The Development of Human Ex Vivo Models of Inflammatory Skin Conditions
Abstract
:1. Introduction
2. Results
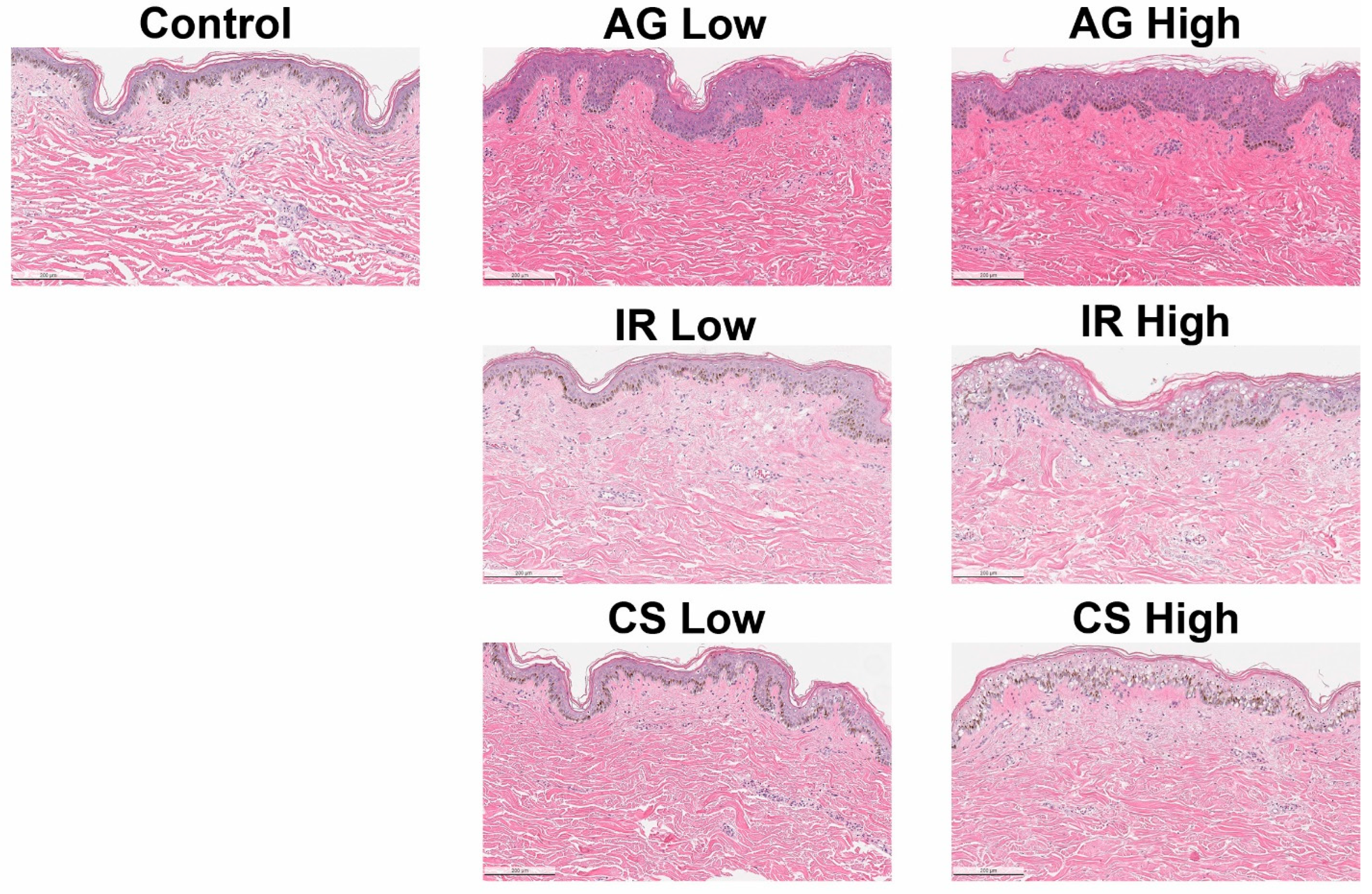
2.1. Inflammatory Triggers Resulted in Compromised Epidermal Barrier
2.2. The Inflammatory Phenotype Is a Result of Different Immune Cell Populations
2.3. The Inflammatory Skin Models Are Characterized by Different Cytokine Profiles
2.4. Inhibition of Inflammatory Responses with Pharmacological Immunosuppressants
2.5. Stratum Corneum Lipidomic Profile Revealed Significant Alteration of Skin Barrier Ceramides and Fatty Acid Groups
2.5.1. Evaluation of the Epidermal Lipidome in Inflamed Skin Models
2.5.2. Alteration to the Ceramide Subclass Distribution
2.5.3. Alteration to the Ceramide Chain Length Distribution
3. Discussion
4. Material and Methods
4.1. Reagents and Preparation of Inflammatory Stimulants
4.2. Inflammation-Induced Angiogenesis (AG) Model
4.3. Skin Irritation (IR) Model
4.4. Chronic Inflammation (CS) Model
4.5. Human Ex Vivo Tissue Processing
4.6. Tissue Treatment and Culture
4.7. Histology and Immunohistochemistry
4.8. Tissue Clearing for 3D Imaging
4.9. Lipidomic of Epidermal Ceramides
4.10. Multiplex ELISA Assays
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ujiie, H.; Rosmarin, D.; Schön, M.P.; Ständer, S.; Boch, K.; Metz, M.; Maurer, M.; Thaci, D.; Schmidt, E.; Cole, C.; et al. Unmet Medical Needs in Chronic, Non-communicable Inflammatory Skin Diseases. Front. Med. 2022, 9, 875492. [Google Scholar] [CrossRef]
- Hadi, H.A.; Tarmizi, A.I.; Khalid, K.A.; Gajdács, M.; Aslam, A.; Jamshed, S. The Epidemiology and Global Burden of Atopic Dermatitis: A Narrative Review. Life 2021, 11, 936. [Google Scholar] [CrossRef]
- Bylund, S.; Kobyletzki, L.; Svalstedt, M. Prevalence and Incidence of Atopic Dermatitis: A Systematic Review. Acta Derm. Venereol. 2020, 100, 320–329. [Google Scholar] [CrossRef]
- Alexis, A.F.; Callender, V.D.; Baldwin, H.E.; Desai, S.R.; Rendon, M.I.; Taylor, S.C. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: Review and clinical practice experience. J. Am. Acad. Dermatol. 2019, 80, 1722–1729.e7. [Google Scholar] [CrossRef]
- Rainer, B.M.; Kang, S.; Chien, A.L. Rosacea: Epidemiology, pathogenesis, and treatment. Derm. Endocrinol. 2017, 9, e1361574. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Mehta, M.D.; Schupp, C.W.; Gondo, G.C.; Bell, S.J.; Griffiths, C.E.M. Psoriasis Prevalence in Adults in the United States. JAMA Dermatol. 2021, 157, 940–946. [Google Scholar] [CrossRef]
- Nazir, Z.; Strunk, A.; Garg, A. Age- and sex-adjusted prevalence estimates among adults with psoriasis in the United States. J. Am. Acad. Dermatol. 2022, 86, 703–705. [Google Scholar] [CrossRef]
