Preparation and Activity of Hemostatic and Antibacterial Dressings with Greige Cotton/Zeolite Formularies Having Silver and Ascorbic Acid Finishes
Abstract
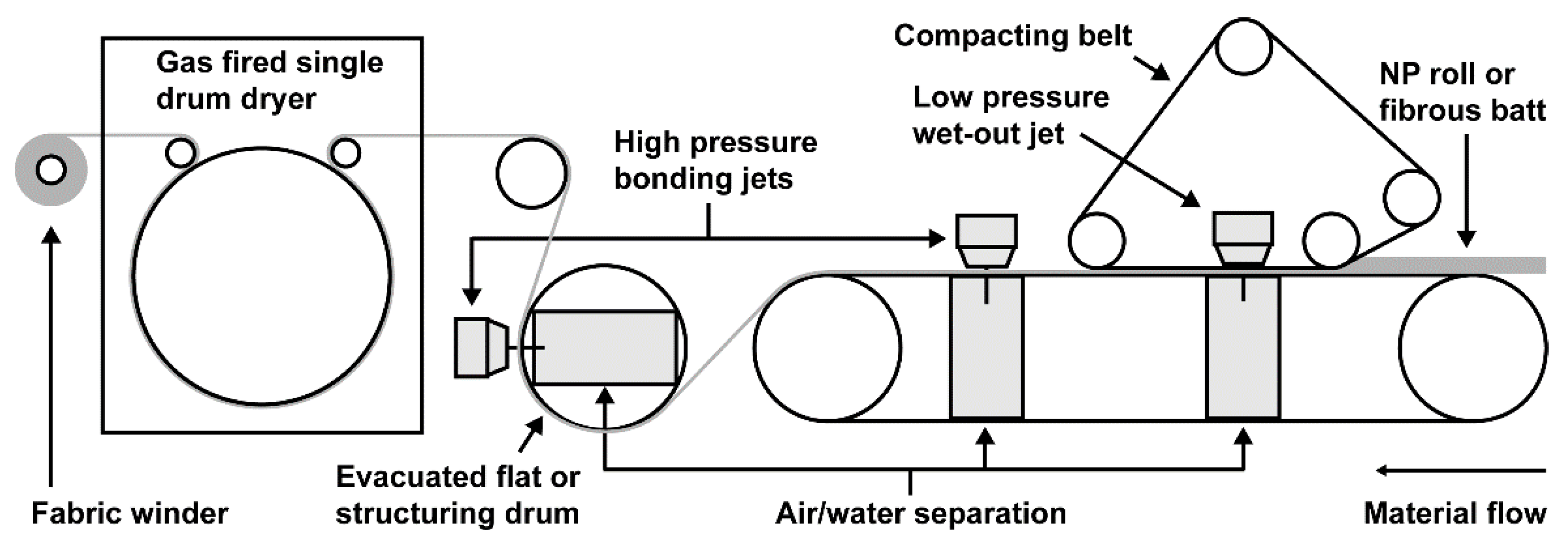
:1. Introduction
2. Results
2.1. Antibacterial Activity
| Fabric Name | Abbreviation | Fabric Treatment |
|---|---|---|
| TACGauze | TGz | None |
| BIOGauze | BGz | Ascorbic acid on TACGauze applied using the pad-dry-cure method |
| Citrate TACGauze | CXTGz | Crosslinking citric acid and ascorbic acid to TACGauze using the pad-dry-cure method |
| Silver “TACGauze” | AgTGz | Silver “TACGauze” version manufactured at SRRC using cotton fibers embedded with silver nanoparticles 1 |
| BIOGauze with zeolite | BGz +Y | Pad-dry application of ammonium Y zeolite (NH4Y) formulation to BIOGauze |
| Citrate TACGauze with zeolite | CXTGz +Y | Pad-dry application of NH4Y zeolite formulation to citrate gauze |
| Silver “TACGauze” with zeolite | AgTGz +Y | Pad-dry application of NH4Y zeolite formulation to AgTGz |
| TACGauze with zeolite | TGz +Y | Pad-dry application of NH4Y zeolite formulation to TACGauze |
| Sample | K. pneumoniae (4352) | S. aureus (6538) |
|---|---|---|
| % Reduction after 24 h | ||
| TGz | 0 | 0 |
| BGz | >99.99 | >99.99 |
| CXTGz | >99.99 | >99.99 |
| AgTGz | >99.99 | >99.99 |
| BGz +Y | >99.99 | >99.99 |
| CXTGz +Y | >99.99 | >99.99 |
| AgTGz +Y | >99.99 | >99.99 |
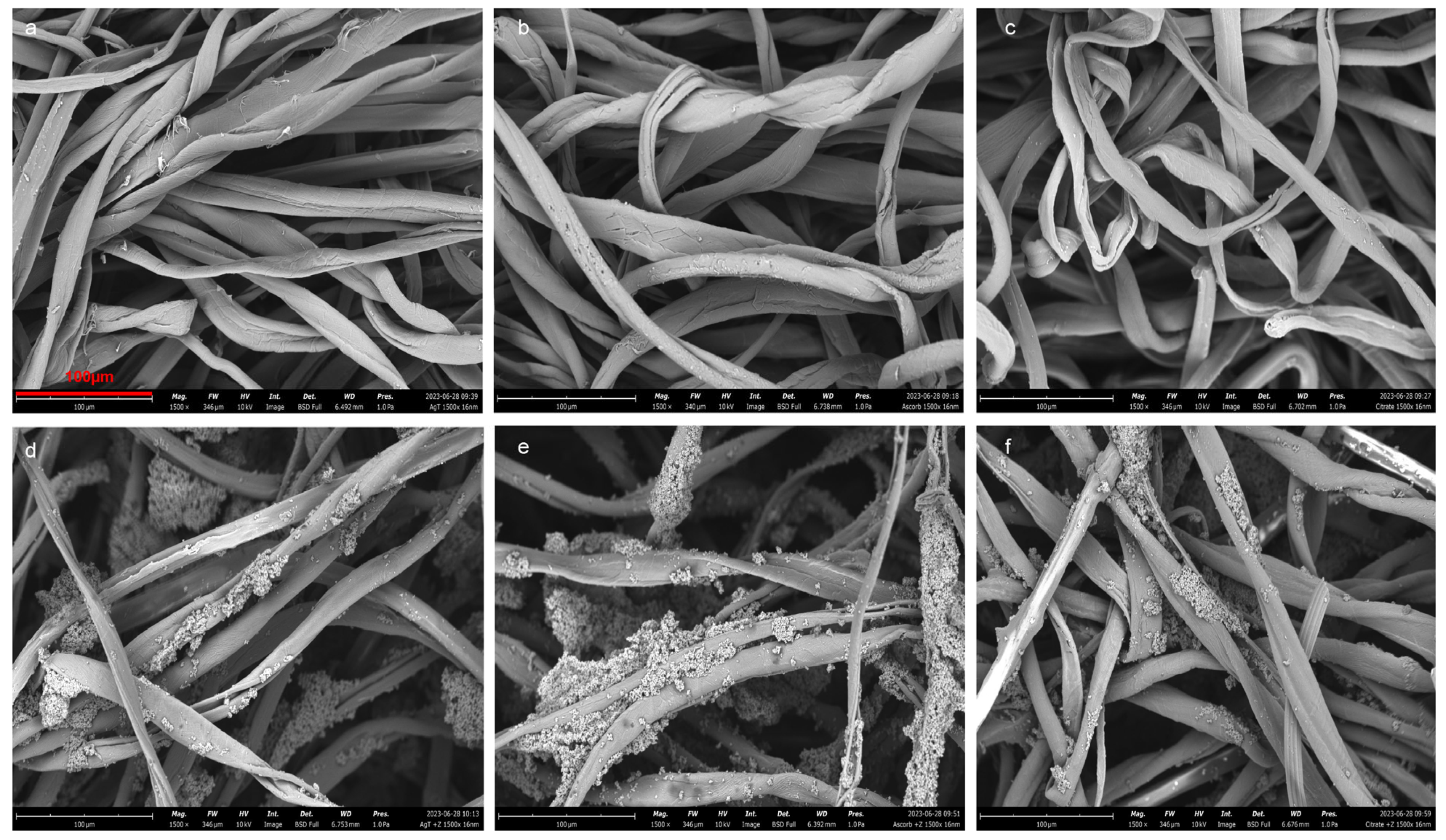
2.2. Scanning Electron Microscopy (SEM)
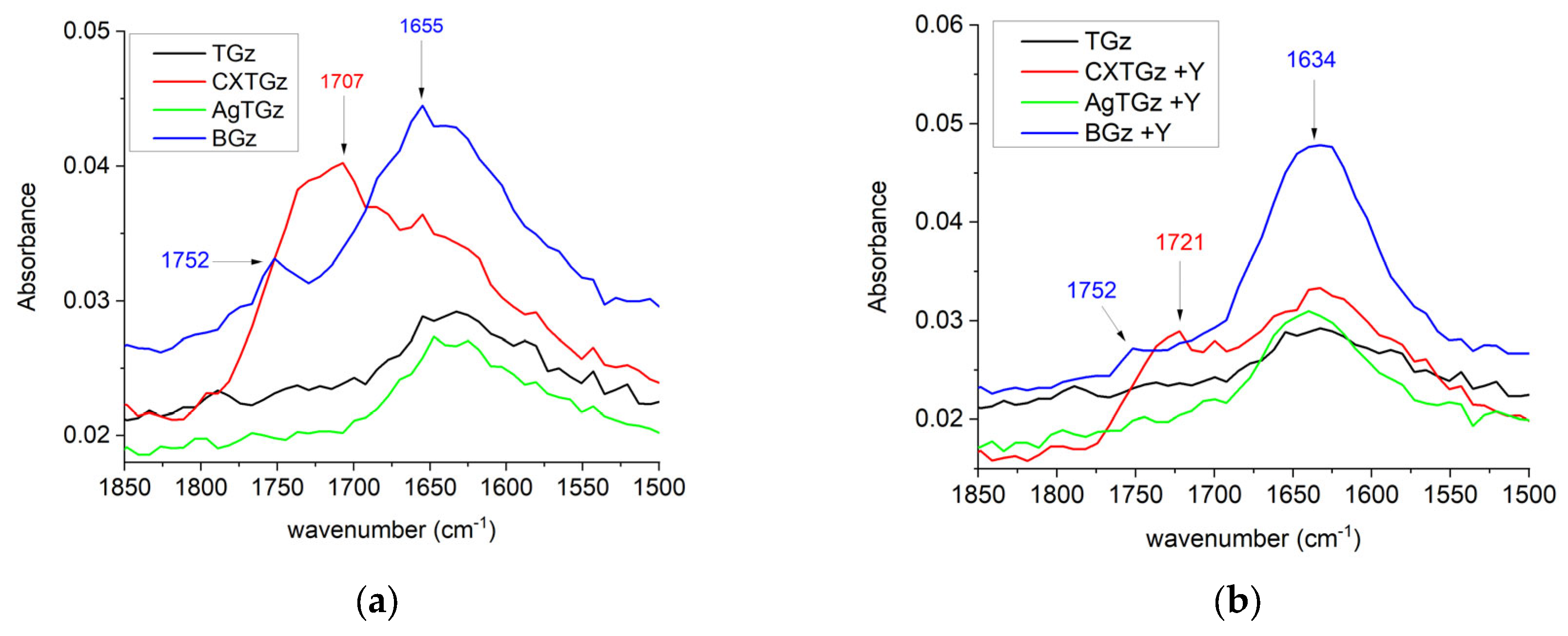
2.3. Fourier-Transform Infrared Spectroscopy (FT-IR)
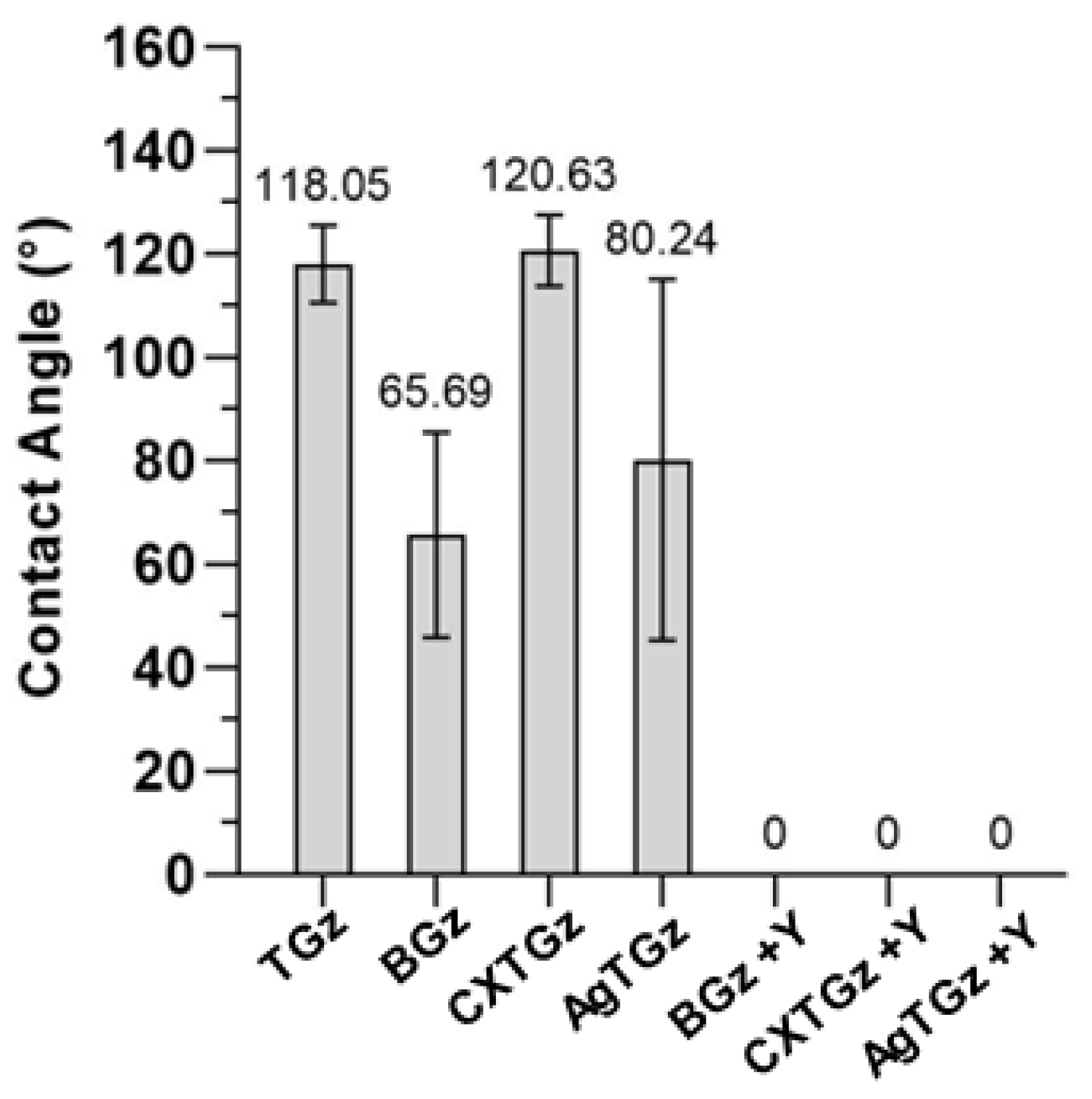
2.4. Contact Angle
2.5. Hydrogen Peroxide Generation
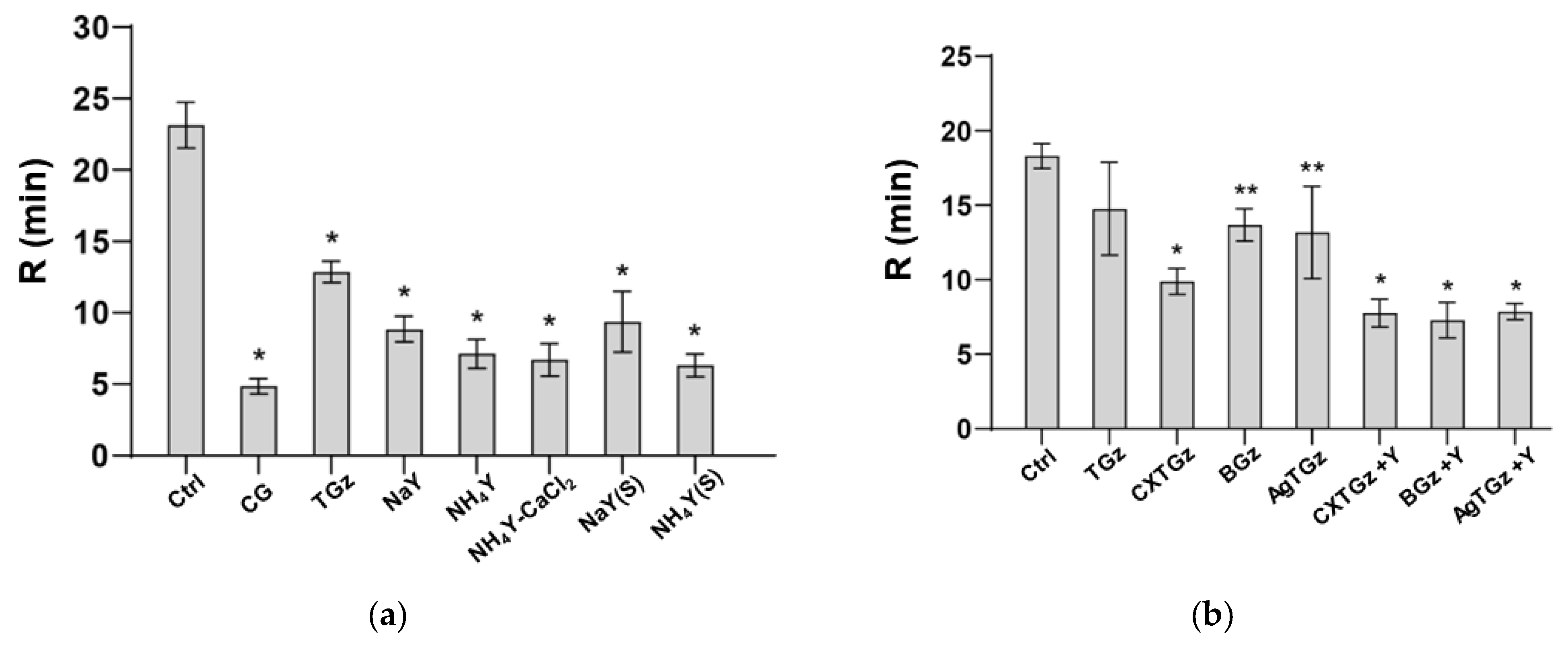
2.6. Thromboelastography-Monitored Fibrin Formation and Absorption Capacity
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Production of Silver TACGauze (AgTGz) Fabric
4.2.1. Synthesis of Silver Nanoparticle-Embedded Cotton Fibers
4.2.2. Nonwoven Fabric Production of Silver Nanoparticle-Embedded TGz
4.3. Fabric Treatment Methods
4.3.1. BIOGauze (BGz) and Citrate TACGauze (CXTGz) Production
4.3.2. Zeolite-Treated Gauze
