Role of Mitochondria in Inflammatory Bowel Diseases: A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
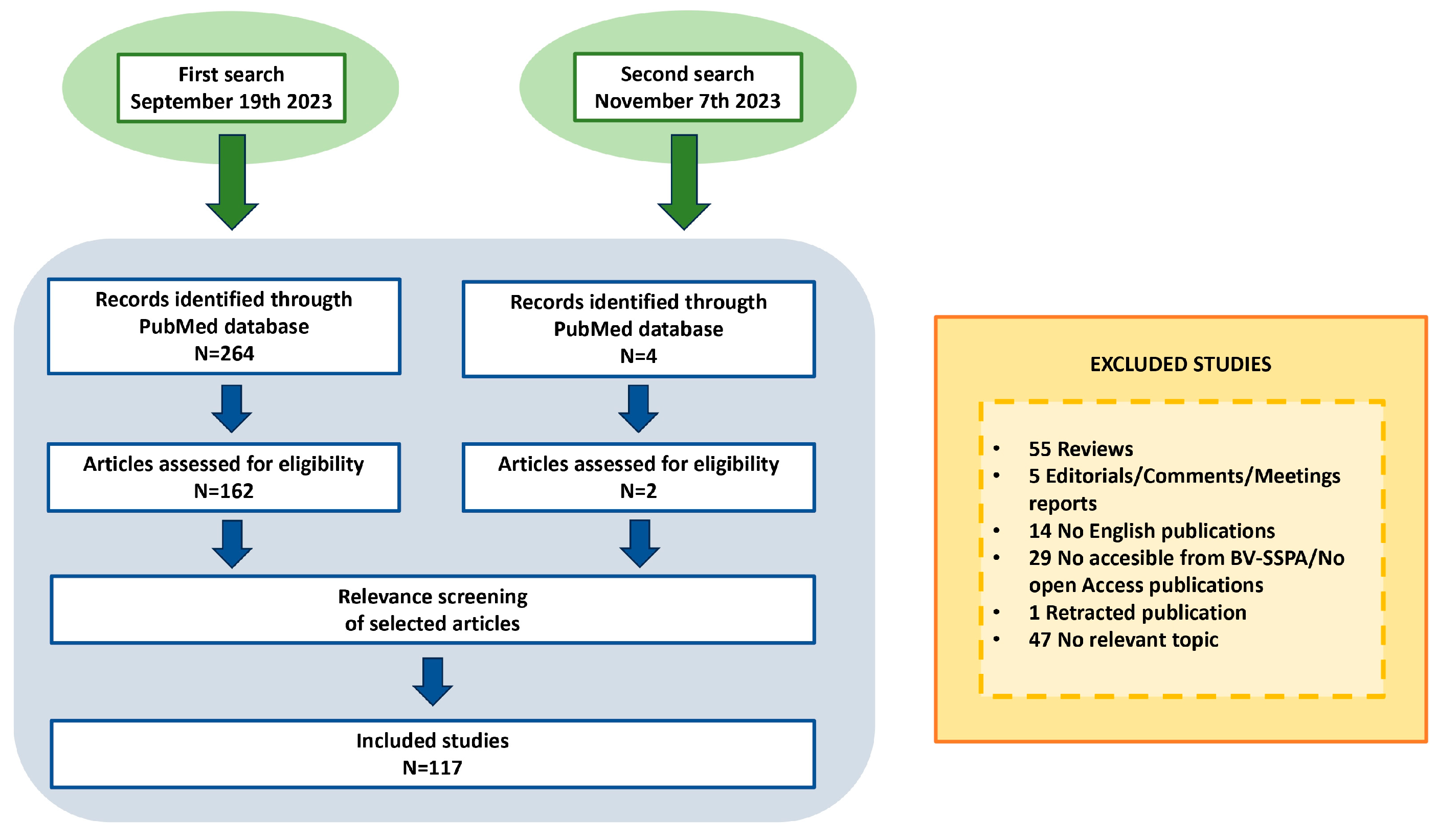
3.1. Literature Search
3.2. Omics Signatures as Evidence of the Role of Mitochondria in IBD
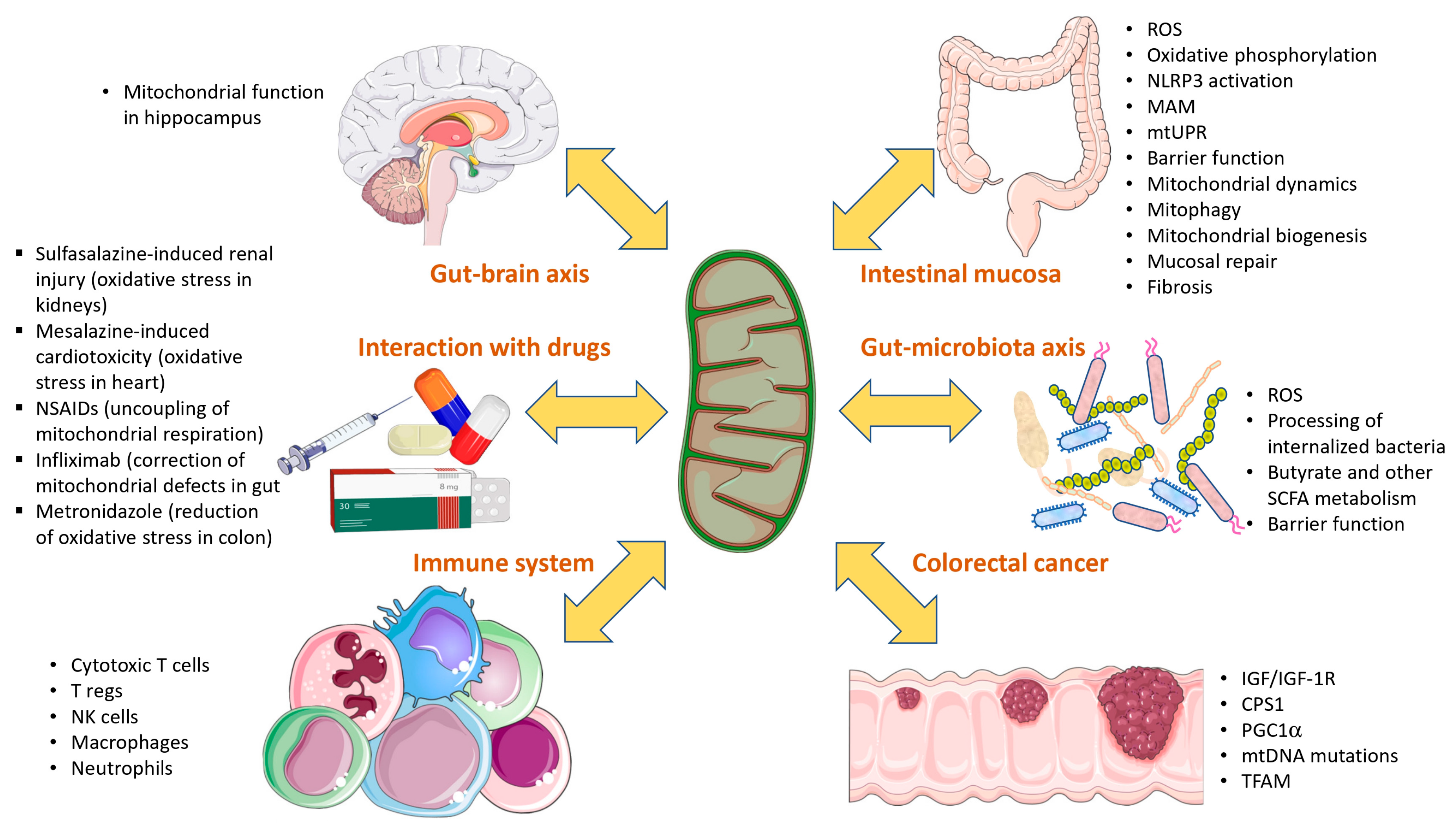
3.3. Role of Mitochondria within Intestinal Mucosa in the Development of IBD and Pathological Mechanisms
3.3.1. Defects in the Oxidative Phosphorylation and Mitochondrial Respiration
3.3.2. Mitochondrial ROS and Oxidative Stress
3.3.3. Mitochondrial Response to Stress
3.3.4. Intestinal Permeability
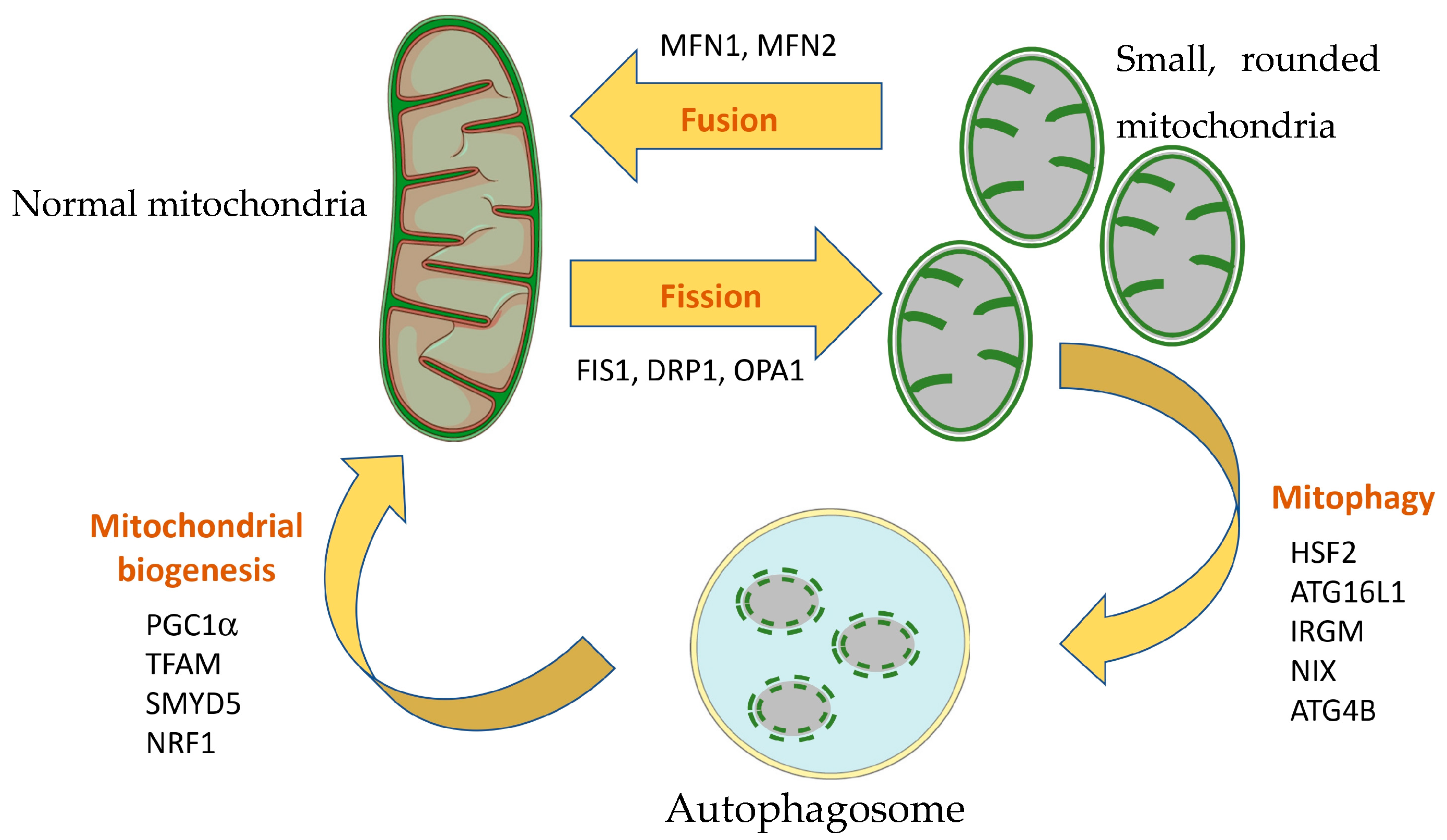
3.4. Mitochondrial Dynamics, Mitophagy and Mitochondrial Biogenesis in IBD
3.5. Involvement of Mitochondria in Mucosal Repair and Fibrosis
3.6. Role of Mitochondria in the Immune System
3.7. Role of Mitochondria in Gut–Microbiota Axis
3.8. Role of Mitochondria in Gut–Brain Axis
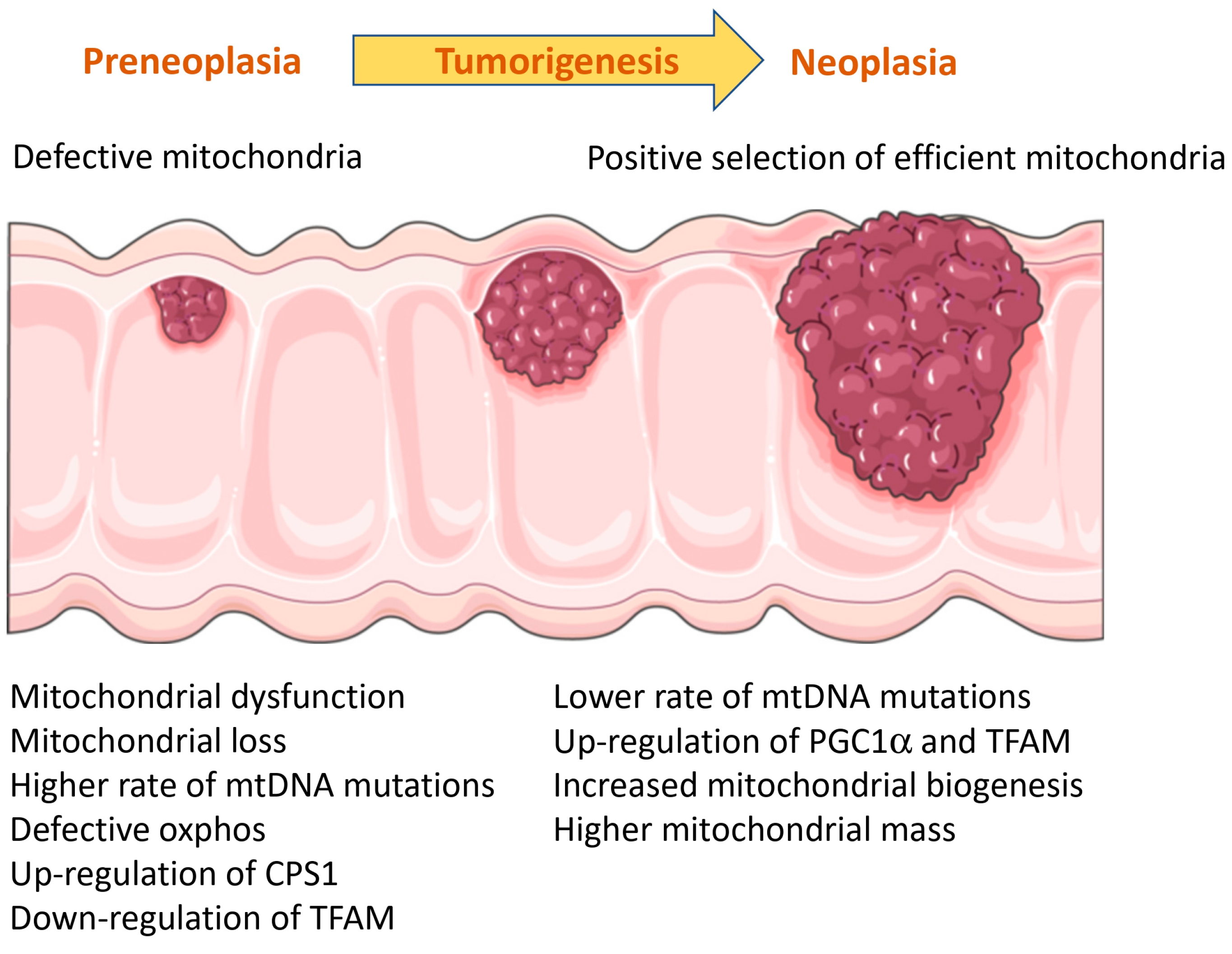
3.9. Role of Mitochondria in Colitis-Associated Colorectal Cancer (CRC)
3.10. Role of Mitochondria in IBD-Associated Arthritis and Sarcopenia
3.11. Interaction with Drugs
3.12. Mitochondria as a Therapeutic Target
3.13. Future Perspectives
3.14. Limitations of This Review
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide Incidence and Prevalence of Inflammatory Bowel Disease in the 21st Century: A Systematic Review of Population-Based Studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Shekhawat, P.S.; Srinivas, S.R.; Matern, D.; Bennett, M.J.; Boriack, R.; George, V.; Xu, H.; Prasad, P.D.; Roon, P.; Ganapathy, V. Spontaneous Development of Intestinal and Colonic Atrophy and Inflammation in the Carnitine-Deficient Jvs (OCTN2(−/−)) Mice. Mol. Genet. Metab. 2007, 92, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Nasser, J.; Bergman, D.T.; Fulco, C.P.; Guckelberger, P.; Doughty, B.R.; Patwardhan, T.A.; Jones, T.R.; Nguyen, T.H.; Ulirsch, J.C.; Lekschas, F.; et al. Genome-Wide Enhancer Maps Link Risk Variants to Disease Genes. Nature 2021, 593, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Dankowski, T.; Schröder, T.; Möller, S.; Yu, X.; Ellinghaus, D.; Bär, F.; Fellermann, K.; Lehnert, H.; Schreiber, S.; Franke, A.; et al. Male-Specific Association between MT-ND4 11719 A/G Polymorphism and Ulcerative Colitis: A Mitochondria-Wide Genetic Association Study. BMC Gastroenterol. 2016, 16, 118. [Google Scholar] [CrossRef] [PubMed]
- Haberman, Y.; Karns, R.; Dexheimer, P.J.; Schirmer, M.; Somekh, J.; Jurickova, I.; Braun, T.; Novak, E.; Bauman, L.; Collins, M.H.; et al. Ulcerative Colitis Mucosal Transcriptomes Reveal Mitochondriopathy and Personalized Mechanisms Underlying Disease Severity and Treatment Response. Nat. Commun. 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Vatn, S.S.; Lindstrøm, J.C.; Moen, A.E.F.; Brackmann, S.; Tannæs, T.M.; Olbjørn, C.; Bergemalm, D.; Keita, Å.V.; Gomollon, F.; Detlie, T.E.; et al. Mucosal Gene Transcript Signatures in Treatment Naïve Inflammatory Bowel Disease: A Comparative Analysis of Disease to Symptomatic and Healthy Controls in the European IBD-Character Cohort. Clin. Exp. Gastroenterol. 2022, 15, 5–25. [Google Scholar] [CrossRef] [PubMed]
- McQueen, P.; Busman-Sahay, K.; Rieder, F.; Noël-Romas, L.; McCorrister, S.; Westmacott, G.; Estes, J.D.; Burgener, A. Intestinal Proteomic Analysis of a Novel Non-Human Primate Model of Experimental Colitis Reveals Signatures of Mitochondrial and Metabolic Dysfunction. Mucosal Immunol. 2019, 12, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Bredell, B.X.; Anderson, E.R.; Martin, A.; Mays, C.; Nagao-Kitamoto, H.; Huang, S.; Győrffy, B.; Greenson, J.K.; Hardiman, K.; et al. Quantitative Proteomics Identifies STEAP4 as a Critical Regulator of Mitochondrial Dysfunction Linking Inflammation and Colon Cancer. Proc. Natl. Acad. Sci. USA 2017, 114, E9608–E9617. [Google Scholar] [CrossRef]
- Wang, J.-M.; Lin, S.-R.; Zhu, Y.-B.; Yuan, J.; Wang, Y.-M.; Zhang, Q.; Xie, L.-S.; Li, S.-H.; Liu, S.-Q.; Yu, S.-G.; et al. Proteomic Analysis of Lysine Acetylation Reveals That Metabolic Enzymes and Heat Shock Proteins May Be Potential Targets for DSS-Induced Mice Colitis. Int. Immunopharmacol. 2021, 101, 108336. [Google Scholar] [CrossRef]
- Novak, E.A.; Crawford, E.C.; Mentrup, H.L.; Griffith, B.D.; Fletcher, D.M.; Flanagan, M.R.; Schneider, C.; Firek, B.; Rogers, M.B.; Morowitz, M.J.; et al. Epithelial NAD+ Depletion Drives Mitochondrial Dysfunction and Contributes to Intestinal Inflammation. Front. Immunol. 2023, 14, 1231700. [Google Scholar] [CrossRef]
- Vincent, G.; Novak, E.A.; Siow, V.S.; Cunningham, K.E.; Griffith, B.D.; Comerford, T.E.; Mentrup, H.L.; Stolz, D.B.; Loughran, P.; Ranganathan, S.; et al. Nix-Mediated Mitophagy Modulates Mitochondrial Damage during Intestinal Inflammation. Antioxid. Redox Signal 2020, 33, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.H.; Tucker, S.A.; Quevedo, S.F.; Inal, A.; Korzenik, J.R.; Haigis, M.C. Metabolic Analyses Reveal Dysregulated NAD+ Metabolism and Altered Mitochondrial State in Ulcerative Colitis. PLoS ONE 2022, 17, e0273080. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Sun, R.; Ji, J.; Yang, F.; Tian, W.; Ji, W.; Huang, Q. A Broad Cuproptosis Landscape in Inflammatory Bowel Disease. Front. Immunol. 2022, 13, 1031539. [Google Scholar] [CrossRef] [PubMed]