- Avci, P.; Sadasivam, M.; Gupta, A.; De Melo, W.C.; Huang, Y.-Y.; Yin, R.; Chandran, R.; Kumar, R.; Otufowora, A.; Nyame, T.; et al. Animal models of skin disease for drug discovery. Expert Opin. Drug Discov. 2013, 8, 331–355. [Google Scholar] [CrossRef]
- Kim, D.; Kobayashi, T.; Nagao, K. Research Techniques Made Simple: Mouse Models of Atopic Dermatitis. J. Investig. Dermatol. 2019, 139, 984–990.e1. [Google Scholar] [CrossRef]
- Schön, M.P.; Manzke, V.; Erpenbeck, L. Animal models of psoriasis—Highlights and drawbacks. J. Allergy Clin. Immunol. 2021, 147, 439–455. [Google Scholar] [CrossRef]
- Flamand, N.; Marrot, L.; Belaidi, J.P.; Bourouf, L.; Dourille, E.; Feltes, M.; Meunier, J.R. Development of genotoxicity test procedures with Episkin, a reconstructed human skin model: Towards new tools for in vitro risk assessment of dermally applied compounds? Mutat. Res. 2006, 606, 39–51. [Google Scholar] [CrossRef]
- Cadau, S.; Gault, M.; Berthélémy, N.; Hsu, C.-Y.; Danoux, L.; Pelletier, N.; Goudounèche, D.; Pons, C.; Leprince, C.; Andre-frei, V.; et al. An Inflamed and Infected Reconstructed Human Epidermis to Study Atopic Dermatitis and Skin Care In-gredients. Int. J. Mol. Sci. 2022, 23, 12880. [Google Scholar] [CrossRef]
- Hall, M.J.; Lopes-Ventura, S.; Neto, M.V.; Charneca, J.; Zoio, P.; Seabra, M.C.; Oliva, A.; Barral, D.C. Reconstructed human pigmented skin/epidermis models achieve epidermal pigmentation through melanocore transfer. Pigment Cell Melanoma Res. 2022, 35, 425–435. [Google Scholar] [CrossRef]
- Hubaux, R.; Bastin, C.; Salmon, M. On the relevance of an in vitro reconstructed human epidermis model for drug screening in atopic dermatitis. Exp. Dermatol. 2018, 27, 1403–1407. [Google Scholar] [CrossRef]
- Liu, X.; Michael, S.; Bharti, K.; Ferrer, M.; Song, M.J. A biofabricated vascularized skin model of atopic dermatitis for preclinical studies. Biofabrication 2020, 12, 035002. [Google Scholar] [CrossRef]
- Shin, J.U.; Abaci, H.E.; Herron, L.; Guo, Z.; Sallee, B.; Pappalardo, A.; Jackow, J.; Wang, E.H.C.; Doucet, Y.; Christiano, A.M. Recapitulating T cell infiltration in 3D psoriatic skin models for patient-specific drug testing. Sci. Rep. 2020, 10, 4123. [Google Scholar] [CrossRef]
- Eberlin, S.; Silva, M.S.D.; Facchini, G.; Silva, G.H.D.; Pinheiro, A.L.T.A.; Eberlin, S.; Pinheiro, A.D.S. The Ex Vivo Skin Model as an Alternative Tool for the Efficacy and Safety Evaluation of Topical Products. Altern. Lab. Anim. 2020, 48, 10–22. [Google Scholar] [CrossRef]
- Hofmann, E.; Fink, J.; Eberl, A.; Prugger, E.-M.; Kolb, D.; Luze, H.; Schwingenschuh, S.; Birngruber, T.; Magnes, C.; Mautner, S.I.; et al. A novel human ex vivo skin model to study early local responses to burn injuries. Sci. Rep. 2021, 11, 364. [Google Scholar] [CrossRef]
