The Role of the FGF19 Family in the Pathogenesis of Gestational Diabetes: A Narrative Review
Abstract
:1. Introduction
2. Scope and Methodology
3. Fibroblast Growth Factors
3.1. FGF19
Mechanisms of Action
3.2. FGF21
Mechanisms of Action
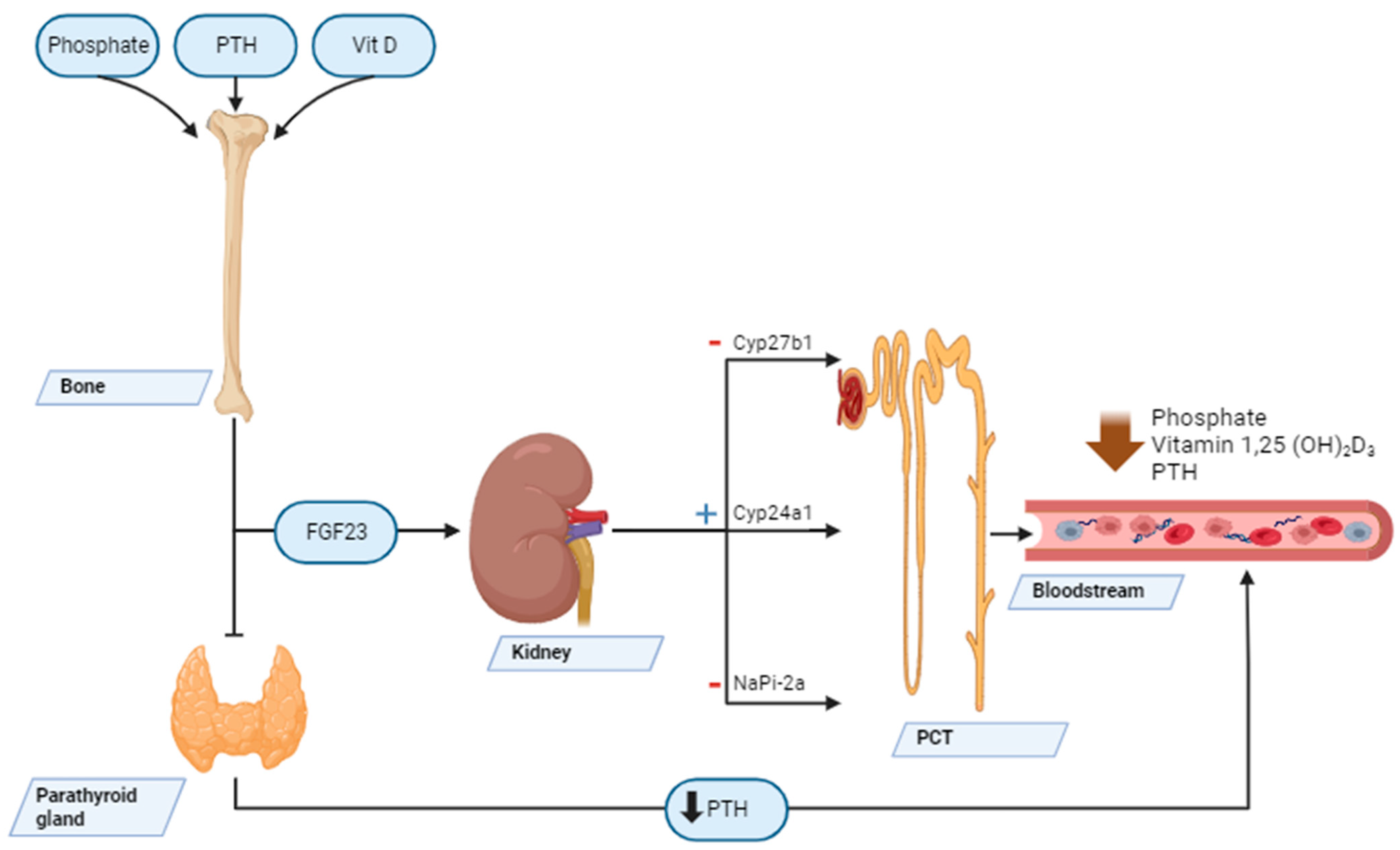
3.3. FGF23
Mechanisms of Action
4. FGF19 Family in GDM
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sadikot, S.; Purandare, C.N.; Cho, N.H.; Hod, M. FIGO-IDF joint statement and declaration on hyperglycemia in pregnancy. Diabetes Res. Clin. Pract. 2018, 145, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ge, H.; Weiszmann, J.; Hecht, R.; Li, Y.S.; Véniant, M.M.; Xu, J.; Wu, X.; Lindberg, R.; Li, Y. Inhibition of lipolysis may contribute to the acute regulation of plasma FFA and glucose by FGF21 in ob/ob mice. FEBS Lett. 2009, 583, 3230–3234. [Google Scholar] [CrossRef]
- Hsuchou, H.; Pan, W.; Kastin, A.J. The fasting polypeptide FGF21 can enter brain from blood. Peptides 2007, 28, 2382–2386. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.K.; Hallschmid, M.; Adya, R.; Kern, W.; Lehnert, H.; Randeva, H.S. Fibroblast Growth Factor 21 (FGF21) in Human Cerebrospinal Fluid: Relationship with Plasma FGF21 and Body Adiposity. Diabetes 2011, 60, 2758–2762. [Google Scholar] [CrossRef] [PubMed]
- Paulo, M.S.; Abdo, N.M.; Bettencourt-Silva, R.; Al-Rifai, R.H. Gestational Diabetes Mellitus in Europe: A Systematic Review and Meta-Analysis of Prevalence Studies. Front. Endocrinol. 2021, 12, 691033. [Google Scholar] [CrossRef] [PubMed]
- Bookout, A.L.; de Groot, M.H.M.; Owen, B.M.; Lee, S.; Gautron, L.; Lawrence, H.L.; Ding, X.; Elmquist, J.K.; Takahashi, J.S.; Mangelsdorf, D.J.; et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 2013, 19, 1147–1152. [Google Scholar] [CrossRef]
- Liang, Q.; Zhong, L.; Zhang, J.; Wang, Y.; Bornstein, S.R.; Triggle, C.R.; Ding, H.; Lam, K.S.L.; Xu, A. FGF21 Maintains Glucose Homeostasis by Mediating the Cross Talk Between Liver and Brain During Prolonged Fasting. Diabetes 2014, 63, 4064–4075. [Google Scholar] [CrossRef]
- Plows, J.; Stanley, J.; Baker, P.; Reynolds, C.; Vickers, M. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- WHO TEAM Guidelines Review Committee. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy: A World Health Organization Guideline. Diabetes Res. Clin. Pract. 2014, 103, 341–363. [Google Scholar] [CrossRef]
- Henkel, A.S.; Anderson, K.A.; Dewey, A.M.; Kavesh, M.H.; Green, R.M. A chronic high-cholesterol diet paradoxically suppresses hepatic CYP7A1 expression in FVB/NJ mice. J. Lipid Res. 2011, 52, 289–298. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Holmstrom, S.R.; Fon Tacer, K.; Bookout, A.L.; Kliewer, S.A.; Mangelsdorf, D.J. Regulation of Bile Acid Synthesis by Fat-soluble Vitamins A and D. J. Biol. Chem. 2010, 285, 14486–14494. [Google Scholar] [CrossRef]
- Qiu, C.; Williams, M.A.; Vadachkoria, S.; Frederick, I.O.; Luthy, D.A. Increased maternal plasma leptin in early pregnancy and risk of gestational diabetes mellitus. Obstet. Gynecol. 2004, 103, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Tam, V.; Patel, N.; Turcotte, M.; Bossé, Y.; Paré, G.; Meyre, D. Benefits and limitations of genome-wide association studies. Nat Rev. Genet. 2019, 20, 467–484. [Google Scholar] [CrossRef]
- Kwak, S.H.; Kim, S.H.; Cho, Y.M.; Go, M.J.; Cho, Y.S.; Choi, S.H.; Moon, M.K.; Jung, H.S.; Shin, H.D.; Kang, H.M.; et al. A genome-wide association study of gestational diabetes mellitus in Korean women. Diabetes 2012, 61, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Pervjakova, N.; Moen, G.H.; Borges, M.C.; Ferreira, T.; Cook, J.P.; Allard, C.; Beaumont, R.N.; Canouil, M.; Hatem, G.; Heiskala, A.; et al. Multi-ancestry genome-wide association study of gestational diabetes mellitus highlights genetic links with type 2 diabetes. Hum. Mol. Genet. 2022, 31, 3377–3391. [Google Scholar] [CrossRef]
- Beenken, A.; Mohammadi, M. The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009, 8, 235–253. [Google Scholar] [CrossRef]
- Lain, K.Y.; Daftary, A.R.; Ness, R.B.; Roberts, J.M. First trimester adipocytokine concentrations and risk of developing gestational diabetes later in pregnancy. Clin. Endocrinol. 2008, 69, 407–411. [Google Scholar] [CrossRef]
- Wang, D.; Zhu, W.; Li, J.; An, C.; Wang, Z. Serum Concentrations of Fibroblast Growth Factors 19 and 21 in Women with Gestational Diabetes Mellitus: Association with Insulin Resistance, Adiponectin, and Polycystic Ovary Syndrome History. PLoS ONE 2013, 8, e81190. [Google Scholar] [CrossRef]
- Izaguirre, M.; Gil, M.J.; Monreal, I.; Montecucco, F.; Frühbeck, G.; Catalán, V. The Role and Potential Therapeutic Implications of the Fibroblast Growth Factors in Energy Balance and Type 2 Diabetes. Curr. Diabetes Rep. 2017, 17, 43. [Google Scholar] [CrossRef]
- Fon Tacer, K.; Bookout, A.L.; Ding, X.; Kurosu, H.; John, G.B.; Wang, L.; Goetz, R.; Mohammadi, M.; Kuro-O, M.; Mangelsdorf, D.J.; et al. Research Resource: Comprehensive Expression Atlas of the Fibroblast Growth Factor System in Adult Mouse. Mol. Endocrinol. 2010, 24, 2050–2064. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Investig. 2005, 115, 1627–1635. [Google Scholar] [CrossRef]