- O’Morain, C.; Smethurst, P.; Levi, A.J.; Peters, T.J. Organelle Pathology in Ulcerative and Crohn’s Colitis with Special Reference to the Lysosomal Alterations. Gut 1984, 25, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Restivo, N.L.; Srivastava, M.D.; Schafer, I.A.; Hoppel, C.L. Mitochondrial Dysfunction in a Patient with Crohn Disease: Possible Role in Pathogenesis. J. Pediatr. Gastroenterol. Nutr. 2004, 38, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zarate, N.; Soderholm, J.D.; Bourgeois, J.M.; Liu, L.W.C.; Huizinga, J.D. Ultrastructural Injury to Interstitial Cells of Cajal and Communication with Mast Cells in Crohn’s Disease. Neurogastroenterol. Motil. 2007, 19, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Sifroni, K.G.; Damiani, C.R.; Stoffel, C.; Cardoso, M.R.; Ferreira, G.K.; Jeremias, I.C.; Rezin, G.T.; Scaini, G.; Schuck, P.F.; Dal-Pizzol, F.; et al. Mitochondrial Respiratory Chain in the Colonic Mucosal of Patients with Ulcerative Colitis. Mol. Cell Biochem. 2010, 342, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Schneider, A.M.; Özsoy, M.; Zimmermann, F.A.; Brunner, S.M.; Feichtinger, R.G.; Mayr, J.A.; Kofler, B.; Neureiter, D.; Klieser, E.; Aigner, E.; et al. Expression of Oxidative Phosphorylation Complexes and Mitochondrial Mass in Pediatric and Adult Inflammatory Bowel Disease. Oxid. Med. Cell Longev. 2022, 2022, 9151169. [Google Scholar] [CrossRef]
- Santhanam, S.; Rajamanickam, S.; Motamarry, A.; Ramakrishna, B.S.; Amirtharaj, J.G.; Ramachandran, A.; Pulimood, A.; Venkatraman, A. Mitochondrial Electron Transport Chain Complex Dysfunction in the Colonic Mucosa in Ulcerative Colitis. Inflamm. Bowel Dis. 2012, 18, 2158–2168. [Google Scholar] [CrossRef]
- Damiani, C.R.; Benetton, C.A.F.; Stoffel, C.; Bardini, K.C.; Cardoso, V.H.; Di Giunta, G.; Pinho, R.A.; Dal-Pizzol, F.; Streck, E.L. Oxidative Stress and Metabolism in Animal Model of Colitis Induced by Dextran Sulfate Sodium. J. Gastroenterol. Hepatol. 2007, 22, 1846–1851. [Google Scholar] [CrossRef]
- Fujiwara, H.; Seike, K.; Brooks, M.D.; Mathew, A.V.; Kovalenko, I.; Pal, A.; Lee, H.J.; Peltier, D.; Kim, S.; Liu, C.; et al. Mitochondrial Complex II in Intestinal Epithelial Cells Regulates T Cell-Mediated Immunopathology. Nat. Immunol. 2021, 22, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Hoang, N.; Edwards, K. Sex-Specic Colonic Mitochondrial Dysfunction in the Indomethacin-Induced Inflammatory Bowel Disease Model in Rats. Res. Sq. 2023, rs.3.rs-2626257. [Google Scholar] [CrossRef]
- Sosnovski, K.E.; Braun, T.; Amir, A.; Moshel, D.; Benshoshan, M.; Vandussen, K.L.; Levhar, N.; Abbas-Egbariya, H.; Beider, K.; Ben-Yishay, R.; et al. GATA6-AS1 Regulates Intestinal Epithelial Mitochondrial Functions, and Its Reduced Expression Is Linked to Intestinal Inflammation and Less Favourable Disease Course in Ulcerative Colitis. J. Crohns Colitis 2023, 17, 960–971. [Google Scholar] [CrossRef] [PubMed]
- Cha, H.; Lee, S.; Hwan Kim, S.; Kim, H.; Lee, D.S.; Lee, H.S.; Lee, J.H.; Park, J.W. Increased Susceptibility of IDH2-Deficient Mice to Dextran Sodium Sulfate-Induced Colitis. Redox Biol. 2017, 13, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kim, Y.R.; Jang, S.; Wang, S.G.; Cho, E.; Mun, S.J.; Jeon, H.I.; Kim, H.K.; Min, S.J.; Yang, C.S. Mito-TIPTP Increases Mitochondrial Function by Repressing the Rubicon-P22phox Interaction in Colitis-Induced Mice. Antioxidants 2021, 10, 1954. [Google Scholar] [CrossRef] [PubMed]
- Theiss, A.L.; Idell, R.D.; Srinivasan, S.; Klapproth, J.; Jones, D.P.; Merlin, D.; Sitaraman, S.V. Prohibitin Protects against Oxidative Stress in Intestinal Epithelial Cells. FASEB J. 2007, 21, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Kathiria, A.S.; Butcher, L.D.; Feagins, L.A.; Souza, R.F.; Boland, C.R.; Theiss, A.L. Prohibitin 1 Modulates Mitochondrial Stress-Related Autophagy in Human Colonic Epithelial Cells. PLoS ONE 2012, 7, e31231. [Google Scholar] [CrossRef]
- Han, J.; Yu, C.; Souza, R.F.; Theiss, A.L. Prohibitin 1 Modulates Mitochondrial Function of Stat3. Cell Signal 2014, 26, 2086–2095. [Google Scholar] [CrossRef]
- Jackson, D.N.; Panopoulos, M.; Neumann, W.L.; Turner, K.; Cantarel, B.L.; Thompson-Snipes, L.; Dassopoulos, T.; Feagins, L.A.; Souza, R.F.; Mills, J.C.; et al. Mitochondrial Dysfunction during Loss of Prohibitin 1 Triggers Paneth Cell Defects and Ileitis. Gut 2020, 69, 1928–1938. [Google Scholar] [CrossRef]
- Alula, K.M.; Jackson, D.N.; Smith, A.D.; Kim, D.S.; Turner, K.; Odstrcil, E.; Kaipparettu, B.A.; Dassopoulos, T.; Venuprasad, K.; Feagins, L.A.; et al. Targeting Mitochondrial Damage as a Therapeutic for Ileal Crohn’s Disease. Cells 2021, 10, 1349. [Google Scholar] [CrossRef]
