Anti-Inflammatory Effects of Alphitolic Acid Isolated from Agrimonia coreana Nakai Extracts Are Mediated via the Inhibition of ICRAC Activity in T Cells
Abstract
:1. Introduction
2. Results
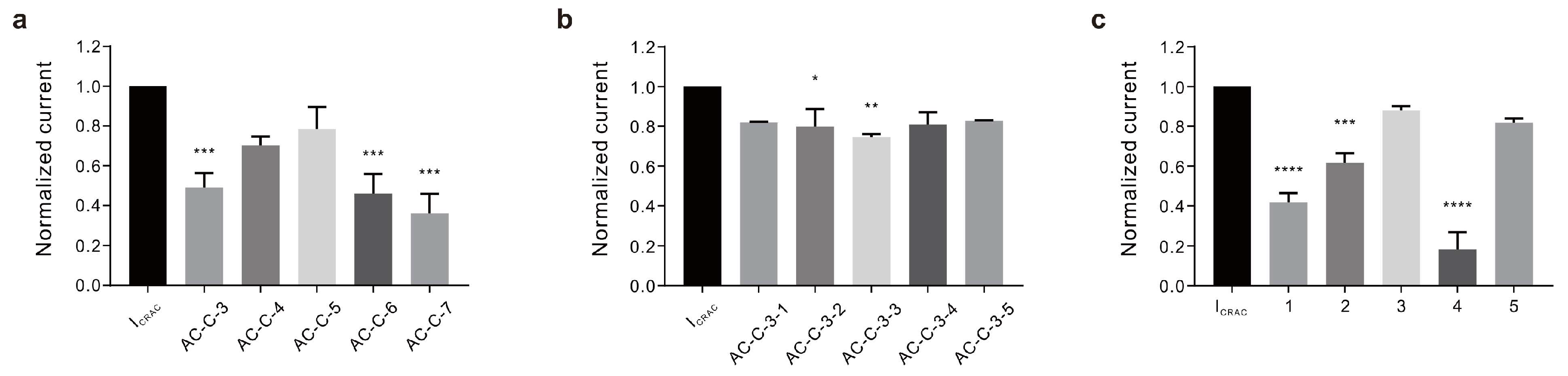
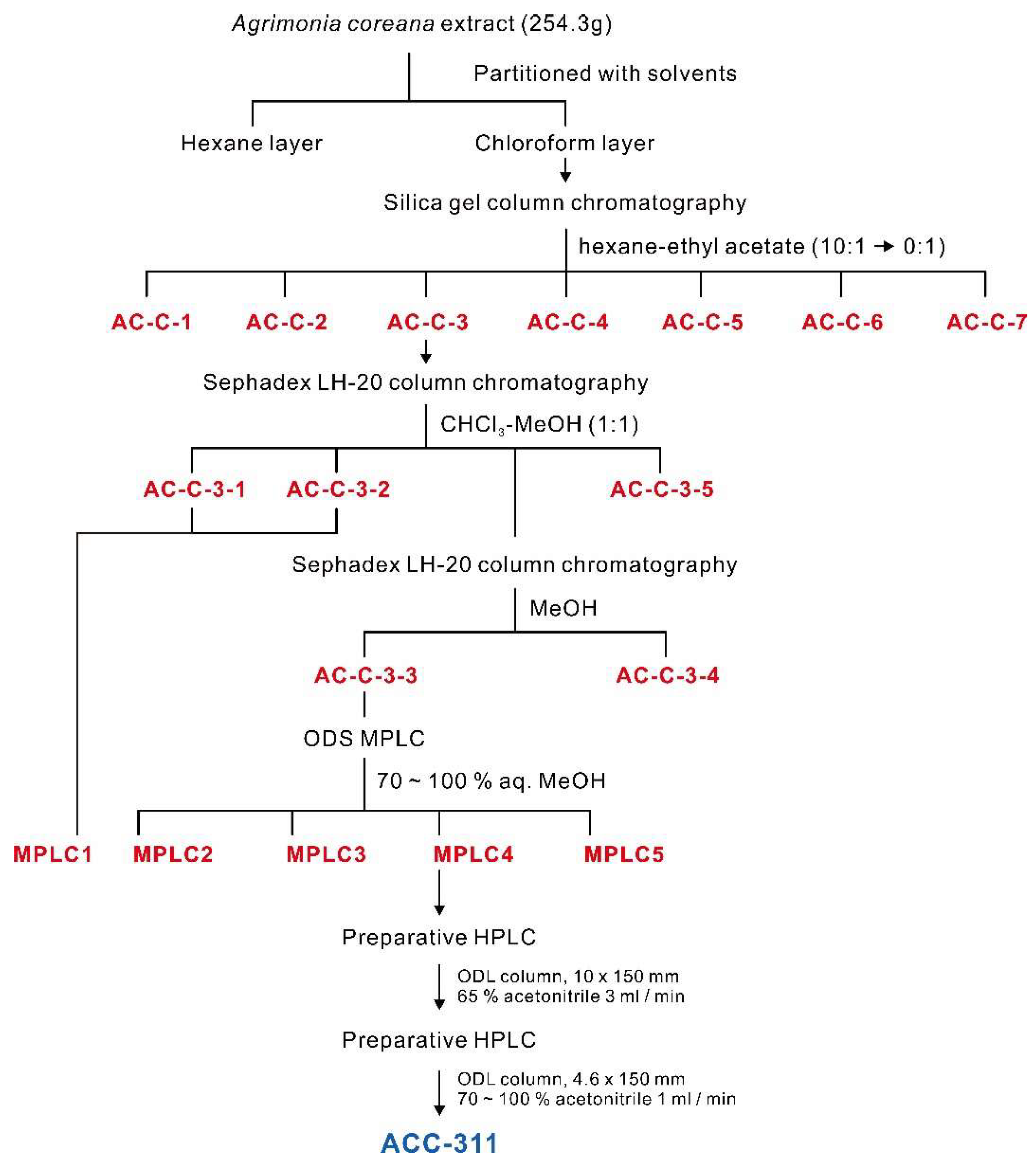
2.1. Preparation of A. coreana Extracts and Isolation of Active Fractions
2.2. Structural Analysis of ALA Using Spectroscopic Methods
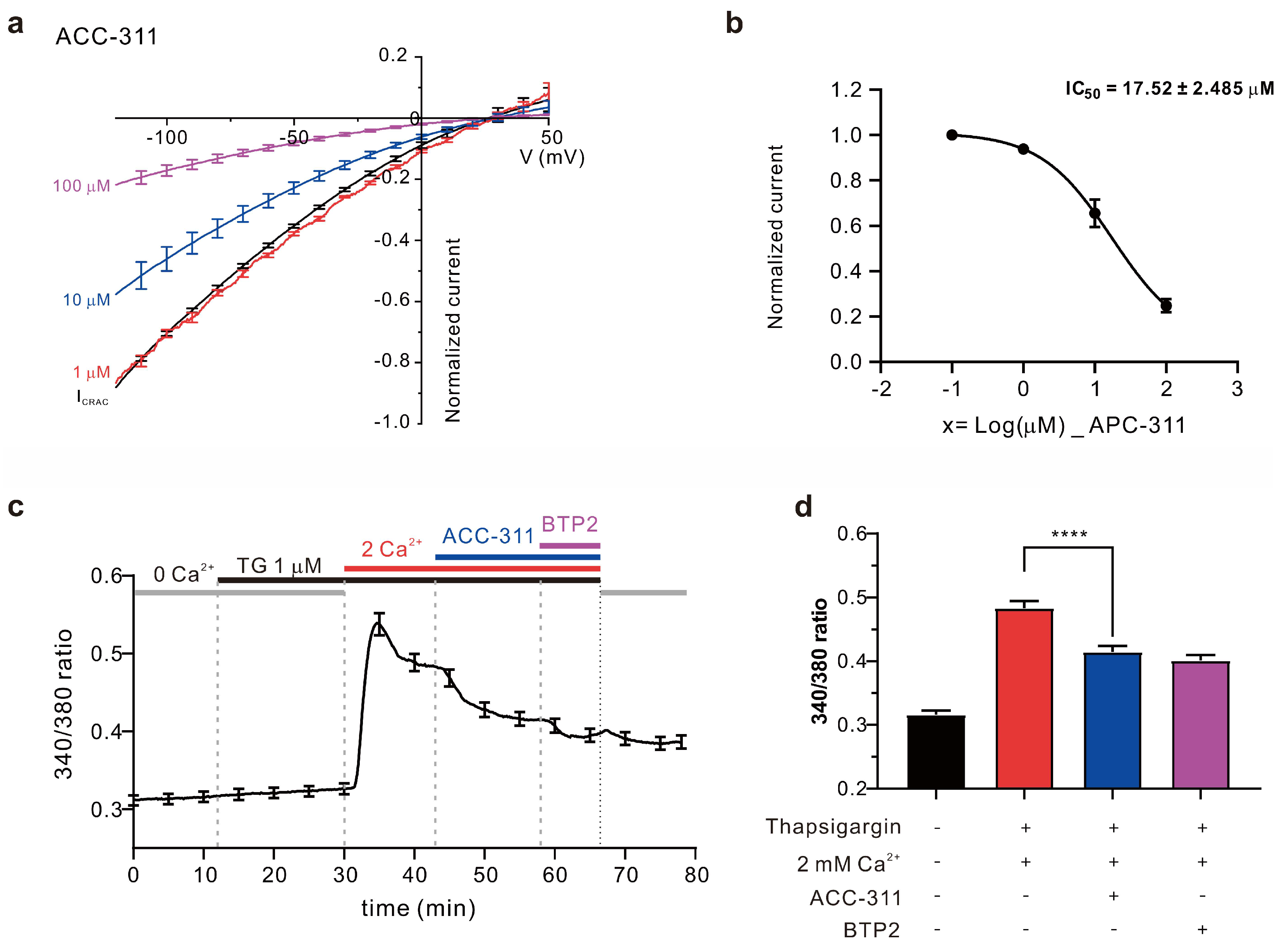
2.3. Inhibitory Effects of Active Constituents on ICRAC and Ca2+ Influx
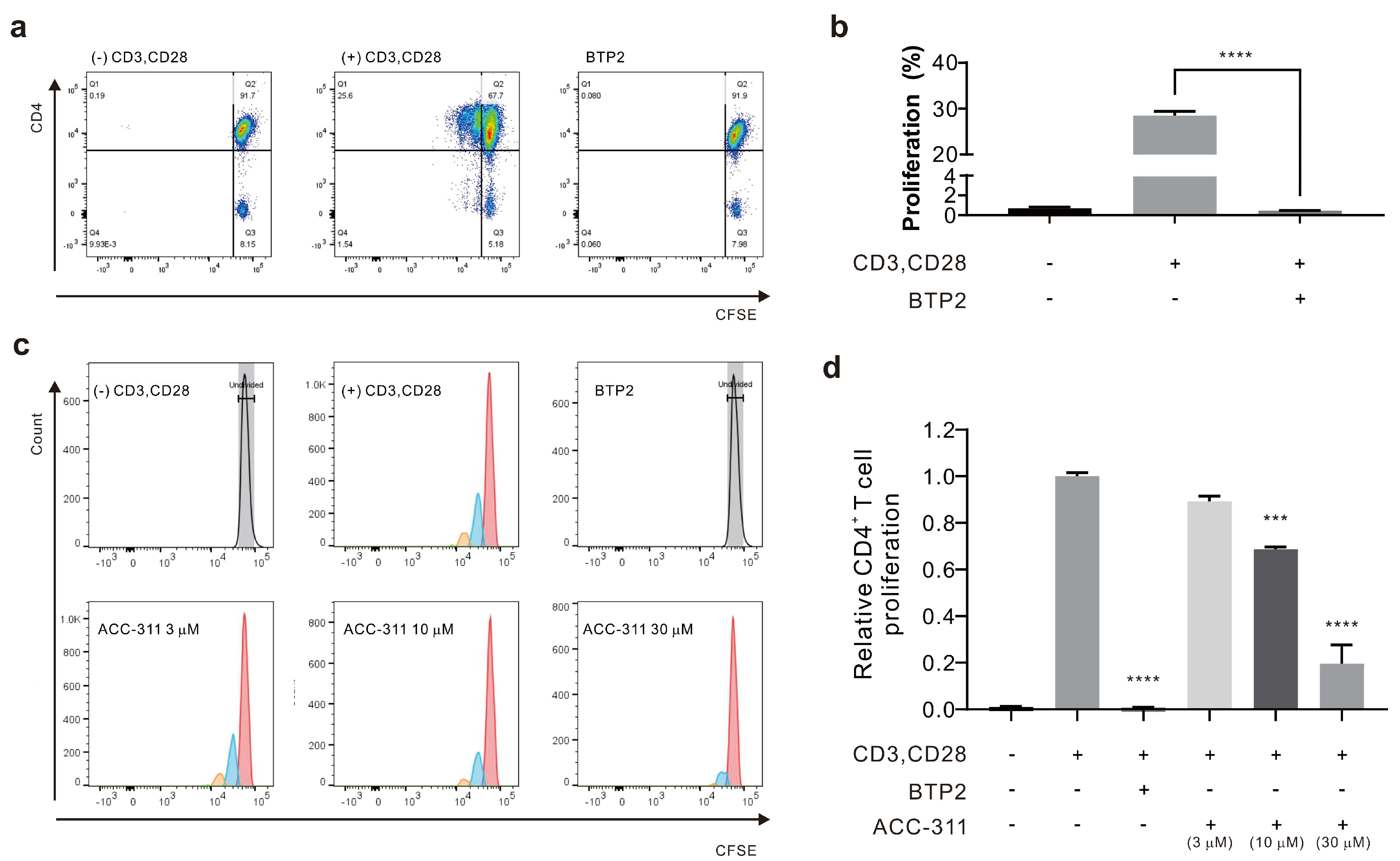
2.4. Inhibitory Effects of Active Constituents on CD4+ T Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. General Experimental Procedures
4.3. Extraction and Isolation
4.4. HPLC
4.5. Nuclear Magnetic Resonance Spectrometry
4.6. Cell Culture
4.7. Electrophysiology
4.8. Calcium Imaging
4.9. Human CD4+ T Cell Proliferation Assay
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Georas, S.N.; Guo, J.; De Fanis, U.D.; Casolaro, V. T-helper cell type-2 degulation in allergic disease. Eur. Respir. J. 2005, 26, 1119–1137. [Google Scholar] [CrossRef]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Gwack, Y.; Prakriya, M.; Srikanth, S.; Puppel, S.H.; Tanasa, B.; Hogan, P.G.; Lewis, R.S.; Daly, M.; Rao, A. A mutation in ORAI1 causes immune deficiency by abrogating CRAC channel function. Nature 2006, 441, 179–185. [Google Scholar] [CrossRef]
- Park, Y.J.; Yoo, S.A.; Kim, M.; Kim, W.U. The role of calcium–calcineurin–NFAT signaling pathway in health and autoimmune diseases. Front. Immunol. 2020, 11, 195. [Google Scholar] [CrossRef]
