Emerging Relevance of Ghrelin in Programmed Cell Death and Its Application in Diseases
Abstract
:1. Introduction
2. Ghrelin and Apoptosis
3. Ghrelin and Pyroptosis
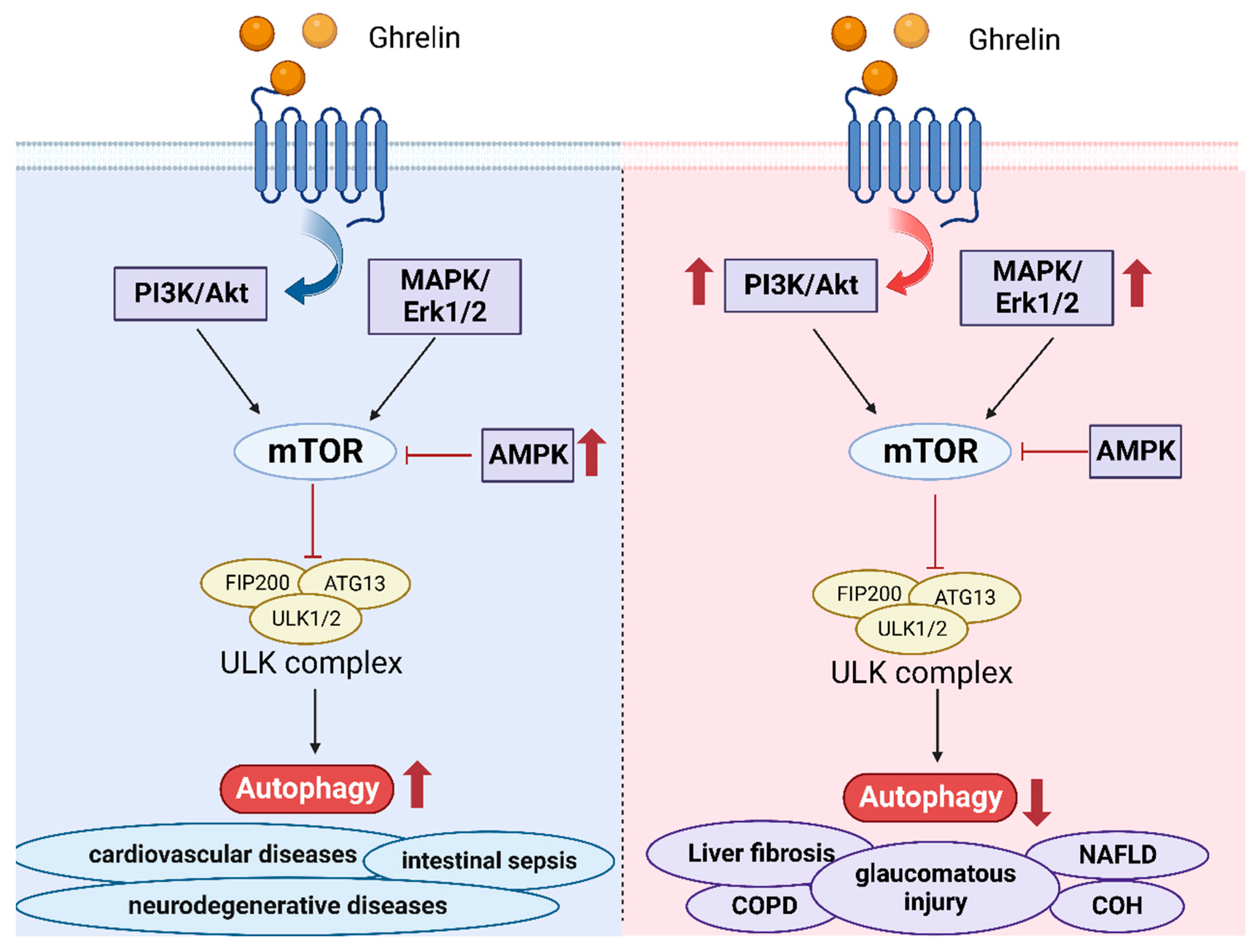
4. Ghrelin and Autophagy
5. Ghrelin and Necroptosis
6. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Kojima, M.; Kangawa, K. Ghrelin: Structure and function. Physiol. Rev. 2005, 85, 495–522. [Google Scholar] [CrossRef]
- Poher, A.L.; Tschöp, M.H.; Müller, T.D. Ghrelin regulation of glucose metabolism. Peptides 2018, 100, 236–242. [Google Scholar] [CrossRef]
- Yamada, C. Involvement of Ghrelin Dynamics in Stress-Induced Eating Disorder: Effects of Sex and Aging. Int. J. Mol. Sci. 2021, 22, 11695. [Google Scholar] [CrossRef]
- Davis, T.R.; Pierce, M.R.; Novak, S.X.; Hougland, J.L. Ghrelin octanoylation by ghrelin O-acyltransferase: Protein acylation impacting metabolic and neuroendocrine signalling. Open Biol. 2021, 11, 210080. [Google Scholar] [CrossRef]
- Jiao, Z.T.; Luo, Q. Molecular Mechanisms and Health Benefits of Ghrelin: A Narrative Review. Nutrients 2022, 14, 4191. [Google Scholar] [CrossRef]
- Yuan, M.J.; Li, W.; Zhong, P. Research progress of ghrelin on cardiovascular disease. Biosci. Rep. 2021, 41, BSR20203387. [Google Scholar] [CrossRef]
- Ukkola, O. Ghrelin in Type 2 diabetes mellitus and metabolic syndrome. Mol. Cell. Endocrinol. 2011, 340, 26–28. [Google Scholar] [CrossRef]
- Delhanty, P.J.; van der Lely, A.J. Ghrelin and glucose homeostasis. Peptides 2011, 32, 2309–2318. [Google Scholar] [CrossRef]
- Churm, R.; Davies, J.S.; Stephens, J.W.; Prior, S.L. Ghrelin function in human obesity and type 2 diabetes: A concise review. Obes. Rev. 2017, 18, 140–148. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Higuchi, J.; Honda, N.; Komura, N. Pharmacological profile and clinical efficacy of anamorelin HCl (ADLUMIZ® Tablets), the first orally available drug for cancer cachexia with ghrelin-like action in Japan. Nihon Yakurigaku Zasshi 2021, 156, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, H.; Pietra, C.; Giuliano, C.; Garcia-Rubio, S.; Xu, X.; Yakabi, S.; Taché, Y.; Wang, L. New ghrelin agonist, HM01 alleviates constipation and L-dopa-delayed gastric emptying in 6-hydroxydopamine rat model of Parkinson’s disease. Neurogastroenterol. Motil. 2014, 26, 1771–1782. [Google Scholar] [CrossRef] [PubMed]
- Tower, J. Programmed cell death in aging. Ageing Res. Rev. 2015, 23 Pt A, 90–100. [Google Scholar] [CrossRef]
- Bedoui, S.; Herold, M.J.; Strasser, A. Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol. 2020, 21, 678–695. [Google Scholar] [CrossRef]
- Gibellini, L.; Moro, L. Programmed Cell Death in Health and Disease. Cells 2021, 10, 1765. [Google Scholar] [CrossRef]
- Wei, X.; Xie, F.; Zhou, X.; Wu, Y.; Yan, H.; Liu, T.; Huang, J.; Wang, F.; Zhou, F.; Zhang, L. Role of pyroptosis in inflammation and cancer. Cell. Mol. Immunol. 2022, 19, 971–992. [Google Scholar] [CrossRef]
- Newton, K. RIPK1 and RIPK3: Critical regulators of inflammation and cell death. Trends Cell Biol. 2015, 25, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Ezquerro, S.; Frühbeck, G.; Rodríguez, A. Ghrelin and autophagy. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 402–408. [Google Scholar] [CrossRef]
- Ugwu, F.N.; Yu, A.P.; Sin, T.K.; Tam, B.T.; Lai, C.W.; Wong, S.C.; Siu, P.M. Protective Effect of Unacylated Ghrelin on Compression-Induced Skeletal Muscle Injury Mediated by SIRT1-Signaling. Front. Physiol. 2017, 8, 962. [Google Scholar] [CrossRef]