4.4. Hydrogen Peroxide Assays
4.5. Scanning Electron Microscopy (SEM)
4.6. Fourier-Transform Infrared Spectroscopy (FTIR)
4.7. Contact Angle
4.8. Thromboelastography
4.9. Liquid Absorbent Capacity
4.10. Antibacterial Testing of Fabrics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eguia, E.; Bunn, C.; Kulshrestha, S.; Markossian, T.; Durazo-Arvizu, R.; Baker, M.S.; Gonzalez, R.; Behzadi, F.; Churpek, M.; Joyce, C.; et al. Trends, Cost, and Mortality from Sepsis After Trauma in the United States: An Evaluation of the National Inpatient Sample of Hospitalizations, 2012–2016. Crit. Care Med. 2020, 48, 1296–1303. [Google Scholar] [CrossRef] [PubMed]
- Katzenell, U.; Ash, N.; Tapia, A.L.; Campino, G.A.; Glassberg, E. Analysis of the Causes of Death of Casualties in Field Military Setting. Mil. Med. 2012, 177, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Keenan, S.; Riesberg, J.C. Prolonged Field Care: Beyond the “Golden Hour”. Wilderness Environ. Med. 2017, 28, S135–S139. [Google Scholar] [CrossRef] [PubMed]
- Crosby, H.A.; Kwiecinski, J.; Horswill, A.R. Staphylococcus aureus Aggregation and Coagulation Mechanisms, and Their Function in Host-Pathogen Interactions. Adv. Appl. Microbiol. 2016, 96, 1–41. [Google Scholar]
- Kwiecinski, J.M.; Horswill, A.R. Staphylococcus aureus bloodstream infections: Pathogenesis and regulatory mechanisms. Curr. Opin. Microbiol. 2020, 53, 51–60. [Google Scholar] [CrossRef]
- Liesenborghs, L.; Verhamme, P.; Vanassche, T. Staphylococcus aureus, master manipulator of the human hemostatic system. J. Thromb. Haemost. 2018, 16, 441–454. [Google Scholar] [CrossRef]
- Rapp, J.; Plackett, T.P.; Crane, J.; Lu, J.; Hall, A.; Hardin, D.M.; Loos, P.E.; Kelly, R.; Murray, C.K.; Keenan, S.; et al. Acute Traumatic Wound Management in the Prolonged Field Care Setting. J. Spec. Oper. Med. A Peer Rev. J. SOF Med. Prof. 2017, 17, 132–149. [Google Scholar] [CrossRef]
- Khan, M.A.; Mujahid, M. A review on recent advances in chitosan based composite for hemostatic dressings. Int. J. Biol. Macromol. 2019, 124, 138–147. [Google Scholar] [CrossRef]