- Tomlinson, E.; Fu, L.; John, L.; Hultgren, B.; Huang, X.; Renz, M.; Stephan, J.P.; Tsai, S.P.; Powell-Braxton, L.; French, D.; et al. Transgenic Mice Expressing Human Fibroblast Growth Factor-19 Display Increased Metabolic Rate and Decreased Adiposity. Endocrinology 2002, 143, 1741–1747. [Google Scholar] [CrossRef]
- Kuro-O, M. A potential link between phosphate and aging—Lessons from Klotho-deficient mice. Mech. Ageing Dev. 2010, 131, 270–275. [Google Scholar] [CrossRef]
- Goetz, R.; Beenken, A.; Ibrahimi, O.A.; Kalinina, J.; Olsen, S.K.; Eliseenkova, A.V.; Xu, C.; Neubert, T.A.; Zhang, F.; Linhardt, R.J.; et al. Molecular Insights into the Klotho-Dependent, Endocrine Mode of Action of Fibroblast Growth Factor 19 Subfamily Members. Mol. Cell Biol. 2007, 27, 3417–3428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y. Fibroblast Growth Factor 21 Analogs for Treating Metabolic Disorders. Front. Endocrinol. 2015, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, F.F. A short story of Klotho and FGF23: A deuce of dark side or the savior? Int. Urol. Nephrol. 2014, 46, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Urakawa, I.; Yamazaki, Y.; Shimada, T.; Iijima, K.; Hasegawa, H.; Okawa, K.; Fujita, T.; Fukumoto, S.; Yamashita, T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006, 444, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S. Actions and Mode of Actions of FGF19 Subfamily Members. Endocr. J. 2008, 55, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Kuro-O, M. Klotho and the Aging Process. Korean J. Intern. Med. 2011, 26, 113. [Google Scholar] [CrossRef] [PubMed]
- Kuro-O, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Kuro-O, M. Klotho and aging. Biochim. Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Manya, H.; Akasaka-Manya, K.; Endo, T. Klotho protein deficiency and aging: Function of α-klotho protein. Geriatr. Gerontol. Int. 2010, 10, S80–S87. [Google Scholar] [CrossRef]
- Kurosu, H.; Choi, M.; Ogawa, Y.; Dickson, A.S.; Goetz, R.; Eliseenkova, A.V.; Mohammadi, M.; Rosenblatt, K.P.; Kliewer, S.A.; Kuro-O, M. Tissue-specific Expression of βKlotho and Fibroblast Growth Factor (FGF) Receptor Isoforms Determines Metabolic Activity of FGF19 and FGF21. J. Biol. Chem. 2007, 282, 26687–26695. [Google Scholar] [CrossRef]
- Bartke, A. Long-lived Klotho mice: New insights into the roles of IGF-1 and insulin in aging. Trends Endocrinol. Metab. 2006, 17, 33–35. [Google Scholar] [CrossRef]
- Dolegowska, K.; Marchelek-Mysliwiec, M.; Nowosiad-Magda, M.; Slawinski, M.; Dolegowska, B. FGF19 subfamily members: FGF19 and FGF21. J. Physiol. Biochem. 2019, 75, 229–240. [Google Scholar] [CrossRef]
- Harmer, N.J.; Pellegrini, L.; Chirgadze, D.; Fernandez-Recio, J.; Blundell, T.L. The Crystal Structure of Fibroblast Growth Factor (FGF) 19 Reveals Novel Features of the FGF Family and Offers a Structural Basis for Its Unusual Receptor Affinity. Biochemistry 2004, 43, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Beenken, A.; Mohammadi, M. The Structural Biology of the FGF19 Subfamily. In Endocrine FGFs and Klothos; Kuro-o, M., Ed.; Advances in Experimental Medicine and Biology Book Series; Springer: New York, NY, USA, 2012; Volume 728, pp. 1–24. [Google Scholar] [CrossRef]
- Nishimura, T.; Utsunomiya, Y.; Hoshikawa, M.; Ohuchi, H.; Itoh, N. Structure and expression of a novel human FGF, FGF-19, expressed in the fetal brain. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 1999, 1444, 148–151. [Google Scholar] [CrossRef]
- Wu, X.; Lemon, B.; Li, X.; Gupte, J.; Weiszmann, J.; Stevens, J.; Hawkins, N.; Shen, W.; Lindberg, R.; Chen, J.L.; et al. C-terminal Tail of FGF19 Determines Its Specificity toward Klotho Co-receptors. J. Biol. Chem. 2008, 283, 33304–33309. [Google Scholar] [CrossRef]
- Vergnes, L.; Lee, J.M.; Chin, R.G.; Auwerx, J.; Reue, K. Diet1 Functions in the FGF15/19 Enterohepatic Signaling Axis to Modulate Bile Acid and Lipid Levels. Cell Metab. 2013, 17, 916–928. [Google Scholar] [CrossRef]
- Kir, S.; Beddow, S.A.; Samuel, V.T.; Miller, P.; Previs, S.F.; Suino-Powell, K.; Xu, H.E.; Shulman, G.I.; Kliewer, S.A.; Mangelsdorf, D.J. FGF19 as a Postprandial, Insulin-Independent Activator of Hepatic Protein and Glycogen Synthesis. Science 2011, 331, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Potthoff, M.J.; Boney-Montoya, J.; Choi, M.; He, T.; Sunny, N.E.; Satapati, S.; Suino-Powell, K.; Xu, H.E.; Gerard, R.D.; Finck, B.N.; et al. FGF15/19 Regulates Hepatic Glucose Metabolism by Inhibiting the CREB-PGC-1α Pathway. Cell Metab. 2011, 13, 729–738. [Google Scholar] [CrossRef]
- Vohra, M.S.; Benchoula, K.; Serpell, C.J.; Hwa, W.E. AgRP/NPY and POMC neurons in the arcuate nucleus and their potential role in treatment of obesity. Eur. J. Pharmacol. 2022, 915, 174611. [Google Scholar] [CrossRef] [PubMed]
