M.; Nacu, V. Chondrocytes isolation from hyaline cartilage by continuous monitoring method. Mold. Med. J. 2021, 64, 13–19. [Google Scholar] [CrossRef]
- Jelodari, S.; Ebrahimi Sadrabadi, A.; Zarei, F.; Jahangir, S.; Azami, M.; Sheykhhasan, M. New insights into cartilage tissue engineering: Improvement of tissue-scaffold integration to enhance cartilage regeneration. BioMed Res. Int. 2022, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Jahr, H.; Gunes, S.; Kuhn, A.R.; Nebelung, S. Bioreactor-controlled physoxia regulates TGF-β signaling to alter extracellular matrix synthesis by human chondrocytes. Int. J. Mol. Sci. 2019, 20, 1715. [Google Scholar] [CrossRef]
- Norby, D.P.; Malemud, C.J.; Sokoloff, L. Differences in the collagen types synthesized by lapine articular chondrocytes in spinner and monolayer culture. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1977, 20, 709–716. [Google Scholar] [CrossRef]
- Bichara, D.A.; O'Sullivan, N.A.; Pomerantseva, I.; Zhao, X.; Sundback, C.A.; Vacanti, J.P. The tissue-engineered auricle: Past, present, and future. Tissue Eng. Part B Rev. 2012, 18, 51–61. [Google Scholar] [CrossRef]
- Bittencourt, R.A.D.C.; Pereira, H.R.; Felisbino, S.L.; Ferreira, R.R. Chondrocyte cultures in tridimensional scaffold: Alginate hydrogel. Acta Ortopédica Bras. 2009, 17, 242–246. [Google Scholar] [CrossRef]
- Lee, C.S.; Gleghorn, J.P.; Choi, N.W.; Cabodi, M.; Stroock, A.D.; Bonassar, L.J. Integration of layered chondrocyte-seeded alginate hydrogel scaffolds. Biomaterials 2007, 28, 2987–2993. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Bian, L.; Zhai, D.Y.; Tous, E.; Rai, R.; Mauck, R.L.; Burdick, J.A. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials 2011, 32, 6425–6434. [Google Scholar] [CrossRef]
- Chan, L.W.; Lee, H.Y.; Heng, P.W. Mechanisms of external and internal gelation and their impact on the functions of alginate as a coat and delivery system. Carbohydr. Polym. 2006, 63, 176–187. [Google Scholar] [CrossRef]
- Abraham, L.C.; Zuena, E.; Perez-Ramirez, B.; Kaplan, D.L. Guide to collagen characterization for biomaterial studies. J. Biomed. Mater. Res. 2008, 87, 264–285. [Google Scholar] [CrossRef]
- Chan, W.W.; Yeo, D.C.L.; Tan, V.; Singh, S.; Choudhury, D.; Naing, M.W. Additive biomanufacturing with collagen inks. Bioengineering 2020, 7, 66. [Google Scholar] [CrossRef]
- Schwab, A.; Hélary, C.; Richards, R.G.; Alini, M.; Eglin, D.; d’Este, M. Tissue mimetic hyaluronan bioink containing collagen fibers with controlled orientation modulating cell migration and alignment. Mater. Today Bio. 2020, 7, 100058. [Google Scholar] [CrossRef]
- Akmal, M.; Singh, A.; Anand, A. The effects of hyaluronic acid on articular chondrocytes. J. Bone Jt. Surg. Br. Vol. 2005, 87, 1143–1149. [Google Scholar] [CrossRef]
- Chen, G.; Lv, Y.; Dong, C.; Yang, L. Effect of internal structure of collagen/hydroxyapatite scaffold on the osteogenic differentiation of mesenchymal stem cells. Curr. Stem Cell Res. Ther. 2015, 10, 99–108. [Google Scholar] [CrossRef]
- Amann, E.; Wolff, P.; Breel, E. Hyaluronic acid facilitates chondrogenesis and matrix deposition of human adipose derived mesenchymal stem cells and human chondrocytes co-cultures. Acta Biomater. 2017, 52, 130–144. [Google Scholar] [CrossRef]
- Patino, M.G.; Neiders, M.E.; Andreana, S.; Noble, B.; Cohen, R.E. Collagen: An overview. Implant. Dent. 2002, 11, 280–285. [Google Scholar] [CrossRef]
- Xia, T.; Liu, W.; Yang, L. A review of gradient stiffness hydrogels used in tissue engineering and regenerative medicine. J. Biomed. Mater. Res. Part A 2017, 105, 1799–1812. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Li, J.; Wang, X.; Zhang, J. 3D culture of chondrocytes in gelatin hydrogels with different stiffness. Polymers 2016, 8, 269. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Kawazoe, N. Fabrication of highly crosslinked gelatin hydrogel and its influence on chondrocyte proliferation and phenotype. Polymers 2017, 9, 309. [Google Scholar] [CrossRef]
- Pandolfi, V.; Pereira, U.; Dufresne, M.; Legallais, C. Alginate-based cell microencapsulation for tissue engineering and regenerative medicine. Curr. Pharm. Des. 2017, 23, 3833–3844. [Google Scholar] [CrossRef]