- Lewis, K.M.; Spazierer, D.; Urban, M.D.; Lin, L.; Redl, H.; Goppelt, A. Comparison of regenerated and non-regenerated oxidized cellulose hemostatic agents. Eur. Surg. 2013, 45, 213–220. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, F.; Huang, Y. Comparative Evaluation of Biological Performance, Biosecurity, and Availability of Cellulose-Based Absorbable Hemostats. Clin. Appl. Thromb. Hemost. 2018, 24, 566–574. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Gaware, V.S.; Lieder, R.; Jónsdóttir, S.; Hjálmarsdóttir, M.Á.; Sigurjonsson, O.E.; Másson, M. The Effect of Substituent, Degree of Acetylation and Positioning of the Cationic Charge on the Antibacterial Activity of Quaternary Chitosan Derivatives. Mar. Drugs 2014, 12, 4635–4658. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Su, Z.; Hu, Y.; Meng, L.; Zhu, F.; Xie, B.; Wan, J.; Wu, Q. Mussel-inspired methacrylated gelatin-dopamine/quaternized chitosan/glycerin sponges with self-adhesion, antibacterial activity, and hemostatic ability for wound dressings. Int. J. Biol. Macromol. 2023, 241, 124102. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Dai, L.; Si, C.; Zeng, Z. Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers. Carbohydr. Polym. 2018, 195, 63–70. [Google Scholar] [CrossRef]
- Goodarz, M.; Behzadnia, A.; Mohammadi, H. Development of cellulosic-based hemostatic dressing with antibacterial activity. Fash. Text. 2022, 9, 30. [Google Scholar] [CrossRef]
- Brand, C.L.; Galsgaard, E.D.; Tornehave, D.; Rømer, J.; Gotfredsen, C.F.; Wassermann, K.; Knudsen, L.B.; Vølund, A.; Sturis, J. Synergistic effect of the human GLP-1 analogue liraglutide and a dual PPARalpha/gamma agonist on glycaemic control in Zucker diabetic fatty rats. Diabetes Obes. Metab. 2009, 11, 795–803. [Google Scholar] [CrossRef]
- Yang, H.; Liang, Y.; Wang, J.; Li, Q.; Li, Q.; Tang, A.; Liu, Y.; Liu, H.-B. Multifunctional wound dressing for rapid hemostasis, bacterial infection monitoring and photodynamic antibacterial therapy. Acta Biomater. 2021, 135, 179–190. [Google Scholar] [CrossRef]
