Histidine-Bound Dinitrosyl Iron Complexes: Antioxidant and Antiradical Properties
Abstract
:1. Introduction
2. Results
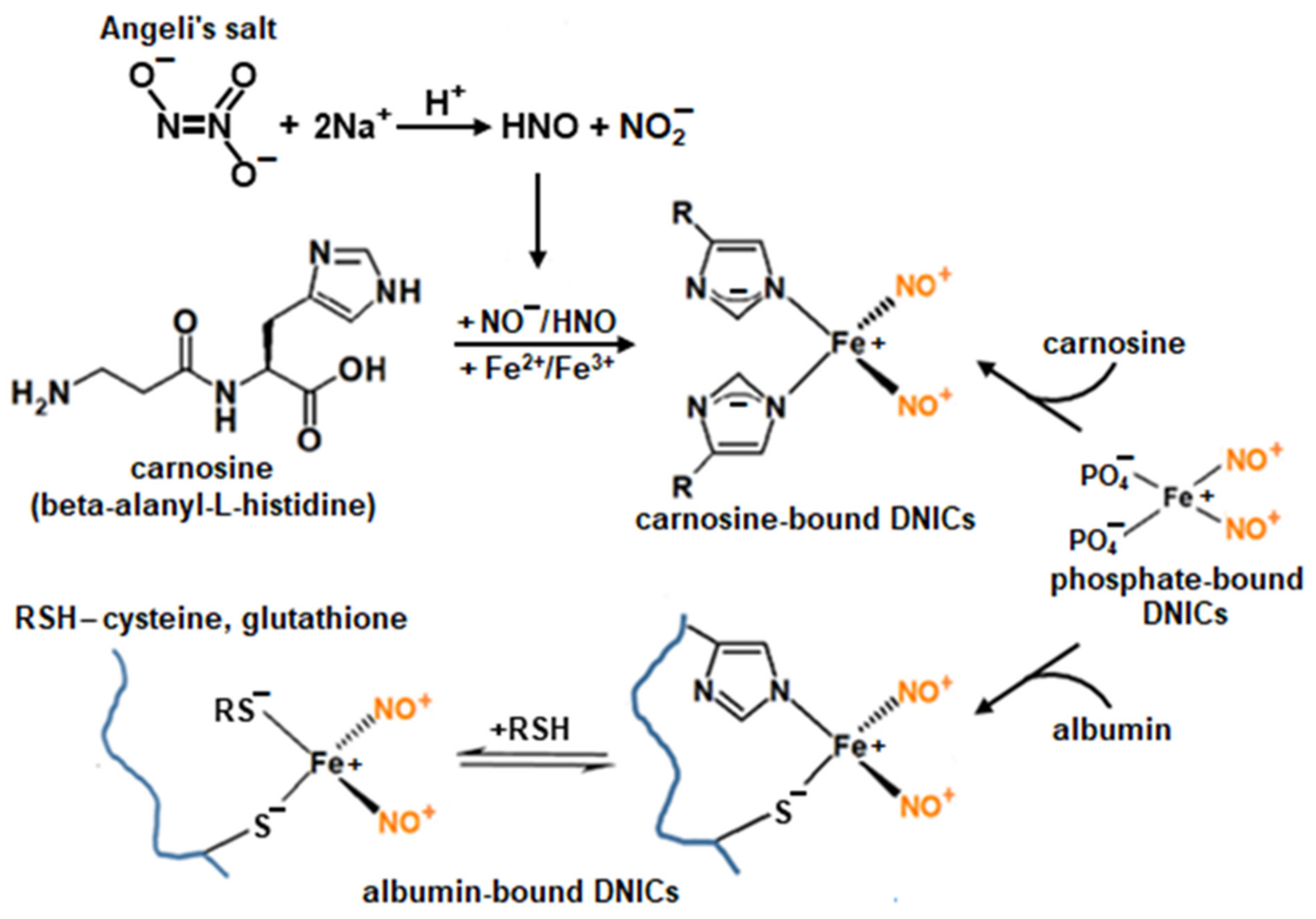
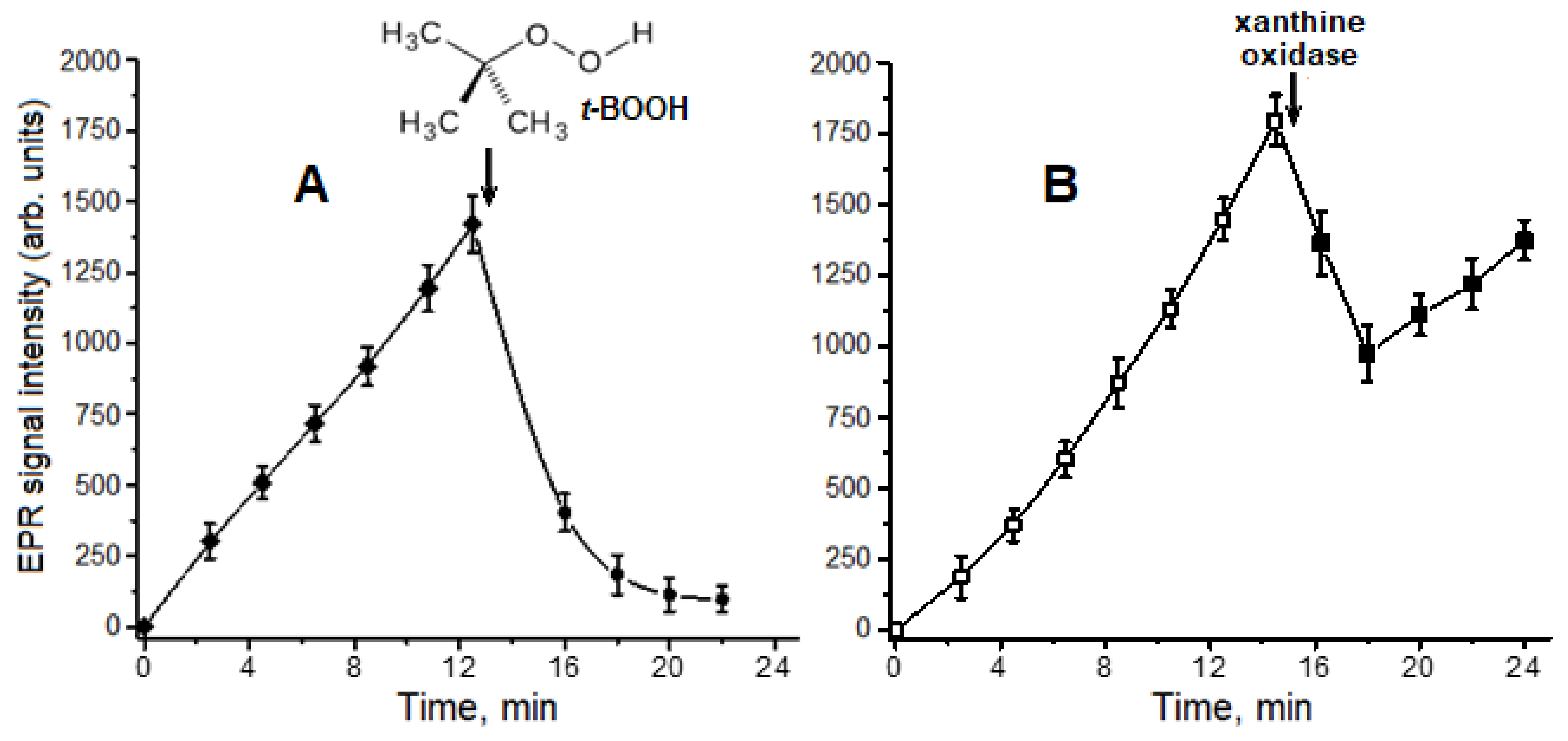
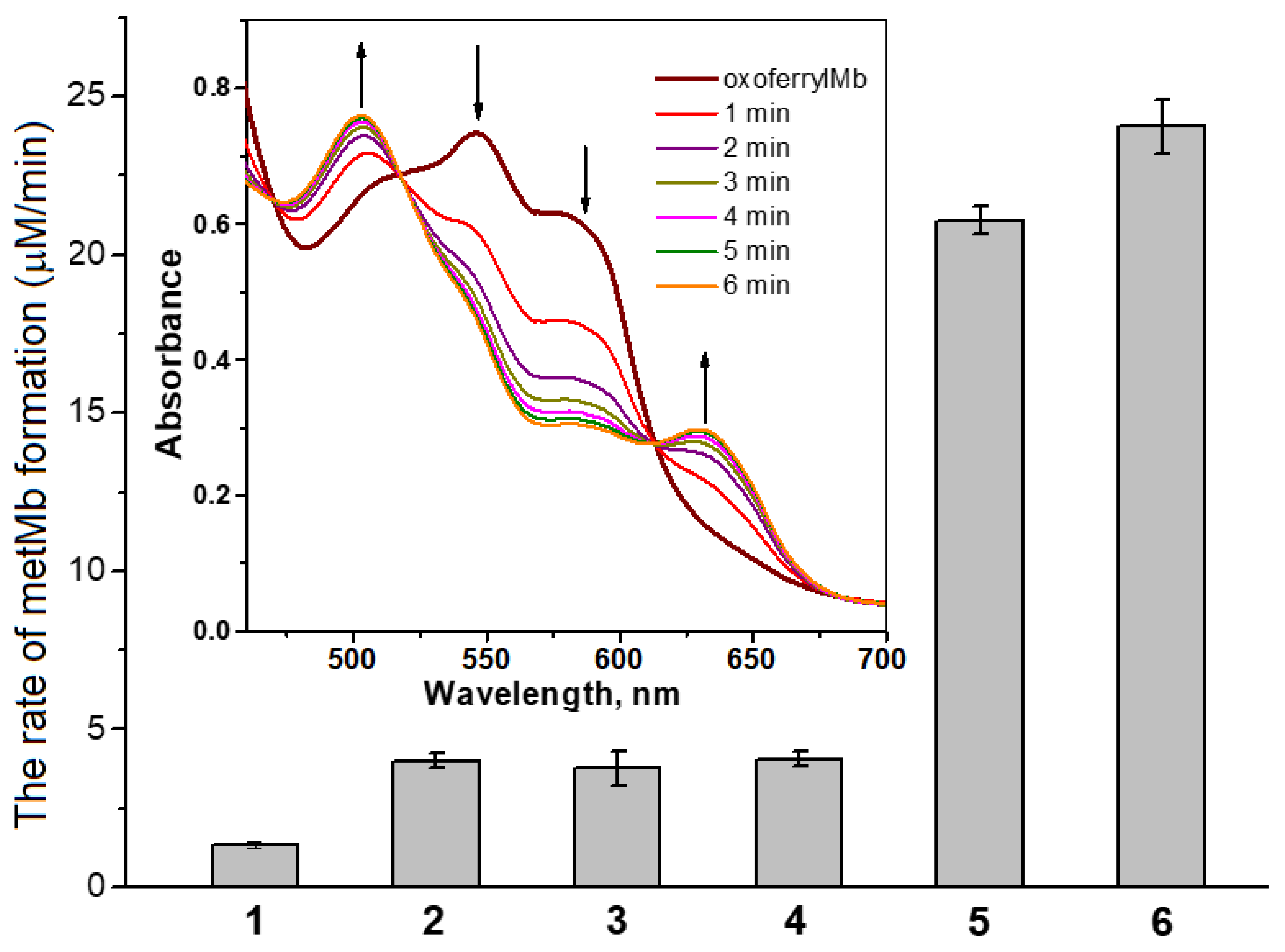
2.1. Interaction of Carnosine-Bound DNICs with Free Radicals and Other Oxidants in Model Systems
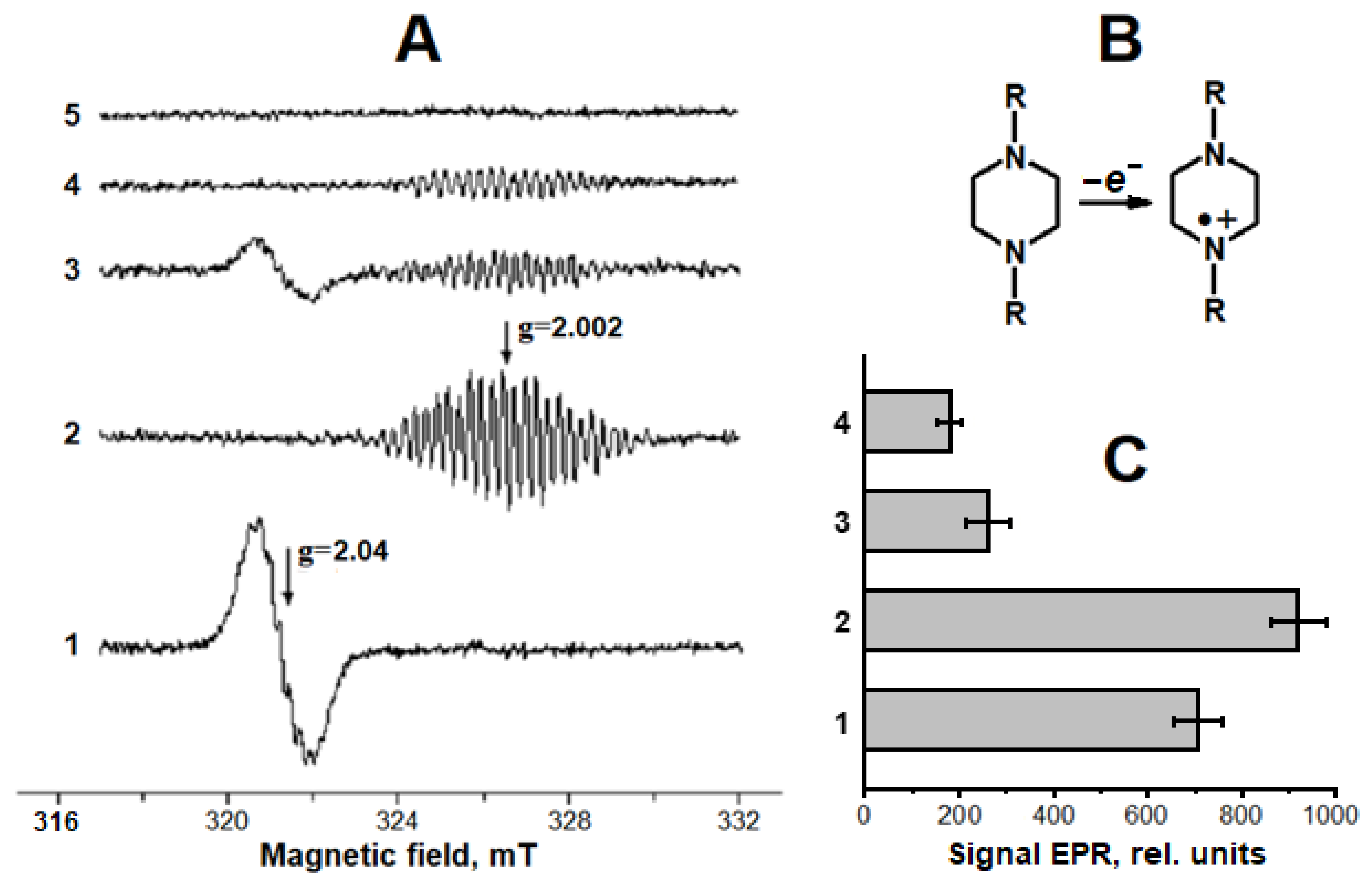
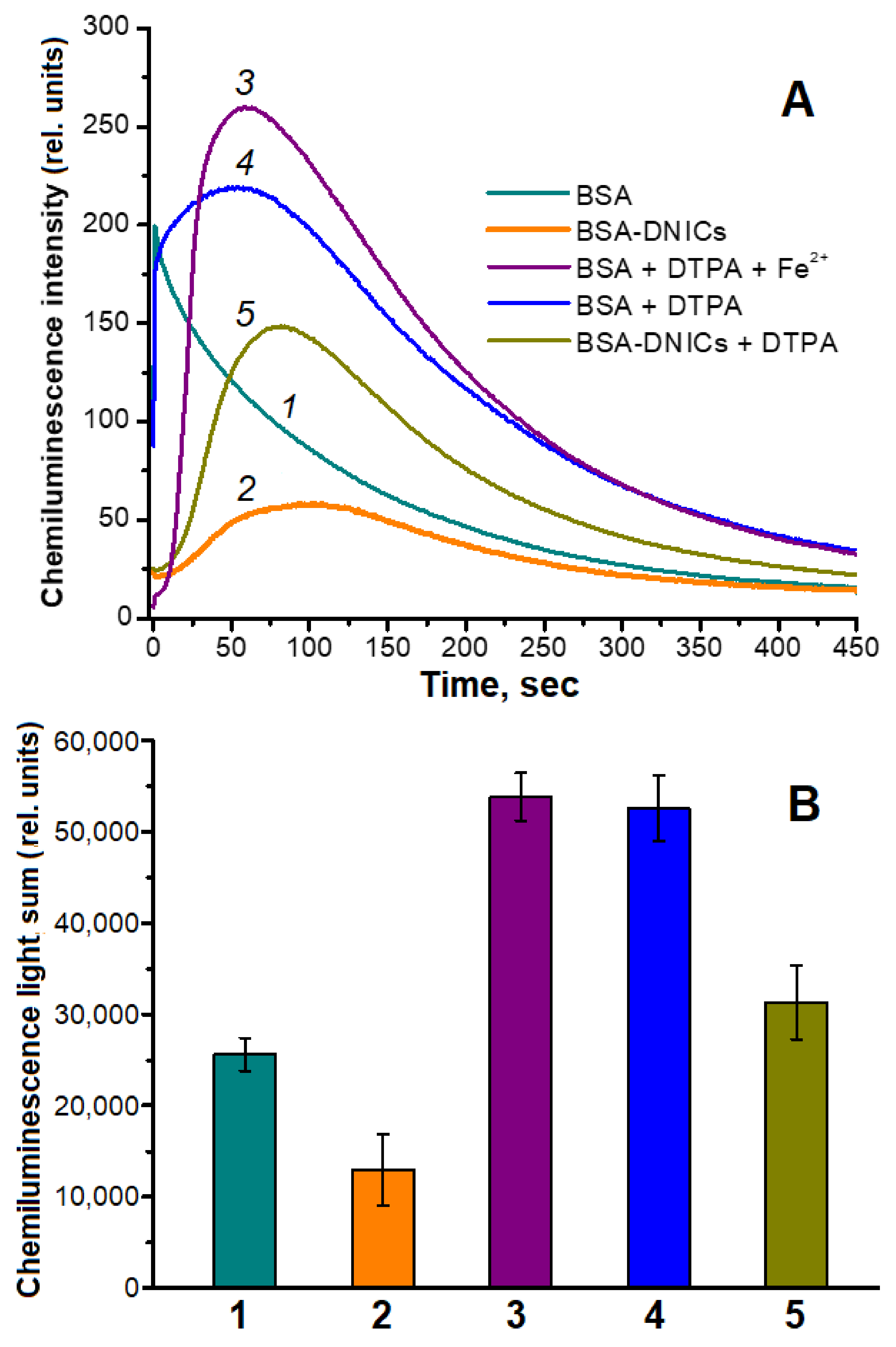
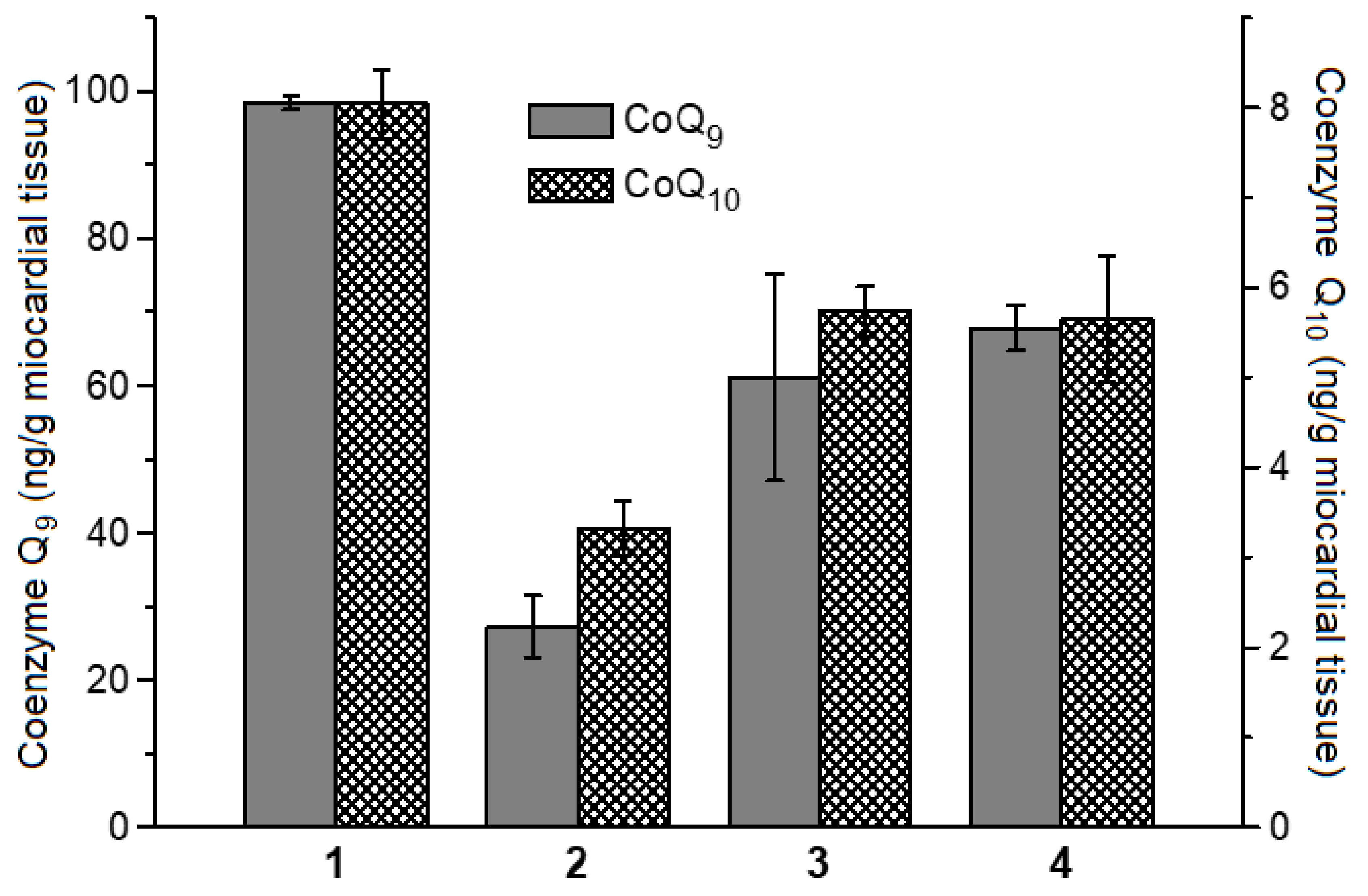
2.2. Antioxidant and Antiradical Properties of DNICs Associated with Albumin
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of DNICs
4.3. EPR Measurements
4.4. Production of Superoxide Anion Radical
4.5. Obtaining and Registration of Oxoferrylmyoglobin (OxoferrylMb)
4.6. Work with Laboratory Animals
4.7. Preparation and Oxidation of Rat Heart Homogenate
4.8. Determination of Coenzymes Q9 and Q10 Contents in Rat Myocardial Homogenate
4.9. Determination of DNICs’ Antioxidant Activity with Chemiluminescence
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miranda, K.M. The chemistry of nitroxyl (HNO) and implications in biology. Coord. Chem. Rev. 2005, 249, 433–455. [Google Scholar] [CrossRef]
- Martínez, M.C.; Andriantsitohaina, R. Reactive nitrogen species: Molecular mechanisms and potential significance in health and disease. Antioxid. Redox Signal. 2009, 11, 669–772. [Google Scholar] [CrossRef] [PubMed]
- Vanin, A.F. Physico-chemistry of dinitrosyl iron complexes as a determinant of their biological activity. Int. J. Mol. Sci. 2021, 22, 10356. [Google Scholar] [CrossRef] [PubMed]
- Kosmachevskaya, O.V.; Nasybullina, E.I.; Pugachenko, I.S.; Novikova, N.N.; Topunov, A.F. Antiglycation and antioxidant effect of nitroxyl towards hemoglobin. Antioxidants 2022, 11, 2007. [Google Scholar] [CrossRef]
- Shumaev, K.B.; Petrova, N.E.; Zabbarova, I.V.; Vanin, A.F.; Topunov, A.F.; Lankin, V.Z.; Ruuge, E.K. Interaction of oxoferrylmyoglobin and dinitrosyl iron complexes. Biochemistry 2004, 69, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Shumaev, K.B.; Gubkin, A.A.; Serezhenkov, V.A.; Lobysheva, I.I.; Kosmachevskaya, O.V.; Ruuge, E.K.; Lankin, V.Z.; Topunov, A.F.; Vanin, A.F. Interaction of reactive oxygen and nitrogen species with albumin- and hemoglobin-bound dinitrosyl-iron complexes. Nitric Oxide 2008, 18, 37–46. [Google Scholar] [CrossRef]
- Shumaev, K.B.; Gorudko, I.V.; Kosmachevskaya, O.V.; Grigoryeva, D.V.; Panasenko, O.M.; Vanin, A.F.; Topunov, A.F.; Terekhova, M.S.; Sokolov, A.V.; Cherenkevich, S.N.; et al. Protective effect of dinitrosyl iron complexes with glutathione in red blood cell lysis induced by hypochlorous acid. Oxid. Med. Cell. Longev. 2019, 2019, e2798154. [Google Scholar] [CrossRef] [PubMed]
- Akentieva, N.P.; Sanina, N.A.; Gizatullin, A.R.; Shkondina, N.I.; Prikhodchenko, T.R.; Shram, S.I.; Zhelev, N.; Aldoshin, S.M. Cytoprotective effects of dinitrosyl iron complexes on viability of human fibroblasts and cardiomyocytes. Front. Pharmacol. 2019, 10, 1277. [Google Scholar] [CrossRef]