- Marcelin, G.; Jo, Y.H.; Li, X.; Schwartz, G.J.; Zhang, Y.; Dun, N.J.; Lyu, R.M.; Blouet, C.; Chang, J.K.; Chua, S. Central action of FGF19 reduces hypothalamic AGRP/NPY neuron activity and improves glucose metabolism. Mol. Metab. 2014, 3, 19–28. [Google Scholar] [CrossRef]
- Morton, G.J.; Matsen, M.E.; Bracy, D.P.; Meek, T.H.; Nguyen, H.T.; Stefanovski, D.; Bergman, R.N.; Wasserman, D.H.; Schwartz, M.W. FGF19 action in the brain induces insulin-independent glucose lowering. J. Clin. Investig. 2013, 123, 4799–4808. [Google Scholar] [CrossRef] [PubMed]
- Ryan, K.K.; Kohli, R.; Gutierrez-Aguilar, R.; Gaitonde, S.G.; Woods, S.C.; Seeley, R.J. Fibroblast Growth Factor-19 Action in the Brain Reduces Food Intake and Body Weight and Improves Glucose Tolerance in Male Rats. Endocrinology 2013, 154, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.T.; Fang, Q.C.; Jia, W.P. Role of fibroblast growth factor 19 in maintaining nutrient homeostasis and disease. Biomed. Environ. Sci. 2014, 27, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Owen, B.M.; Mangelsdorf, D.J.; Kliewer, S.A. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol. Metab. 2015, 26, 22–29. [Google Scholar] [CrossRef]
- Inagaki, T.; Choi, M.; Moschetta, A.; Peng, L.; Cummins, C.L.; McDonald, J.G.; Luo, G.; Jones, S.A.; Goodwin, B.; Richardson, J.A.; et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005, 2, 217–225. [Google Scholar] [CrossRef]
- Kalaany, N.Y.; Mangelsdorf, D.J. LXRS and FXR: The Yin and Yang of Cholesterol and Fat Metabolism. Annu. Rev. Physiol. 2006, 68, 159–191. [Google Scholar] [CrossRef]
- Chiang, J.Y.L. Bile Acid Metabolism and Signaling. In Comprehensive Physiology, 1st ed.; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2013; pp. 1191–1212. [Google Scholar] [CrossRef]
- Vaquero, J.; Monte, M.J.; Dominguez, M.; Muntané, J.; Marin, J.J.G. Differential activation of the human farnesoid X receptor depends on the pattern of expressed isoforms and the bile acid pool composition. Biochem. Pharmacol. 2013, 86, 926–939. [Google Scholar] [CrossRef]
- Kuro-O, M. Klotho and βKlotho. In Endocrine FGFs and Klothos; Kuro-o, M., Ed.; Advances in Experimental Medicine and Biology Book Series; Springer: New York, NY, USA, 2012; Volume 728, pp. 25–40. [Google Scholar] [CrossRef]
- Holt, J.A.; Luo, G.; Billin, A.N.; Bisi, J.; McNeill, Y.Y.; Kozarsky, K.F.; Donahee, M.; Wang, D.Y.; Mansfield, T.A.; Kliewer, S.A.; et al. Definition of a novel growth factor-dependent signal cascade for the suppression of bile acid biosynthesis. Genes. Dev. 2003, 17, 1581–1591. [Google Scholar] [CrossRef]
- Potthoff, M.J.; Kliewer, S.A.; Mangelsdorf, D.J. Endocrine fibroblast growth factors 15/19 and 21: From feast to famine. Genes. Dev. 2012, 26, 312–324. [Google Scholar] [CrossRef]
- Ito, S.; Fujimori, T.; Furuya, A.; Satoh, J.; Nabeshima, Y.; Nabeshima, Y.I. Impaired negative feedback suppression of bile acid synthesis in mice lacking βKlotho. J. Clin. Investig. 2005, 115, 2202–2208. [Google Scholar] [CrossRef]
- von Holstein-Rathlou, S.; BonDurant, L.D.; Peltekian, L.; Naber, M.C.; Yin, T.C.; Claflin, K.E.; Urizar, A.I.; Madsen, A.N.; Ratner, C.; Holst, B.; et al. FGF21 Mediates Endocrine Control of Simple Sugar Intake and Sweet Taste Preference by the Liver. Cell Metab. 2016, 23, 335–343. [Google Scholar] [CrossRef]
- Zhang, F.; Yu, L.; Lin, X.; Cheng, P.; He, L.; Li, X.; Lu, X.; Tan, Y.; Yang, H.; Cai, L.; et al. Minireview: Roles of Fibroblast Growth Factors 19 and 21 in Metabolic Regulation and Chronic Diseases. Mol. Endocrinol. 2015, 29, 1400–1413. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xia, F.; Lam, K.S.; Wang, Y.; Bao, Y.; Zhang, J.; Gu, Y.; Zhou, P.; Lu, J.; Jia, W.; et al. Circadian Rhythm of Circulating Fibroblast Growth Factor 21 Is Related to Diurnal Changes in Fatty Acids in Humans. Clin. Chem. 2011, 57, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, T.; Nakatake, Y.; Konishi, M.; Itoh, N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim. Biophys. Acta (BBA) Gene Struct. Expr. 2000, 1492, 203–206. [Google Scholar] [CrossRef]
- Cuevas-Ramos, D.; Almeda-Valdes, P.; Aguilar-Salinas, C.; Cuevas-Ramos, G.; Cuevas-Sosa, A.; Gomez-Perez, F. The Role of Fibroblast Growth Factor 21 (FGF21) on Energy Balance, Glucose and Lipid Metabolism. CDR 2009, 5, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Eto, K. FGF-21, a newcomer in the field of hypertension research. J. Hum. Hypertens. 2013, 27, 343–344. [Google Scholar] [CrossRef]
- Reinehr, T.; Woelfle, J.; Wunsch, R.; Roth, C.L. Fibroblast Growth Factor 21 (FGF-21) and Its Relation to Obesity, Metabolic Syndrome, and Nonalcoholic Fatty Liver in Children: A Longitudinal Analysis. J. Clin. Endocrinol. Metab. 2012, 97, 2143–2150. [Google Scholar] [CrossRef]
- Hondares, E.; Iglesias, R.; Giralt, A.; Gonzalez, F.J.; Giralt, M.; Mampel, T.; Villarroya, F. Thermogenic Activation Induces FGF21 Expression and Release in Brown Adipose Tissue. J. Biol. Chem. 2011, 286, 12983–12990. [Google Scholar] [CrossRef]
