Serotonin and Interleukin 10 Can Influence the Blood and Urine Viscosity in Gestational Diabetes Mellitus and Pregnancy-Specific Urinary Incontinence
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
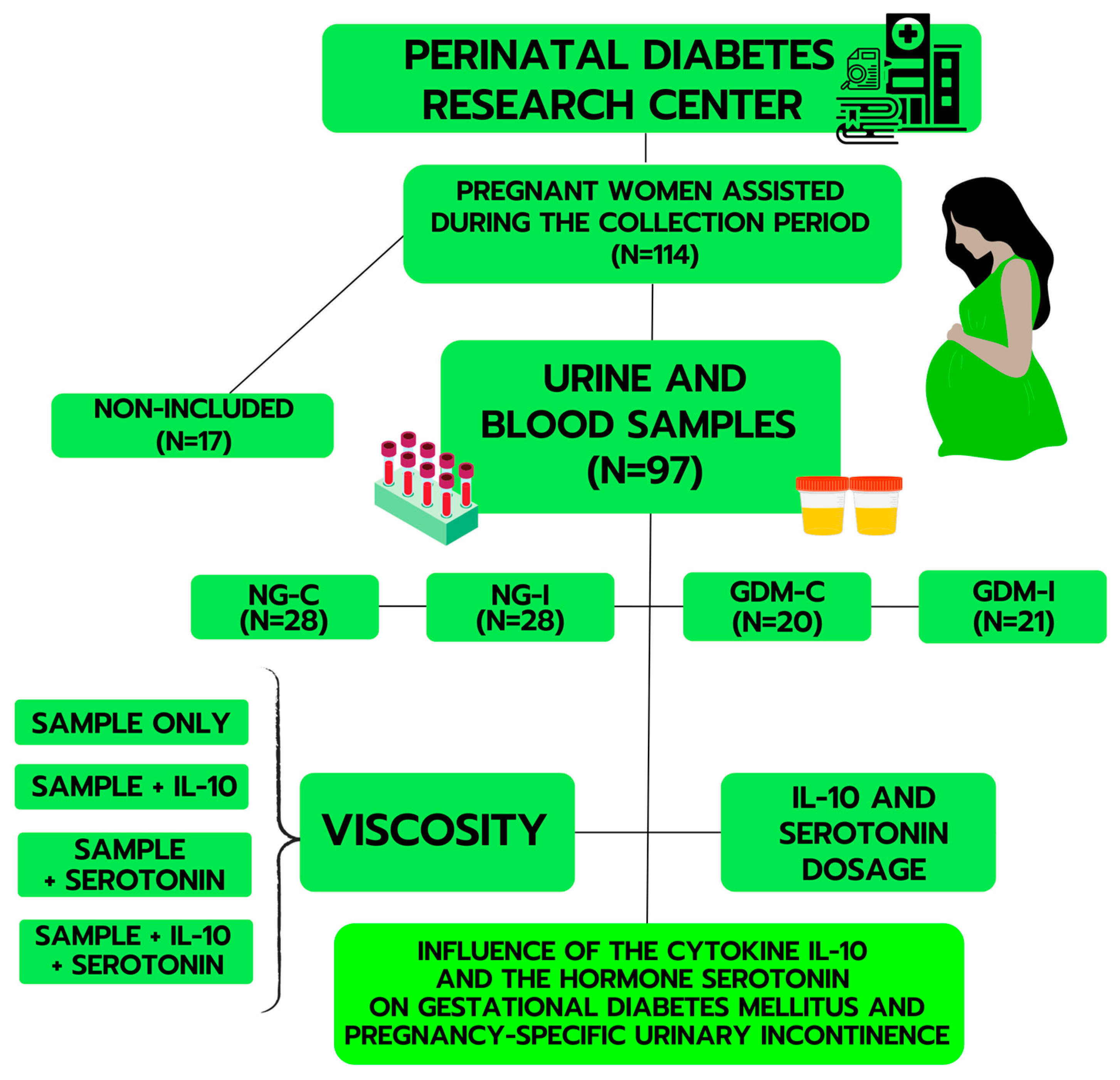
4.1. Study Design and Participants
4.2. Blood and Plasma Separation
4.3. Urine Separation
4.4. Plasma Glucose Determination
4.5. Hematological Parameters
4.6. Serotonin Determination
4.7. IL-10 Cytokine Determination
4.8. Hemorheological Parameters
4.9. Treatment of Blood with IL-10 and Serotonin
4.10. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fagundes, D.L.G.; França, E.L.; Fernandes, R.T.S.; Hara, C.C.P.; Morceli, G.; Honorio-França, A.C.; Calderon, I.M.P. Changes in T-cell phenotype and cytokines profile in maternal blood, cord blood and colostrum of diabetic mothers. J. Matern. Fetal Neonatal Med. 2016, 29, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Perić, M.; Horvatiček, M.; Tandl, V.; Bečeheli, I.; Majali-Martinez, A.; Desoye, G.; Štefulj, J. Glucose, Insulin and Oxygen Modulate Expression of Serotonin-Regulating Genes in Human First-Trimester Trophoblast Cell Line ACH-3P. Biomedicines 2023, 11, 1619. [Google Scholar] [CrossRef]
- França, D.C.H.; França, E.L.; Sobrevia, L.; Barbosa, A.M.P.; Honorio-França, A.C.; Rudge, M.V.C. Integration of nutrigenomics, melatonin, serotonin and inflammatory cytokines in the pathophysiology of pregnancy-specific urinary incontinence in women with gestational diabetes mellitus. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2023, 1869, 166737. [Google Scholar] [CrossRef]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012, 37, 137–162. [Google Scholar] [CrossRef]
- Pillalamarri, N.; Shalom, D.F.; Pilkinton, M.L.; Winkler, H.A.; Chatterjee, P.K.; Solanki, M.; Metz, C.N. Inflammatory Urinary Cytokine Expression and Quality of Life in Patients with Overactive Bladder. Female Pelvic. Med. Reconstr. Surg. 2018, 24, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Chen, X.; Chen, H. Association of Single Nucleotide Polymorphisms of the IL-6, IL-10, and TNF-α Genes with Susceptibility to Gestational Diabetes Mellitus. Genet. Test. Mol. Biomark. 2020, 7, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Herr, N.; Bode, C.; Duerschmied, D. The effects of serotonin in immune cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef]
- Romih, R.; Winder, M.; Lee, G. Recent advances in the biology of the urothelium and applications for urinary bladder dysfunction. BioMed Res. Int. 2014, 2014, 341787. [Google Scholar] [CrossRef]
- Sakai, T.; Kasahara, K.; Tomita, K.; Ikegaki, I.; Kuriyama, H. 5-Hydroxytryptamine-induced bladder hyperactivity via the 5-HT2Areceptor in partial bladder outlet obstruction in rats. Am. J. Physiol. Renal Physiol. 2013, 7, F1020–F1027. [Google Scholar] [CrossRef]