- Shumaev, K.B.; Kosmachevskaya, O.V.; Grachev, D.I.; Timoshin, A.A.; Topunov, A.F.; Lankin, V.Z.; Ruuge, E.K. A possible mechanism of the antioxidant action of dinitrosyl iron complexes. Biochem. Suppl. Ser. B Biomed. Chem. 2021, 15, 313–319. [Google Scholar] [CrossRef]
- Igrunkova, A.; Fayzullin, A.; Churbanov, S.; Shevchenko, P.; Serejnikova, N.; Chepelova, N.; Pahomov, D.; Blinova, E.; Mikaelyan, K.; Zaborova, V.; et al. Spray with nitric oxide donor accelerates wound healing: Potential off-the-shelf solution for therapy? Drug Des. Dev. Ther. 2022, 16, 349–362. [Google Scholar] [CrossRef]
- Vanin, A.F.; Serezhenkov, V.A.; Mikoyan, V.D.; Genkin, M.V. The 2.03 signal as an indicator of dinitrosyl–iron complexes with thiol-containing ligands. Nitric Oxide. 1998, 2, 224–234. [Google Scholar] [CrossRef]
- Lu, T.-T.; Wang, Y.-M.; Hung, C.-H.; Chiou, S.-J.; Liaw, W.-F. Bioinorganic chemistry of the natural [Fe(NO)2] motiv: Evolution of a functional model for NO-related biomedical application and revolutionary development of a translational model. Inorg. Chem. 2018, 57, 12425–12443. [Google Scholar] [CrossRef] [PubMed]
- Truzzi, D.R.; Medeiros, N.M.; Augusto, O.; Ford, P.C. Dinitrosyl iron complexes (DNICs). From spontaneous assembly to biological roles. Inorg. Chem. 2021, 60, 15835–15845. [Google Scholar] [CrossRef] [PubMed]
- Shipovalov, A.V.; Vanin, A.F.; Pyankov, O.V.; Bagryanskaya, E.G.; Mikoyan, V.D.; Tkachev, N.A.; Asanbaeva, N.A.; Popkova, V.Y. Antiviral activity of nitrosonium cations against SARS-CoV-2 on a syrian hamster model. Biophysics 2022, 67, 785–795. [Google Scholar] [CrossRef]
- Boese, M.; Mordvintcev, P.I.; Vanin, A.F.; Busse, R.; Mülsch, A. S-nitrosation of serum albumin by dinitrosyl-iron complex. J. Biol. Chem. 1995, 270, 29244–29249. [Google Scholar] [CrossRef]
- Shumaev, K.B.; Kosmachevskaya, O.V.; Nasybullina, E.I.; Gromov, S.V.; Novikov, A.A.; Topunov, A.F. New dinitrosyl iron complexes bound with physiologically active dipeptide carnosine. J. Biol. Inorg. Chem. 2017, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Stvolinsky, S.L.; Dobrota, D. Anti-ischemic activity of carnosine. Biochemistry 2000, 65, 849–855. [Google Scholar] [PubMed]
- Guiotto, A.; Calderan, A.; Ruzza, P.; Borin, G. Carnosine and carnosine-related antioxidants: A review. Curr. Med. Chem. 2005, 12, 2293–2315. [Google Scholar] [CrossRef]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef]
- Prokopieva, V.D.; Yarygina, E.G.; Bokhan, N.A.; Ivanova, S.A. Use of carnosine for oxidative stress reduction in different pathologies. Oxid. Med. Cell. Longev. 2016, 2016, 2939087. [Google Scholar] [CrossRef] [PubMed]