- Rogan, H.I. Microribbon-hydrogel composite scaffold accelerates cartilage regeneration in vivo with enhanced mechanical properties using mixed stem cells and chondrocytes. Biomaterials 2020, 228, 119579. [Google Scholar] [CrossRef]
- Varghese, S.; Hwang, N.S.; Canver, A.C. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biol. 2008, 27, 12–21. [Google Scholar] [CrossRef]
- Balogová, A.F.; Trebuňová, M.; Bačenková, D.; Kohan, M.; Hudák, R.; Tóth, T.; Živčák, J. Impact of In Vitro Degradation on the Properties of Samples Produced by Additive Production from PLA/PHB-Based Material and Ceramics. Polymers 2022, 14, 5441. [Google Scholar] [CrossRef]
- Trebuňová, M.; Petroušková, P.; Balogová, A.F.; Ižaríková, G.; Horňak, P.; Bačenková, D.; Demeterová, J.; Živčák, J. Evaluation of Biocompatibility of PLA/PHB/TPS Polymer Scaffolds with Different Additives of ATBC and OLA Plasticizers. J. Funct. Biomater. 2023, 14, 412. [Google Scholar] [CrossRef]
- Messaoudi, O.; Henrionnet, C.; Bourge, K.; Loeuille, D.; Gillet, P.; Pinzano, A. Stem cells and extrusion 3D printing for hyaline cartilage engineering. Cells 2020, 10, 2. [Google Scholar] [CrossRef]
- He, Y.; Liu, W.; Guan, L.; Chen, J.; Duan, L.; Jia, Z.; Wang, D. A 3D-printed PLCL scaffold coated with collagen type I and its biocompatibility. BioMed. Res. Int. 2018, 2018, 10. [Google Scholar] [CrossRef]
- Müller, M.; Öztürk, E.; Arlov, O.; Gatenholm, P. Alginate sulfate–nanocellulose bioinks for cartilage bioprinting applications. Ann. Biomed. Eng. 2017, 45, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Kesti, M.; Müller, M.; Becher, J.; Schnabelrauch, M.; D’Este, M.; Eglin, D. A versatile bioink for three-dimensional printing of cellular scaffolds based on thermally and photo-triggered tandem gelation. Acta Biomater. 2015, 11, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.; Song, P.; Peng, Z.; Zhang, B.; Gui, X.; Wang, Y. Chondrocyte-laden GelMA hydrogel combined with 3D printed PLA scaffolds for auricle regeneration. Mater. Sci. Eng. 2021, 130, 112423. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef]
- Zheng, M.H.; Willers, C.; Kirilak, L.; Yates, P. Matrix-induced autologous chondrocyte implantation (MACI®): Biological and histological assessment. Tissue Eng. 2007, 13, 737–746. [Google Scholar] [CrossRef]
- Pavesio, A.; Abatangelo, G.; Borrione, A. Hyaluronan-Based Scaffolds, Hyalograft® C in the Treatment of Knee Cartilage Defects: Preliminary Clinical Findings. In Tissue Engineering of Cartilage and Bone: Novartis Foundation Symposium; John Wiley & Sons, Ltd.: Chichester, UK, 2003; Volume 249, pp. 203–217. [Google Scholar]
- Katsube, K.; Ochi, M.; Uchio, Y.; Maniwa, S. Repair of articular cartilage defects with cultured chondrocytes in atelocollagen gel: Comparison with cultured chondrocytes in suspension. Arch. Orthop. Trauma Surg. 2000, 120, 121–127. [Google Scholar] [CrossRef]
- Tohyama, H.; Yasuda, K.; Minami, A. Atelocollagen-associated autologous chondrocyte implantation for the repair of chondral defects of the knee: A prospective multicenter clinical trial in Japan. J. Orthop. Sci. 2009, 14, 579–588. [Google Scholar] [CrossRef]
- Kuo, C.K.; Li, W.J.; Mauck, R.L.; Tuan, R. Cartilage tissue engineering: Its potential and uses. Curr. Opin. Rheumatol. 2006, 18, 64–73. [Google Scholar] [CrossRef]
- Felson, D.T.; Neogi, T. Osteoarthritis: Is it a disease of cartilage or of bone? Arthritis Rheum. 2004, 50, 341–344. [Google Scholar] [CrossRef]
- Steadman, J.R.; Rodkey, W.G.; Rodrigo, J.J. Microfracture: Surgical technique and rehabilitation to treat chondral defects. Clin. Orthop. Relat. Res. 2001, 391, S362–S369. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Jones, K.J.; Cash, B.M. Matrix-induced autologous chondrocyte implantation with autologous bone grafting for osteochondral lesions of the femoral trochlea. Arthrosc. Tech. 2019, 8, 259–266. [Google Scholar] [CrossRef]
- Kon, E.; Roffi, A.; Filardo, G.; Tesei, G.; Marcacci, M. Scaffold-based cartilage treatments: With or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy 2015, 31, 767–775. [Google Scholar] [CrossRef]