- Ezquerro, S.; Mocha, F.; Frühbeck, G.; Guzmán-Ruiz, R.; Valentí, V.; Mugueta, C.; Becerril, S.; Catalán, V.; Gómez-Ambrosi, J.; Silva, C.; et al. Ghrelin Reduces TNF-α-Induced Human Hepatocyte Apoptosis, Autophagy, and Pyroptosis: Role in Obesity-Associated NAFLD. J. Clin. Endocrinol. Metab. 2019, 104, 21–37. [Google Scholar] [CrossRef]
- Pistritto, G.; Trisciuoglio, D.; Ceci, C.; Garufi, A.; D’Orazi, G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging 2016, 8, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A. Targeting the extrinsic apoptotic pathway in cancer: Lessons learned and future directions. J. Clin. Investig. 2015, 125, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, R.; Kheirollahi, A.; Davoodi, J. Apaf-1: Regulation and function in cell death. Biochimie 2017, 135, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Moujalled, D.; Strasser, A.; Liddell, J.R. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021, 28, 2029–2044. [Google Scholar] [CrossRef] [PubMed]
- Gon, S.; Gatanaga, T.; Sendo, F. Involvement of two types of TNF receptor in TNF-alpha induced neutrophil apoptosis. Microbiol. Immunol. 1996, 40, 463–465. [Google Scholar] [CrossRef] [PubMed]
- Vanden Berghe, T.; van Loo, G.; Saelens, X.; Van Gurp, M.; Brouckaert, G.; Kalai, M.; Declercq, W.; Vandenabeele, P. Differential signaling to apoptotic and necrotic cell death by Fas-associated death domain protein FADD. J. Biol. Chem. 2004, 279, 7925–7933. [Google Scholar] [CrossRef]
- Kurowska, P.; Mlyczynska, E.; Rak, A. Effect of ghrelin on the apoptosis of various cells. A critical review. J. Physiol. Pharmacol. 2019, 70, 3–13. [Google Scholar]
- Irmler, M.; Thome, M.; Hahne, M.; Schneider, P.; Hofmann, K.; Steiner, V.; Bodmer, J.L.; Schröter, M.; Burns, K.; Mattmann, C.; et al. Inhibition of death receptor signals by cellular FLIP. Nature 1997, 388, 190–195. [Google Scholar] [CrossRef]
- Li, H.; Zhu, H.; Xu, C.J.; Yuan, J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998, 94, 491–501. [Google Scholar] [CrossRef]
- Gross, A.; Yin, X.M.; Wang, K.; Wei, M.C.; Jockel, J.; Milliman, C.; Erdjument-Bromage, H.; Tempst, P.; Korsmeyer, S.J. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 1999, 274, 1156–1163. [Google Scholar] [CrossRef]
- Castañeda, T.R.; Tong, J.; Datta, R.; Culler, M.; Tschöp, M.H. Ghrelin in the regulation of body weight and metabolism. Front. Neuroendocrinol. 2010, 31, 44–60. [Google Scholar] [CrossRef] [PubMed]
- Dodington, D.W.; Desai, H.R.; Woo, M. JAK/STAT—Emerging Players in Metabolism. Trends Endocrinol. Metab. 2018, 29, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.G.; Cai, H.Q.; Li, Y.H.; Sui, Y.B.; Zhang, J.S.; Chang, J.R.; Ning, M.; Wu, Y.; Tang, C.S.; Qi, Y.F.; et al. Ghrelin protects heart against ERS-induced injury and apoptosis by activating AMP-activated protein kinase. Peptides 2013, 48, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, Y.; Liu, X.; Wang, X. Ghrelin protected neonatal rat cardiomyocyte against hypoxia/reoxygenation injury by inhibiting apoptosis through Akt-mTOR signal. Mol. Biol. Rep. 2017, 44, 219–226. [Google Scholar] [CrossRef]
- Jin, L.; Cai, C.L.; Lang, X.L.; Li, B.L. Ghrelin inhibits inflammatory response and apoptosis of myocardial injury in septic rats through JAK/STAT signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 11740–11746. [Google Scholar] [PubMed]