- Yokoyama, T.; Saino, T.; Nakamuta, N.; Yamamoto, Y. Topographic distribution of serotonin-immunoreactive urethral endocrine cells and their relationship with calcitonin gene-related peptide-immunoreactive nerves in male rats. Acta Histochem. 2017, 119, 78–83. [Google Scholar] [CrossRef]
- Sacramento, P.M.; Monteiro, C.; Dias, A.S.O.; Kasahara, T.M.; Ferreira, T.B.; Hygino, J.; Wing, A.C.; Andrade, R.M.; Rueda, F.; Sales, M.C.; et al. Serotonin decreases the production of Th1/Th17 cytokines and elevates the frequency of regulatory CD4+T-cell subsets in multiple sclerosis patients. Eur. J. Immunol. 2018, 48, 1376–1388. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, A.; Sakai-Saito, A.; Hattori, T.; Kanno-Saito, S.; Katano, Y.; Okada, T. Differences between young and aged rats in voiding frequency and detrusor muscle serotonergic contraction. Exp. Gerontol. 2019, 124, 110642. [Google Scholar] [CrossRef]
- Coelho, A.; Oliveira, R.; Cavaleiro, H.; Cruz, C.D.; Cruz, F. Evidence for an urethro-vesical crosstalk mediated by serotonin. Neurourol. Urodyn. 2018, 37, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Latorre, E.; Mendoza, C.; Matheus, N.; Castro, M.; Grasa, L.; Mesonero, J.E.; Alcalde, A.I. IL-10 modulates serotonin transporter activity and molecular expression in intestinal epithelial cells. Cytokine 2013, 3, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Huang, J.; Ni, J.; Si, J.; Gu, B.; Wang, Z.; Andersson, K.-E. Streptozotocin-induced diabetes causes upregulation of serotonin (5-HT)2A/C receptors in lumbosacral cord motoneurons and down regulation of serotonergic paraneurons in the urethra. Brain Res. 2019, 1715, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, K.; Mahmoudi, F. Association of the Effect of SLC6A4 Gene Polymorphisms on the Risk of Diabetes. Gene Cell Tissue 2023, 10, e127469. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging roles for serotonin in regulating metabolism: New implications for an ancient molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Abi-Chahin, T.C.; Hausen, M.d.A.; Mansano-Marques, C.M.; Halfoun, V.L. Microvascular reactivity in type 1 diabetics. Arq. Bras. Endocrinol. Metabol. 2009, 6, 741–746. [Google Scholar] [CrossRef]
- Walker, R.T.; Matrai, A.; Bogar, L.; Dormandy, J.A. Serotonin and the flow properties of blood. J. Cardiovasc. Pharmacol. 1985, 7, 35–37. [Google Scholar] [CrossRef]
- França, E.L.; Ribeiro, E.B.; Scherer, E.F.; Cantarini, D.G.; Pessôa, R.S.; França, F.L.; Honorio-França, A.C. Effects of Momordica charantia L. on the Blood Rheological Properties in Diabetic Patients. BioMed Res. Int. 2014, 2014, e840379. [Google Scholar] [CrossRef]