- Dashdorj, A.; KR, J.; Lim, S.; Jo, A.; Nguyen, M.N.; Ha, J.; Yoon, K.S.; Kim, H.J.; Park, J.H.; Murphy, M.P.; et al. Mitochondria-Targeted Antioxidant MitoQ Ameliorates Experimental Mouse Colitis by Suppressing NLRP3 Inflammasome-Mediated Inflammatory Cytokines. BMC Med. 2013, 11, 178. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, A.V.; Chelombitko, M.A.; Chernyavskij, D.A.; Galkin, I.I.; Pletjushkina, O.Y.; Vasilieva, T.V.; Zinovkin, R.A.; Chernyak, B.V. Mitochondria-Targeted Antioxidant SkQ1 Prevents the Development of Experimental Colitis in Mice and Impairment of the Barrier Function of the Intestinal Epithelium. Cells 2022, 11, 3441. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, W.; Sun, S.; Xu, X.; Fei, J.; Zhou, Q.; Qin, C.; Ou, S.; Wu, F.; Wu, F.t.; et al. Advanced Oxidation Protein Products Induce Paneth Cells Defects by Endoplasmic Reticulum Stress in Crohn’s Disease. iScience 2023, 26, 107312. [Google Scholar] [CrossRef] [PubMed]
- Rath, E.; Berger, E.; Messlik, A.; Nunes, T.; Liu, B.; Kim, S.C.; Hoogenraad, N.; Sans, M.; Sartor, R.B.; Haller, D. Induction of DsRNA-Activated Protein Kinase Links Mitochondrial Unfolded Protein Response to the Pathogenesis of Intestinal Inflammation. Gut 2012, 61, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S.; Wang, M.; Harrington, J.C.; Chuang, B.M.; Eckmann, L.; Kaufman, R.J. Phosphorylation of Eif2α Is Dispensable for Differentiation but Required at a Posttranscriptional Level for Paneth Cell Function and Intestinal Homeostasis in Mice. Inflamm. Bowel Dis. 2014, 20, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Khaloian, S.; Rath, E.; Hammoudi, N.; Gleisinger, E.; Blutke, A.; Giesbertz, P.; Berger, E.; Metwaly, A.; Waldschmitt, N.; Allez, M.; et al. Mitochondrial Impairment Drives Intestinal Stem Cell Transition into Dysfunctional Paneth Cells Predicting Crohn’s Disease Recurrence. Gut 2020, 69, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Van Haaften-Visser, D.Y.; Harakalova, M.; Mocholi, E.; Van Montfrans, J.M.; Elkadri, A.; Rieter, E.; Fiedler, K.; Van Hasselt, P.M.; Triffaux, E.M.M.; Van Haelst, M.M.; et al. Ankyrin Repeat and Zinc-Finger Domain-Containing 1 Mutations Are Associated with Infantile-Onset Inflammatory Bowel Disease. J. Biol. Chem. 2017, 292, 7904–7920. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.C.; Davison, J.M.; Stuckenholz, C.; Lu, L.; Bahary, N. Dysregulated Phosphatidylinositol Signaling Promotes Endoplasmic-Reticulum- Stress-Mediated Intestinal Mucosal Injury and Inflammation in Zebrafish. DMM Dis. Models Mech. 2014, 7, 93–106. [Google Scholar] [CrossRef]
- Söderholm, J.D.; Olaison, G.; Peterson, K.H.; Franzén, L.E.; Lindmark, T.; Wirén, M.; Tagesson, C.; Sjódahl, R. Augmented Increase in Tight Junction Permeability by Luminal Stimuli in the Non-Inflamed Ileum of Crohn’s Disease. Gut 2002, 50, 307–313. [Google Scholar] [CrossRef]
- Wang, A.; Keita, Å.V.; Phan, V.; McKay, C.M.; Schoultz, I.; Lee, J.; Murphy, M.P.; Fernando, M.; Ronaghan, N.; Balce, D.; et al. Targeting Mitochondria-Derived Reactive Oxygen Species to Reduce Epithelial Barrier Dysfunction and Colitis. Am. J. Pathol. 2014, 184, 2516–2527. [Google Scholar] [CrossRef]
- Ho, G.T.; Aird, R.E.; Liu, B.; Boyapati, R.K.; Kennedy, N.A.; Dorward, D.A.; Noble, C.L.; Shimizu, T.; Carter, R.N.; Chew, E.T.S.; et al. MDR1 Deficiency Impairs Mitochondrial Homeostasis and Promotes Intestinal Inflammation. Mucosal Immunol. 2018, 11, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.H.T.; Lee, J.S.; Murphy, E.M.; Gerich, M.E.; Dran, R.; Glover, L.E.; Abdulla, Z.I.; Skelton, M.R.; Colgan, S.P. Creatine Transporter, Reduced in Colon Tissues From Patients With Inflammatory Bowel Diseases, Regulates Energy Balance in Intestinal Epithelial Cells, Epithelial Integrity, and Barrier Function. Gastroenterology 2020, 159, 984–998.e1. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, A.K.; Navaneetha Krishnan, S.; Jijon, H.; Shutt, T.E.; Colarusso, P.; McKay, D.M. Tissue Imaging Reveals Disruption of Epithelial Mitochondrial Networks and Loss of Mitochondria-Associated Cytochrome-C in Inflamed Human and Murine Colon. Mitochondrion 2023, 68, 44–59. [Google Scholar] [CrossRef]
- Fu, S.-C.; Qu, J.-Y.; Li, L.-X.; Yang, X.-X.; Li, Y.-Q.; Zuo, X.-L. Excessive Mitochondrial Fission Suppresses Mucosal Repair by Impairing Butyrate Metabolism in Colonocytes. Inflamm. Bowel Dis. 2023, izad132. [Google Scholar] [CrossRef] [PubMed]
- Lan, A.; Guerbette, T.; Andriamihaja, M.; Magnin, B.; Bordet, M.; Ferron, P.J.; Burel, A.; Viel, R.; Fromenty, B.; Corlu, A.; et al. Mitochondrial Remodeling and Energy Metabolism Adaptations in Colonic Crypts during Spontaneous Epithelial Repair after Colitis Induction in Mice. Free Radic. Biol. Med. 2023, 205, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Mancini, N.L.; Goudie, L.; Xu, W.; Sabouny, R.; Rajeev, S.; Wang, A.; Esquerre, N.; Al Rajabi, A.; Jayme, T.S.; van Tilburg Bernandes, E.; et al. Perturbed Mitochondrial Dynamics Is a Novel Feature of Colitis That Can Be Targeted to Lessen Disease. CMGH 2020, 10, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Zhang, F.; Wang, W.; Zhao, W.; Zhou, J.; Feng, Y.; Wu, J.; Li, M.; Bai, X.; Zeng, Z.; et al. Heat Shock Transcription Factor 2 Promotes Mitophagy of Intestinal Epithelial Cells Through PARL/PINK1/Parkin Pathway in Ulcerative Colitis. Front. Pharmacol. 2022, 13, 893426. [Google Scholar] [CrossRef]