- Demaurex, N.; Nunes, P. The role of STIM and ORAI proteins in phagocytic immune cells. Am. J. Physiol. Cell Physiol. 2016, 310, C496–C508. [Google Scholar] [CrossRef] [PubMed]
- Ashmole, I.; Duffy, S.M.; Leyland, M.L.; Morrison, V.S.; Begg, M.; Bradding, P. CRACM/Orai ion channel expression and function in human lung mast cells. J. Allergy Clin. Immunol. 2012, 129, 1628–1635.e2. [Google Scholar] [CrossRef]
- Feske, S.; Picard, C.; Fischer, A. Immunodeficiency due to mutations in ORAI1 and STIM1. Clin. Immunol. 2010, 135, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Kappel, S.; Christine, C. Targeting CRAC channels in inflammatory bowel disease. EMBO. Mol. Med. 2022, 14, e16489. [Google Scholar] [CrossRef]
- Chalmers, S.B.; Monteith, G.R. ORAI channels and cancer. Cell Calcium 2018, 74, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Haba, S.; Katherine, N.; Chew, W.C.; Richard, F.; David, J.B.; Marc, A.B. ORAI1 Ca2+ channel as a therapeutic target in pathological vascular remodelling. Front. Cell. Dev. Biol. 2021, 9, 653812. [Google Scholar] [CrossRef]
- Khan, H.Y.; Mazahir, I.; Reddy, S.; Fazili, F.; Azmi, A. Role of CRAC channel in cancer: Implications for therapeutic development. Expert. Rev. Precis. Med. Drug. Dev. 2020, 5, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Stauderman, K.A. CRAC channels as targets for drug discovery and development. Cell Calcium 2018, 74, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Nam, Y.R.; Kim, E.J.; Nam, J.H.; Kim, W.K. Spirodela polyrhiza and its chemical constituent vitexin exert anti-allergic effect via ORAI1 channel inhibition. Am. J. Chin. Med. 2018, 46, 1243–1261. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.T.L.; Kim, H.J.; Kim, W.K.; Nam, J.H. Flos magnoliae constituent fargesin has an anti-allergic effect via ORAI1 channel inhibition. Korean J. Physiol. Pharmacol. 2021, 25, 251–258. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.; Shin, H.Y.; Nam, Y.R.; Phan, H.T.L.; Chin, Y.W.; Nam, J.H.; Kim, W.K. Inhibitory effects of α-Mangostin on T cell cytokine secretion via ORAI1 calcium channel and K+ channels inhibition. PeerJ. 2021, 9, e10973. [Google Scholar] [CrossRef]
- Shin, D.H.; Seo, E.Y.; Pang, B.; Nam, J.H.; Kim, H.S.; Kim, W.K.; Kim, S.J. Inhibition of Ca2+-release-activated Ca2+- channel (CRAC) and K+ channels by curcumin in Jurkat-T cells. J. Pharmacol. Sci. 2011, 115, 144–154. [Google Scholar] [CrossRef]
- Shi, S.; Zhao, Q.; Ke, C.; Long, S.; Zhang, F.; Zhang, X.; Li, Y.; Liu, X.; Hu, H.; Yin, S. Loureirin B exerts its immunosuppressive effects by inhibiting STIM1/Orai1 and KV1.3 channels. Front. Pharmacol. 2021, 25, 685092. [Google Scholar] [CrossRef]
- Teichert, R.W.; Olivera, B.M. Natural products and ion channel pharmacology. Future Med. Chem. 2011, 2, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Ju Eun, G.; Ho, S.H.; Jung Hee, C.; Yeo, G.; In, L. Comparison of Main Components in Agrimonia pilosa Ledebour and Agrimonia coreana Nakai by HPLC. Korean Soc. Med. Crop Sci. 2016, 2016, 103. [Google Scholar]
- Wen, S.; Zhang, X.; Wu, Y.; Yu, S.; Zhang, W.; Liu, D.; Yang, K.; Sun, J. Agrimonia pilosa Ledeb.: A review of its traditional uses, botany, phytochemistry, pharmacology, and toxicology. Heliyon 2022, 8, e09972. [Google Scholar] [CrossRef]