- de Ménonville, S.T.; Rosignoli, C.; Soares, E.; Roquet, M.; Bertino, B.; Chappuis, J.; Defoin-Platel, C.; Piwnica, D. Topical Treatment of Rosacea with Ivermectin Inhibits Gene Expression of Cathelicidin Innate Immune Mediators, LL-37 and KLK5, in Reconstructed and Ex Vivo Skin Models. Dermatol. Ther. 2017, 7, 213–225. [Google Scholar] [CrossRef]
- Cho, H.; Won, C.H.; Chang, S.E.; Lee, M.W.; Park, G.-H. Usefulness and Limitations of Skin Explants to Assess Laser Treatment. Med. Lasers 2013, 2, 58–63. [Google Scholar] [CrossRef]
- Neves, L.M.G.; Wilgus, T.A.; Bayat, A. In Vitro, Ex Vivo, and In Vivo Approaches for Investigation of Skin Scarring: Human and Animal Models. Adv. Wound Care 2021, 12, 97–116. [Google Scholar] [CrossRef]
- Barresi, R.; Chen, E.; Liao, I.-C.; Liu, X.; Baalbaki, N.; Lynch, S.; Brieva, P.; Wang, M.; Zheng, Q.; Bouez, C. ARTICLE: Alteration to the Skin Barrier Integrity Following Broad-Spectrum UV Exposure in an Ex Vivo Tissue Model. J. Drugs Dermatol. 2021, 20, 23s–28s. [Google Scholar] [CrossRef]
- Barresi, R.; Dumbuya, H.; Liao, I.-C.; Chen, Y.; Yan, X.; Wangari-Olivero, J.; Baalbaki, N.; Lynch, S.; Brieva, P.; Wang, M.; et al. Alteration to the Skin Ceramide Profile Following Broad-Spectrum UV Exposure. J. Drugs Dermatol. 2022, 21, 77–85. [Google Scholar] [CrossRef]
- Medgyesi, B.; Dajnoki, Z.; Béke, G.; Gáspár, K.; Szabó, I.L.; Janka, E.A.; Póliska, S.; Hendrik, Z.; Méhes, G.; Törőcsik, D.; et al. Rosacea Is Characterized by a Profoundly Diminished Skin Barrier. J. Investig. Dermatol. 2020, 140, 1938–1950.e5. [Google Scholar] [CrossRef]
- Farshchian, M.; Daveluy, S. Rosacea. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lee, S.G.; Yoon, M.S.; Kim, D.H.; Shin, J.U.; Lee, H.J. Hyaluronan Oligosaccharides Improve Rosacea-Like Phenotype through Anti-Inflammatory and Epidermal Barrier-Improving Effects. Ann. Dermatol. 2020, 32, 189–196. [Google Scholar] [CrossRef]
- Saarnilehto, M.; Chapman, H.; Savinko, T.; Lindstedt, K.; Lauerma, A.I.; Koivisto, A. Contact sensitizer 2,4-dinitrochlorobenzene is a highly potent human TRPA1 agonist. Allergy 2014, 69, 1424–1427. [Google Scholar] [CrossRef]
- Bieber, T. Atopic dermatitis. Ann. Dermatol. 2010, 22, 125–137. [Google Scholar] [CrossRef]
- Girolomoni, G.; de Bruin-Weller, M.; Aoki, V.; Kabashima, K.; Deleuran, M.; Puig, L.; Bansal, A.; Rossi, A.B. Nomenclature and clinical phenotypes of atopic dermatitis. Ther. Adv. Chronic Dis. 2021, 12, 20406223211002979. [Google Scholar] [CrossRef]
- Fiset, P.-O.; Leung, D.Y.; Hamid, Q. Immunopathology of atopic dermatitis. J. Allergy Clin. Immunol. 2006, 118, 287–290. [Google Scholar] [CrossRef]