- Matsuzawa-Ishimoto, Y.; Shono, Y.; Gomez, L.E.; Hubbard-Lucey, V.M.; Cammer, M.; Neil, J.; Dewan, M.Z.; Lieberman, S.R.; Lazrak, A.; Marinis, J.M.; et al. Autophagy Protein ATG16L1 Prevents Necroptosis in the Intestinal Epithelium. J. Exp. Med. 2017, 214, 3687–3705. [Google Scholar] [CrossRef]
- Singh, S.B.; Ornatowski, W.; Vergne, I.; Naylor, J.; Delgado, M.; Roberts, E.; Ponpuak, M.; Master, S.; Pilli, M.; White, E.; et al. Human IRGM Regulates Autophagy and Cell-Autonomous Immunity Functions through Mitochondria. Nat. Cell Biol. 2010, 12, 1154–1165. [Google Scholar] [CrossRef]
- Liu, B.; Gulati, A.S.; Cantillana, V.; Henry, S.C.; Schmidt, E.A.; Daniell, X.; Grossniklaus, E.; Schoenborn, A.A.; Balfour Sartor, R.; Taylor, G.A. Irgm1-Deficient Mice Exhibit Paneth Cell Abnormalities and Increased Susceptibility to Acute Intestinal Inflammation. Am. J. Physiol. Gastro-Intest. Liver Physiol. 2013, 305, 573–584. [Google Scholar] [CrossRef]
- Magalhaes-Novais, S.; Blecha, J.; Naraine, R.; Mikesova, J.; Abaffy, P.; Pecinova, A.; Milosevic, M.; Bohuslavova, R.; Prochazka, J.; Khan, S.; et al. Mitochondrial Respiration Supports Autophagy to Provide Stress Resistance during Quiescence. Autophagy 2022, 18, 2409–2426. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Peng, C.; Gan, Y.; Wang, Y.; Chen, Z.; Han, X.; Chen, Y. Differently Surface-Labeled Polystyrene Nanoplastics at an Environmentally Relevant Concentration Induced Crohn’s Ileitis-like Features via Triggering Intestinal Epithelial Cell Necroptosis. Environ. Int. 2023, 176, 107968. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Sun, X.; Gheinani, P.T.; Guan, X.; Sharma, S.; Zhou, Y.; Jin, C.; Yang, Z.; Naren, A.P.; Yin, J.; et al. Epithelial SMYD5 Exaggerates IBD by Down-Regulating Mitochondrial Functions via Post-Translational Control of PGC-1α Stability. CMGH 2022, 14, 375–403. [Google Scholar] [CrossRef] [PubMed]
- Reifen, R.; Levy, E.; Berkovich, Z.; Tirosh, O. Vitamin A Exerts Its Antiinflammatory Activities in Colitis through Preservation of Mitochondrial Activity. Nutrition 2015, 31, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wu, H.; Wang, J.; Chen, X.; Pan, J.; Liu, P.; Zhang, J.; Chen, Y.; Zhu, W.; Tang, C.; et al. Vitamin D Receptor Inhibits EMT via Regulation of the Epithelial Mitochondrial Function in Intestinal Fibrosis. J. Biol. Chem. 2021, 296, 100531. [Google Scholar] [CrossRef] [PubMed]
- Jurickova, I.; Bonkowski, E.; Angerman, E.; Novak, E.; Huron, A.; Akers, G.; Iwasawa, K.; Braun, T.; Hadar, R.; Hooker, M.; et al. Eicosatetraynoic Acid and Butyrate Regulate Human Intestinal Organoid Mitochondrial and Extracellular Matrix Pathways Implicated in Crohn’s Disease Strictures. Inflamm. Bowel Dis. 2022, 28, 988–1003. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yu, T.; Zhou, G.; Yao, S.; Wakamiya, M.; Hu, H.; Paessler, S.; Sun, J.; Cong, Y. Intrinsic STING Switches off Pathogenetic Programs of Th1 Cells to Inhibit Colitis. CMGH 2023, 15, 1161–1179. [Google Scholar] [CrossRef]
- Bamidele, A.O.; Mishra, S.K.; Hirsova, P.; Fehrenbach, P.J.; Valenzuela-Pérez, L.; Kim Lee, H.S. Interleukin-21 Drives a Hypermetabolic State and CD4+ T Cell-Associated Pathogenicity in Chronic Intestinal Inflammation. bioRxiv 2023. 2023.06.02.543518. [Google Scholar] [CrossRef]
- Schenk, U.; Westendorf, A.M.; Radaelli, E.; Casati, A.; Ferro, M.; Fumagalli, M.; Verderio, C.; Buer, J.; Scanziani, E.; Grassi, F. Purinergic Control of T Cell Activation by ATP Released through Pannexin-1 Hemichannels. Sci. Signal 2008, 1, ra6. [Google Scholar] [CrossRef]
- Zaiatz Bittencourt, V.; Jones, F.; Tosetto, M.; Doherty, G.A.; Ryan, E.J. Dysregulation of Metabolic Pathways in Circulating Natural Killer Cells Isolated from Inflammatory Bowel Disease Patients. J. Crohns Colitis 2021, 15, 1316–1325. [Google Scholar] [CrossRef]
- Ip, W.K.E.; Hoshi, N.; Shouval, D.S.; Snapper, S.; Medzhitov, R. Anti-Inflammatory Effect of IL-10 Mediated by Metabolic Reprogramming of Macrophages. Science 2017, 356, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.J.; Reyes, C.N.; Liang, W.; Becker, C.; Shimada, K.; Wheeler, M.L.; Cho, H.C.; Popescu, N.I.; Coggeshall, K.M.; Arditi, M.; et al. Hexokinase Is an Innate Immune Receptor for the Detection of Bacterial Peptidoglycan. Cell 2016, 166, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Wang, X.; Yang, Y.; Yan, Y.; Yu, C.; Zhou, R.; Jiang, W. Plant Lectins Activate the NLRP3 Inflammasome to Promote Inflammatory Disorders. J. Immunol. 2017, 198, 2082–2092. [Google Scholar] [CrossRef] [PubMed]
- Riffelmacher, T.; Giles, D.A.; Zahner, S.; Dicker, M.; Andreyev, A.Y.; McArdle, S.; Perez-Jeldres, T.; van der Gracht, E.; Murray, M.P.; Hartmann, N.; et al. Metabolic Activation and Colitis Pathogenesis Is Prevented by Lymphotoxin β Receptor Expression in Neutrophils. Mucosal Immunol. 2021, 14, 679–690. [Google Scholar] [CrossRef] [PubMed]