- Hop, N.Q.; Son, N.T. The medicinal plant Agrimonia pilosa Ledeb.: Botanical description, traditional use, phytochemistry and pharmacology. Comb. Chem. High Throughput Screen. 2023, 26, 1660–1688. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.E.; Lee, Y.G.; Kang, J.H.; Bai, Y.F.; Jeong, Y.J.; Baek, N.I.; Seo, Y.J.; Kang, S.C. Anti-viral activity of compounds from Agrimonia pilosa and Galla Rhois extract mixture. Bioorg. Chem. 2019, 93, 103320. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Park, K.E.; Kwak, Y.J.; Bae, M.K.; Bae, S.K.; Jang, I.S.; Jang, H.O. Agrimonia pilosa Ledeb root extract: Anti-inflammatory activities of the medicinal herb in LPS-induced inflammation. Am. J. Chin. Med. 2020, 48, 1875–1893. [Google Scholar] [CrossRef]
- Joo, Y.H.; Lee, Y.G.; Lim, Y.; Jeon, H.; Lee, I.G.; Cho, Y.B.; Hong, S.H.; Kim, E.H.; Choi, S.H.; Kim, J.W.; et al. Anti-influenza A virus activity by Agrimonia pilosa and Galla Rhois extract mixture. Biomed. Pharmacother. 2022, 155, 113773. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.J.; Lee, K.H.; Park, M.H.; Seong, B.L. Broad-spectrum antiviral effect of Agrimonia pilosa extract on influenza viruses. Microbiol. Immunol. 2010, 54, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Branch, G.; Burgess, D.; Dunstan, P.; Foo, L.; Green, G.; Mack, J.; Ritchie, E.; Taylor, W. Constituents of Alphitonia Species. III. Alphitexolide, a new triterpene, and other extractives. Aust. J. Chem. 1972, 25, 2209–2216. [Google Scholar] [CrossRef]