- Kim, B.E.; Leung, D.Y.; Boguniewicz, M.; Howell, M.D. Loricrin and involucrin expression is down-regulated by Th2 cytokines through STAT-6. Clin. Immunol. 2008, 126, 332–337. [Google Scholar] [CrossRef]
- Kim, B.E.; Howell, M.D.; Guttman, E.; Gilleaudeau, P.M.; Cardinale, I.R.; Boguniewicz, M.; Krueger, J.G.; Leung, D.Y. TNF-alpha downregulates filaggrin and loricrin through c-Jun N-terminal kinase: Role for TNF-alpha antag-onists to improve skin barrier. J. Investig. Dermatol. 2011, 131, 1272–1279. [Google Scholar] [CrossRef]
- Steinhoff, M.; Buddenkotte, J.; Aubert, J.; Sulk, M.; Novak, P.; Schwab, V.D.; Mess, C.; Cevikbas, F.; Rivier, M.; Carlavan, I.; et al. Clinical, Cellular, and Molecular Aspects in the Pathophysiology of Rosacea. J. Investig. Dermatol. Symp. Proc. 2011, 15, 2–11. [Google Scholar] [CrossRef]
- van der Does, A.M.; Beekhuizen, H.; Ravensbergen, B.; Vos, T.; Ottenhoff, T.H.; van Dissel, J.T.; Drijfhout, J.W.; Hiemstra, P.S.; Nibbering, P.H. LL-37 directs macrophage differentiation toward macrophages with a proinflammatory signature. J. Immunol. 2010, 185, 1442–1449. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.; Jeong, S.; Jo, H.; Woo, Y.R.; Park, H.J. Inhibition of mast cell infiltration in an LL-37-induced rosacea mouse model using topical brimonidine tartrate 0.33% gel. Exp. Dermatol. 2017, 26, 1143–1145. [Google Scholar] [CrossRef]
- Facheris, P.; Jeffery, J.; Del Duca, E.; Guttman-Yassky, E. The translational revolution in atopic dermatitis: The paradigm shift from pathogenesis to treatment. Cell. Mol. Immunol. 2023, 20, 448–474. [Google Scholar] [CrossRef]
- Riedl, R.; Kühn, A.; Rietz, D.; Hebecker, B.; Glowalla, K.-G.; Peltner, L.K.; Jordan, P.M.; Werz, O.; Lorkowski, S.; Wiegand, C.; et al. Establishment and Characterization of Mild Atopic Dermatitis in the DNCB-Induced Mouse Model. Int. J. Mol. Sci. 2023, 24, 12325. [Google Scholar] [CrossRef]
- Yang, C.-C.; Hung, Y.-L.; Ko, W.-C.; Tsai, Y.-J.; Chang, J.-F.; Liang, C.-W.; Chang, D.-C.; Hung, C.-F. Effect of Neferine on DNCB-Induced Atopic Dermatitis in HaCaT Cells and BALB/c Mice. Int. J. Mol. Sci. 2021, 22, 8237. [Google Scholar] [CrossRef]
- Lazarski, C.A.; Ford, J.; Katzman, S.D.; Rosenberg, A.F.; Fowell, D.J. IL-4 Attenuates Th1-Associated Chemokine Expression and Th1 Trafficking to Inflamed Tissues and Limits Pathogen Clearance. PLoS ONE 2013, 8, e71949. [Google Scholar] [CrossRef]
- Mitchell, R.E.; Hassan, M.; Burton, B.R.; Britton, G.; Hill, E.V.; Verhagen, J.; Wraith, D.C. IL-4 enhances IL-10 production in Th1 cells: Implications for Th1 and Th2 regulation. Sci. Rep. 2017, 7, 11315. [Google Scholar] [CrossRef]
- Ai, W.; Li, H.; Song, N.; Li, L.; Chen, H. Optimal Method to Stimulate Cytokine Production and Its Use in Immunotoxicity Assessment. Int. J. Environ. Res. Public Health 2013, 10, 3834–3842. [Google Scholar] [CrossRef]