- Formentini, L.; Santacatterina, F.; Núñez de Arenas, C.; Stamatakis, K.; López-Martínez, D.; Logan, A.; Fresno, M.; Smits, R.; Murphy, M.P.; Cuezva, J.M. Mitochondrial ROS Production Protects the Intestine from Inflammation through Functional M2 Macrophage Polarization. Cell Rep. 2017, 19, 1202–1213. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Itoiz, M.A.; Peña-Cearra, A.; Martín-Ruiz, I.; Lavín, J.L.; Simó, C.; Rodríguez, H.; Atondo, E.; Flores, J.M.; Carreras-González, A.; Tomás-Cortázar, J.; et al. The Mitochondrial Negative Regulator MCJ Modulates the Interplay between Microbiota and the Host during Ulcerative Colitis. Sci. Rep. 2020, 10, 572. [Google Scholar] [CrossRef] [PubMed]
- Boyapati, R.K.; Dorward, D.A.; Tamborska, A.; Kalla, R.; Ventham, N.T.; Doherty, M.K.; Whitfield, P.D.; Gray, M.; Loane, J.; Rossi, A.G.; et al. Mitochondrial DNA Is a Pro-Inflammatory Damage-Associated Molecular Pattern Released during Active IBD. Inflamm. Bowel Dis. 2018, 24, 2113–2122. [Google Scholar] [CrossRef]
- Verma, A.; Pittala, S.; Alhozeel, B.; Shteinfer-Kuzmine, A.; Ohana, E.; Gupta, R.; Chung, J.H.; Shoshan-Barmatz, V. The Role of the Mitochondrial Protein VDAC1 in Inflammatory Bowel Disease: A Potential Therapeutic Target. Mol. Ther. 2022, 30, 726–744. [Google Scholar] [CrossRef]
- Ostuni, M.A.; Issop, L.; Péranzi, G.; Walker, F.; Fasseu, M.; Elbim, C.; Papadopoulos, V.; Lacapere, J.J. Overexpression of Translocator Protein in Inflammatory Bowel Disease: Potential Diagnostic and Treatment Value. Inflamm. Bowel Dis. 2010, 16, 1476–1487. [Google Scholar] [CrossRef]
- Jimenez, I.A.; Stilin, A.P.; Morohaku, K.; Hussein, M.H.; Koganti, P.P.; Selvaraj, V. Mitochondrial Translocator Protein Deficiency Exacerbates Pathology in Acute Experimental Ulcerative Colitis. Front. Physiol. 2022, 13, 896951. [Google Scholar] [CrossRef]
- Lahiri, A.; Hedl, M.; Yan, J.; Abraham, C. Human LACC1 Increases Innate Receptor-Induced Responses and a LACC1 Disease-Risk Variant Modulates These Outcomes. Nat. Commun. 2017, 8, 15614. [Google Scholar] [CrossRef] [PubMed]
- Cader, M.Z.; de Almeida Rodrigues, R.P.; West, J.A.; Sewell, G.W.; Md-Ibrahim, M.N.; Reikine, S.; Sirago, G.; Unger, L.W.; Inglesias-Romero, A.B.; Ramshorn, K.; et al. FAMIN Is a Multifunctional Purine Enzyme Enabling the Purine Nucleotide Cycle. Cell 2020, 180, 278–295.e23. [Google Scholar] [CrossRef] [PubMed]
- Danne, C.; Michaudel, C.; Skerniskyte, J.; Planchais, J.; Magniez, A.; Agus, A.; Michel, M.L.; Lamas, B.; Da Costa, G.; Spatz, M.; et al. CARD9 in Neutrophils Protects from Colitis and Controls Mitochondrial Metabolism and Cell Survival. Gut 2023, 72, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Nazli, A.; Yang, P.-C.; Jury, J.; Howe, K.; Watson, J.L.; Söderholm, J.D.; Sherman, P.M.; Perdue, M.H.; McKay, D.M. Epithelia under Metabolic Stress Perceive Commensal Bacteria as a Threat. Am. J. Pathol. 2004, 164, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Ra, E.A.; Lee, T.A.; Won Kim, S.; Park, A.; Choi, H.J.; Jang, I.; Kang, S.; Hee Cheon, J.; Cho, J.W.; Eun Lee, J.; et al. TRIM31 Promotes Atg5/Atg7-Independent Autophagy in Intestinal Cells. Nat. Commun. 2016, 7, 11726. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Lopes, F.; Poon, K.K.H.; Mckay, D.M. Absence of the NOD2 Protein Renders Epithelia More Susceptible to Barrier Dysfunction Due to Mitochondrial Dysfunction. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, 26–38. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, E.; Penrose, H.M.; Heller, S.; Nakhoul, H.; Baddoo, M.; Flemington, E.F.; Kandil, E.; Savkovic, S.D. Bacterial TLR4 and NOD2 Signaling Linked to Reduced Mitochondrial Energy Function in Active Inflammatory Bowel Disease. Gut Microbes 2020, 11, 350–363. [Google Scholar] [CrossRef]
- Lopes, F.; Keita, Å.V.; Saxena, A.; Reyes, J.L.; Mancini, N.L.; Al Rajabi, A.; Wang, A.; Baggio, C.H.; Dicay, M.; Van Dalen, R.; et al. ER-Stress Mobilization of Death-Associated Protein Kinase-1-Dependent Xenophagy Counteracts Mitochondria Stress-Induced Epithelial Barrier Dysfunction. J. Biol. Chem. 2018, 293, 3073–3087. [Google Scholar] [CrossRef]
- Mancini, N.L.; Rajeev, S.; Jayme, T.S.; Wang, A.; Keita, Å.V.; Workentine, M.L.; Hamed, S.; Söderholm, J.D.; Lopes, F.; Shutt, T.E.; et al. Crohn’s Disease Pathobiont Adherent-Invasive E Coli Disrupts Epithelial Mitochondrial Networks with Implications for Gut Permeability. CMGH 2021, 11, 551–571. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.Y.; Shin, S.G.; Kim, J.K.; Silwal, P.; Kim, Y.J.; Shin, N.R.; Kim, P.S.; Won, M.; Lee, S.H.; et al. ESRRA (Estrogen Related Receptor Alpha) Is a Critical Regulator of Intestinal Homeostasis through Activation of Autophagic Flux via Gut Microbiota. Autophagy 2021, 17, 2856–2875. [Google Scholar] [CrossRef]