- Sakna, S.T.; Maghraby, Y.R.; Abdelfattah, M.S.; Farag, M.A. Phytochemical diversity and pharmacological effects of triterpenes from genus Ziziphus: A comprehensive review. Phytochem. Rev. 2022. [Google Scholar] [CrossRef]
- Raju, R.; Gunawardena, D.; Ahktar, M.A.; Low, M.; Reddell, P.; Münch, G. Anti-inflammatory chemical profiling of the australian rainforest tree Alphitonia Petriei (Rhamnaceae). Molecules 2016, 21, 1521. [Google Scholar] [CrossRef]
- Bai, L.-Y.; Chiu, C.-F.; Chiu, S.-J.; Chen, Y.-W.; Hu, J.-L.; Wu, C.-Y.; Weng, J.-R. Alphitolic acid, an anti-inflammatory triterpene, induces apoptosis and autophagy in oral squamous cell carcinoma cells, in part, through a p53-dependent pathway. J. Funct. Foods 2015, 18, 368–378. [Google Scholar] [CrossRef]
- Damiano, S.; Forino, M.; De, A.; Vitali, L.A.; Lupidi, G.; Taglialatela-Scafati, O. Antioxidant and antibiofilm activities of secondary metabolites from Ziziphus jujuba leaves used for infusion preparation. Food Chem. 2017, 230, 24–29. [Google Scholar] [CrossRef]
- Aguirre, M.C.; Delporte, C.; Backhouse, N.; Erazo, S.; Letelier, M.E.; Cassels, B.K.; Silva, X.; Alegría, S.; Negrete, R. Topical anti-inflammatory activity of 2α-hydroxy pentacyclic triterpene acids from the leaves of Ugni molinae. Bioorg. Med. Chem. 2006, 14, 5673–5677. [Google Scholar] [CrossRef]
- Sakurai, N.; Yaguchi, Y.; Hirakawa, T.; Nagai, M.; Inoue, T. Two myricanol glycosides from Myrica rubra and revision of the structure of isomyricanone. Phytochemistry 1991, 30, 3077–3079. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Lee, D.-U.; Nam, J.H.; Kim, W.K. Inhibitory effect of Agrimonia pilosa leaf extract on the UV-induced photoaging-related ion channel, ORAI1, and the enzymes tyrosinase and elastase. J. Food Biochem. 2016, 40, 2–9. [Google Scholar] [CrossRef]