- Hou, H.; Zhou, Y.; Yu, J.; Mao, L.; Bosco, M.J.; Wang, J.; Lu, Y.; Mao, L.; Wu, X.; Wang, F.; et al. Establishment of the Reference Intervals of Lymphocyte Function in Healthy Adults Based on IFN-gamma Secretion Assay upon Phorbol-12-Myristate-13-Acetate/Ionomycin Stimulation. Front. Immunol. 2018, 9, 172. [Google Scholar] [CrossRef]
- Dijkgraaf, F.E.; Toebes, M.; Hoogenboezem, M.; Mertz, M.; Vredevoogd, D.W.; Matos, T.R.; Teunissen, M.B.M.; Luiten, R.M.; Schumacher, T.N. Labeling and tracking of immune cells in ex vivo human skin. Nat. Protoc. 2020, 16, 791–811. [Google Scholar] [CrossRef]
- Bauer, T.; Gubi, D.; Klufa, J.; Novoszel, P.; Holcmann, M.; Sibilia, M. Ex-Vivo Skin Explant Culture Is a Model for TSLP-Mediated Skin Barrier Immunity. Life 2021, 11, 1237. [Google Scholar] [CrossRef]
- Biedermann, T.; Röcken, M.; Carballido, J.M. TH1 and TH2 Lymphocyte Development and Regulation of TH Cell–Mediated Immune Responses of the Skin. J. Investig. Dermatol. Symp. Proc. 2004, 9, 5–14. [Google Scholar] [CrossRef]
- Wurtz, O.; Bajenoff, M.; Guerder, S. IL-4-mediated inhibition of IFN-gamma production by CD4+ T cells proceeds by several developmentally regulated mechanisms. Int. Immunol. 2004, 16, 501–508. [Google Scholar] [CrossRef]
- Fujii, M. The Pathogenic and Therapeutic Implications of Ceramide Abnormalities in Atopic Dermatitis. Cells 2021, 10, 2386. [Google Scholar] [CrossRef]
- Di Nardo, A.; Wertz, P.; Giannetti, A.; Seidenari, S. Ceramide and cholesterol composition of the skin of patients with atopic dermatitis. Acta Derm. Venereol. 1998, 78, 27–30. [Google Scholar] [CrossRef]
- Toncic, R.J.; Jakasa, I.; Hadzavdic, S.L.; Goorden, S.M.; Ghauharali-van der Vlugt, K.J.M.; Stet, F.S.; Balic, A.; Petkovic, M.; Pavicic, B.; Zuzul, K.; et al. Altered Levels of Sphingosine, Sphinganine and Their Ceramides in Atopic Dermatitis Are Related to Skin Barrier Function, Disease Severity and Local Cytokine Milieu. Int. J. Mol. Sci. 2020, 21, 1958. [Google Scholar] [CrossRef]
- van Smeden, J.; Janssens, M.; Gooris, G.; Bouwstra, J. The important role of stratum corneum lipids for the cutaneous barrier function. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2014, 1841, 295–313. [Google Scholar] [CrossRef]
- Yokose, U.; Ishikawa, J.; Morokuma, Y.; Naoe, A.; Inoue, Y.; Yasuda, Y.; Tsujimura, H.; Fujimura, T.; Murase, T.; Hatamochi, A. The ceramide [NP]/[NS] ratio in the stratum corneum is a potential marker for skin properties and epidermal differentiation. BMC Dermatol. 2020, 20, 6. [Google Scholar] [CrossRef]
- Holleran, W.M.; Takagi, Y.; Uchida, Y. Epidermal sphingolipids: Metabolism, function, and roles in skin disorders. Am. J. Clin. Dermatol. 2003, 4, 107–129. [Google Scholar] [CrossRef]