- Creighton, J.V.; Gonҫalves, L.S.; Artioli, G.G.; Tan, D.; Elliott-Sale, K.J.; Turner, M.D.; Doig, C.L.; Sale, C. Physiological roles of carnosine in myocardial function and health. Adv. Nutr. 2022, 13, 1914–1929. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.P.; Garrett, M.R.; Ferry, G.; Smith, M.A. Carnosine: A versatile antioxidant and antiglycating agent. Sci. Aging Knowl. Environ. 2005, 2005, pe12. [Google Scholar] [CrossRef] [PubMed]
- Cripps, M.J.; Hanna, K.; Lavilla Jr, C.; Sayers, S.R.; Caton, P.W.; Sims, C.; De Girolamo, L.; Sale, C.; Turner, M.D. Carnosine scavenging of glucolipotoxic free radicals enhances insulin secretion and glucose uptake. Sci. Rep. 2017, 7, 13313. [Google Scholar] [CrossRef]
- Wu, H.-C.; Shiau, C.-Y.; Chen, H.-M.; Chiou, T.-K. Antioxidant activities of carnosine, anserine, some free amino acids and their combination. J. Food Drug Anal. 2003, 11, 148–153. [Google Scholar] [CrossRef]
- Caruso, G.; Fresta, C.G.; Fidilio, A.; O’Donnell, F.; Musso, N.; Lazzarino, G.; Grasso, M.; Amorini, A.M.; Tascedda, F.; Bucolo, C.; et al. Carnosine decreases PMA-induced oxidative stress and inflammation in murine macrophages. Antioxidants 2019, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Evran, B.; Karpuzoğlu, H.; Develi, S.; Kalaz, E.B.; Soluk-Tekkeşin, M.; Olgaç, V.; Doğru-Abbasoğlu, S.; Uysal, M. Effects of carnosine on prooxidant–antioxidant status in heart tissue, plasma and erythrocytes of rats with isoproterenol-induced myocardial infarction. Pharmacol. Rep. 2014, 66, 81–86. [Google Scholar] [CrossRef]
- Solana-Manrique, C.; Sanz, F.J.; Martínez-Carrión, G.; Paricio, N. Antioxidant and neuroprotective effects of carnosine: Therapeutic implications in neurodegenerative diseases. Antioxidants 2022, 11, 848. [Google Scholar] [CrossRef]
- Babizhayev, M.A.; Yegorov, Y.E. An “enigmatic” L-carnosine (β-alanyl-L-histidine)? Cell proliferative activity as a fundamental property of a natural dipeptide inherent to traditional antioxidant, anti-aging biological activities: Balancing and a hormonally correct agent, novel patented oral therapy dosage formulation for mobility, skeletal muscle power and functional performance, hypothalamic-pituitary- brain relationship in health, aging and stress studies. Recent Pat. Drug Deliv. Formul. 2015, 9, 1–64. [Google Scholar] [CrossRef]
- Chmielewska, K.; Dzierzbicka, K.; Inkielewicz-Stępniak, I.; Przybyłowska, M. Therapeutic potential of carnosine and its derivatives in the treatment of human diseases. Chem. Res. Toxicol. 2020, 33, 1561–1578. [Google Scholar] [CrossRef]
- Jukić, I.; Kolobarić, N.; Stupin, A.; Matić, A.; Kozina, N.; Mihaljević, Z.; Mihalj, M.; Šušnjara, P.; Stupin, M.; Ćurić, Ž.B.; et al. Carnosine, small but mighty-prospect of use as functional ingredient for functional food formulation. Antioxidants 2021, 10, 1037. [Google Scholar] [CrossRef]
- Menini, S.; Iacobini, C.; Fantauzzi, C.B.; Pugliese, G. L-carnosine and its derivatives as new therapeutic agents for the prevention and treatment of vascular complications of diabetes. Curr. Med. Chem. 2020, 27, 1744–1763. [Google Scholar] [CrossRef] [PubMed]