- Muise, E.S.; Azzolina, B.; Kuo, D.W.; El-Sherbeini, M.; Tan, Y.; Yuan, X.; Mu, J.; Thompson, J.R.; Berger, J.P.; Wong, K.K. Adipose Fibroblast Growth Factor 21 Is Up-Regulated by Peroxisome Proliferator-Activated Receptor γ and Altered Metabolic States. Mol. Pharmacol. 2008, 74, 403–412. [Google Scholar] [CrossRef]
- Iglesias, P.; Selgas, R.; Romero, S.; Díez, J.J. Mechanisms in Endocrinology: Biological role, clinical significance, and therapeutic possibilities of the recently discovered metabolic hormone fibroblastic growth factor 21. Eur. J. Endocrinol. 2012, 167, 301–309. [Google Scholar] [CrossRef]
- Ding, X.; Boney-Montoya, J.; Owen, B.M.; Bookout, A.L.; Coate, K.C.; Mangelsdorf, D.J.; Kliewer, S.A. βKlotho Is Required for Fibroblast Growth Factor 21 Effects on Growth and Metabolism. Cell Metab. 2012, 16, 387–393. [Google Scholar] [CrossRef]
- Adams, A.C.; Cheng, C.C.; Coskun, T.; Kharitonenkov, A. FGF21 Requires βklotho to Act In Vivo. PLoS ONE 2012, 7, e49977. [Google Scholar] [CrossRef]
- Lee, J.H.; Giannikopoulos, P.; Duncan, S.A.; Wang, J.; Johansen, C.T.; Brown, J.D.; Plutzky, J.; Hegele, R.A.; Glimcher, L.H.; Lee, A.H. The transcription factor cyclic AMP–responsive element–binding protein H regulates triglyceride metabolism. Nat. Med. 2011, 17, 812–815. [Google Scholar] [CrossRef]
- Boutant, M.; Cantó, C. SIRT1 metabolic actions: Integrating recent advances from mouse models. Mol. Metab. 2014, 3, 5–18. [Google Scholar] [CrossRef]
- Singh, V.; Ubaid, S. Role of Silent Information Regulator 1 (SIRT1) in Regulating Oxidative Stress and Inflammation. Inflammation 2020, 43, 1589–1598. [Google Scholar] [CrossRef]
- Mac-Marcjanek, K.; Zieleniak, A.; Zurawska-Klis, M.; Cypryk, K.; Wozniak, L.; Wojcik, M. Expression Profile of Diabetes-Related Genes Associated with Leukocyte Sirtuin 1 Overexpression in Gestational Diabetes. Int. J. Mol. Sci. 2018, 19, 3826. [Google Scholar] [CrossRef]
- Wojcik, M.; Mac-Marcjanek, K.; Wozniak, L. Physiological and Pathophysiological Functions of SIRT1. Mini-Reviews Med. Chem. 2009, 9, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Jiang, X.; Ma, H.; Wang, Y.; Xue, P.; Liu, Y. SIRT1 and insulin resistance. J. Diabetes Complicat. 2016, 30, 178–183. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Wroblewski, V.J.; Koester, A.; Chen, Y.F.; Clutinger, C.K.; Tigno, X.T.; Hansen, B.C.; Shanafelt, A.B.; Etgen, G.J. The Metabolic State of Diabetic Monkeys Is Regulated by Fibroblast Growth Factor-21. Endocrinology 2007, 148, 774–781. [Google Scholar] [CrossRef]
- Badman, M.K.; Pissios, P.; Kennedy, A.R.; Koukos, G.; Flier, J.S.; Maratos-Flier, E. Hepatic Fibroblast Growth Factor 21 Is Regulated by PPARα and Is a Key Mediator of Hepatic Lipid Metabolism in Ketotic States. Cell Metab. 2007, 5, 426–437. [Google Scholar] [CrossRef]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine Regulation of the Fasting Response by PPARα-Mediated Induction of Fibroblast Growth Factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef]
- Lundåsen, T.; Hunt, M.C.; Nilsson, L.M.; Sanyal, S.; Angelin, B.; Alexson, S.E.H.; Rudling, M. PPARα is a key regulator of hepatic FGF21. Biochem. Biophys. Res. Commun. 2007, 360, 437–440. [Google Scholar] [CrossRef]
- Laeger, T.; Henagan, T.M.; Albarado, D.C.; Redman, L.M.; Bray, G.A.; Noland, R.C.; Münzberg, H.; Hutson, S.M.; Gettys, T.W.; Schwartz, M.W.; et al. FGF21 is an endocrine signal of protein restriction. J. Clin. Investig. 2014, 124, 3913–3922. [Google Scholar] [CrossRef]
- De Sousa-Coelho, A.L.; Relat, J.; Hondares, E.; Pérez-Martí, A.; Ribas, F.; Villarroya, F.; Marrero, P.F.; Haro, D. FGF21 mediates the lipid metabolism response to amino acid starvation. J. Lipid Res. 2013, 54, 1786–1797. [Google Scholar] [CrossRef]
- Wente, W.; Efanov, A.M.; Brenner, M.; Kharitonenkov, A.; Köster, A.; Sandusky, G.E.; Sewing, S.; Treinies, I.; Zitzer, H.; Gromada, J. Fibroblast Growth Factor-21 Improves Pancreatic β-Cell Function and Survival by Activation of Extracellular Signal–Regulated Kinase 1/2 and Akt Signaling Pathways. Diabetes 2006, 55, 2470–2478. [Google Scholar] [CrossRef]
- Patel, R.; Bookout, A.L.; Magomedova, L.; Owen, B.M.; Consiglio, G.P.; Shimizu, M.; Zhang, Y.; Mangelsdorf, D.J.; Kliewer, S.A.; Cummins, C.L. Glucocorticoids Regulate the Metabolic Hormone FGF21 in a Feed-Forward Loop. Mol. Endocrinol. 2015, 29, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Gälman, C.; Lundåsen, T.; Kharitonenkov, A.; Bina, H.A.; Eriksson, M.; Hafström, I.; Dahlin, M.; Åmark, P.; Angelin, B.; Rudling, M. The Circulating Metabolic Regulator FGF21 Is Induced by Prolonged Fasting and PPARα Activation in Man. Cell Metab. 2008, 8, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, C.; Dyson, P.; Sprecher, D.; Tsintzas, K.; Karpe, F. Circulating Fibroblast Growth Factor 21 Is Induced by Peroxisome Proliferator-Activated Receptor Agonists but Not Ketosis in Man. J. Clin. Endocrinol. Metab. 2009, 94, 3594–3601. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.W.; Li, L.; Yang, G.Y.; Li, K.; Qi, X.Y.; Zhu, W.; Tang, Y.; Liu, H.; Boden, G. Circulating FGF-21 Levels in Normal Subjects and in Newly Diagnose Patients with Type 2 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2007, 116, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Klen, J.; Dolžan, V. Glucagon-like Peptide-1 Receptor Agonists in the Management of Type 2 Diabetes Mellitus and Obesity: The Impact of Pharmacological Properties and Genetic Factors. Int. J. Mol. Sci. 2022, 23, 3451. [Google Scholar] [CrossRef]