- Eid, R.A.; Alkhateeb, M.A.; Al-Shraim, M.; Eleawa, S.M.; Shatoor, A.S.; El-Kott, A.F.; Zaki, M.S.A.; Shatoor, K.A.; Bin-Jaliah, I.; Al-Hashem, F.H. Ghrelin prevents cardiac cell apoptosis during cardiac remodelling post experimentally induced myocardial infarction in rats via activation of Raf-MEK1/2-ERK1/2 signalling. Arch. Physiol. Biochem. 2019, 125, 93–103. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chiang, C.F.; Chen, L.F.; Liao, S.C.; Hsieh, W.Y.; Lin, W.L. Polymersomes conjugated with des-octanoyl ghrelin for the delivery of therapeutic and imaging agents into brain tissues. Biomaterials 2014, 35, 2051–2065. [Google Scholar] [CrossRef]
- Davis, J. Hunger, ghrelin and the gut. Brain Res. 2018, 1693 Pt B, 154–158. [Google Scholar] [CrossRef]
- Stasi, C.; Milani, S. Functions of Ghrelin in Brain, Gut and Liver. CNS Neurol. Disord. Drug Targets 2016, 15, 956–963. [Google Scholar] [CrossRef]
- Isokawa, M. Ghrelin-O-acyltransferase (GOAT) acylates ghrelin in the hippocampus. In Vitamins and Hormones; Academic Press: Cambridge, MA, USA, 2022; Volume 118, pp. 369–392. [Google Scholar]
- Howick, K.; Griffin, B.T.; Cryan, J.F.; Schellekens, H. From Belly to Brain: Targeting the Ghrelin Receptor in Appetite and Food Intake Regulation. Int. J. Mol. Sci. 2017, 18, 273. [Google Scholar] [CrossRef] [PubMed]
- de Barros, C.T.; Rios, A.C.; Alves, T.F.R.; Batain, F.; Crescencio, K.M.M.; Lopes, L.J.; Zielińska, A.; Severino, P.; Mazzola, P.G.; Souto, E.B.; et al. Cachexia: Pathophysiology and Ghrelin Liposomes for Nose-to-Brain Delivery. Int. J. Mol. Sci. 2020, 21, 5974. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Burney, B.O.; Robinson, S.M. Effects of triglycerides, obesity, and starvation on ghrelin transport across the blood-brain barrier. Peptides 2008, 29, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Tschöp, M.; Robinson, S.M.; Heiman, M.L. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J. Pharmacol. Exp. Ther. 2002, 302, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Stanzione, R.; Volpe, M. Mitochondrial Dysfunction Contributes to Hypertensive Target Organ Damage: Lessons from an Animal Model of Human Disease. Oxid. Med. Cell. Longev. 2016, 2016, 1067801. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liu, W.; Doycheva, D.M.; Gamdzyk, M.; Lu, W.; Tang, J.; Zhang, J.H. Ghrelin attenuates oxidative stress and neuronal apoptosis via GHSR-1α/AMPK/Sirt1/PGC-1α/UCP2 pathway in a rat model of neonatal HIE. Free Radic. Biol. Med. 2019, 141, 322–337. [Google Scholar] [CrossRef]
- Zhu, K.; Zhang, M.L.; Liu, S.T.; Li, X.Y.; Zhong, S.M.; Li, F.; Xu, G.Z.; Wang, Z.; Miao, Y. Ghrelin Attenuates Retinal Neuronal Autophagy and Apoptosis in an Experimental Rat Glaucoma Model. Investig. Ophthalmol. Vis. Sci. 2017, 58, 6113–6122. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiao, Q.; Zhao, K.; Gao, Y. Ghrelin inhibits high glucose-induced PC12 cell apoptosis by regulating TLR4/NF-κB pathway. Inflammation 2013, 36, 1286–1294. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, T. Ghrelin inhibits cisplatin-induced MDA-MB-231 breast cancer cell apoptosis via PI3K/Akt/mTOR signaling. Exp. Ther. Med. 2020, 19, 1633–1640. [Google Scholar] [CrossRef]
- Bai, J.; Jiang, G.; Zhao, M.; Wang, S. Ghrelin Mitigates High-Glucose-Induced Oxidative Damage and Apoptosis in Lens Epithelial Cells. J. Diabetes Res. 2022, 2022, 1373533. [Google Scholar] [CrossRef]