- Bertinaria, M.; Rolando, B.; Giorgis, M.; Montanaro, G.; Marini, E.; Collino, M.; Benetti, E.; Daniele, P.G.; Fruttero, R.; Gasco, A. Carnosine analogues containing NO-donor substructures: Synthesis, physico-chemical characterization and preliminary pharmacological profile. Eur. J. Med. Chem. 2012, 54, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.B.; Hammo, B.; Barry, J.; Radhakrishnan, K. Overview of albumin physiology and its role in pediatric diseases. Curr. Gastroenterol. Rep. 2021, 23, 11. [Google Scholar] [CrossRef]
- Shevtsova, A.; Gordiienko, I.; Tkachenko, V.; Ushakova, G. Ischemia-modified albumin: Origins and clinical implications. Oxid. Med. Cell. Longev. 2021, 2021, 9945424. [Google Scholar] [CrossRef] [PubMed]
- Allakhverdiev, E.S.; Maksimov, G.V.; Rodnenkov, O.V.; Luneva, O.G.; Tsoraev, G.V.; Ivanov, A.D.; Yusipovich, A.I.; Martynyuk, T.V. Effect of dinitrosyl iron complex on albumin conformation. Biochemistry 2021, 86, 533–539. [Google Scholar] [CrossRef]
- Figueroa, S.M.; Araos, P.; Reyes, J.; Gravez, B.; Barrera-Chimal, J.; Amador, C.A. Oxidized albumin as a mediator of kidney disease. Antioxidants 2021, 10, 404. [Google Scholar] [CrossRef]
- Ellidag, H.Y.; Eren, E.; Yılmaz, N.; Cekin, Y. Oxidative stress and ischemia-modified albumin in chronic ischemic heart failure. Redox Rep. 2014, 19, 118–123. [Google Scholar] [CrossRef]
- Gurumurthy, P.; Borra, S.K.; Yeruva, R.K.R.; Victor, D.; Babu, S.; Cherian, K.M. Estimation of ischemia modified albumin (IMA) levels in patients with acute coronary syndrome. Ind. J. Clin. Biochem. 2014, 29, 367–371. [Google Scholar] [CrossRef]
- Tampa, M.; Mitran, C.I.; Mitran, M.I.; Amuzescu, A.; Matei, C.; Georgescu, S.R. Ischemia-modified albumin—A potential new marker of oxidative stress in dermatological diseases. Medicina 2022, 58, 669. [Google Scholar] [CrossRef]
- Hallström, S.; Franz, M.; Gasser, H.; Vodrazka, M.; Semsroth, S.; Losert, U.M.; Haisjackl, M.; Podesser, B.K.; Malinski, T. S-nitroso human serum albumin reduces ischaemia/reperfusion injury in the pig heart after unprotected warm ischaemia. Cardiovasc. Res. 2007, 77, 506–514. [Google Scholar] [CrossRef]
- Pokidova, O.V.; Luzhkov, V.B.; Emel’yanova, N.S.; Krapivin, V.B.; Kotelnikov, A.I.; Sanina, N.A.; Aldoshin, S.M. Effect of albumin on the transformation of dinitrosyl iron complexes with thiourea ligands. Dalton Trans. 2020, 49, 12674–12685. [Google Scholar] [CrossRef] [PubMed]
- Faingold, I.I.; Poletaeva, D.A.; Soldatova, Y.V.; Smolina, A.V.; Pokidova, O.V.; Kulikov, A.V.; Sanina, N.A.; Kotelnikova, R.A. Effects of albumin-bound nitrosyl iron complex with thiosulfate ligands on lipid peroxidation and activities of mitochondrial enzymes in vitro. Nitric Oxide 2021, 117, 46–52. [Google Scholar] [CrossRef]