- Tamer, T.M.; Alsehli, M.H.; Omer, A.M.; Afifi, T.H.; Sabet, M.M.; Mohy-Eldin, M.S.; Hassan, M.A. Development of Polyvinyl Alcohol/Kaolin Sponges Stimulated by Marjoram as Hemostatic, Antibacterial, and Antioxidant Dressings for Wound Healing Promotion. Int. J. Mol. Sci. 2021, 22, 13050. [Google Scholar] [CrossRef]
- Gerling, K.A.; Kersey, A.J.; Lauria, A.L.; Mares, J.A.; Hutzler, J.D.; White, P.W.; Abel, B.; Burmeister, D.M.; Propper, B.; White, J.M. Evaluation of novel hemostatic agents in a coagulopathic swine model of junctional hemorrhage. J. Trauma Acute Care Surg. 2023, 95, S144–S151. [Google Scholar] [CrossRef]
- Peng, H.T. Hemostatic agents for prehospital hemorrhage control: A narrative review. Mil. Med. Res. 2020, 7, 13. [Google Scholar] [CrossRef]
- Boulton, A.J.; Lewis, C.T.; Naumann, D.N.; Midwinter, M.J. Prehospital haemostatic dressings for trauma: A systematic review. Emerg. Med. J. 2018, 35, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Graves, E.; Prevost, N.; Condon, B.; Yager, D.; Dacorta, J.; Bopp, A. Development of a Nonwoven Hemostatic Dressing Based on Unbleached Cotton: A De Novo Design Approach. Pharmaceutics 2020, 12, 609. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Prevost, N.; Yager, D.; Nam, S.; Graves, E.; Santiago, M.; Condon, B.; Dacorta, J. Antimicrobial and Hemostatic Activities of Cotton-Based Dressings Designed to Address Prolonged Field Care Applications. Mil. Med. 2021, 186, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Prevost, N.T.; Cintron, M.S. A Comparison of Hemostatic Activities of Zeolite-Based Formulary Finishes on Cotton Dressings. J. Funct. Biomater. 2023, 14, 255. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.T.; Yager, D.; Mackin, R.; Santiago, M.; Chang, S.; Condon, B.; Dacorta, J. Ascorbic Acid as an Adjuvant to Unbleached Cotton Promotes Antimicrobial Activity in Spunlace Nonwovens. Int. J. Mol. Sci. 2022, 23, 3598. [Google Scholar] [CrossRef]
- Nam, S.; Hillyer, M.B.; He, Z.; Chang, S.; Edwards, J.V. Self-induced transformation of raw cotton to a nanostructured primary cell wall for a renewable antimicrobial surface. Nanoscale Adv. 2022, 4, 5404–5416. [Google Scholar] [CrossRef]