- Wu, C.R.; Yang, Q.Y.; Chen, Q.W.; Li, C.Q.; He, W.Y.; Zhao, Y.P.; Wang, L. Ghrelin attenuate cerebral microvascular leakage by regulating inflammation and apoptosis potentially via a p38 MAPK-JNK dependent pathway. Biochem. Biophys. Res. Commun. 2021, 552, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Sirini, M.A.; Anchordoquy, J.M.; Anchordoquy, J.P.; Pascua, A.M.; Nikoloff, N.; Carranza, A.; Relling, A.E.; Furnus, C.C. The presence of acylated ghrelin during in vitro maturation of bovine oocytes induces cumulus cell DNA damage and apoptosis, and impairs early embryo development. Zygote 2017, 25, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, X.; Lv, T.; Liu, J.; Ren, Y.; Zhang, J.; Zhang, Y. Effects of Ghrelin on the Apoptosis of Rheumatoid Arthritis Fibroblast-Like Synoviocyte MH7A Cells. Biol. Pharm. Bull. 2019, 42, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Belloni, A.S.; Macchi, C.; Rebuffat, P.; Conconi, M.T.; Malendowicz, L.K.; Parnigotto, P.P.; Nussdorfer, G.G. Effect of ghrelin on the apoptotic deletion rate of different types of cells cultured in vitro. Int. J. Mol. Med. 2004, 14, 165–167. [Google Scholar] [CrossRef]
- Fang, Y.; Tian, S.; Pan, Y.; Li, W.; Wang, Q.; Tang, Y.; Yu, T.; Wu, X.; Shi, Y.; Ma, P.; et al. Pyroptosis: A new frontier in cancer. Biomed. Pharmacother. 2020, 121, 109595. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.K.; Li, C.Y.; Lin, I.L.; Syue, W.J.; Chen, Y.F.; Cheng, K.C.; Teng, Y.N.; Lin, Y.H.; Yen, C.H.; Chiu, C.C. Inflammation-related pyroptosis, a novel programmed cell death pathway, and its crosstalk with immune therapy in cancer treatment. Theranostics 2021, 11, 8813–8835. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, S.B.; Miao, E.A. Gasdermins: Effectors of Pyroptosis. Trends Cell Biol. 2017, 27, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, M.; Qi, L.; Wu, Y.; Song, D.; Gan, J.; Li, Y.; Bai, Y. Pyroptosis, a new bridge to tumor immunity. Cancer Sci. 2021, 112, 3979–3994. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef]
- Liston, A.; Masters, S.L. Homeostasis-altering molecular processes as mechanisms of inflammasome activation. Nat. Rev. Immunol. 2017, 17, 208–214. [Google Scholar]
- Lamkanfi, M. Emerging inflammasome effector mechanisms. Nat. Rev. Immunol. 2011, 11, 213–220. [Google Scholar] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [PubMed]
- Shi, J.; Zhao, Y.; Wang, K.; Shi, X.; Wang, Y.; Huang, H.; Zhuang, Y.; Cai, T.; Wang, F.; Shao, F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 2015, 526, 660–665. [Google Scholar] [PubMed]
- Zheng, X.; Chen, W.; Gong, F.; Chen, Y.; Chen, E. The Role and Mechanism of Pyroptosis and Potential Therapeutic Targets in Sepsis: A Review. Front. Immunol. 2021, 12, 711939. [Google Scholar] [PubMed]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar]
- Qu, R.; Dong, L.; Zhang, J.; Yu, X.; Wang, L.; Zhu, S. Cryo-EM structure of human heptameric Pannexin 1 channel. Cell Res. 2020, 30, 446–448. [Google Scholar]
- Li, L.; He, L.; Wu, D.; Chen, L.; Jiang, Z. Pannexin-1 channels and their emerging functions in cardiovascular diseases. Acta Biochim. Biophys. Sin. 2015, 47, 391–396. [Google Scholar]
- Yang, D.; He, Y.; Muñoz-Planillo, R.; Liu, Q.; Núñez, G. Caspase-11 Requires the Pannexin-1 Channel and the Purinergic P2X7 Pore to Mediate Pyroptosis and Endotoxic Shock. Immunity 2015, 43, 923–932. [Google Scholar]
- Karmakar, M.; Katsnelson, M.A.; Dubyak, G.R.; Pearlman, E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1β secretion in response to ATP. Nat. Commun. 2016, 7, 10555. [Google Scholar] [CrossRef]
- Shao, X.F.; Li, B.; Shen, J.; Wang, Q.F.; Chen, S.S.; Jiang, X.C.; Qiang, D. Ghrelin alleviates traumatic brain injury-induced acute lung injury through pyroptosis/NF-κB pathway. Int. Immunopharmacol. 2020, 79, 106175. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, Z.; He, X.; Yu, H.; Feng, J. Ghrelin Attenuates Neuroinflammation and Demyelination in Experimental Autoimmune Encephalomyelitis Involving NLRP3 Inflammasome Signaling Pathway and Pyroptosis. Front. Pharmacol. 2019, 10, 1320. [Google Scholar] [CrossRef]