- Kirsch, M.; Lomonosova, E.E.; Korth, H.G.; Sustmann, R.; de Groot, H. Hydrogen peroxide formation by reaction of peroxynitrite with HEPES and related tertiary amines. implications for a general mechanism. J. Biol. Chem. 1998, 273, 12716–12724. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Kozlov, A.V.; Tyurina, Y.Y.; Shvedova, A.A.; Yalowich, J.C. Antioxidant mechanisms of nitric oxide against iron catalyzed oxidative stress in cells. Antioxid. Redox Signal. 2001, 3, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, C.; Mahtani, H.K.; Du, J.; Patel, A.R.; Lancaster, J.R. Nitrosothiol formation and protection against fenton chemistry by nitric oxide-induced dinitrosyliron complex formation from anoxia-initiated cellular chelatable iron. J. Biol. Chem. 2014, 289, 19917–19927. [Google Scholar] [CrossRef] [PubMed]
- Shumaev, K.B.; Gubkina, S.A.; Kumskova, E.M.; Shepelkova, G.S.; Ruuge, E.K.; Lankin, V.Z. Superoxide formation as a result of interaction of L-lysine with dicarbonyl compounds and its possible mechanism. Biochemistry 2009, 74, 461–466. [Google Scholar] [CrossRef]
- Magenta, A.; Greco, S.; Capogrossi, M.C.; Gaetano, C.; Martelli, F. Nitric Oxide, Oxidative Stress, and p66Shc Interplay in Diabetic Endothelial Dysfunction. BioMed Res. Int. 2014, 2014, 193095. [Google Scholar] [CrossRef] [PubMed]
- Vanin, A.F.; Huisman, A.; Stroes, E.S.G.; de Ruijter-Heijstek, F.C.; Rabelink, T.J.; Faassen, E.E. Antioxidant capacity of mononitrosyl-iron-dithiocarbamate complexes: Implications for NO trapping. Free Rad. Biol. Med. 2001, 30, 813–824. [Google Scholar] [CrossRef]
- Sumaev, K.B.; Kosmachevskaya, O.V.; Timoshin, A.A.; Vanin, A.F.; Topunov, A.F. Dinitrosyl iron complexes bind with hemoglobin as markers of oxidative stress. Methods Enzymol. 2008, 436, 445–461. [Google Scholar] [CrossRef]
- Reeder, B.J.; Svistunenko, D.A.; Sharpe, M.A.; Wilson, M.T. Characteristics and mechanism of formation of peroxide-induced heme to protein cross-linking in myoglobin. Biochemistry 2002, 41, 367–375. [Google Scholar] [CrossRef]
- Baron, C.P.; Andersen, H.J. Myoglobin-induced lipid oxidation. A review. J. Agric. Food Chem. 2002, 50, 3887–3897. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.T.; Reeder, B.J. The peroxidatic activities of Myoglobin and Hemoglobin, their pathological consequences and possible medical interventions. Mol. Asp. Med. 2022, 84, 101045. [Google Scholar] [CrossRef]
- Herold, S.; Rehmann, F.-J.K. Kinetic and mechanistic studies of the reactions of nitrogen monoxide and nitrite with ferryl myoglobin. J. Biol. Inorg. Chem. 2001, 6, 543–555. [Google Scholar] [CrossRef]
- Herold, S.; Rehmann, F.-J.K. Kinetics of the reactions of nitrogen monoxide and nitrite with ferryl hemoglobin. Free Rad. Biol. Med. 2003, 34, 531–545. [Google Scholar] [CrossRef] [PubMed]
- Kitagishi, H.; Tamaki, M.; Ueda, T.; Hirota, S.; Ohta, T.; Naruta, Y.; Kano, K. Oxoferryl porphyrin/hydrogen peroxide system whose behavior is equivalent to hydroperoxoferric porphyrin. J. Am. Chem. Soc. 2010, 132, 16730–16732. [Google Scholar] [CrossRef] [PubMed]