- Nam, S.; Hinchliffe, D.J.; Hillyer, M.B.; Gary, L.; He, Z. Washable Antimicrobial Wipes Fabricated from a Blend of Nanocomposite Raw Cotton Fiber. Molecules 2023, 28, 1051. [Google Scholar] [CrossRef]
- Bichara, L.C.; Lanús, H.E.; Brandán, S.A. First oxidation product of vitamin c, the dehydro-l-ascorbic acid dimer. study based on FTIR-Raman and DFT calculations. J. Chem. Chem. Eng. Data 2011, 5, 936–945. [Google Scholar]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.T.; Santiago, M.; von Hoven, T.; Condon, B.D.; Qureshi, H.; Yager, D.R. Hydrogen Peroxide Generation of Copper/Ascorbate Formulations on Cotton: Effect on Antibacterial and Fibroblast Activity for Wound Healing Application. Molecules 2018, 23, 2399. [Google Scholar] [CrossRef]
- Montagna, M.T.; Triggiano, F.; Barbuti, G.; Bartolomeo, N.; De Giglio, O.; Diella, G.; Lopuzzo, M.; Rutigliano, S.; Serio, G.; Caggiano, G. Study on the In Vitro Activity of Five Disinfectants against Nosocomial Bacteria. Int. J. Environ. Res. Public Health 2019, 16, 1895. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Ma, J.; Li, N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly(AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr. Polym. 2011, 84, 76–82. [Google Scholar] [CrossRef]
- Pusateri, A.E.; Holcomb, J.B.; Kheirabadi, B.S.; Alam, H.B.; Wade, C.E.; Ryan, K.L. Making sense of the preclinical literature on advanced hemostatic products. J. Trauma 2006, 60, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Derakhshankhah, H.; Hosseini, A.; Taghavi, F.; Jafari, S.; Lotfabadi, A.; Ejtehadi, M.R.; Shahbazi, S.; Fattahi, A.; Ghasemi, A.; Barzegari, E.; et al. Molecular interaction of fibrinogen with zeolite nanoparticles. Sci. Rep. 2019, 9, 1558. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Graves, E.; Bopp, A.; Prevost, N.; Santiago, M.; Condon, B. Electrokinetic and Hemostatic Profiles of Nonwoven Cellulosic/Synthetic Fiber Blends with Unbleached Cotton. J. Funct. Biomater. 2014, 5, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.V.; Mao, N.; Russell, S.; Carus, E.; Condon, B.; Hinchliffe, D.; Gary, L.; Graves, E.; Bopp, A.; Wang, Y. Fluid handling and fabric handle profiles of hydroentangled greige cotton and spunbond polypropylene nonwoven topsheets. Proc. IMechE Part L J. Mater. Des. Appl. 2015, 230, 847–859. [Google Scholar]