- Thiel, M.; Wolfs, M.J.; Bauer, S.; Wenning, A.S.; Burckhart, T.; Schwarz, E.C.; Scott, A.M.; Renner, C.; Hoth, M. Efficiency of T-Cell costimulation by CD80 and CD86 cross-linking correlates with calcium entry. Immunology 2010, 129, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, E.C.; Kummerow, C.; Wenning, A.S.; Wagner, K.; Sappok, A.; Waggershauser, K.; Griesemer, D.; Strauss, B.; Wolfs, M.J.; Quintana, A.; et al. Calcium dependence of T cell proliferation following focal stimulation. Eur. J. Immunol. 2007, 37, 2723–2733. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Noyer, L.; Kahlfuss, S.; Raphael, D.; Tao, A.Y.; Kaufmann, U.; Zhu, J.; Mitchell-Flack, M.; Sidhu, I.; Zhou, F.; et al. Distinct roles of ORAI1 in T cell–mediated allergic airway inflammation and immunity to influenza A virus infection. Sci. Adv. 2022, 8, eabn6552. [Google Scholar] [CrossRef]
- Qu, B.; Al-Ansary, D.; Kummerow, C.; Hoth, M.; Schwarz, E.C. ORAI-mediated calcium influx in T cell proliferation, apoptosis and tolerance. Cell Calcium 2011, 50, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.U.; Kim, L.K.; Choi, J.M. Revisiting the concept of targeting NFAT to control T cell immunity and autoimmune diseases. Front. Immunol. 2018, 9, 2747. [Google Scholar] [CrossRef]
- Hogan, P.G. Calcium-NFAT transcriptional signalling in T cell activation and T cell exhaustion. Cell Calcium 2017, 63, 66–69. [Google Scholar] [CrossRef]