- Peña-Cearra, A.; Song, D.; Castelo, J.; Palacios, A.; Lavín, J.L.; Azkargorta, M.; Elortza, F.; Fuertes, M.; Pascual-Itoiz, M.A.; Barriales, D.; et al. Mitochondrial Dysfunction Promotes Microbial Composition That Negatively Impacts on Ulcerative Colitis Development and Progression. NPJ Biofilms Microbiomes 2023, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Hayasaki, T.; Nishimura, Y.; Nakamura, M.; Takeda, T.; Tabuchi, Y.; Obinata, M.; Hanawa, T.; Yamada, H. Butyrate Induces Necrotic Cell Death in Murine Colonic Epithelial Cell MCE301. Biol. Pharm. Bull. 2006, 29, 2041–2045. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, S.; Venkatraman, A.; Ramakrishna, B.S. Impairment of Mitochondrial Acetoacetyl CoA Thiolase Activity in the Colonic Mucosa of Patients with Ulcerative Colitis. Gut 2007, 56, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.; Lutgendorff, F.; Phan, V.; Söderholm, J.D.; Sherman, P.M.; McKay, D.M. Enhanced Translocation of Bacteria across Metabolically Stressed Epithelia Is Reduced by Butyrate. Inflamm. Bowel Dis. 2010, 16, 1138–1148. [Google Scholar] [CrossRef] [PubMed]
- Mottawea, W.; Chiang, C.K.; Mühlbauer, M.; Starr, A.E.; Butcher, J.; Abujamel, T.; Deeke, S.A.; Brandel, A.; Zhou, H.; Shokralla, S.; et al. Altered Intestinal Microbiota-Host Mitochondria Crosstalk in New Onset Crohn’s Disease. Nat. Commun. 2016, 7, 13419. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Ogawa, S.A.; Chau, L.; Whelan, K.A.; Hamilton, K.E.; Chen, J.; Tan, L.; Chen, E.Z.; Keilbaugh, S.; Fogt, F.; et al. Mitochondrial Dysfunction in Inflammatory Bowel Disease Alters Intestinal Epithelial Metabolism of Hepatic Acylcarnitines. J. Clin. Investig. 2021, 131, e133371. [Google Scholar] [CrossRef] [PubMed]
- Safwat El-Deeb, O.; El-Esawy, R.O.; Al-Shenawy, H.A.; Ghanem, H.B. Modulating Gut Dysbiosis and Mitochondrial Dysfunction in Oxazolone-Induced Ulcerative Colitis: The Restorative Effects of β-Glucan and/or Celastrol. Redox Rep. 2022, 27, 60–69. [Google Scholar] [CrossRef] [PubMed]
- El-Deeb, O.S.; Elesawy, R.O.; Eltokhy, A.K.; Al-Shenawy, H.A.; Ghanem, H.B.; Rizk, F.H.; Barhoma, R.A.E.; Shalaby, R.H.; Abdelsattar, A.M.; Mashal, S.S.; et al. Moderating Gut Microbiome/Mitochondrial Axis in Oxazolone Induced Ulcerative Colitis: The Evolving Role of β-Glucan and/or, Aldose Reductase Inhibitor, Fidarestat. Int. J. Mol. Sci. 2023, 24, 2711. [Google Scholar] [CrossRef]
- Lee, J.Y.; Cevallos, S.A.; Byndloss, M.X.; Tiffany, C.R.; Olsan, E.E.; Butler, B.P.; Young, B.M.; Rogers, A.W.L.; Nguyen, H.; Kim, K.; et al. High-Fat Diet and Antibiotics Cooperatively Impair Mitochondrial Bioenergetics to Trigger Dysbiosis That Exacerbates Pre-Inflammatory Bowel Disease. Cell Host Microbe 2020, 28, 273–284.e6. [Google Scholar] [CrossRef]
- Haj-Mirzaian, A.; Amiri, S.; Amini-Khoei, H.; Hosseini, M.J.; Haj-Mirzaian, A.; Momeny, M.; Rahimi-Balaei, M.; Dehpour, A.R. Anxiety- and Depressive-Like Behaviors Are Associated with Altered Hippocampal Energy and Inflammatory Status in a Mouse Model of Crohn’s Disease. Neuroscience 2017, 366, 124–137. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, Q.; Xie, L.; Li, C.; Zhuang, Z.; Lin, S.; Lv, P.; Liu, Y.; Wu, Q.; Yu, S. Electroacupuncture and Moxibustion Regulate Hippocampus Glia and Mitochondria Activation in DSS-Induced Colitis Mice. Evid.-Based Complement. Altern. Med. 2020, 2020, 2530253. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Yang, X.Y.; Cui, S.X.; Gao, Z.H.; Qu, X.J. Heterozygous Knockout Insulin-like Growth Factor-1 Receptor (IGF-1R) Regulates Mitochondrial Functions and Prevents Colitis and Colorectal Cancer. Free Radic. Biol. Med. 2019, 134, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, T.A.; Pan, S.; Bronner, M.P.; Crispin, D.A.; Mirzaei, H.; Cooke, K.; Tamura, Y.; Nikolskaya, T.; Jebailey, L.; Goodlett, D.R.; et al. Proteins That Underlie Neoplastic Progression of Ulcerative Colitis. Proteom. Clin. Appl. 2009, 3, 1326. [Google Scholar] [CrossRef] [PubMed]
- Ussakli, C.H.; Ebaee, A.; Binkley, J.; Brentnall, T.A.; Emond, M.J.; Rabinovitch, P.S.; Risques, R.A. Mitochondria and Tumor Progression in Ulcerative Colitis. J. Natl. Cancer Inst. 2013, 105, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Baker, K.T.; Nachmanson, D.; Kumar, S.; Emond, M.J.; Ussakli, C.; Brentnall, T.A.; Kennedy, S.R.; Risques, R.A. Mitochondrial DNA Mutations Are Associated with Ulcerative Colitis Preneoplasia but Tend to Be Negatively Selected in Cancer. Mol. Cancer Res. 2019, 17, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kobunai, T.; Yamamoto, Y.; Murono, K.; Emoto, S.; Hiyoshi, M.; Kaneko, M.; Sasaki, K.; Shuno, Y.; Nishikawa, T.; et al. Assessment of the Changes in Mitochondrial Gene Polymorphism in Ulcerative Colitis and the Etiology of Ulcerative Colitis-Associated Colorectal Cancer. Anticancer. Res. 2020, 40, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; He, X.; Zhao, J.; Wang, D.; Guo, S.; Gao, T.; Wang, G.; Jin, C.; Yan, Z.; Wang, N.; et al. Mitochondrial Transcription Factor A Plays Opposite Roles in the Initiation and Progression of Colitis-Associated Cancer. Cancer Commun. 2021, 41, 695–714. [Google Scholar] [CrossRef]
- Qin, Y.; Yu, Y.; Yang, C.; Wang, Z.; Yang, Y.; Wang, C.; Zheng, Q.; Li, D.; Xu, W. Atractylenolide I Inhibits NLRP3 Inflammasome Activation in Colitis-Associated Colorectal Cancer via Suppressing Drp1-Mediated Mitochondrial Fission. Front. Pharmacol. 2021, 12, 674340. [Google Scholar] [CrossRef]