- Nauck, M.A.; Meier, J.J. Incretin hormones: Their role in health and disease. Diabetes Obes. Metab. 2018, 20 (Suppl. S1), 5–21. [Google Scholar] [CrossRef]
- Liu, J.; Yang, K.; Yang, J.; Xiao, W.; Le, Y.; Yu, F.; Gu, L.; Lang, S.; Tian, Q.; Jin, T.; et al. Liver-derived fibroblast growth factor 21 mediates effects of glucagon-like peptide-1 in attenuating hepatic glucose output. eBioMedicine 2019, 41, 73–84. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, L.; Wang, C.; Liu, H.; Boden, G.; Yang, G.; Li, L. Liraglutide increases FGF-21 activity and insulin sensitivity in high fat diet and adiponectin knockdown induced insulin resistance. PLoS ONE 2012, 7, e48392. [Google Scholar] [CrossRef]
- Pan, Q.; Lin, S.; Li, Y.; Liu, L.; Li, X.; Gao, X.; Yan, J.; Gu, B.; Chen, X.; Li, W.; et al. A novel GLP-1 and FGF21 dual agonist has therapeutic potential for diabetes and non-alcoholic steatohepatitis. eBioMedicine 2021, 63, 103202. [Google Scholar] [CrossRef]
- Domouzoglou, E.M.; Naka, K.K.; Vlahos, A.P.; Papafaklis, M.I.; Michalis, L.K.; Tsatsoulis, A.; Maratos-Flier, E. Fibroblast growth factors in cardiovascular disease: The emerging role of FGF21. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1029–H1038. [Google Scholar] [CrossRef]
- Lakhani, I.; Gong, M.; Wong, W.T.; Bazoukis, G.; Lampropoulos, K.; Wong, S.H.; Wu, W.K.K.; Wong, M.C.S.; Ong, K.L.; Liu, T.; et al. Fibroblast growth factor 21 in cardio-metabolic disorders: A systematic review and meta-analysis. Metabolism 2018, 83, 11–17. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, Z.; Yin, X.; Liu, Y.; Yan, X.; Lin, S.; Xiao, J.; Wang, X.; Feng, W.; Li, X. Serum Levels of FGF-21 Are Increased in Coronary Heart Disease Patients and Are Independently Associated with Adverse Lipid Profile. PLoS ONE 2010, 5, e15534. [Google Scholar] [CrossRef]
- Zhang, W.; Chu, S.; Ding, W.; Wang, F. Serum Level of Fibroblast Growth Factor 21 Is Independently Associated with Acute Myocardial Infarction. PLoS ONE 2015, 10, e0129791. [Google Scholar] [CrossRef]
- Han, X.; Chen, C.; Cheng, G.; Xie, C.; Yang, M.; Shou, X.; Sun, C. Serum fibroblast growth factor 21 levels are increased in atrial fibrillation patients. Cytokine 2015, 73, 176–180. [Google Scholar] [CrossRef]
- Lenart-Lipińska, M.; Matyjaszek-Matuszek, B.; Gernand, W.; Nowakowski, A.; Solski, J. Serum fibroblast growth factor 21 is predictive of combined cardiovascular morbidity and mortality in patients with type 2 diabetes at a relatively short-term follow-up. Diabetes Res. Clin. Pract. 2013, 101, 194–200. [Google Scholar] [CrossRef]
- Ong, K.L.; Januszewski, A.S.; O’Connell, R.; Jenkins, A.J.; Xu, A.; Sullivan, D.R.; Barter, P.J.; Hung, W.T.; Scott, R.S.; Taskinen, M.R.; et al. The relationship of fibroblast growth factor 21 with cardiovascular outcome events in the Fenofibrate Intervention and Event Lowering in Diabetes study. Diabetologia 2015, 58, 464–473. [Google Scholar] [CrossRef]
- Planavila, A.; Redondo, I.; Hondares, E.; Vinciguerra, M.; Munts, C.; Iglesias, R.; Gabrielli, L.A.; Sitges, M.; Giralt, M.; van Bilsen, M.; et al. Fibroblast growth factor 21 protects against cardiac hypertrophy in mice. Nat. Commun. 2013, 4, 2019. [Google Scholar] [CrossRef]
- Liu, S.Q.; Roberts, D.; Kharitonenkov, A.; Zhang, B.; Hanson, S.M.; Li, Y.C.; Zhang, L.Q.; Wu, Y.H. Endocrine Protection of Ischemic Myocardium by FGF21 from the Liver and Adipose Tissue. Sci. Rep. 2013, 3, 2767. [Google Scholar] [CrossRef]
- Joki, Y.; Ohashi, K.; Yuasa, D.; Shibata, R.; Ito, M.; Matsuo, K.; Kambara, T.; Uemura, Y.; Hayakawa, S.; Hiramatsu-Ito, M.; et al. FGF21 attenuates pathological myocardial remodeling following myocardial infarction through the adiponectin-dependent mechanism. Biochem. Biophys. Res. Commun. 2015, 459, 124–130. [Google Scholar] [CrossRef]
- Tanajak, P.; Sa-nguanmoo, P.; Wang, X.; Liang, G.; Li, X.; Jiang, C.; Chattipakorn, S.C.; Chattipakorn, N. Fibroblast growth factor 21 (FGF21) therapy attenuates left ventricular dysfunction and metabolic disturbance by improving FGF21 sensitivity, cardiac mitochondrial redox homoeostasis and structural changes in pre-diabetic rats. Acta Physiol. 2016, 217, 287–299. [Google Scholar] [CrossRef]