- Berdyshev, E.; Goleva, E.; Bronova, I.; Dyjack, N.; Rios, C.; Jung, J.; Taylor, P.; Jeong, M.; Hall, C.F.; Richers, B.N.; et al. Lipid abnormalities in atopic skin are driven by type 2 cytokines. J. Clin. Investig. 2018, 3, e98006. [Google Scholar] [CrossRef]
- Danso, M.O.; Berkers, T.; Mieremet, A.; Hausil, F.; Bouwstra, J.A. An ex vivo human skin model for studying skin barrier repair. Exp. Dermatol. 2015, 24, 48–54. [Google Scholar] [CrossRef]
- Berkers, T.; Boiten, W.; Absalah, S.; van Smeden, J.; Lavrijsen, A.; Bouwstra, J. Compromising human skin in vivo and ex vivo to study skin barrier repair. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2019, 1864, 1103–1108. [Google Scholar] [CrossRef]
- Park, Y.H.; Jang, W.H.; Seo, J.A.; Park, M.; Lee, T.R.; Park, Y.H.; Kim, D.K.; Lim, K.M. Decrease of ceramides with very long-chain fatty acids and downregulation of elongases in a murine atopic dermatitis model. J. Investig. Dermatol. 2012, 132, 476–479. [Google Scholar] [CrossRef]
- Školová, B.; Janůšová, B.; Zbytovská, J.; Gooris, G.; Bouwstra, J.; Slepička, P.; Berka, P.; Roh, J.; Palát, K.; Hrabálek, A.; et al. Ceramides in the Skin Lipid Membranes: Length Matters. Langmuir 2013, 29, 15624–15633. [Google Scholar] [CrossRef]
- Norlén, L.; Nicander, I.; Lundsjö, A.; Cronholm, T.; Forslind, B. A new HPLC-based method for the quantitative analysis of inner stratum corneum lipids with special reference to the free fatty acid fraction. Arch. Dermatol. Res. 1998, 290, 508–516. [Google Scholar] [CrossRef]
- Lim, H.W.; Collins, S.A.; Resneck, J.S.; Bolognia, J.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A.; et al. A risk adjustment approach to estimating the burden of skin disease in the United States. J. Am. Acad. Dermatol. 2018, 78, 129–140. [Google Scholar] [CrossRef]
- Lim, H.W.; Collins, S.A.; Resneck, J.S., Jr.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A.; et al. The burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017, 76, 958–972.e2. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Wang, A.-P.; Shi, T.-Y.; Zhang, J.; Xu, H.; Wang, D.-Q.; Feng, L. The psychosocial adaptation of patients with skin disease: A scoping review. BMC Public Health 2019, 19, 1404. [Google Scholar] [CrossRef]
- Farzanfar, D.; Dowlati, Y.; French, L.E.; Lowes, M.A.; Alavi, A. Inflammation: A Contributor to Depressive Comorbidity in Inflammatory Skin Disease. Ski. Pharmacol. Physiol. 2018, 31, 246–251. [Google Scholar] [CrossRef]
- Wolff, K.; Johnson, R. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology, 8th ed.; McGraw-Hill Education: New York, NY, USA, 2017. [Google Scholar]