- Scherer, E.F.; Cantarini, D.G.; Siqueira, R.; Ribeiro, E.B.; Braga, É.M.; Honório-França, A.C.; França, E.L. Cytokine modulation of human blood viscosity from vivax malaria patients. Acta Trop. 2016, 158, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Arihan, O.; Yabanoglu, S.C.; Ucar, G.; Falkmarken, N.D. Effects of two selected SSRIs on hemorheological parameters in rats. Clin. Hemorheol. Microcirc. 2019, 1, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, C.J.; Hamilton, P.K.; McVeigh, K.A.; McVeigh, G.E. A cardiologist view of vascular disease in diabetes. Diabetes Obes. Metab. 2008, 4, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Fioretto, P.M.; Dodson, P. Does microvascular disease predict macrovascular events in type 2 diabetes? Atherosclerosis 2011, 1, 13–18. [Google Scholar] [CrossRef]
- Schwanke, C.H.A.; Bittencourt, L.; Noronha, J.A.P.; Augustin, S.A.J.; Jung, I.E.; Cruz, I.B.M. Is there an association between T102C polymorphism of the serotonin receptor 2A gene and urinary incontinence? Braz. J. Med. Biol. Res. 2007, 40, 1315–1322. [Google Scholar] [CrossRef]
- Leitner, M.; Fragner, L.; Danner, S.; Holeschofsky, N.; Leitner, K.; Tischler, S.; Doerfler, H.; Bachmann, G.; Sun, X.; Jaeger, W.; et al. Combined Metabolomic Analysis of Plasma and Urine Reveals AHBA, Tryptophan and Serotonin Metabolism as Potential Risk Factors in Gestational Diabetes Mellitus (GDM). Front. Mol. Biosci. 2017, 4, 84. [Google Scholar] [CrossRef]
- Barbosa, A.M.P.; Enriquez, E.M.A.; Rodrigues, M.R.K.; Prudencio, C.B.; Atallah, Á.N.; Reyes, D.R.A.; Hallur, R.L.S.; Nunes, S.K.; Pinheiro, F.A.; Filho, C.I.S.; et al. Effectiveness of the pelvic floor muscle training on muscular dysfunction and pregnancy specific urinary incontinence in pregnant women with gestational diabetes mellitus: A systematic review protocol. PLoS ONE 2020, 12, e0241962. [Google Scholar] [CrossRef]
- Reyes, D.R.A.; Barbosa, A.M.P.; Juliana, F.F.; Sofia, Q.B.C.V.; Costa, S.M.B.; Hallur, R.L.S.; Enriquez, E.M.A.; Oliveira, R.G.; Rossignolli, P.d.S.; Pedroni, C.R.; et al. Viability of ex-vivo myography as a diagnostic tool for rectus abdominis muscle electrical activity collected at Cesarean section within a diamater cohort study. Biomed. Eng. Online 2022, 1, 76. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, P.; Huang, Y.; Yang, X.; Sun, F.; Ye, Y.; Lai, Y.; Ouyang, J.; Wu, L.; Li, Y.; et al. BMI and lipidomic biomarkers with risk of gestational diabetes in pregnant women. Obesity 2022, 10, 2044–2054. [Google Scholar] [CrossRef]
- Song, X.; Wang, C.; Wang, T.; Zhang, S.; Qin, J. Obesity and risk of gestational diabetes mellitus: A two-sample Mendelian randomization study. Diabetes Res. Clin. Pract. 2023, 197, 110561. [Google Scholar] [CrossRef]
- Eidgahi, E.S.; Nasiri, M.; Kariman, N.; Ardebili, N.S.; Salehi, M.; Kazemi, M.; Zayeri, F. Diagnostic accuracy of first and early second trimester multiple biomarkers for prediction of gestational diabetes mellitus: A multivariate longitudinal approach. BMC Pregnancy Childbirth 2022, 1, 13. [Google Scholar] [CrossRef]