- Fu, T.; Wang, L.; Zeng, Q.; Zhang, Y.; Sheng, B.; Han, L. Ghrelin Ameliorates Asthma by Inhibiting Endoplasmic Reticulum Stress. Am. J. Med. Sci. 2017, 354, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, B.; Xie, W.; Chen, Z.; Yang, G.; Cai, Y.; Shang, H.; Zhao, W. Ghrelin attenuates secondary brain injury following intracerebral hemorrhage by inhibiting NLRP3 inflammasome activation and promoting Nrf2/ARE signaling pathway in mice. Int. Immunopharmacol. 2020, 79, 106180. [Google Scholar] [CrossRef] [PubMed]
- Coll, R.C.; Schroder, K.; Pelegrín, P. NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol. Sci. 2022, 43, 653–668. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, P.; Li, P.; Feng, L.; Ren, Q.; Xie, X.; Xu, J. Ghrelin protects the heart against ischemia/reperfusion injury via inhibition of TLR4/NLRP3 inflammasome pathway. Life Sci. 2017, 186, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Niu, F.; Chen, J.; Cao, X.; Liu, Z.; Bao, X.; Xu, Y. Ghrelin improves muscle function in dystrophin-deficient mdx mice by inhibiting NLRP3 inflammasome activation. Life Sci. 2019, 232, 116654. [Google Scholar] [CrossRef]
- Han, W.; Cui, C.; Zhang, H.; Guo, Y.; Xie, D.; Zhang, W.; Wang, C.; Yang, M.; Jiang, P. Ghrelin ameliorates diabetes-associated behavioral deficits and NLRP3 inflammasome activation via autophagic flux enhancement. Pharmacol. Res. 2022, 179, 106224. [Google Scholar] [CrossRef]
- Ling, L.; Yang, M.; Ding, W.; Gu, Y. Ghrelin attenuates UUO-induced renal fibrosis via attenuation of Nlrp3 inflammasome and endoplasmic reticulum stress. Am. J. Transl. Res. 2019, 11, 131–141. [Google Scholar]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [PubMed]
- Ueno, T.; Komatsu, M. Autophagy in the liver: Functions in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, Y. Yoshinori Ohsumi: Autophagy from beginning to end. Interview by Caitlin Sedwick. J. Cell Biol. 2012, 197, 164–165. [Google Scholar] [PubMed]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B. Autophagy in Human Diseases. N. Engl. J. Med. 2020, 383, 1564–1576. [Google Scholar] [CrossRef] [PubMed]
- Marzella, L.; Ahlberg, J.; Glaumann, H. Autophagy, heterophagy, microautophagy and crinophagy as the means for intracellular degradation. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 1981, 36, 219–234. [Google Scholar] [CrossRef]
- Wang, L.; Klionsky, D.J.; Shen, H.M. The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell Biol. 2023, 24, 186–203. [Google Scholar]
- Griffey, C.J.; Yamamoto, A. Macroautophagy in CNS health and disease. Nat. Rev. Neurosci. 2022, 23, 411–427. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, R.; Zhu, L. Chaperone-Mediated Autophagy. Adv. Exp. Med. Biol. 2019, 1206, 435–452. [Google Scholar]
- Wang, Y.; Zhang, H. Regulation of Autophagy by mTOR Signaling Pathway. Adv. Exp. Med. Biol. 2019, 1206, 67–83. [Google Scholar]
- Ge, Y.; Zhou, M.; Chen, C.; Wu, X.; Wang, X. Role of AMPK mediated pathways in autophagy and aging. Biochimie 2022, 195, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, S.; Ma, B. Autophagy and autophagy-related proteins in cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Sun, G.; Li, E.; Kiselyov, K.; Sun, D. ER stress and impaired autophagy flux in neuronal degeneration and brain injury. Ageing Res. Rev. 2017, 34, 3–14. [Google Scholar] [CrossRef]