- Mannino, M.H.; Patel, R.S.; Eccardt, A.M.; Janowiak, B.E.; Wood, D.S.; He, F.; Fisher, J.S. Reversible oxidative modifications in myoglobin and functional implications. Antioxidants 2020, 9, 549. [Google Scholar] [CrossRef]
- Nagababu, E.; Rifkind, J.M. Reaction of hydrogen peroxide with ferrylhemoglobin: Superoxide production and heme degradation. Biochemistry 2000, 39, 12503–12511. [Google Scholar] [CrossRef] [PubMed]
- Zee, J. Formation of peroxide- and globin-derived radicals from the reaction of methemoglobin and metmyoglobin with t-butyl hydroperoxide: An ESR spin-trapping investigation. Biochem. J. 1997, 322, 633–639. [Google Scholar] [CrossRef]
- Reeder, B.J.; Svistunenko, D.A.; Cooper, C.E.; Wilson, M.T. The radical and redox chemistry of myoglobin and hemoglobin: From in vitro studies to human pathology. Antioxid. Redox Signal. 2004, 6, 954–966. [Google Scholar] [CrossRef]
- Bastos, E.L.; Romoff, P.; Eckert, C.R.; Baader, W.J. Evaluation of antiradical capacity by H2O2-hemin-induced luminol chemiluminescence. J. Agric. Food Chem. 2003, 51, 7481–7488. [Google Scholar] [CrossRef]
- Mishima, E.; Conrad, M. Nutritional and metabolic control of ferroptosis. Annu. Rev. Nutr. 2022, 42, 275–309. [Google Scholar] [CrossRef]
- Kucharská, J.; Braunová, Z.; Ulicná, O.; Zlatos, L.; Gvozdjáková, A. Deficit of coenzyme Q in heart and liver mitochondria of rats with streptozotocin-induced diabetes. Physiol. Res. 2000, 49, 411–418. [Google Scholar] [PubMed]
- Zhang, Y.; Turunen, M.; Appelkvist, E.L. Restricted uptake of dietary coenzyme Q is in contrast to the unrestricted uptake of alpha-tocopherol into rat organs and cells. J. Nutr. 1996, 126, 2089–2097. [Google Scholar] [CrossRef] [PubMed]
- Bhagavan, H.N.; Chopra, R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetic. Free Radic. Res. 2006, 40, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.A.; Tyurina, Y.Y.; Gorbunov, N.V.; Tyurin, V.A.; Castranova, V.; Kommineni, C.; Ojimba, J.; Gandley, R.; McLaughlin, M.K.; Kagan, V.E. Tert-butyl hydroperoxide/hemoglobin-induced oxidative stress and damage to vascular smooth muscle cells: Different effects of nitric oxide and nitrosothiols. Biochem. Pharmacol. 1999, 57, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Rubbo, H.; Radi, R.; Anselmi, D.; Kirk, M.; Barnes, S.; Butler, J.; Eiserich, J.P.; Freeman, B.A. Nitric oxide reaction with lipid peroxyl radicals spares alpha-tocopherol during lipid peroxidation. J. Biol. Chem. 2000, 275, 10812–10818. [Google Scholar] [CrossRef] [PubMed]
- Chamulitrat, W. EPR studies of nitric oxide interactions of alkoxyl and peroxyl radicals in in vitro and ex vivo model systems. Antioxid. Redox Signal. 2001, 3, 177–187. [Google Scholar] [CrossRef]