- Lyu, X.; Jia, J.; Yang, H.; Deng, Y.; Wu, H.; Wang, S.; Zhao, C.; Lei, J.; Zou, X.; Yang, Y.; et al. Hematological Parameters in the First Trimester and the Risk of Gestational Diabetes Mellitus—Beijing, China, 2017–2020. China CDC Wkly. 2023, 9, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Faerch, K.; Carstensen-Kirberg, M.; Lowe, G.D.; Haapakoski, R.; Witte, D.R.; Brunner, E.J.; Roden, M.; Tabák, A.G.; Kivimäki, M.; et al. Biomarkers of subclinical inflammation and increases in glycaemia, insulin resistance and beta-cell function in non-diabetic individuals: The Whitehall II study. Eur. J. Endocrinol. 2016, 175, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; Gomez-Pinilla, P.J.; Camello-Almaraz, C.; Martin-Cano, F.E.; Pascua, P.; Rol, M.A.; Acuna-Castroviejo, D.; Camello, P.J. Melatonin, a potential therapeutic agent for smooth muscle-related pathological conditions and aging. Curr. Med. Chem. 2010, 34, 4150–4165. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, B.R.; Liu, G.; Arioglu-Inan, E.; Michel, M.C. Established and emerging treatments for diabetes-associated lower urinary tract dysfunction. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 8, 887–906. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Chehrehgosha, M.; Sajjadi-Jazi, S.M.; Meftah, A.M. Tryptophan and serotonin levels as potent biomarkers in diabetes mellitus complications: A new approach of diagnostic role. J. Diabetes Metab. Disord. 2022, 2, 1923–1934. [Google Scholar] [CrossRef]
- de Groat, W.C.; Griffiths, D.; Yoshimura, N. Neural control of the lower urinary tract. Compr. Physiol. 2015, 1, 327–396. [Google Scholar] [CrossRef]
- Poonia, M.K.; Kaur, G.; Chintamaneni, M.; Changela, I. New insights into molecular targets for urinary incontinence. Indian J. Pharmacol. 2010, 5, 261–266. [Google Scholar] [CrossRef]
- Lee, K.-S.; NA, Y.-G.; Dean-Mckinney, T.; Klausner, A.P.; Tuttle, J.B.; Steers, W.D. Alterations in voiding frequency and cystometry in the clomipramine induced model of endogenous depression and reversal with fluoxetine. J. Urol. 2003, 5, 2067–2071. [Google Scholar] [CrossRef]
- William, D.S. Management of urinary incontinence in women. Drug Discov. Today Ther. Strateg. 2004, 2, 267–273. [Google Scholar]
- Michel, M.C.; Peters, S.L. Role of serotonin and noradrenaline in stress urinary incontinence. BJU Int. 2004, 94 (Suppl. S1), 23–30. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Almorós, A.; Hang, T.; Peiró, C.; Soriano-Guillén, L.; Egido, J.; Tuñón, J.; Lorenzo, Ó. Predictive and diagnostic biomarkers for gestational diabetes and its associated metabolic and cardiovascular diseases. Cardiovasc. Diabetol. 2019, 1, 140. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, P.; Fields, A.M.; Falcone, S.; Mesaros, C.; Susiarjo, M. Susceptibility to Low Vitamin B6 Diet-Induced Gestational Diabetes Is Modulated by Strain Differences in Mice. Endocrinology 2023, 10, bqad130. [Google Scholar] [CrossRef]