- Kaess, B.M.; Barnes, T.A.; Stark, K.; Charchar, F.J.; Waterworth, D.; Song, K.; Wang, W.Y.S.; Vollenweider, P.; Waeber, G.; Mooser, V.; et al. FGF21 signalling pathway and metabolic traits—Genetic association analysis. Eur. J. Hum. Genet. 2010, 18, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Adya, R.; Chen, J.; Ramanjaneya, M.; Bari, M.F.; Bhudia, S.K.; Hillhouse, E.W.; Tan, B.K.; Randeva, H.S. Novel Insights into the Cardio-Protective Effects of FGF21 in Lean and Obese Rat Hearts. PLoS ONE 2014, 9, e87102. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, Y.; Gu, J.; Wang, S.; Zhou, S.; Wang, Y.; Tan, Y.; Feng, W.; Fu, Y.; Mellen, N.; et al. Fenofibrate increases cardiac autophagy via FGF21/SIRT1 and prevents fibrosis and inflammation in the hearts of Type 1 diabetic mice. Clin. Sci. 2016, 130, 625–641. [Google Scholar] [CrossRef]
- Cummins, P. Fibroblast and transforming growth factor expression in the cardiac myocyte. Cardiovasc. Res. 1993, 27, 1150–1154. [Google Scholar] [CrossRef]
- Saito, H.; Maeda, A.; Ohtomo, S.; Hirata, M.; Kusano, K.; Kato, S.; Ogata, E.; Segawa, H.; Miyamoto, K.; Fukushima, N. Circulating FGF-23 Is Regulated by 1α,25-Dihydroxyvitamin D3 and Phosphorus in Vivo. J. Biol. Chem. 2005, 280, 2543–2549. [Google Scholar] [CrossRef]
- Hanks, L.J.; Casazza, K.; Judd, S.E.; Jenny, N.S.; Gutiérrez, O.M. Associations of Fibroblast Growth Factor-23 with Markers of Inflammation, Insulin Resistance and Obesity in Adults. PLoS ONE 2015, 10, e0122885. [Google Scholar] [CrossRef] [PubMed]
- Grethen, E.; Hill, K.M.; Jones, R.; Cacucci, B.M.; Gupta, C.E.; Acton, A.; Considine, R.V.; Peacock, M. Serum Leptin, Parathyroid Hormone, 1,25-Dihydroxyvitamin D, Fibroblast Growth Factor 23, Bone Alkaline Phosphatase, and Sclerostin Relationships in Obesity. J. Clin. Endocrinol. Metab. 2012, 97, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.; Dolezal-Oltarzewska, K.; Janus, D.; Drozdz, D.; Sztefko, K.; Starzyk, J.B. FGF23 contributes to insulin sensitivity in obese adolescents—Preliminary results: FGF23 contributes to insulin sensitivity in obese adolescents. Clin. Endocrinol. 2012, 77, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Kanbay, M.; Vervloet, M.; Cozzolino, M.; Siriopol, D.; Covic, A.; Goldsmith, D.; Solak, Y. Novel Faces of Fibroblast Growth Factor 23 (FGF23): Iron Deficiency, Inflammation, Insulin Resistance, Left Ventricular Hypertrophy, Proteinuria and Acute Kidney Injury. Calcif. Tissue Int. 2017, 100, 217–228. [Google Scholar] [CrossRef]
- Garland, J.S.; Holden, R.M.; Ross, R.; Adams, M.A.; Nolan, R.L.; Hopman, W.M.; Morton, A.R. Insulin resistance is associated with Fibroblast Growth Factor-23 in stage 3–5 chronic kidney disease patients. J. Diabetes Complicat. 2014, 28, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Wahl, P.; Xie, H.; Scialla, J.; Anderson, C.A.M.; Bellovich, K.; Brecklin, C.; Chen, J.; Feldman, H.; Gutierrez, O.M.; Lash, J.; et al. Earlier onset and greater severity of disordered mineral metabolism in diabetic patients with chronic kidney disease. Diabetes Care 2012, 35, 994–1001. [Google Scholar] [CrossRef]
- Adema, A.Y.; van Ittersum, F.J.; Hoenderop, J.G.; de Borst, M.H.; Nanayakkara, P.W.; Ter Wee, P.M.; Heijboer, A.C.; Vervloet, M.G. NIGRAM Consortium. Reduction of Oxidative Stress in Chronic Kidney Disease Does Not Increase Circulating α-Klotho Concentrations. PLoS ONE 2016, 11, e0144121. [Google Scholar] [CrossRef]
- Kurosu, H.; Ogawa, Y.; Miyoshi, M.; Yamamoto, M.; Nandi, A.; Rosenblatt, K.P.; Baum, M.G.; Schiavi, S.; Hu, M.C.; Moe, O.W.; et al. Regulation of Fibroblast Growth Factor-23 Signaling by Klotho. J. Biol. Chem. 2006, 281, 6120–6123. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Hasegawa, H.; Yamazaki, Y.; Muto, T.; Hino, R.; Takeuchi, Y.; Fujita, T.; Nakahara, K.; Fukumoto, S.; Yamashita, T. FGF-23 Is a Potent Regulator of Vitamin D Metabolism and Phosphate Homeostasis. J. Bone Miner. Res. 2003, 19, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Gosse, P. Left ventricular hypertrophy as a predictor of cardiovascular risk. J. Hypertens. 2005, 23 (Suppl. S1), S27–S33. [Google Scholar] [CrossRef] [PubMed]
- Samms, R.J.; Smith, D.P.; Cheng, C.C.; Antonellis, P.P.; Perfield, J.W.; Kharitonenkov, A.; Gimeno, R.E.; Adams, A.C. Discrete Aspects of FGF21 In Vivo Pharmacology Do Not Require UCP1. Cell Rep. 2015, 11, 991–999. [Google Scholar] [CrossRef]
- Lanske, B.; Razzaque, M.S. Molecular interactions of FGF23 and PTH in phosphate regulation. Kidney Int. 2014, 86, 1072–1074. [Google Scholar] [CrossRef]
- Gutiérrez, O.M.; Januzzi, J.L.; Isakova, T.; Laliberte, K.; Smith, K.; Collerone, G.; Sarwar, A.; Hoffmann, U.; Coglianese, E.; Christenson, R.; et al. Fibroblast Growth Factor 23 and Left Ventricular Hypertrophy in Chronic Kidney Disease. Circulation 2009, 119, 2545–2552. [Google Scholar] [CrossRef]
- Xie, J.; Cha, S.K.; An, S.W.; Kuro-O, M.; Birnbaumer, L.; Huang, C.L. Cardioprotection by Klotho through downregulation of TRPC6 channels in the mouse heart. Nat. Commun. 2012, 3, 1238. [Google Scholar] [CrossRef]
- Norman, P.E.; Powell, J.T. Vitamin D and Cardiovascular Disease. Circ. Res. 2014, 114, 379–393. [Google Scholar] [CrossRef]