- Rosso, J.D.; Zeichner, J.; Alexis, A.; Cohen, D.; Berson, D. Understanding the Epidermal Barrier in Healthy and Compromised Skin: Clinically Relevant Information for the Dermatology Practitioner: Proceedings of an Expert Panel Roundtable Meeting. J. Clin. Aesthet. Dermatol. 2016, 9, S2–S8. [Google Scholar] [PubMed]
- Schwingen, J.; Kaplan, M.; Kurschus, F.C. Review-Current Concepts in Inflammatory Skin Diseases Evolved by Tran-scriptome Analysis: In-Depth Analysis of Atopic Dermatitis and Psoriasis. Int. J. Mol. Sci. 2020, 21, 699. [Google Scholar] [CrossRef]
- Park, Y.-G.; Sohn, C.H.; Chen, R.; McCue, M.; Yun, D.H.; Drummond, G.T.; Ku, T.; Evans, N.B.; Oak, H.C.; Trieu, W.; et al. Protection of tissue physicochemical properties using polyfunctional crosslinkers. Nat. Biotechnol. 2018, 37, 73–83. [Google Scholar] [CrossRef]
- Yun, D.H.; Park, Y.G.; Cho, J.H.; Kamentsky, L.; Evans, N.B.; Albanese, A.; Xie, K.; Swaney, J.; Sohn, C.H.; Tian, Y.; et al. Ultrafast immunostaining of organ-scale tissues for scalable proteomic phenotyping. bioRxiv 2019. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Cho, J.H.; Murray, E.; Bakh, N.; Choi, H.; Ohn, K.; Ruelas, L.; Hubbert, A.; McCue, M.; Vassallo, S.L.; et al. Stochastic electrotransport selectively enhances the transport of highly electromobile molecules. Proc. Natl. Acad. Sci. USA 2015, 112, E6274–E6283. [Google Scholar] [CrossRef]
- Murray, E.; Cho, J.H.; Goodwin, D.; Ku, T.; Swaney, J.; Kim, S.-Y.; Choi, H.; Park, Y.-G.; Park, J.-Y.; Hubbert, A.; et al. Simple, Scalable Proteomic Imaging for High-Dimensional Profiling of Intact Systems. Cell 2015, 163, 1500–1514. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, E.H.C.; Barresi-Thornton, R.; Chen, L.-C.; Senna, M.M.; Liao, I.-C.; Chen, Y.; Zheng, Q.; Bouez, C. The Development of Human Ex Vivo Models of Inflammatory Skin Conditions. Int. J. Mol. Sci. 2023, 24, 17255. https://doi.org/10.3390/ijms242417255
Wang EHC, Barresi-Thornton R, Chen L-C, Senna MM, Liao I-C, Chen Y, Zheng Q, Bouez C. The Development of Human Ex Vivo Models of Inflammatory Skin Conditions. International Journal of Molecular Sciences. 2023; 24(24):17255. https://doi.org/10.3390/ijms242417255
Chicago/Turabian StyleWang, Eddy Hsi Chun, Rebecca Barresi-Thornton, Li-Chi Chen, Maryanne Makredes Senna, I-Chien Liao, Ying Chen, Qian Zheng, and Charbel Bouez. 2023. "The Development of Human Ex Vivo Models of Inflammatory Skin Conditions" International Journal of Molecular Sciences 24, no. 24: 17255. https://doi.org/10.3390/ijms242417255
APA StyleWang, E. H. C., Barresi-Thornton, R., Chen, L.-C., Senna, M. M., Liao, I.-C., Chen, Y., Zheng, Q., & Bouez, C. (2023). The Development of Human Ex Vivo Models of Inflammatory Skin Conditions. International Journal of Molecular Sciences, 24(24), 17255. https://doi.org/10.3390/ijms242417255