- Koopman, N.; Katsavelis, D.; Ten Hove, A.S.; Brul, S.; de Jonge, W.J.; Seppen, J. The Multifaceted Role of Serotonin in Intestinal Homeostasis. Int. J. Mol. Sci. 2021, 22, 9487. [Google Scholar] [CrossRef] [PubMed]
- Almaça, J.; Molina, J.; Menegaz, D.; Pronin, A.N.; Tamayo, A.; Slepak, V.; Berggren, P.-O.; Caicedo, A. Human Beta Cells Produce and Release Serotonin to Inhibit Glucagon Secretion from Alpha Cells. Cell Rep. 2016, 12, 3281–3291. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Ferchaud-Roucher, V.; Kaeffer, B.; Poupeau, G.; Castellano, B.; Darmaun, D. Maternal and fetal tryptophan metabolism in gestating rats: Effects of intrauterine growth restriction. Amino Acids 2016, 1, 281–290. [Google Scholar] [CrossRef]
- Glover, V. Prenatal stress and its effects on the fetus and the child: Possible underlying biological mechanisms. Adv. Neurobiol. 2015, 10, 269–283. [Google Scholar] [CrossRef]
- Tagoma, A.; Haller-Kikkatalo, K.; Oras, A.; Roos, K.; Kirss, A.; Uibo, R. Plasma cytokines during pregnancy provide insight into the risk of diabetes in the gestational diabetes risk group. J. Diabetes Investig. 2022, 9, 1596–1606. [Google Scholar] [CrossRef]
- Bahar, A.; Akha, O.; Bordbar, M.; Abediankenari, S.; Mohammadpoor, R.; Ehsani, Z.; Kashi, Z. Anti-Inflammatory Markers IL-10 and IL-35: Role in Developing Gestational Diabetes Mellitus. J. Clin. Diagn. Res. 2020, 8, OC01–OC03. [Google Scholar] [CrossRef]
- Fagundes, D.L.G.; França, E.L.; Morceli, G.; Rudge, M.V.C.; Calderon, I.d.M.P.; Honorio-França, A.C. The role of cytokines in the functional activity of phagocytes in blood and colostrum of diabetic mothers. Clin. Dev. Immunol. 2013, 2013, 590190. [Google Scholar] [CrossRef]
- Queiroz, A.A.; França, E.L.; Hara, C.C.P.; Honorio, M.S.; Fagundes, D.L.G.; Calderon, I.M.P.; Honorio-França, A.C. Phenotypic characterization of regulatory T cells populations in maternal blood, cord blood and placenta from diabetic mothers. J. Matern. Fetal Neonatal Med. 2019, 7, 1098–1104. [Google Scholar] [CrossRef] [PubMed]
- Louis, M.B.P.; França, D.C.H.; Queiroz, A.A.; Calderon, I.M.P.; França, E.L.; Honorio-França, A.C. Melatonin Hormone Acts on Cells of Maternal Blood and Placenta from Diabetic Mothers. Front. Physiol. 2022, 12, 765928. [Google Scholar] [CrossRef] [PubMed]
- Del Rosario-Capellan, M.L.; Carlos-Raboca, J.; Litonjua, A.D. Total sialic acid and other inflammatory markers as predictors of gestational diabetes. Philos. J. Intern. Med. 2009, 47, 878. [Google Scholar] [CrossRef]