- Reich, N.; Hölscher, C. Acylated Ghrelin as a Multi-Targeted Therapy for Alzheimer’s and Parkinson’s Disease. Front. Neurosci. 2020, 14, 614828. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. Vascular calcification: Mechanisms of vascular smooth muscle cell calcification. Trends Cardiovasc. Med. 2015, 25, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Liu, L.; Song, C.; Chen, W.; Gui, S. Ghrelin improves vascular autophagy in rats with vascular calcification. Life Sci. 2017, 179, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.X.; Shi, B.; Lou, X.L.; Liu, J.Q.; Ma, G.G.; Liang, D.Y.; Ma, S. Ghrelin protects small intestinal epithelium against sepsis-induced injury by enhancing the autophagy of intestinal epithelial cells. Biomed. Pharmacother. 2016, 83, 1315–1320. [Google Scholar] [CrossRef]
- Uwagboe, I.; Adcock, I.M.; Lo Bello, F.; Caramori, G.; Mumby, S. New drugs under development for COPD. Minerva Medica 2022, 113, 471–496. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Yan, X.; Li, R.; Zhang, H. Ghrelin ameliorates chronic obstructive pulmonary disease-associated infllammation and autophagy. Biotechnol. Appl. Biochem. 2021, 68, 356–365. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, S.; Yu, F.; Li, H.; Guo, C.; Fan, X. Ghrelin Attenuates Liver Fibrosis through Regulation of TGF-β1 Expression and Autophagy. Int. J. Mol. Sci. 2015, 16, 21911–21930. [Google Scholar] [CrossRef]
- Kobayashi, S.; Volden, P.; Timm, D.; Mao, K.; Xu, X.; Liang, Q. Transcription factor GATA4 inhibits doxorubicin-induced autophagy and cardiomyocyte death. J. Biol. Chem. 2010, 285, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, X.L.; Chen, H.L.; Wu, D.; Chen, J.X.; Wang, X.X.; Li, R.L.; He, J.H.; Mo, L.; Cen, X.; et al. Ghrelin inhibits doxorubicin cardiotoxicity by inhibiting excessive autophagy through AMPK and p38-MAPK. Biochem. Pharmacol. 2014, 88, 334–350. [Google Scholar] [CrossRef] [PubMed]
- Amiri, F.; Halabian, R.; Salimian, M.; Shokrgozar, M.A.; Soleimani, M.; Jahanian-Najafabadi, A.; Roudkenar, M.H. Induction of multipotency in umbilical cord-derived mesenchymal stem cells cultivated under suspension conditions. Cell Stress Chaperones 2014, 19, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, W. Preconditioning of mesenchymal stem cells with ghrelin exerts superior cardioprotection in aged heart through boosting mitochondrial function and autophagy flux. Eur. J. Pharmacol. 2021, 903, 174142. [Google Scholar] [CrossRef] [PubMed]
- Ferro, F.; Servais, S.; Besson, P.; Roger, S.; Dumas, J.F.; Brisson, L. Autophagy and mitophagy in cancer metabolic remodelling. Semin. Cell Dev. Biol. 2020, 98, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.M.M.; Towers, C.G.; Thorburn, A. Targeting autophagy in cancer. Nat. Rev. Cancer 2017, 17, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.; Kanehisa, A.; Martins, I.; Senovilla, L.; Chargari, C.; Dugue, D.; Mariño, G.; Kepp, O.; Michaud, M.; Perfettini, J.L.; et al. Autophagy inhibition radiosensitizes in vitro, yet reduces radioresponses in vivo due to deficient immunogenic signalling. Cell Death Differ. 2014, 21, 92–99. [Google Scholar] [CrossRef]
- Bonfili, L.; Cuccioloni, M.; Cecarini, V.; Mozzicafreddo, M.; Palermo, F.A.; Cocci, P.; Angeletti, M.; Eleuteri, A.M. Ghrelin induces apoptosis in colon adenocarcinoma cells via proteasome inhibition and autophagy induction. Apoptosis 2013, 18, 1188–1200. [Google Scholar] [CrossRef]
