Neuron and Brain Maturation 2.0
Funding
Conflicts of Interest
References
- Obernier, K.; Alvarez-Buylla, A. Neural stem cells: Origin, heterogeneity and regulation in the adult mammalian brain. Development 2019, 146, dev156059. [Google Scholar] [CrossRef] [PubMed]
- Kast, R.J.; Levitt, P. Precision in the development of neocortical architecture: From progenitors to cortical networks. Prog. Neurobiol. 2019, 175, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Bond, A.M.; Ming, G.; Song, H. Adult mammalian neural stem cells and neurogenesis: Five decades later. Cell Stem Cell 2015, 17, 385–395. [Google Scholar] [CrossRef] [PubMed]
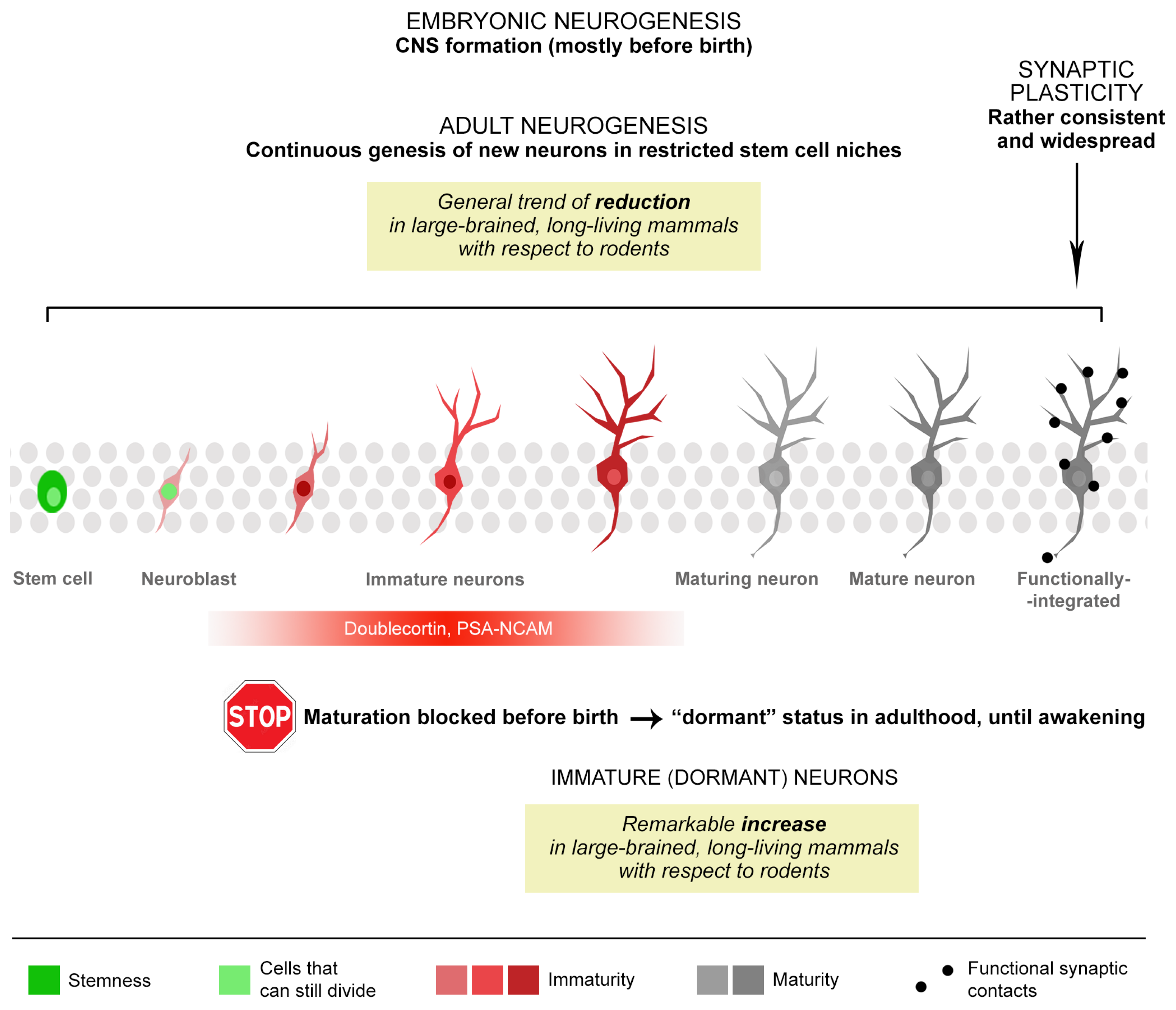
- Bonfanti, L.; Charvet, C.J. Brain plasticity in humans and model systems: Advances, challenges, and future directions. Int. J. Mol. Sci. 2021, 22, 9358. [Google Scholar] [CrossRef] [PubMed]
- Cushman, J.D.; Drew, M.R.; Krasne, F.B. The environmental sculpting hypothesis of juvenile and adult hippocampal neurogenesis. Prog. Neurobiol. 2021, 199, 101961. [Google Scholar] [CrossRef] [PubMed]
- Semënov, M.V. Adult hippocampal neurogenesis is a developmental process involved in cognitive development. Front. Neurosci. 2019, 13, 159. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G. Environmental enrichment, new neurons and the neurobiology of individuality. Nat. Rev. Neurosci. 2019, 20, 235–245. [Google Scholar] [CrossRef]
- Bao, H.; Song, J. Treating brain disorders by targeting adult neural stem cells. Trends Mol. Med. 2018, 24, 991–1006. [Google Scholar] [CrossRef]
- Bonfanti, L. Adult neurogenesis 50 years later: Limits and opportunities in mammals. Front. Neurosci. 2016, 10, 44. [Google Scholar] [CrossRef]
- Meyer, H.C.; Lee, F.S. Translating developmental neuroscience to understand risk for psychiatric disorders. Am. J. Psychiatry 2023, 180, 540–547. [Google Scholar] [CrossRef]
- Tooley, U.A.; Bassett, D.S.; Mackey, A.P. Environmental influences on the pace of brain development. Nat. Rev. Neurosci. 2021, 22, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Climent, M.A.; Castillo-Gómez, E.; Varea, E.; Guirado, R.; Blasco-Ibáñez, J.M.; Crespo, C.; Martínez-Guijarro, F.J.; Nácher, J. A population of prenatally generated cells in the rat paleocortex maintains an immature neuronal phenotype into adulthood. Cereb. Cortex 2008, 18, 2229–2240. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, B.; Reisinger, M.; Hochwartner, M.; Gabriele, G.; Jakubecova, D.; Benedetti, A.; Bonfanti, L.; Couillard-Despres, S. The awakening of dormant neuronal precursors in the adult and aged brain. Aging Cell 2023, 30, e13974. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Nguyen, T.; Ihrie, R.A.; Mirzadeh, Z.; Tsai, H.-H.; Wong, M.; Gupta, N.; Berger, M.S.; Huang, E.; Garcia-Verdugo, J.M.; et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature 2011, 478, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Paredes, M.F.; Sorrells, S.F.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Brain size and limits to adult neurogenesis. J. Comp. Neurol. 2016, 524, 646–664. [Google Scholar] [CrossRef]
- Brus, M.; Keller, M.; Lévy, F. Temporal features of adult neurogenesis: Differences and similarities across mammalian species. Front. Neurosci. 2013, 7, 135–144. [Google Scholar] [CrossRef]