- Cowan, A.Q.; Cho, D.J.; Rosenson, R.S. Importance of blood rheology in the pathophysiology of atherothrombosis. Cardiovasc. Drugs. Ther. 2012, 4, 339–348. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.H.; Ribeiro, A.A.L.; Deluque, A.L.; Cotrim, A.C.M.; de Marchi, P.G.F.; França, E.L.; Honorio-França, A.C. Effects of barium chloride adsorbed to polyethylene glycol (PEG) microspheres on co-culture of human blood mononuclear cell and breast cancer cell lines (MCF-7). Immunopharmacol. Immunotoxicol. 2018, 8, 18–24. [Google Scholar] [CrossRef]
- Luo, Y.; Acevedo, D.; Baños, N.; Pluma, A.; Castellanos-Moreira, R.; Moreno, E.; Rodríguez-García, S.; Deyà-Martínez, A.; García-García, A.; Quesada-Masachs, E.; et al. Expected impact of immunomodulatory agents during pregnancy: A newborn’s perspective. Pediatr. Allergy Immunol. 2023, 2, e13911. [Google Scholar] [CrossRef]
- Ribeiro, E.B.; Honorio-França, A.C.; França, E.L.; Soler, M.A. Design and Development of Nanoemulsion Systems Containing Interferon Gamma. Protein Pept. Lett. 2016, 7, 626–638. [Google Scholar] [CrossRef]
- Garcia-Prat, M.; Álvarez-Sierra, D.; Aguiló-Cucurull, A.; Salgado-Perandrés, S.; Briongos-Sebastian, S.; Franco-Jarava, C.; Martin-Nalda, A.; Colobran, R.; Montserrat, I.; Hernández-González, M.; et al. Extended immunophenotyping reference values in a healthy pediatric population. Cytom. Part B Clin. Cytom. 2019, 3, 223–233. [Google Scholar] [CrossRef]
- Scharschmidt, T.C.; Vasquez, K.S.; Truong, H.-A.; Gearty, S.V.; Pauli, M.L.; Nosbaum, A.; Gratz, I.K.; Otto, M.; Moon, J.J.; Liese, J.; et al. A Wave of Regulatory T Cells into Neonatal Skin Mediates Tolerance to Commensal Microbes. Immunity 2015, 5, 1011–1021. [Google Scholar] [CrossRef]
- Fagundes, D.L.G.; França, E.L.; Hara, C.C.P.; Honório-França, A.C. Immunomodulatory effects of poly (ethylene glycol) microspheres adsorbed with cortisol on activity of colostrum phagocytes. Int. J. Pharmacol. 2012, 8, 510–518. [Google Scholar] [CrossRef]
- de Quental, O.B.; França, E.L.; Honório-França, A.C.; Morais, T.C.; Daboin, B.E.G.; Bezerra, I.M.P.; Komninakis, S.V.; de Abreu, L.C. Zika Virus Alters the Viscosity and Cytokines Profile in Human Colostrum. J. Immunol. Res. 2019, 2019, 9020519. [Google Scholar] [CrossRef] [PubMed]
- Youngwanichsetha, S. Association of gestational diabetes and hypertensive disorders among pregnant women. Arch. Clin. Hypertens. 2020, 1, 13–14. [Google Scholar] [CrossRef]
- Birukov, A.; Glintborg, D.; Schulze, M.B.; Jensen, T.K.; Kuxhaus, O.; Andersen, L.B.; Kräker, K.; Polemiti, E.; Jensen, B.L.; Jørgensen, J.S.; et al. Elevated blood pressure in pregnant women with gestational diabetes according to the WHO criteria: Importance of overweight. J. Hypertens. 2022, 8, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Marschner, S.; Pant, A.; Henry, A.; Maple-Brown, L.J.; Moran, L.; Cheung, N.W.; Chow, C.K.; Zaman, S. Cardiovascular risk management following gestational diabetes and hypertensive disorders of pregnancy: A narrative review. Med. J. Aust. 2023, 218, 484–491. [Google Scholar] [CrossRef]