- Carey, J.L.; Remmers, A.E.; Flanigan, D.C. Use of MACI (autologous cultured chondrocytes on porcine collagen membrane) in the United States: Preliminary experience. Orthop. J. Sport. Med. 2020, 8, 2325967120941816. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ma, Y.; Min, Y.; Sun, J.; Shi, X.; Gao, G.; Wang, J. Progress and prospect of technical and regulatory challenges on tissue-engineered cartilage as therapeutic combination product. Bioact. Mater. 2023, 20, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Eschweiler, J.; Goetze, C.; Tingart, M.; Maffulli, N. Membrane scaffolds for matrix-induced autologous chondrocyte implantation in the knee: A systematic review. Br. Med. Bull. 2021, 16, 50–61. [Google Scholar] [CrossRef]
- Howell, M.; Liao, Q.; Gee, C.W. Surgical management of osteochondral defects of the knee: An educational review. Curr. Rev. Musculoskelet. Med. 2021, 14, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Baugé, C.; Duval, E.; Ollitrault, D.; Girard, N.; Leclercq, S.; Galéra, P. Type II TGFβ receptor modulates chondrocyte phenotype. Age 2013, 35, 1105–1116. [Google Scholar] [CrossRef]
- Peterson, L.; Cole, B.J. Cartilage Repair Strategies; Springer Science & Business Media: Medford, MA, USA, 2014; Volume 1. [Google Scholar]
| Hyaline Cartilage | Hypertrophic Cartilage | Fibrocartilage |
|---|---|---|
| SOX9, SOX5, SOX6 | COL10 | COL1 |
| COL2, COL9, COL11 | MEF2C | COL3 |
| ACAN | RUNX2 | α-Smooth muscle actin (α-SMA) |
| RUNX1 | RUNX3 | Fibroblast-specific protein 1 (FSP1/S100A4) |
| Small novel rich in cartilage (SNORC) | - | TIMP1, Cell migration-inducing protein (CEMIP) |
| WW domain-containing protein 2 (WWP2), MicroRNA-140 (miR-140) | - | - |
| Abbreviation Glossary | Acronym |
|---|---|
| Advanced glycation end products | AGE |
| Aggrecan | ACAN |
| Ascorbic acid | AA |
| Autologous chondrocyte implantation | ACI |
| Bone morphogenetic protein | BMP |
| Chondroitin sulfate | CS |
| Collagen type I | COL1 |
| Collagen type II | COL2 |
| Collagen type II alpha-1 gene | Col2A1 |
| Drosophila mothers against decapentaplegic proteins | Smad |
| Extracellular matrix | ECM |
| Fibroblast growth factor | FGF |
| Gelatin methacryloyl | GelMA |
| Glycosaminoglycans | GAGs |
| High-mobility group box | HMGB |
| Intercellular adhesion molecule 1 | ICAM-1 (CD54) |
| Keratan sulfate | KS |
| Matrix metalloproteinases | MMPs |
| Matrix-induced autologous chondrocyte implantation | MACI |
| Mesenchymal stem cells | MSCs |
| Mitogen-activated protein kinases | MAPK |
| Osteoarthritis | OA |
| Synthetic polyethylene glycol | PEG |
| Polycaprolactone | PLC |
| Polyglycolic acid | PGA |
| Proteoglycan-4 | PRG4 |
| Reactive oxygen species | ROS |
| Retinoid acid | RA |
| Retinoid acid receptor | RAR |
| Runt-related transcription factor 1 | RUNX11 |
| Small Novel Rich in Cartilage | Snorc |
| SRY-Box Transcription Factor | SOX |
| Tissue metallopeptidase inhibitor 1 | TIMP1 |
| Toll-like receptor | TLR |
| Transforming growth factor beta | TGF-β2 |
| Vascular cell adhesion molecule 1 | VCAM-1 (CD106) |
| Vascular endothelial growth factor | VEGF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bačenková, D.; Trebuňová, M.; Demeterová, J.; Živčák, J. Human Chondrocytes, Metabolism of Articular Cartilage, and Strategies for Application to Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 17096. https://doi.org/10.3390/ijms242317096
Bačenková D, Trebuňová M, Demeterová J, Živčák J. Human Chondrocytes, Metabolism of Articular Cartilage, and Strategies for Application to Tissue Engineering. International Journal of Molecular Sciences. 2023; 24(23):17096. https://doi.org/10.3390/ijms242317096
Chicago/Turabian StyleBačenková, Darina, Marianna Trebuňová, Jana Demeterová, and Jozef Živčák. 2023. "Human Chondrocytes, Metabolism of Articular Cartilage, and Strategies for Application to Tissue Engineering" International Journal of Molecular Sciences 24, no. 23: 17096. https://doi.org/10.3390/ijms242317096
APA StyleBačenková, D., Trebuňová, M., Demeterová, J., & Živčák, J. (2023). Human Chondrocytes, Metabolism of Articular Cartilage, and Strategies for Application to Tissue Engineering. International Journal of Molecular Sciences, 24(23), 17096. https://doi.org/10.3390/ijms242317096