- Kohler, S.J.; Williams, N.I.; Stanton, G.B.; Cameron, J.L.; Greenough, W.T. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc. Natl. Acad. Sci. USA 2011, 108, 10326–10331. [Google Scholar] [CrossRef]
- Sherwood, C.C.; Miller, S.B.; Molly, M.; Stimpson, C.D.; Phillips, K.A.; Jacobs, B.; Hof, P.R.; Raghanti, M.A.; Smaers, J.B. Invariant synapse density and neuronal connectivity scaling in primate neocortical evolution. Cereb. Cortex 2020, 30, 5604–5615. [Google Scholar] [CrossRef]
- De Felipe, J.; Alonso-Nanclares, L.; Arellano, J.I. Microstructure of the neocortex: Comparative aspects. J. Neurocytol. 2002, 31, 299–316. [Google Scholar] [CrossRef]
- Wang, L.; Pang, K.; Zhou, L.; Cebrián-Silla, A.; González-Granero, S.; Wang, S.; Bi, Q.; White, M.L.; Ho, B.; Li, J.; et al. A cross-species proteomic map reveals neoteny of human synapse development. Nature 2023, 622, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-N.; Hu, D.-D.; Cai, X.-L.; Wang, Y.; Yang, C.; Jiang, J.; Zhang, Q.-L.; Tu, T.; Wang, X.-S.; Wang, H.; et al. Doublecortin expressing neurons in human cerebral cortex layer II and amygdala from infancy to 100 year-old. Mol. Neurobiol. 2023, 60, 3464–3485. [Google Scholar] [CrossRef] [PubMed]
- Bradke, F.; Di Giovanni, S.; Fawcett, J. Neuronal maturation: Challenges and opportunities in a nascent field. Trends Neurosci. 2020, 43, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, F.; Eisinger, B.E.; Kelnhofer, L.E.; Jobe, E.M.; Zhao, X. Integrative single-cell transcriptomics reveals molecular networks defining neuronal maturation during postnatal neurogenesis. Cereb. Cortex 2017, 27, 2064–2077. [Google Scholar] [CrossRef]
- Maffezzini, C.; Calvo-Garrido, J.; Wredenberg, A.; Freyer, C. Metabolic regulation of neurodifferentiation in the adult brain. Cell. Mol. Life Sci. 2020, 77, 2483–2496. [Google Scholar] [CrossRef]
- Gunz, P.; Neubauer, S.; Falk, D.; Tafforeau, P.; Le Cabec, A.; Smith, T.M.; Kimbel, W.H.; Spoor, F.; Alemseged, Z. Australopithecus afarensis endocasts suggest ape-like brain organization and prolonged brain growth. Sci. Adv. 2020, 6, eaaz4729. [Google Scholar] [CrossRef]
- de Sousa, A.A.; Rigby Dames, B.A.; Graff, E.C.; Mohamedelhassan, R.; Vassilopoulos, T.; Charvet, C.J. Going beyond established model systems of Alzheimer’s disease: Companion animals provide novel insights into the neurobiology of aging. Commun. Biol. 2023, 6, 655. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonfanti, L.; Couillard-Després, S. Neuron and Brain Maturation 2.0. Int. J. Mol. Sci. 2023, 24, 17113. https://doi.org/10.3390/ijms242317113
Bonfanti L, Couillard-Després S. Neuron and Brain Maturation 2.0. International Journal of Molecular Sciences. 2023; 24(23):17113. https://doi.org/10.3390/ijms242317113
Chicago/Turabian StyleBonfanti, Luca, and Sébastien Couillard-Després. 2023. "Neuron and Brain Maturation 2.0" International Journal of Molecular Sciences 24, no. 23: 17113. https://doi.org/10.3390/ijms242317113
APA StyleBonfanti, L., & Couillard-Després, S. (2023). Neuron and Brain Maturation 2.0. International Journal of Molecular Sciences, 24(23), 17113. https://doi.org/10.3390/ijms242317113