- Wang, S.Q.; Cui, S.X.; Qu, X.J. Metformin Inhibited Colitis and Colitis-Associated Cancer (CAC) through Protecting Mitochondrial Structures of Colorectal Epithelial Cells in Mice. Cancer Biol. Ther. 2019, 20, 338–348. [Google Scholar] [CrossRef]
- Morris, C.J.; Farr, M.; Hollywell, C.A.; Hawkins, C.F.; Scott, D.L.; Walton, K.W. Ultrastructure of the Synovial Membrane in Seronegative Inflammatory Arthropathies. J. R. Soc. Med. 1983, 76, 27–31. [Google Scholar] [CrossRef]
- Briet, F.; Twomey, C.; Jeejeebhoy, K.N. Effect of Malnutrition and Short-Term Refeeding on Peripheral Blood Mononuclear Cell Mitochondrial Complex I Activity in Humans. Am. J. Clin. Nutr. 2003, 77, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Niknahad, H.; Heidari, R.; Mohammadzadeh, R.; Ommati, M.M.; Khodaei, F.; Azarpira, N.; Abdoli, N.; Zarei, M.; Asadi, B.; Rasti, M.; et al. Sulfasalazine Induces Mitochondrial Dysfunction and Renal Injury. Ren. Fail. 2017, 39, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Bahreini, F.; Jamali, Z.; Pourahmad, J. Mesalazine Induces Oxidative Stress and Cytochrome c Release in Isolated Rat Heart Mitochondria: An Analysis of Cardiotoxic Effects. Int. J. Toxicol. 2020, 39, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, S.; Sigthorsson, G.; Simpson, R.J.; Watts, J.; Jacob, M.; Tavares, I.A.; Rafi, S.; Roseth, A.; Foster, R.; Price, A.B.; et al. Uncoupling of Intestinal Mitochondrial Oxidative Phosphorylation and Inhibition of Cyclooxygenase Are Required for the Development of NSAID-Enteropathy in the Rat. Aliment. Pharmacol. Ther. 2000, 14, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Fratila, O.C.; Craciun, C. Ultrastructural Evidence of Mucosal Healing after Infliximab in Patients with Ulcerative Colitis. J. Gastrointestin. Liver Dis. 2010, 19, 147–153. [Google Scholar] [PubMed]
- Pélissier, M.A.; Marteau, P.; Pochart, P. Antioxidant Effects of Metronidazole in Colonic Tissue. Dig. Dis. Sci. 2007, 52, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Liu, W.; Jin, B.; Geng, J.; Li, J.; Ding, H.; Wu, X.; Xu, Q.; Sun, Y.; Gao, J. Asiatic Acid Ameliorates Dextran Sulfate Sodium-Induced Murine Experimental Colitis via Suppressing Mitochondria-Mediated NLRP3 Inflammasome Activation. Int. Immunopharmacol. 2015, 24, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Saito, R.; Tamura, M.; Matsui, H.; Nagano, Y.; Suzuki, H.; Kaneko, T.; Mizokami, Y.; Hyodo, I. Qing Dai Attenuates Nonsteroidal Antii Inflammatory Drugginduced Mitochondrial Reactive Oxygen Species in Gastrointestinal Epithelial Cells. J. Clin. Biochem. Nutr. 2015, 56, 8–14. [Google Scholar] [CrossRef]
- Han, J.; Zhao, Q.; Basmadjian, C.; Désaubry, L.; Theiss, A.L. Flavaglines Ameliorate Experimental Colitis and Protect Against Intestinal Epithelial Cell Apoptosis and Mitochondrial Dysfunction. Inflamm. Bowel Dis. 2016, 22, 55–67. [Google Scholar] [CrossRef]
- Taya, S.; Kakehashi, A.; Wongpoomchai, R.; Gi, M.; Ishii, N.; Wanibuchi, H. Preventive Effects of Spirogyra Neglecta and a Polysaccharide Extract against Dextran Sodium Sulfate Induced Colitis in Mice. Asian Pac. J. Cancer Prev. 2016, 17, 2235–2245. [Google Scholar] [CrossRef]
- Yeganeh, P.R.; Leahy, J.; Spahis, S.; Patey, N.; Desjardins, Y.; Roy, D.; Delvin, E.; Garofalo, C.; Leduc-Gaudet, J.P.; St-Pierre, D.; et al. Apple Peel Polyphenols Reduce Mitochondrial Dysfunction in Mice with DSS-Induced Ulcerative Colitis. J. Nutr. Biochem. 2018, 57, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, Q.; Zhu, Q.; Tan, R.; Bai, D.; Bu, X.; Lin, B.; Zhao, K.; Pan, C.; Chen, H.; et al. Flavonoid VI-16 Protects against DSS-Induced Colitis by Inhibiting Txnip-Dependent NLRP3 Inflammasome Activation in Macrophages via Reducing Oxidative Stress. Mucosal Immunol. 2019, 12, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Liu, Q.; Wang, M.; Wei, H.-K.; Peng, J. GPA Peptide-Induced Nur77 Localization at Mitochondria Inhibits Inflammation and Oxidative Stress through Activating Autophagy in the Intestine. Oxid. Med. Cell Longev. 2020, 2020, 4964202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, W.; Shen, C.; Wang, X.; Pu, Z.; Yin, Q. Network Pharmacology for Systematic Understanding of Schisandrin B Reduces the Epithelial Cells Injury of Colitis through Regulating Pyroptosis by AMPK/Nrf2/NLRP3 Inflammasome. Aging 2021, 13, 23193–23209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, P.; Zhang, Y.; Jiang, H.; Luan, H.; Xu, Y.; Zhang, Y.; Li, R. Demethyleneberberine Blocked the Maturation of IL-1β in Inflammation by Inhibiting TLR4-Mitochondria Signaling. Int. Immunopharmacol. 2022, 113, 109319. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Xiang, Q.; Shi, G.; Xu, Z.; Ma, X.; Wang, Y.; Xuan, Z.; Xu, F. Licorice Protects against Ulcerative Colitis via the Nrf2/PINK1-Mediated Mitochondrial Autophagy. Immun. Inflamm. Dis. 2023, 11, e757. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, W.; Shi, G.; Huang, Y.; Sun, X.; Sun, N.; Jiang, D. Atractylenolide III Improves Mitochondrial Function and Protects Against Ulcerative Colitis by Activating AMPK/SIRT1/PGC-1 α. Mediat. Inflamm. 2022, 2022, 9129984. [Google Scholar] [CrossRef]