- Evans-Nguyen, K.M.; Tolles, L.R.; Gorkun, O.V.; Lord, S.T.; Schoenfisch, M.H. Interactions of Thrombin with Fibrinogen Adsorbed on Methyl-, Hydroxyl-, Amine-, and Carboxyl-Terminated Self-Assembled Monolayers. Biochemistry 2005, 44, 15561–15568. [Google Scholar] [CrossRef]
- Rodrigues, S.N.; Gonçalves, I.C.; Martins, M.C.; Barbosa, M.A.; Ratner, B.D. Fibrinogen adsorption, platelet adhesion and activation on mixed hydroxyl-/methyl-terminated self-assembled monolayers. Biomaterials 2006, 27, 5357–5367. [Google Scholar] [CrossRef]
- Sperling, C.; Fischer, M.; Maitz, M.F.; Werner, C. Blood coagulation on biomaterials requires the combination of distinct activation processes. Biomaterials 2009, 30, 4447–4456. [Google Scholar] [CrossRef]
- Johne, J.; Blume, C.; Benz, P.M.; Pozgajová, M.; Ullrich, M.; Schuh, K.; Nieswandt, B.; Walter, U.; Renné, T. Platelets promote coagulation factor XII-mediated proteolytic cascade systems in plasma. Biol. Chem. 2006, 387, 173–178. [Google Scholar] [CrossRef]
- Naudin, C.; Burillo, E.; Blankenberg, S.; Butler, L.; Renné, T. Factor XII Contact Activation. Semin. Thromb. Hemost. 2017, 43, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Behrens, A.M.; Sikorski, M.J.; Kofinas, P. Hemostatic strategies for traumatic and surgical bleeding. J. Biomed. Mater. Res. A 2014, 102, 4182–4194. [Google Scholar] [CrossRef] [PubMed]
- Vogler, E.A.; Siedlecki, C.A. Contact activation of blood-plasma coagulation. Biomaterials 2009, 30, 1857–1869. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Travers, R.J.; Morrissey, J.H. How it all starts: Initiation of the clotting cascade. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, R.; Siedlecki, C.A.; Vogler, E.A. Autoactivation of blood factor XII at hydrophilic and hydrophobic surfaces. Biomaterials 2006, 27, 4325–4332. [Google Scholar] [CrossRef] [PubMed]