- Hermann-Kleiter, N.; Baier, G. NFAT pulls the strings during CD4+ T helper cell effector functions. Blood 2010, 115, 2989–2997. [Google Scholar] [CrossRef]
- Ruan, J.; Sun, F.; Hao, M.; Han, L.; Yu, H.; Lin, F.; Wang, L.; Cao, G.; Zhang, Y.; Wang, T. Structurally diverse triterpenes obtained from the fruits of Ziziphus jujuba Mill. as inflammation inhibitors by NF-κB signaling pathway. Food Funct. 2021, 12, 4496–4503. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]


| Code Name | Inhibition Rate (%) |
|---|---|
| Fractionalized | |
| HEX | 76.03 ± 5.654% |
| CHCl3 | 92.84 ± 1.901% |
| EA | 43.31 ± 3.439% |
| BuOH | 26.67 ± 11.930% |
| H2O | 22.49 ± 6.832% |
| Silica gel column | |
| AC-C-3 | 50.96 ± 7.190% |
| AC-C-4 | 29.77 ± 4.434% |
| AC-C-5 | 21.65 ± 11.199% |
| AC-C-6 | 53.98 ± 9.776% |
| AC-C-7 | 63.91 ± 9.654% |
| Sephadex LH-20 | |
| AC-C-3-1 | 18.16 ± 0.360% |
| AC-C-3-2 | 20.19 ± 8.809% |
| AC-C-3-3 | 25.40 ± 1.414% |
| AC-C-3-4 | 19.06 ± 6.094% |
| AC-C-3-5 | 17.21 ± 0.292% |
| ODS-MPLC | |
| MPLC1 | 58.32 ± 4.681% |
| MPLC2 | 38.36 ± 4.840% |
| MPLC3 | 12.04 ± 2.072% |
| MPLC4 | 81.86 ± 8.631% |
| MPLC5 | 18.22 ± 2.106% |
| No. | Alphitolic Acid (1) | |
|---|---|---|
| δC | δH | |
| 1 | 48.4 | 1.99 (m), 0.83 (m) |
| 2 | 69.8 | 3.60 (ddd, 11.5, 9.5, 5.0) |
| 3 | 84.4 | 2.88 (d, 9.5) |
| 4 | 40.5 | |
| 5 | 56.8 | 0.79 (m) |
| 6 | 19.5 | 1.53 (m), 1.42 (m) |
| 7 | 35.5 | 1.44 (m), 1.38 (m) |
| 8 | 42.0 | |
| 9 | 52.0 | 1.38 (m) |
| 10 | 39.5 | |
| 11 | 22.2 | 1.45 (m), 1.29 (m) |
| 12 | 26.8 | 1.73 (m), 1.07 (m) |
| 13 | 39.6 | 2.33 (td, 11.5, 9.0) |
| 14 | 43.6 | |