- Hummel, S.G.; Fischer, A.J.; Martin, S.M.; Schafer, F.Q.; Buettner, G.R. Nitric oxide as a cellular antioxidant: A little goes a long way. Free Radic. Biol. Med. 2006, 40, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.G.; Kalyvas, H.; Skodje, K.M.; Hayashi, T.; Moënne-Loccoz, P.; Callan, P.E.; Shearer, J.; Kirschenbaum, L.J.; Kim, E. Phenol nitration induced by an {Fe(NO)2}10 dinitrosyl iron complex. J. Am. Chem. Soc. 2011, 133, 1184–1187. [Google Scholar] [CrossRef]
- Su, L.-J.; Zhang, J.-H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.-Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Tang, D. Ferroptosis by Lipid Peroxidation: The Tip of the Iceberg? Front. Cell Dev. Biol. 2021, 9, 646890. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Liu, X.; Guo, S.; Jiang, L.; Huang, Y.; Wu, Y. Carnosine alleviates kidney tubular epithelial injury by targeting NRF2 mediated ferroptosis in diabetic nephropathy. Amino Acids 2023, 55, 1141–1155. [Google Scholar] [CrossRef] [PubMed]
- Kehl, D.W.; Iqbal, N.; Fard, A.; Kipper, B.A.; De La Parra Landa, A.; Maisel, A.S. Biomarkers in acute myocardial injury. Transl. Res. 2012, 159, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Erenler, A.K.; Yardan, T.; Kati, C.; Altuntaş, M.; Turedi, S. Role of ischemia-modified albumin in clinical practice. J. Lab. Med. 2015, 39, 241–247. [Google Scholar] [CrossRef]
- Kroger-Ohlsen, M.V.; Ostdal, H.; Andersen, M.L. The effect of pH on the oxidation of bovine serum albumin by hypervalent myoglobin species. Arch. Biochem. Biophys. 2003, 416, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Giannoglou, G.D.; Chatzizisis, Y.S.; Misirli, G. The syndrome of rhabdomyolysis: Pathophysiology and diagnosis. Eur. J. Intern. Med. 2007, 18, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Troxler, M.; Thompson, D.; Homer-Vanniasinkam, S. Ischaemic skeletal muscle increases serum ischaemia modified albumin. Eur.J. Vasc. Endovasc. Surg. 2006, 31, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.P.; Mason, R.P. Mechanism of radical production from the reaction of cytochrome c with organic hydroperoxides. J. Biol. Chem. 1995, 270, 12709–12716. [Google Scholar] [CrossRef]
- Fukuto, J.M.; Dutton, A.S.; Houk, K.N. The chemistry and biology of nitroxyl (HNO): A chemically unique species with novel and important biological activity. Chembiochem 2005, 6, 612–619. [Google Scholar] [CrossRef]
- Lopez, B.E.; Shinyashiki, M.; Han, T.H.; Fukuto, J.M. Antioxidant actions of nitroxyl (HNO). Free Radic. Biol. Med. 2007, 42, 482–491. [Google Scholar] [CrossRef]
- Gorbunov, N.V.; Yalowich, J.C.; Gaddam, A.; Thampatty, P.; Ritov, V.B.; Kisin, E.R.; Elsayed, N.M.; Kagan, V.E. Nitric oxide prevents oxidative damage produced by tert-butyl hydroperoxide in erythroleukemia cells via nitrosylation of heme and non-heme iron. Electron paramagnetic resonance evidence. J. Biol. Chem. 1997, 272, 12328–12341. [Google Scholar] [CrossRef] [PubMed]
- Fedotcheva, T.; Shimanovsky, N.; Fedotcheva, N. Specific features of mitochondrial dysfunction under conditions of ferroptosis induced by t-butylhydroperoxide and iron: Protective role of the inhibitors of lipid peroxidation and mitochondrial permeability transition pore opening. Membranes 2023, 13, 372. [Google Scholar] [CrossRef]