- Rudge, M.V.; Calderon, I.M.; Ramos, M.D.; Abbade, J.F.; Rugolo, L.M.S. Perinatal outcome of pregnancies complicated by diabetes and by maternal daily hyperglycemia not related to diabetes. a retrospective 10-year analysis. Gynecol. Obstet. Investig. 2000, 2, 108–112. [Google Scholar] [CrossRef]
- Tamanini, J.T.N.; Dambros, M.; D’Ancona, C.A.L.; Palma, P.C.R.; Netto, R., Jr. Validação para o português do “International Consultation on Incontinence Questionnaire-Short Form” (ICIQ-SF). Rev. Saude Publica 2004, 38, 438–444. [Google Scholar] [CrossRef]
- Pereira, V.S.; Santos, J.Y.C.; Correia, G.N.; Driusso, P. Tradução e validação para a línguaportuguesa de um questionário para avaliação da gravidade da incontinênciaurinária. Rev. Bras. Gynecol. E Obs. 2011, 4, 182–187. [Google Scholar] [CrossRef]
- Hara, C.C.P.; França, E.L.; Fagundes, D.L.G.; Queiroz, A.A.; Rudge, M.V.C.; Honorio-França, A.C.; Calderon, I.M.P. Characterization of Natural Killer Cells and Cytokines in Maternal Placenta and Fetus of Diabetic Mothers. J. Immunol. Res. 2016, 2016, 7154524. [Google Scholar] [CrossRef]
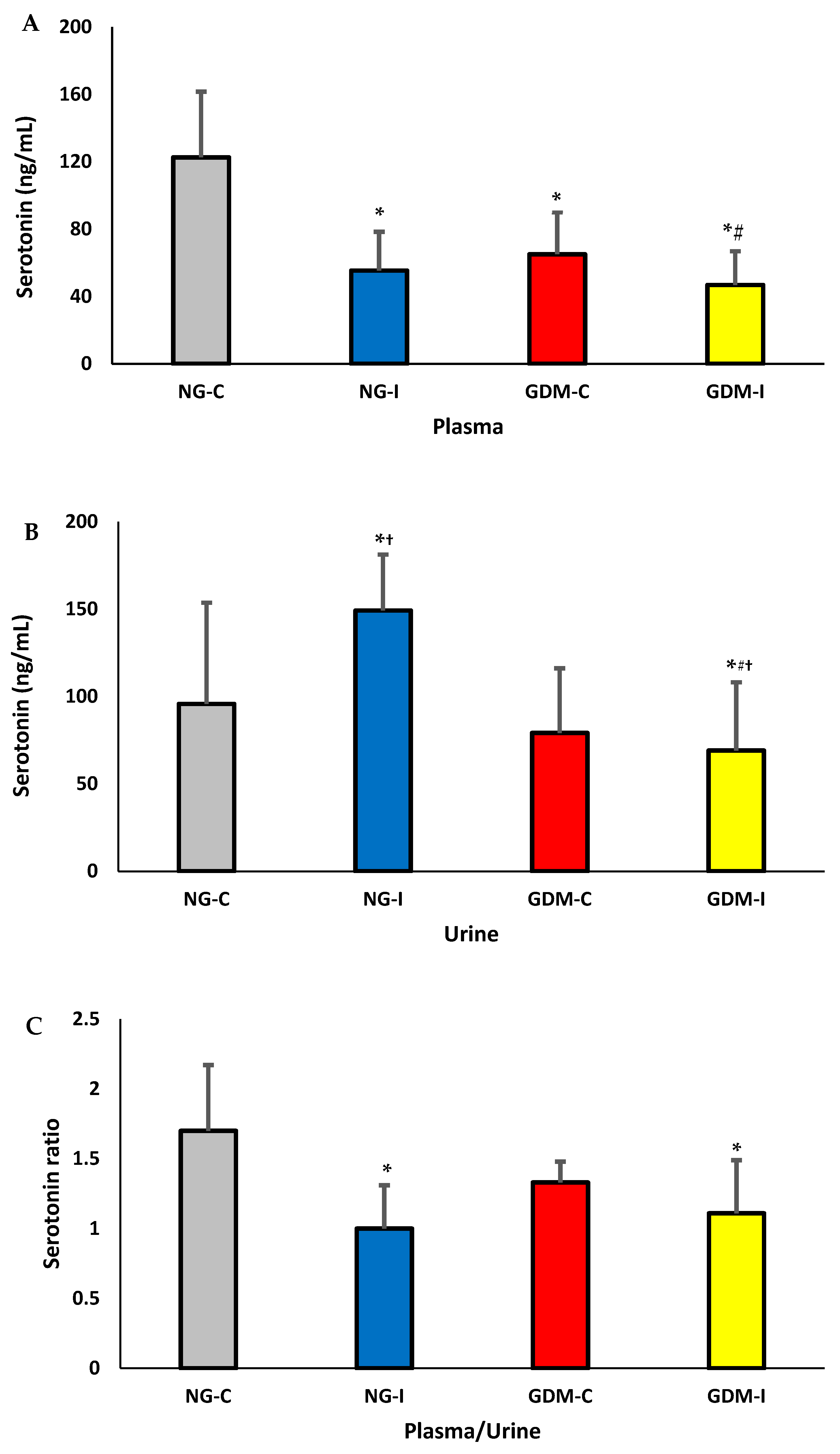

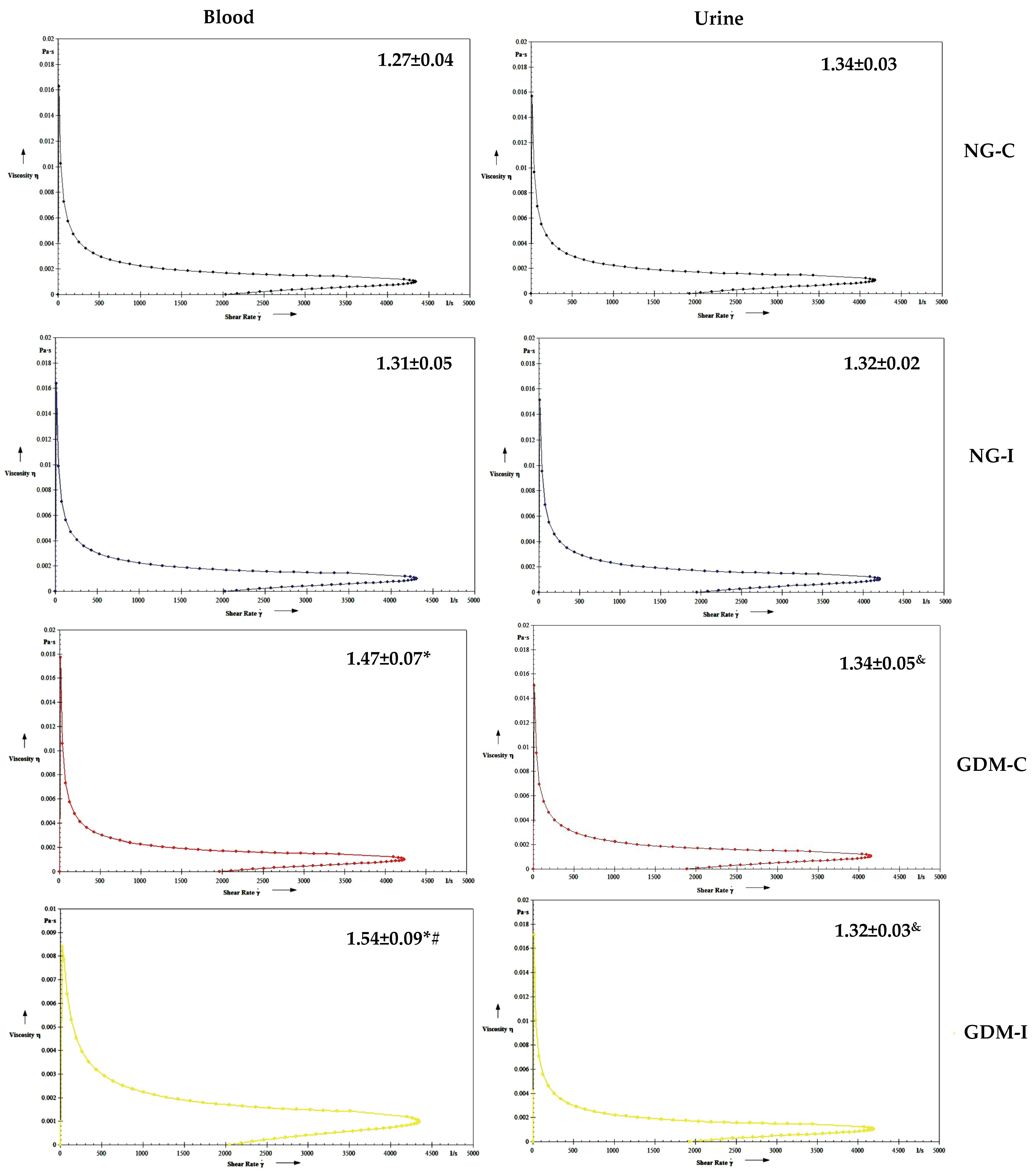
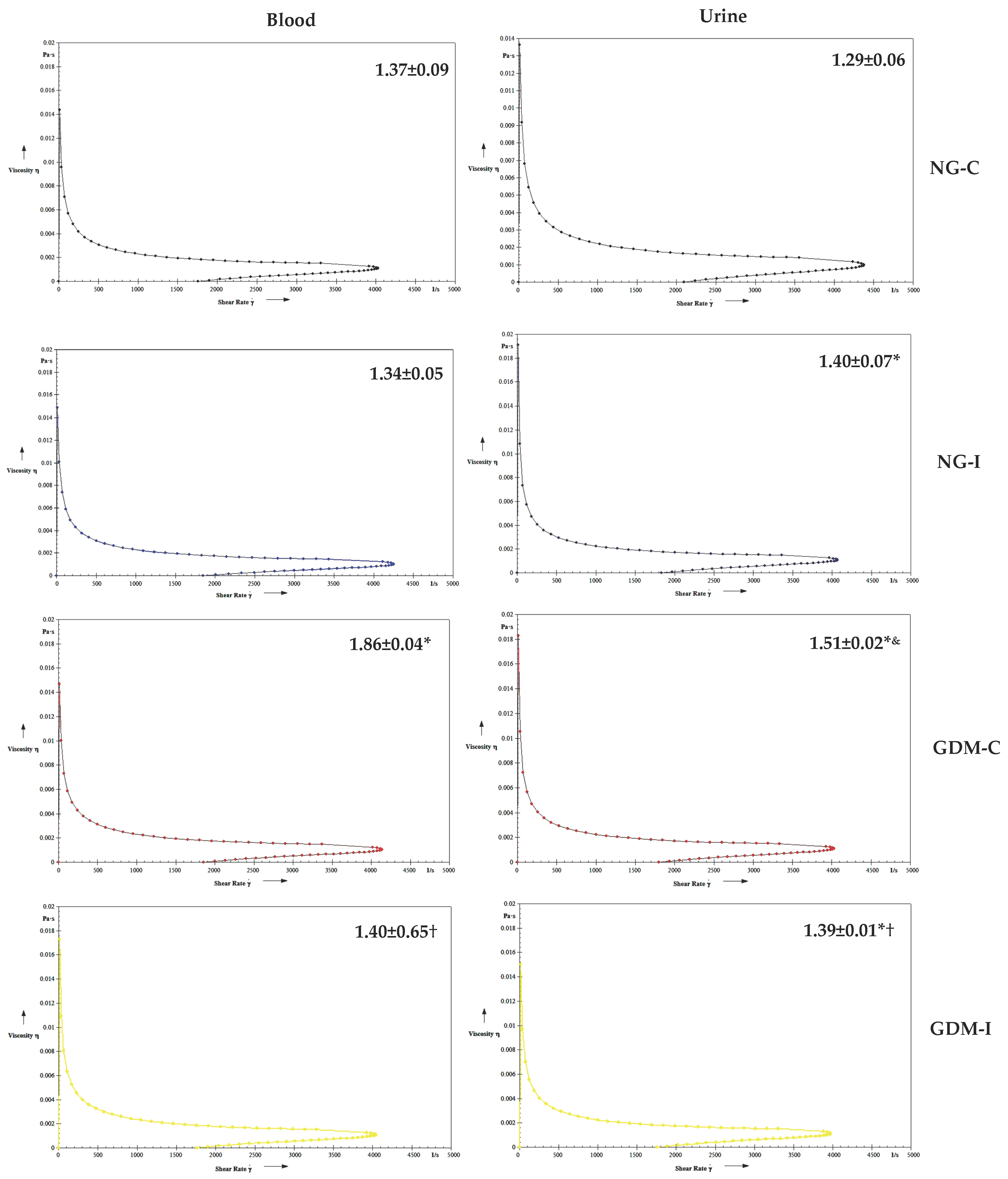
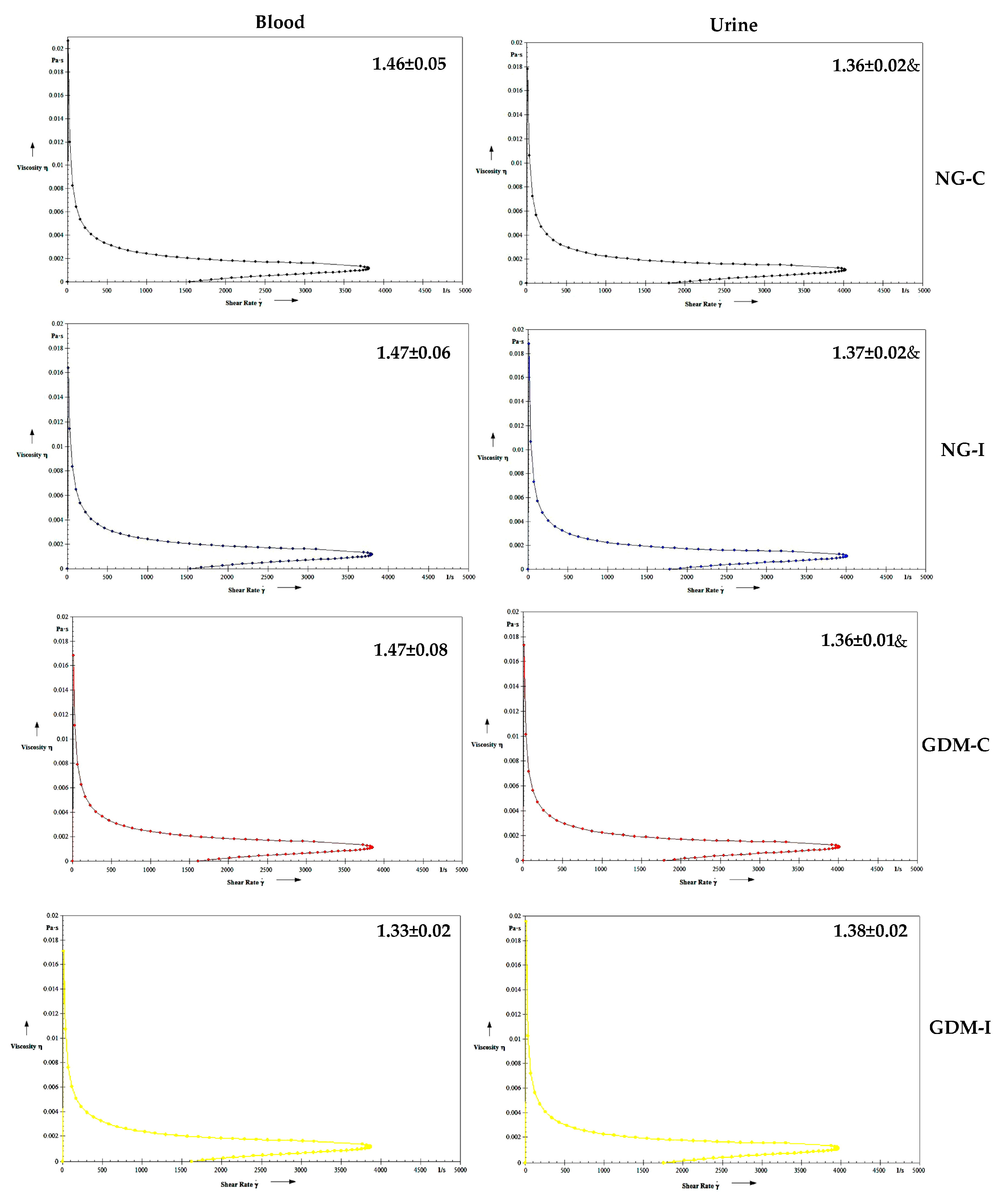

| Groups | NG-C (N = 28) | NG-UI (N = 28) | GDM-C (N = 20) | GDM-I (N = 21) |
|---|---|---|---|---|
| Age | 25.14 ± 4.68 | 26.53 ± 6.91 | 30.40 ± 6.42 | 30.47 ± 6.56 |
| BMI categories (%): | ||||
| Normal weight | 58.8 | 51.7 | 38.6 | 36.7 |
| Overweight | 41.2 | 48.3 | 61.4 | 63.3 |
| Obesity | 0 | 0 | 0 | 0 |
| Glucose level (mg/dL) | 75.28 ± 6.87 | 75.18 ± 7.37 | 92.12 ± 9.88 * | 90.32 ± 12.18 *# |
| Urine Density | 1.01 ± 0.011 | 1.01 ± 0.008 | 1.01 ± 0.006 | 1.01 ± 0.006 |
| Parameters | NG-C | NG-I | GDM-C | GDM-I | p |
|---|---|---|---|---|---|
| Erythrocytes (106/μL) | 4.10 ± 0.37 | 4.00 ± 0.41 | 4.37 ± 0.33 * | 4.00 ± 0.43 # | 0.0361 |
| Hemoglobin (g/dL) | 12.57 ± 0.88 | 12.37 ± 1.06 | 13.06 ± 1.04 | 11.91 ± 1.30 *# | 0.0242 |
| Hematocrit (%) | 38.56 ± 2.32 | 38.18 ± 3.08 | 39.11 ± 2.91 | 35.87 ± 3.52 *# | 0.0239 |
| Leukocytes (103/μL) | 9.68 ± 2.15 | 9.26 ± 2.66 | 9.12 ± 1.66 | 10.12 ± 2.60 | 0.5677 |
| Neutrophils (%) | 69.33 ± 5.77 | 69.08 ± 5.54 | 57.56 ± 20.01 * | 68.88 ± 7.17 | 0.0019 |
| Lymphocytes (%) | 21.30 ± 4.92 | 21.91 ± 4.39 | 31.63 ± 17.09 * | 21.30 ± 5.12 | 0.0011 |
| Monocytes (%) | 7.48 ± 1.51 | 7.22 ± 1.55 | 8.56 ± 2.59 | 7.52 ± 2.50 | 0.1236 |
| Eosinophils (%) | 1.80 ± 0.96 | 1.84 ± 1.38 | 1.98 ± 1.12 | 2.17 ± 2.71 | 0.2317 |
| Platelets (103 μL) | 251.32 ± 57.92 | 232.25 ± 60.00 | 284.84 ± 51.89 | 250.67 ± 73.97 | 0.1017 |
| Groups | NG-C | NG-I | GDM-C | GDM-I |
|---|---|---|---|---|
| Plasma/Urine | ||||