| 15 | 30.8 | 1.54 (m), 1.16 (m) |
| 16 | 33.4 | 2.23 (d, 13.0), 1.41 (m) |
| 17 | 50.5 | |
| 18 | 39.6 | 1.61 (m) |
| 19 | 48.5 | 3.03 (m) |
| 20 | 152.1 | |
| 21 | 31.7 | 1.94 (m), 1.38 (m) |
| 22 | 38.2 | 1.89 (m), 1.42 (m) |
| 23 | 29.1 | 0.98 (s) |
| 24 | 17.2 | 0.77 (s) |
| 25 | 17.9 | 0.91 (s) |
| 26 | 16.7 | 0.96 (s) |
| 27 | 15.1 | 1.00 (s) |
| 28 | 180.4 | |
| 29 | 110.1 | 4.70 (d, 2.0), 4.58 (d, 2.0) |
| 30 | 19.6 | 1.69 (s) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.J.; Lee, J.S.; Nam, Y.R.; Lee, J.M.; Ki, D.-W.; Yun, B.-S.; Choi, S.W.; Van, N.T.H.; Nam, J.H.; Kim, H.J.; et al. Anti-Inflammatory Effects of Alphitolic Acid Isolated from Agrimonia coreana Nakai Extracts Are Mediated via the Inhibition of ICRAC Activity in T Cells. Int. J. Mol. Sci. 2023, 24, 17309. https://doi.org/10.3390/ijms242417309
Park SJ, Lee JS, Nam YR, Lee JM, Ki D-W, Yun B-S, Choi SW, Van NTH, Nam JH, Kim HJ, et al. Anti-Inflammatory Effects of Alphitolic Acid Isolated from Agrimonia coreana Nakai Extracts Are Mediated via the Inhibition of ICRAC Activity in T Cells. International Journal of Molecular Sciences. 2023; 24(24):17309. https://doi.org/10.3390/ijms242417309
Chicago/Turabian StylePark, Su Jin, Jin Seok Lee, Yu Ran Nam, Ji Min Lee, Dae-Won Ki, Bong-Sik Yun, Seong Woo Choi, Nhung Thi Hong Van, Joo Hyun Nam, Hyun Jong Kim, and et al. 2023. "Anti-Inflammatory Effects of Alphitolic Acid Isolated from Agrimonia coreana Nakai Extracts Are Mediated via the Inhibition of ICRAC Activity in T Cells" International Journal of Molecular Sciences 24, no. 24: 17309. https://doi.org/10.3390/ijms242417309