- Fathi, P.; Sikorski, M.; Christodoulides, K.; Langan, K.; Choi, Y.S.; Titcomb, M.; Ghodasara, A.; Wonodi, O.; Thaker, H.; Vural, M.; et al. Zeolite-loaded alginate-chitosan hydrogel beads as a topical hemostat. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Khoshmohabat, H.; Paydar, S.; Kazemi, H.M.; Dalfardi, B. Overview of Agents Used for Emergency Hemostasis. Trauma Mon. 2016, 21, e26023. [Google Scholar] [CrossRef]
- Tribble, D.R.; Murray, C.K.; Lloyd, B.A.; Ganesan, A.; Mende, K.; Blyth, D.M.; Petfield, J.L.; McDonald, J. After the Battlefield: Infectious Complications among Wounded Warriors in the Trauma Infectious Disease Outcomes Study. Mil. Med. 2019, 184, 18–25. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Norman, G.; Shi, C.; Westby, M.J.; Price, B.L.; McBain, A.J.; Dumville, J.C.; Cullum, N. Bacteria and bioburden and healing in complex wounds: A prognostic systematic review. Wound Repair Regen. 2021, 29, 466–477. [Google Scholar] [CrossRef]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Lineback, C.B.; Nkemngong, C.A.; Wu, S.T.; Li, X.; Teska, P.J.; Haley, F. Oliver. Hydrogen peroxide and sodium hypochlorite disinfectants are more effective against Staphylococcus aureus and Pseudomonas aeruginosa biofilms than quaternary ammonium compounds. Antimicrob. Resist. Infect. Control 2018, 7, 154. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, P.; Poulose, E. Silver nanoparticles: Mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 994–1003. [Google Scholar] [CrossRef]
- Mahaseth, T.; Kuzminov, A. Potentiation of hydrogen peroxide toxicity: From catalase inhibition to stable DNA-iron complexes. Mutat. Res./Rev. Mutat. Res. 2017, 773, 274–281. [Google Scholar] [CrossRef]
- Hron, R.J.; Hinchliffe, D.J.; Condon, B.D. Optimized hydroentanglement processing parameters for nonwoven fabrics composed entirely of cotton fibers. J. Eng. Fibers Fabr. 2020, 15, 1–11. [Google Scholar] [CrossRef]
- ISO/IEC 17025:2017(en); General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneva, Switzerland; International Electrotechnical Commission: Geneva, Switzerland, 2017.