| Serotonin | r = −0.1617 p = 0.7291 | r = −0.4782 p = 0.2777 | r = −0.8138 * p = 0.0487 | r = 0.1442 p = 0.7578 |
| IL-10 | r = −0.7273 * p = 0.0408 | r = 0.8413 * p = 0.0486 | r = 0.3988 p = 0.3277 | r = 0.2742 p = 0.5110 |
| Serotonin/IL-10 cytokine | ||||
| Plasma | r = −0.4946 p = 0.2127 | r = −0.2316 p = 0.5810 | r = 0.4427 p = 0.2720 | r = −0.1846 p = 0.6616 |
| Urine | r = 0.1782 p = 0.6728 | r = −0.3080 p = 0.4580 | r = 0.0706 p = 0.8680 | r = 0.9379 * p = 0.0331 |
| Viscosity | NG-C | NG-I | GDM-C | GDM-I | |
|---|---|---|---|---|---|
| Blood | Serotonin | r = 0.5933 p = 0.1210 | r = −0.4010 p = 0.3248 | r = −0.4620 p = 0.2490 | r = −0.7082 * p = 0.0493 |
| IL-10 | r = 0.4225 p = 0.2970 | r = −0.7775 * p = 0.0231 | r = 0.6091 p = 0.1089 | r = −0.8069 * p = 0.0155 | |
| Urine | Serotonin | r = 0.3562 p = 0.3865 | r = −0.0682 p = 0.8724 | r = 0.7711 * p = 0.0250 | r = −0.5800 p = 0.1317 |
| IL-10 | r = 0.2602 p = 0.5337 | r = 0.2505 p = 0.5496 | r = −0.3784 p = 0.3553 | r = −0.1908 p = 0.6513 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
França, D.C.H.; Honorio-França, A.C.; Silva, K.M.R.; Alves, F.C.B.; Bueno, G.; Costa, S.M.B.; Cotrim, A.C.d.M.; Barbosa, A.M.P.; França, E.L.; Rudge, M.V.C.; et al. Serotonin and Interleukin 10 Can Influence the Blood and Urine Viscosity in Gestational Diabetes Mellitus and Pregnancy-Specific Urinary Incontinence. Int. J. Mol. Sci. 2023, 24, 17125. https://doi.org/10.3390/ijms242417125
França DCH, Honorio-França AC, Silva KMR, Alves FCB, Bueno G, Costa SMB, Cotrim ACdM, Barbosa AMP, França EL, Rudge MVC, et al. Serotonin and Interleukin 10 Can Influence the Blood and Urine Viscosity in Gestational Diabetes Mellitus and Pregnancy-Specific Urinary Incontinence. International Journal of Molecular Sciences. 2023; 24(24):17125. https://doi.org/10.3390/ijms242417125
Chicago/Turabian StyleFrança, Danielle Cristina Honório, Adenilda Cristina Honorio-França, Kênia Maria Rezende Silva, Fernanda Cristina Bérgamo Alves, Gabriela Bueno, Sarah Maria Barneze Costa, Aron Carlos de Melo Cotrim, Angélica Mércia Pascon Barbosa, Eduardo Luzía França, Marilza Vieira Cunha Rudge, and et al. 2023. "Serotonin and Interleukin 10 Can Influence the Blood and Urine Viscosity in Gestational Diabetes Mellitus and Pregnancy-Specific Urinary Incontinence" International Journal of Molecular Sciences 24, no. 24: 17125. https://doi.org/10.3390/ijms242417125
APA StyleFrança, D. C. H., Honorio-França, A. C., Silva, K. M. R., Alves, F. C. B., Bueno, G., Costa, S. M. B., Cotrim, A. C. d. M., Barbosa, A. M. P., França, E. L., Rudge, M. V. C., & The Diamater Study Group. (2023). Serotonin and Interleukin 10 Can Influence the Blood and Urine Viscosity in Gestational Diabetes Mellitus and Pregnancy-Specific Urinary Incontinence. International Journal of Molecular Sciences, 24(24), 17125. https://doi.org/10.3390/ijms242417125