- Nicoletti, V.G.; Santoro, A.M.; Grasso, G.; Vagliasindi, L.I.; Giuffrida, M.L.; Cuppari, C.; Purrello, V.S.; Stella, A.M.G.; Rizzarelli, E. Carnosine interaction with nitric oxide and astroglial cell protection. J. Neurosci. Res. 2007, 85, 2239–2245. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Colombrita, C.; Guagliano, E.; Sapienza, M.; Ravagna, A.; Cardile, V.; Scapagnini, G.; Santoro, A.M.; Mangiameli, A.; Butterfield, D.A.; et al. Protective effect of carnosine during nitrosative stress in astroglial cell cultures. Neurochem. Res. 2005, 30, 797–807. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Nakashima, Y.; Toda, K. Carnosine facilitates nitric oxide production in endothelial F-2 cells. Biol. Pharm. Bull. 2009, 32, 1836–1839. [Google Scholar] [CrossRef]
- Severina, I.S.; Bussygina, O.G.; Pyatakova, N.V. Carnosine as a regulator of soluble guanylate cyclase. Biochemistry 2000, 65, 783–788. [Google Scholar]
- Liu, T.; Zhang, M.; Terry, M.H.; Schroeder, H.; Wilson, S.M.; Power, G.G.; Li, Q.; Tipple, T.E.; Borchardt, D.; Blood, A.B. Hemodynamic effects of glutathione-liganded binuclear dinitrosyl iron complex: Evidence for nitroxyl generation and modulation by plasma albumin. Mol. Pharmacol. 2018, 93, 427–437. [Google Scholar] [CrossRef]
- Kalenikova, E.I.; Gorodetskaya, E.A.; Kulyak, O.Y.; Kozaeva, L.P.; Makarov, V.G.; Pozharitskaya, O.N.; Shikov, A.N.; Medvedev, O.S. Preclinical study of the pharmacokinetics of a new intravenous dosage form of ubiquinol. Pharm. Chem. J. 2018, 51, 949–953. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shumaev, K.B.; Kosmachevskaya, O.V.; Nasybullina, E.I.; Ruuge, E.K.; Kalenikova, E.I.; Topunov, A.F. Histidine-Bound Dinitrosyl Iron Complexes: Antioxidant and Antiradical Properties. Int. J. Mol. Sci. 2023, 24, 17236. https://doi.org/10.3390/ijms242417236
Shumaev KB, Kosmachevskaya OV, Nasybullina EI, Ruuge EK, Kalenikova EI, Topunov AF. Histidine-Bound Dinitrosyl Iron Complexes: Antioxidant and Antiradical Properties. International Journal of Molecular Sciences. 2023; 24(24):17236. https://doi.org/10.3390/ijms242417236
Chicago/Turabian StyleShumaev, Konstantin B., Olga V. Kosmachevskaya, Elvira I. Nasybullina, Enno K. Ruuge, Elena I. Kalenikova, and Alexey F. Topunov. 2023. "Histidine-Bound Dinitrosyl Iron Complexes: Antioxidant and Antiradical Properties" International Journal of Molecular Sciences 24, no. 24: 17236. https://doi.org/10.3390/ijms242417236
APA StyleShumaev, K. B., Kosmachevskaya, O. V., Nasybullina, E. I., Ruuge, E. K., Kalenikova, E. I., & Topunov, A. F. (2023). Histidine-Bound Dinitrosyl Iron Complexes: Antioxidant and Antiradical Properties. International Journal of Molecular Sciences, 24(24), 17236. https://doi.org/10.3390/ijms242417236