- ISO17034:2016(en); General Requirements for the Competence of Reference Material Producers. International Organization for Standardization: Geneva, Switzerland, 2016.


| Sample | Liquid Absorptive Capacity | |
|---|---|---|
| % | SD 1 | |
| TGz | 1016.40 | 13.37 |
| BGz | 887.59 | 76.66 |
| CXTGz | 1017.16 | 22.48 |
| AgTGz | 787.52 | 12.66 |
| BGz +Y | 610.40 | 25.62 |
| CXTGz +Y | 698.06 | 43.94 |
| AgTGz +Y | 522.63 | 24.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edwards, J.V.; Prevost, N.T.; Hinchliffe, D.J.; Nam, S.; Chang, S.; Hron, R.J.; Madison, C.A.; Smith, J.N.; Poffenberger, C.N.; Taylor, M.M.; et al. Preparation and Activity of Hemostatic and Antibacterial Dressings with Greige Cotton/Zeolite Formularies Having Silver and Ascorbic Acid Finishes. Int. J. Mol. Sci. 2023, 24, 17115. https://doi.org/10.3390/ijms242317115
Edwards JV, Prevost NT, Hinchliffe DJ, Nam S, Chang S, Hron RJ, Madison CA, Smith JN, Poffenberger CN, Taylor MM, et al. Preparation and Activity of Hemostatic and Antibacterial Dressings with Greige Cotton/Zeolite Formularies Having Silver and Ascorbic Acid Finishes. International Journal of Molecular Sciences. 2023; 24(23):17115. https://doi.org/10.3390/ijms242317115
Chicago/Turabian StyleEdwards, J. Vincent, Nicolette T. Prevost, Doug J. Hinchliffe, Sunghyun Nam, SeChin Chang, Rebecca J. Hron, Crista A. Madison, Jade N. Smith, Chelsie N. Poffenberger, Michelle M. Taylor, and et al. 2023. "Preparation and Activity of Hemostatic and Antibacterial Dressings with Greige Cotton/Zeolite Formularies Having Silver and Ascorbic Acid Finishes" International Journal of Molecular Sciences 24, no. 23: 17115. https://doi.org/10.3390/ijms242317115
APA StyleEdwards, J. V., Prevost, N. T., Hinchliffe, D. J., Nam, S., Chang, S., Hron, R. J., Madison, C. A., Smith, J. N., Poffenberger, C. N., Taylor, M. M., Martin, E. J., & Dixon, K. J. (2023). Preparation and Activity of Hemostatic and Antibacterial Dressings with Greige Cotton/Zeolite Formularies Having Silver and Ascorbic Acid Finishes. International Journal of Molecular Sciences, 24(23), 17115. https://doi.org/10.3390/ijms242317115