- Xu, Q.; Guo, J.; Li, X.; Wang, Y.; Wang, D.; Xiao, K.; Zhu, H.; Wang, X.; Hu, C.A.; Zhang, G.; et al. Necroptosis Underlies Hepatic Damage in a Piglet Model of Lipopolysaccharide-Induced Sepsis. Front. Immunol. 2021, 12, 633830. [Google Scholar] [CrossRef]
- Seo, J.; Nam, Y.W.; Kim, S.; Oh, D.B.; Song, J. Necroptosis molecular mechanisms: Recent findings regarding novel necroptosis regulators. Exp. Mol. Med. 2021, 53, 1007–1017. [Google Scholar] [CrossRef]
- Samir, P.; Malireddi, R.K.S.; Kanneganti, T.D. The PANoptosome: A Deadly Protein Complex Driving Pyroptosis, Apoptosis, and Necroptosis (PANoptosis). Front. Cell. Infect. Microbiol. 2020, 10, 238. [Google Scholar] [CrossRef]
- Green, D.R. The Coming Decade of Cell Death Research: Five Riddles. Cell 2019, 177, 1094–1107. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Kim, H.R.; Park, S.Y.; Hwang, S.M.; Hong, S.M.; Park, S.; Kang, H.C.; Morgan, M.J.; Cha, J.H.; Lee, D.; et al. RIPK3 activation induces TRIM28 derepression in cancer cells and enhances the anti-tumor microenvironment. Mol. Cancer 2021, 20, 107. [Google Scholar] [CrossRef] [PubMed]
- Annibaldi, A.; Meier, P. Checkpoints in TNF-Induced Cell Death: Implications in Inflammation and Cancer. Trends Mol. Med. 2018, 24, 49–65. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, X. RIP kinases as modulators of inflammation and immunity. Nat. Immunol. 2018, 19, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Chen, X.; Hu, J.; Fu, Y.; Peng, J.; Li, Y.; Chen, J.; Li, P.; Liu, L.; Cao, J.; et al. Ghrelin protects against contact dermatitis and psoriasiform skin inflammation by antagonizing TNF-α/NF-κB signaling pathways. Sci. Rep. 2019, 9, 1348. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Chen, X.; Wang, W.; Qiu, C.; Ban, M.; Guo, L.; Vasilev, K.; Chen, J.; Li, W.; Zhao, Y. Ghrelin protects against osteoarthritis through interplay with Akt and NF-κB signaling pathways. FASEB J. 2018, 32, 1044–1058. [Google Scholar] [CrossRef]
- Huang, C.X.; Yuan, M.J.; Huang, H.; Wu, G.; Liu, Y.; Yu, S.B.; Li, H.T.; Wang, T. Ghrelin inhibits post-infarct myocardial remodeling and improves cardiac function through anti-inflammation effect. Peptides 2009, 30, 2286–2291. [Google Scholar] [CrossRef]
- Gong, Y.; Fan, Z.; Luo, G.; Yang, C.; Huang, Q.; Fan, K.; Cheng, H.; Jin, K.; Ni, Q.; Yu, X.; et al. The role of necroptosis in cancer biology and therapy. Mol. Cancer 2019, 18, 100. [Google Scholar] [CrossRef]
- Strilic, B.; Yang, L.; Albarrán-Juárez, J.; Wachsmuth, L.; Han, K.; Müller, U.C.; Pasparakis, M.; Offermanns, S. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature 2016, 536, 215–218. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, H.; Guo, W.; Yu, L. Potential role of ghrelin in the regulation of inflammation. FASEB J. 2022, 36, e22508. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Steller, H. Programmed cell death in animal development and disease. Cell 2011, 147, 742–758. [Google Scholar] [CrossRef] [PubMed]
- Strasser, A.; Cory, S.; Adams, J.M. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011, 30, 3667–3683. [Google Scholar] [CrossRef]
- Heshmati, M.; Soltani, A.; Sanaei, M.J.; Nahid-Samiei, M.; Shirzad, H.; Jami, M.S.; GhatrehSamani, M. Ghrelin induces autophagy and CXCR4 expression via the SIRT1/AMPK axis in lymphoblastic leukemia cell lines. Cell. Signal. 2020, 66, 109492. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.; Gómez-Ambrosi, J.; Catalán, V.; Rotellar, F.; Valentí, V.; Silva, C.; Mugueta, C.; Pulido, M.R.; Vázquez, R.; Salvador, J.; et al. The ghrelin O-acyltransferase-ghrelin system reduces TNF-α-induced apoptosis and autophagy in human visceral adipocytes. Diabetologia 2012, 55, 3038–3050. [Google Scholar] [CrossRef]