- Yuan, W.; Pan, W.; Kong, J.; Zheng, W.; Szeto, F.L.; Wong, K.E.; Cohen, R.; Klopot, A.; Zhang, Z.; Li, Y.C. 1,25-Dihydroxyvitamin D3 Suppresses Renin Gene Transcription by Blocking the Activity of the Cyclic AMP Response Element in the Renin Gene Promoter. J. Biol. Chem. 2007, 282, 29821–29830. [Google Scholar] [CrossRef]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D3 is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef]
- Andrukhova, O.; Slavic, S.; Smorodchenko, A.; Zeitz, U.; Shalhoub, V.; Lanske, B.; Pohl, E.E.; Erben, R.G. FGF 23 regulates renal sodium handling and blood pressure. EMBO Mol. Med. 2014, 6, 744–759. [Google Scholar] [CrossRef]
- Wang, D.; Xu, S.; Ding, W.; Zhu, C.; Deng, S.; Qiu, X.; Wang, Z. Decreased placental and muscular expression of the fibroblast growth factor 19 in gestational diabetes mellitus. J. Diabetes Investig. 2019, 10, 171–181. [Google Scholar] [CrossRef]
- Colomiere, M.; Permezel, M.; Riley, C.; Desoye, G.; Lappas, M. Defective insulin signaling in placenta from pregnancies complicated by gestational diabetes mellitus. Eur. J. Endocrinol. 2009, 160, 567–578. [Google Scholar] [CrossRef]
- Schaefer-Graf, U.M.; Graf, K.; Kulbacka, I.; Kjos, S.L.; Dudenhausen, J.; Vetter, K.; Herrera, E. Maternal Lipids as Strong Determinants of Fetal Environment and Growth in Pregnancies with Gestational Diabetes Mellitus. Diabetes Care 2008, 31, 1858–1863. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, D.; Li, Z.; Xu, S.; Chen, H.; Ding, W.; Yang, J.; Zhao, W.; Sun, B.; Wang, Z. FGF15/FGF19 alleviates insulin resistance and upregulates placental IRS1/GLUT expression in pregnant mice fed a high-fat diet. Placenta 2021, 112, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.N.; Huang, R.; Liu, X.; Xu, Y.J.; Wang, W.J.; He, H.; Zhang, G.H.; Zheng, T.; Fang, F.; Fan, J.G.; et al. Fibroblast Growth Factor 19 in Gestational Diabetes Mellitus and Fetal Growth. Front. Endocrinol. 2022, 12, 805722. [Google Scholar] [CrossRef] [PubMed]
- Karasek, D.; Krystynik, O.; Kucerova, V.; Macakova, D.; Cibickova, L.; Schovanek, J.; Haluzik, M. Adipoctin, A-FABP and FGF-19 Levels in Women with Early Diagnosed Gestational Diabetes. J. Clin. Med. 2022, 11, 2417. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.; Stepan, H.; Kratzsch, J.; Verlohren, M.; Verlohren, H.J.; Drynda, K.; Lössner, U.; Blüher, M.; Stumvoll, M.; Fasshauer, M. Serum fibroblast growth factor 21 levels in gestational diabetes mellitus in relation to insulin resistance and dyslipidemia. Metabolism 2010, 59, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M.; Wang, W.F.; Zhou, L.H.; Ma, L.; An, Y.; Xu, W.J.; Li, T.H.; Yu, Y.H.; Li, D.S.; Liu, Y. Fibroblast growth factor 21 expressions in white blood cells and sera of patients with gestational diabetes mellitus during gestation and postpartum. Endocrine 2015, 48, 519–527. [Google Scholar] [CrossRef]
- Jia, X.; Bai, L.; Ma, N.; Lu, Q. The relationship between serum adipokine fibroblast growth factor-21 and gestational diabetes mellitus. J. Diabetes Investig. 2022, 13, 2047–2053. [Google Scholar] [CrossRef]
- Bonakdaran, S.; Khorasani, Z.M.; Jafarzadeh, F. Increased Serum Level of FGF21 in Gestational Diabetes Mellitus. Acta Endocrinol. 2017, 13, 278–281. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Wei, W.; Yu, F.; Liu, R.; Shen, Y.; Zhang, R.; Yuan, G.; Zhou, H. Circulating levels of fibroblast growth factor 21 in gestational diabetes mellitus: A meta-analysis. Endocr. J. 2021, 68, 345–352. [Google Scholar] [CrossRef]
- Wang, Z.; Yuan, M.; Xu, C.; Zhang, Y.; Ying, C.; Xiao, X. FGF21 Serum Levels in the Early Second Trimester Are Positively Correlated with the Risk of Subsequent Gestational Diabetes Mellitus: A Propensity-Matched Nested Case-Control Study. Front. Endocrinol. 2021, 12, 630287. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wu, L.; Wang, Y.; Ye, Y.; Yang, X.; Yuan, J.; Xu, J.; Wang, Y.X.; Song, X.; Yan, S.; et al. Maternal overweight and obesity modify the association of serum fibroblast growth factor 21 levels with gestational diabetes mellitus: A nested case-control study. Diabetes Metab. Res. Rev. 2023, e3717. [Google Scholar] [CrossRef] [PubMed]
- Megia, A.; Gil-Lluis, P.; Näf, S.; Ceperuelo-Mallafré, V.; Gonzalez-Clemente, J.M.; Llauradó, G.; Nuñez-Roa, C.; Roche, K.; Ballesteros, M.; Yañez, R.E.; et al. Cord blood FGF21 in gestational diabetes and its relationship with postnatal growth. Acta Diabetol. 2015, 52, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Han, Z.; Li, P.; Li, X. Fibroblast growth factor-21 is a potential diagnostic factor for patients with gestational diabetes mellitus. Exp. Ther. Med. 2018, 16, 1397–1402. [Google Scholar] [CrossRef]
- Mosavat, M.; Omar, S.Z.; Sthanshewar, P. Serum FGF-21 and FGF-23 in association with gestational diabetes: A longitudinal case-control study. Horm. Mol. Biol. Clin. Investig. 2020, 41, 20190060. [Google Scholar] [CrossRef]
- Kizilgul, M.; Kan, S.; Beysel, S.; Apaydin, M.; Ozcelik, O.; Caliskan, M.; Ozbek, M.; Ozdemir, S.; Cakal, E. Is fibroblast growth factor 23 a new cardiovascular risk marker in gestational diabetes? Arch. Endocrinol. Metab. 2017, 61, 562–566. [Google Scholar] [CrossRef]