- Gwyer Findlay, E.; Sutton, G.; Ho, G.-T. The MARVEL Trial: A Phase 2b Randomised Placebo-Controlled Trial of Oral MitoQ in Moderate Ulcerative Colitis. Immunother. Adv. 2021, 1, ltaa002. [Google Scholar] [CrossRef]
| Gene/Protein/Signature | Type of Study/Sample | Role in Mitochondria | Ref |
|---|---|---|---|
| OCTN2 | GWAS meta-analyses | Carnitine transporters | [2] |
| PPIF | Enhancer screening | Control of the mitochondrial permeability transition and mitochondrial membrane potential | [3] |
| MT-ND4 | GWAS | Subunit of the respiratory electron transport chain Complex I | [4] |
| Down-regulation of nuclear- and mitochondrial-encoded mitochondrial genes, genes from the TCA cycle, and metabolic functions | RNA-seq (rectal samples from pediatric and adult UC) | Mitochondrial function | [5] |
| Down-regulation of genes involved in mitochondrial respiration in non-inflamed and inflamed tissue from UC and CD | Gene expression microarray (mucosal biopsies from UC, CD, and unclassified IBD | Mitochondrial respiration | [6] |
| Decreased expression of proteins related to mitochondrial energy metabolism | Proteomics (colonic tissue from DSS-treated rhesus macaques) | Mitochondrial respiration and metabolism of fatty acids | [7] |
| Increased expression of STEAP4 (a ferrireductase) | Proteomics (colon from DSS-treated mice) | Mitochondrial iron balance | [8] |
| Up-regulation of SIRT3 and SIRT5; differentially acetylated proteins enrichment in the TCA cycle and fatty acid metabolism | Proteomic analysis of lysine-acetylated proteins and acetylation sites (colon from DSS-treated mice) | TCA cycle and metabolism of fatty acids | [9] |
| Decreased expression of PDHA1, DBT, DLAT, LIAS. | Gene expression meta-analysis of genes involved in cuproptosis | TCA cycle, glycolysis, gluconeogenesis, lipid, pyruvate and propanoate metabolism | [13] |
| Compound | Natural Source | Model | Benefits on Colitis | Benefits on Mitochondrial Function | Ref. |
|---|---|---|---|---|---|
| Asiatic acid | Chinese herb Centella asiatica | DSS-induced colitis (C57BL/6J female mice) |
|
| [107] |
| Qing Dai powder | Qing Dai herb | NSAID-induced cell injury in RGM1 and IEC6 cell lines |
|
| [108] |
| FL3 and FL37 | Flavaglines found in medicinal plants of Southeast Asia |
|
|
| [109] |
| FL3 | Found in medicinal plants of Southeast Asia | DSS-induced colitis (C57BL/6J male mice) |
|
| [109] |
| Extract of Spirogyra neglecta | Spirogyra neglecta (freshwater green alga found in Thailand) | DSS-induced colitis (male Crl: CD1 (ICR) mice) |
|
| [110] |
| Dried apple peel powder | Apple | DSS-induced colitis (C57BL/6J male mice) |
|
| [111] |
| Flavonoid VI-16 | Synthetic (although flavonoids are present in plants) | DSS-induced colitis (C57BL/6J male mice) |
|
| [112] |
| Gly-Pro-Ala peptide | Isolated from fish skin gelatin hydrolysate | MODE-K cell line |
|
| [113] |
| Gly-Pro-Ala peptide | Isolated from fish skin gelatin hydrolysate | DSS-induced colitis (C57BL/6J male mice) |
|
| [113] |
| Schisandrin B | Schisandra chinensis | HCT-116 cell line |
|
| [114] |
| Schisandrin B | Schisandra chinensis | DSS-induced colitis (C57BL/6J mice) |
| [114] | |
| Demethyleneberberine | Coptis chinensis Franch | RAW264.7 cell line |
|
| [115] |
| Demethyleneberberine | Coptis chinensis Franch | DSS-induced colitis (C57BL/6J female mice) |
|
| [115] |
| Licorice | Dry roots and rhizomes of the leguminous plants Glycyrrhiza uralensis Fisch, Glycyrrhiza inflata Bata or Glycyrrhiza glabra | DSS-induced colitis (BALB/C mice) |
|
| [116] |
| Atractylenolide III | Root extracts of Atractylodes macrocephala Koidz | DSS-induced colitis (C57BL/6J male mice) |
|
| [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Quintero, M.J.; Rodríguez-Díaz, C.; Rodríguez-González, F.J.; Fernández-Castañer, A.; García-Fuentes, E.; López-Gómez, C. Role of Mitochondria in Inflammatory Bowel Diseases: A Systematic Review. Int. J. Mol. Sci. 2023, 24, 17124. https://doi.org/10.3390/ijms242317124
Sánchez-Quintero MJ, Rodríguez-Díaz C, Rodríguez-González FJ, Fernández-Castañer A, García-Fuentes E, López-Gómez C. Role of Mitochondria in Inflammatory Bowel Diseases: A Systematic Review. International Journal of Molecular Sciences. 2023; 24(23):17124. https://doi.org/10.3390/ijms242317124
Chicago/Turabian StyleSánchez-Quintero, María José, Cristina Rodríguez-Díaz, Francisco J. Rodríguez-González, Alejandra Fernández-Castañer, Eduardo García-Fuentes, and Carlos López-Gómez. 2023. "Role of Mitochondria in Inflammatory Bowel Diseases: A Systematic Review" International Journal of Molecular Sciences 24, no. 23: 17124. https://doi.org/10.3390/ijms242317124
APA StyleSánchez-Quintero, M. J., Rodríguez-Díaz, C., Rodríguez-González, F. J., Fernández-Castañer, A., García-Fuentes, E., & López-Gómez, C. (2023). Role of Mitochondria in Inflammatory Bowel Diseases: A Systematic Review. International Journal of Molecular Sciences, 24(23), 17124. https://doi.org/10.3390/ijms242317124