- Miller, D.R.; Thorburn, A. Autophagy and organelle homeostasis in cancer. Dev. Cell 2021, 56, 906–918. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Barrón, J.C.; Kostova, I.; Becker, S.; Strebhardt, K. Caspase-8: The double-edged sword. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188357. [Google Scholar] [CrossRef]
- Frank, D.; Vince, J.E. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death Differ. 2019, 26, 99–114. [Google Scholar] [CrossRef]
- Tsuchiya, K. Switching from Apoptosis to Pyroptosis: Gasdermin-Elicited Inflammation and Antitumor Immunity. Int. J. Mol. Sci. 2021, 22, 426. [Google Scholar] [CrossRef]
- Luo, B.; Wang, L.; Gao, W.; Su, Y.; Lu, Y.; Zheng, J.; Yin, J.; Zhao, Q.; Li, J.; Da, Y.; et al. Using a Gene Network of Pyroptosis to Quantify the Responses to Immunotherapy and Prognosis for Neuroblastoma Patients. Front. Immunol. 2022, 13, 845757. [Google Scholar] [CrossRef]
- Dagher-Hamalian, C.; Stephan, J.; Zeeni, N.; Harhous, Z.; Shebaby, W.N.; Abdallah, M.S.; Faour, W.H. Ghrelin-induced multi-organ damage in mice fed obesogenic diet. Inflamm. Res. 2020, 69, 1019–1026. [Google Scholar] [CrossRef] [PubMed]


| Affected Cells | Diseases | Involved Pathways | Effects | Reference |
|---|---|---|---|---|
| Cardiomyocytes | Heart diseases caused by ERS | GHS-R1a/CaMKK/AMPK pathway | Inhibitory effects | [28] |
| Heart diseases caused by H/R injury | Akt-mTOR signaling pathway | inhibitory effects | [29] | |
| myocardial injury in sepsis | JAK/STAT signaling pathway | Inhibitory effects | [30] | |
| myocardial infarction | Raf-MEK1/2-ERK1/2 signaling | Inhibitory effects | [31] | |
| Neurons | Hypoxic-ischemic encephalopathy | GHSR-1α/AMPK/Sirt1/PGC-1α/UCP2 pathway | Inhibitory effects | [32] |
| COH | Decreasing caspase3 | Inhibitory effects | [34] | |
| diabetic encephalopathy | TLR4/NF-κB pathway | Inhibitory effects | [35] | |
| human breast cancer cells | Breast cancer | PI3K/Akt/mTOR signaling pathway | Inhibitory effects | [36] |
| human lens epithelial (HLE) cells | Diabetic cataract | Bcl-2 increases Bax decreases | Inhibitory effects | [37] |
| pericytes | atherosclerosis | p38 MAPK-JNK pathway | Promoting effects | [38] |
| cumulus cells | - | Unknown | Promoting effects | [39] |
| MH7A synovial cells | - | cleaved-caspases-8, -9, and -3 increase | Promoting effects | [40] |
| human adrenocortical cells, rat osteoblasts | - | Unknown | No effects | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zeng, Z.; Liu, Y.; Liu, D. Emerging Relevance of Ghrelin in Programmed Cell Death and Its Application in Diseases. Int. J. Mol. Sci. 2023, 24, 17254. https://doi.org/10.3390/ijms242417254
Zhang X, Zeng Z, Liu Y, Liu D. Emerging Relevance of Ghrelin in Programmed Cell Death and Its Application in Diseases. International Journal of Molecular Sciences. 2023; 24(24):17254. https://doi.org/10.3390/ijms242417254
Chicago/Turabian StyleZhang, Xue, Zihan Zeng, Yaning Liu, and Dan Liu. 2023. "Emerging Relevance of Ghrelin in Programmed Cell Death and Its Application in Diseases" International Journal of Molecular Sciences 24, no. 24: 17254. https://doi.org/10.3390/ijms242417254
APA StyleZhang, X., Zeng, Z., Liu, Y., & Liu, D. (2023). Emerging Relevance of Ghrelin in Programmed Cell Death and Its Application in Diseases. International Journal of Molecular Sciences, 24(24), 17254. https://doi.org/10.3390/ijms242417254