- Hocher, C.F.; Chen, X.; Zuo, J.; Horvathova, K.; Hocher, B.; Krämer, B.K.; Chu, C. Fibroblast growth factor 23 is associated with the development of gestational diabetes mellitus. Diabetes Metab. Res Rev. 2023, 39, e3704. [Google Scholar] [CrossRef]
- Tuzun, D.; Oguz, A.; Aydin, M.N.; Kurutas, E.B.; Ercan, O.; Sahin, M.; Ünsal, V.; Ceren, I.; Akçay, A.; Gul, K. Is FGF-23 an early indicator of atherosclerosis and cardiac dysfunction in patients with gestational diabetes? Arch. Endocrinol. Metab. 2018, 62, 506–513. [Google Scholar] [CrossRef]
- Dekker Nitert, M.; Barrett, H.L.; Kubala, M.H.; Scholz Romero, K.; Denny, K.J.; Woodruff, T.M.; McIntyre, H.D.; Callaway, L.K. Increased Placental Expression of Fibroblast Growth Factor 21 in Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2014, 99, E591–E598. [Google Scholar] [CrossRef] [PubMed]
- Durnwald, C.; Mele, L.; Landon, M.B.; Varner, M.W.; Casey, B.M.; Reddy, U.M.; Wapner, R.J.; Rouse, D.J.; Tita, A.T.N.; Thorp, J.M.; et al. Fibroblast Growth Factor 21 and Metabolic Dysfunction in Women with a Prior Glucose-Intolerant Pregnancy. Am. J. Perinatol. 2021, 38, 1380–1385. [Google Scholar] [CrossRef] [PubMed]


| Study | Size of the Groups | Effects | ||
|---|---|---|---|---|
| FGF19 | FGF21 | FGF23 | ||
| Wang et al. [125] | 20 GDM 25 healthy | Significantly lower expression in the placenta and rectus muscle in GDM. | No significant differences in subcutaneous adipose tissue. | X |
| Wang et al. [18] | 30 GDM 60 healthy | Close negative correlation with insulin resistance. Reduced levels in GDM (2nd trimester). | Positive correlation with insulin resistance. Elevated levels in GDM (2nd trimester). | X |
| Yang et al. [129] | 153 GDM 153 healthy | Higher concentration in cord blood in newborns born prematurely and by elective cesarean section. | X | X |
| Karasek et al. [130] | 23 GDM 54 healthy | Concentration levels in early diagnosed GDM do not differ significantly from healthy patients. Negative correlation with BMI and body weight. | X | X |
| Stein et al. [131] | 40 GDM 80 healthy | X | Positive correlation with fasting insulin levels, HOMA-IR, triglyceride levels. Negative correlation with adiponectin and HDL. No difference between GDM and healthy patients. | X |
| Li et al. [132] | 51 GDM 50 healthy | X | Higher levels in white blood cells and serum in GDM. | X |
| Jia et al. [133] | 62 GDM 58 healthy | X | Significantly higher levels of FGF21 concentrations in GDM. Relation to prepregnancy BMI in GDM. | X |
| Bonakdaran et al. [134] | 30 GDM 60 healthy | X | Significantly higher levels of FGF21 concentrations in GDM. No correlation with insulin resistance. | X |
| Jia et al. [135] | 397 GDM 435 healthy | X | Positive correlation of higher FGF21 concentrations with GDM. | X |
| Wang et al. [136] | 133 GDM 133 healthy | X | FGF21 quartiles Q2, Q3, and Q4 were associated with greater odds of GDM occurrence than Q1. | X |
| Wu et al. [137] | 332 GDM 664 healthy | X | Elevated FGF21 concentrations in early pregnancy (6–15 weeks) were associated with an increased risk of developing GDM, especially in overweight and obese women. | X |
| Megia et al. [138] | 79 GDM 78 healthy | X | Maternal concentration higher in GDM. mFGF21 significantly higher than cbFGF21 in GDM. mFGF21 levels positively correlated with HOMA-IR and insulin and glucose levels. Positive correlation between cbFGF21 and BMI of children at 12 and 24 months. | X |
| Kizilgul et al. [141] | 46 GDM 36 healthy | X | X | Higher levels in GDM. |
| Mosavat et al. [140] | 53 GDM 43 healthy | X | Significantly lower concentration in GDM (24–28 weeks). No differences before and after delivery. | Lower concentration in GDM (24–28 weeks) and before delivery. 1 pg/mL decrease in concentration associated with a 1.4× increased risk of GDM. |
| Hocher et al. [142] | 64 GDM 762 healthy | X | X | No difference in iFGF23 and cFGF23 concentrations in the cord blood of newborns of GDM mothers and non-GDM patients. |
| Tuzun et al. [143] | 54 GDM 33 healthy | X | X | Significantly higher concentrations in GDM. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska, A.; Poniedziałek-Czajkowska, E.; Mierzyński, R. The Role of the FGF19 Family in the Pathogenesis of Gestational Diabetes: A Narrative Review. Int. J. Mol. Sci. 2023, 24, 17298. https://doi.org/10.3390/ijms242417298
Sadowska A, Poniedziałek-Czajkowska E, Mierzyński R. The Role of the FGF19 Family in the Pathogenesis of Gestational Diabetes: A Narrative Review. International Journal of Molecular Sciences. 2023; 24(24):17298. https://doi.org/10.3390/ijms242417298
Chicago/Turabian StyleSadowska, Agata, Elżbieta Poniedziałek-Czajkowska, and Radzisław Mierzyński. 2023. "The Role of the FGF19 Family in the Pathogenesis of Gestational Diabetes: A Narrative Review" International Journal of Molecular Sciences 24, no. 24: 17298. https://doi.org/10.3390/ijms242417298





