Genome-Wide Identification, Characterization, and Expression Analysis of the Copper-Containing Amine Oxidase Gene Family in Mangrove Kandelia obovata
Abstract
:1. Introduction
2. Results
2.1. Identification of CuAO Family Members in Kandelia obovata
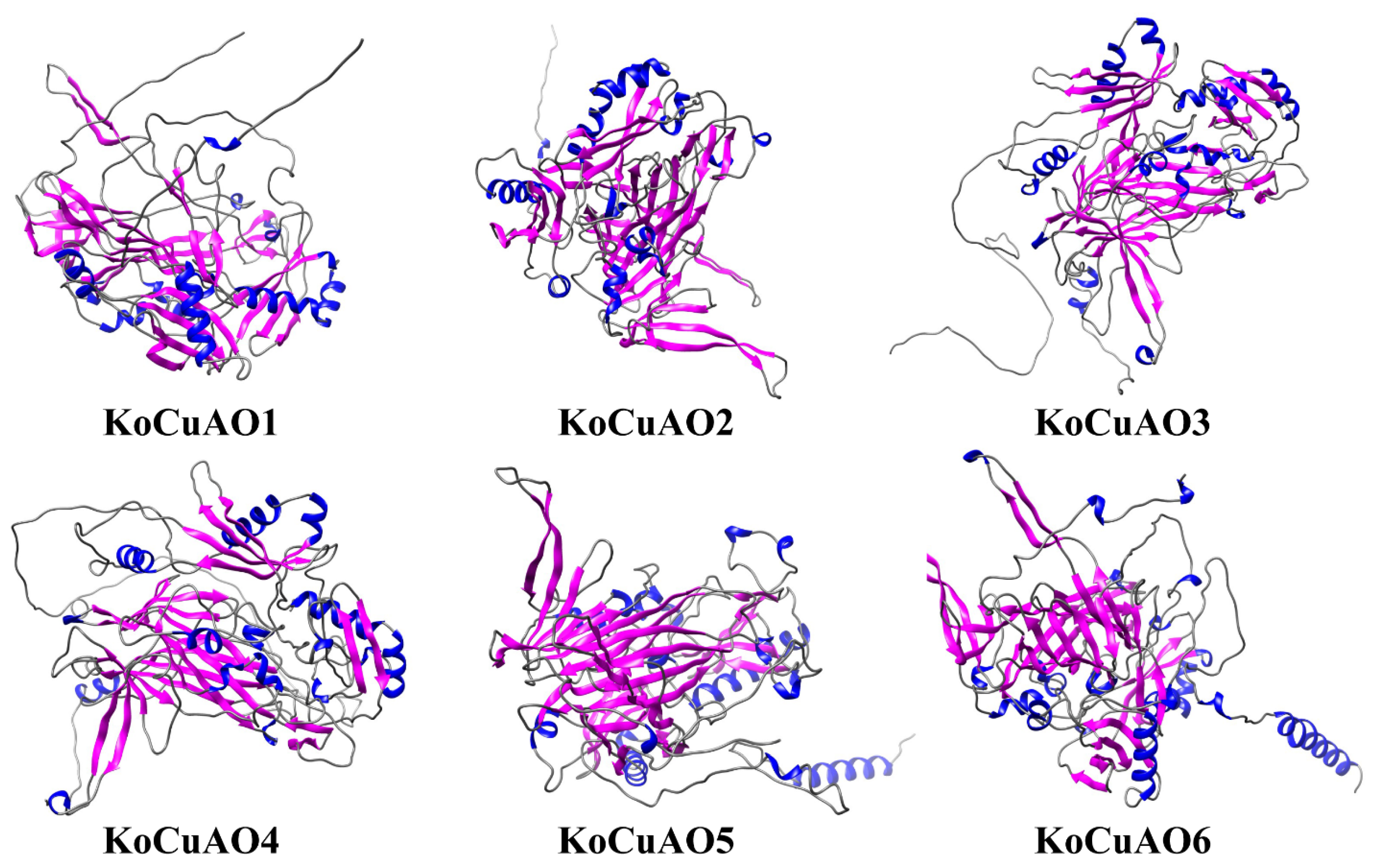
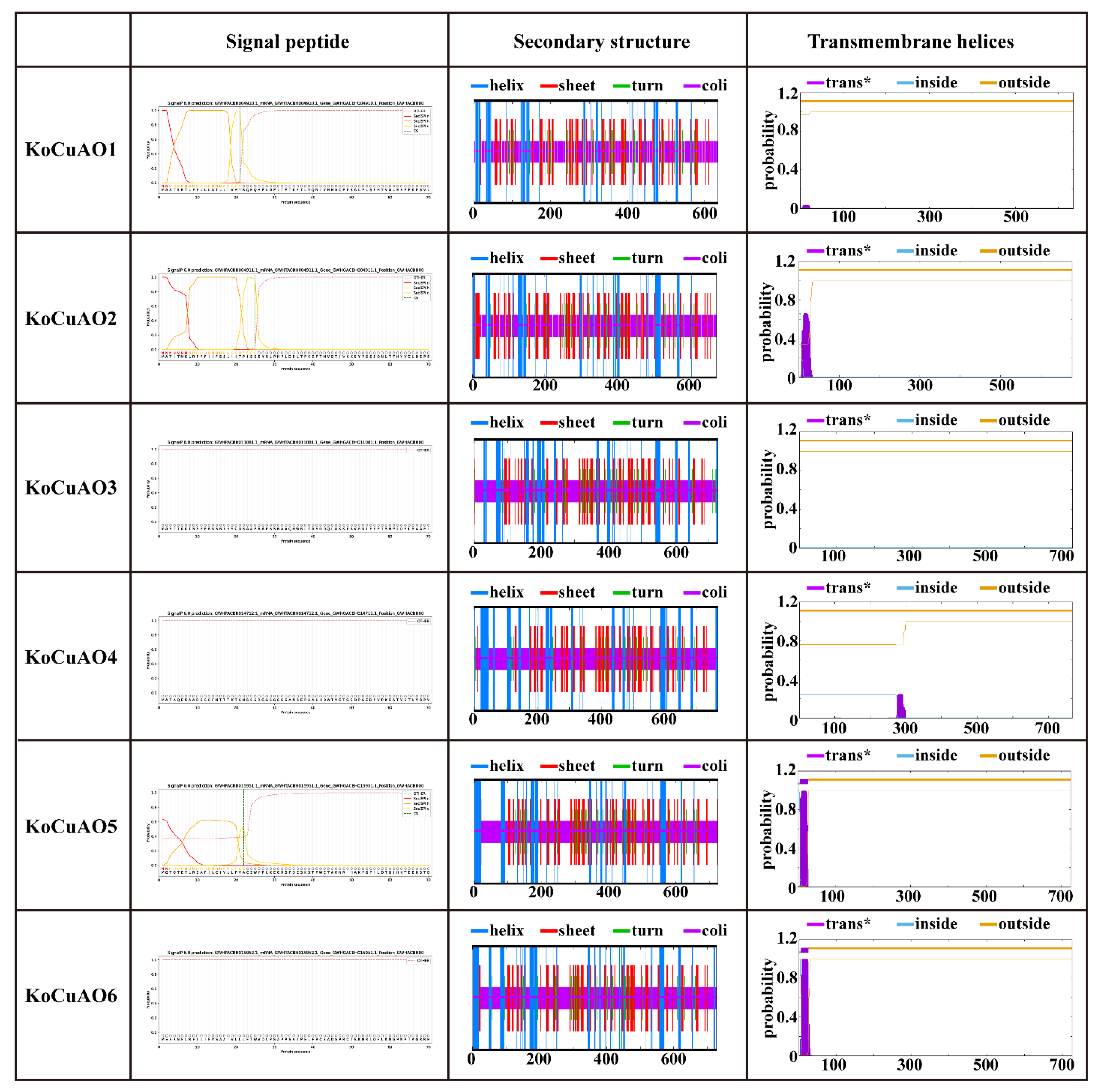
2.2. Diversity within the CuAO Family in Relation to Domain and Three-Dimensional Structure
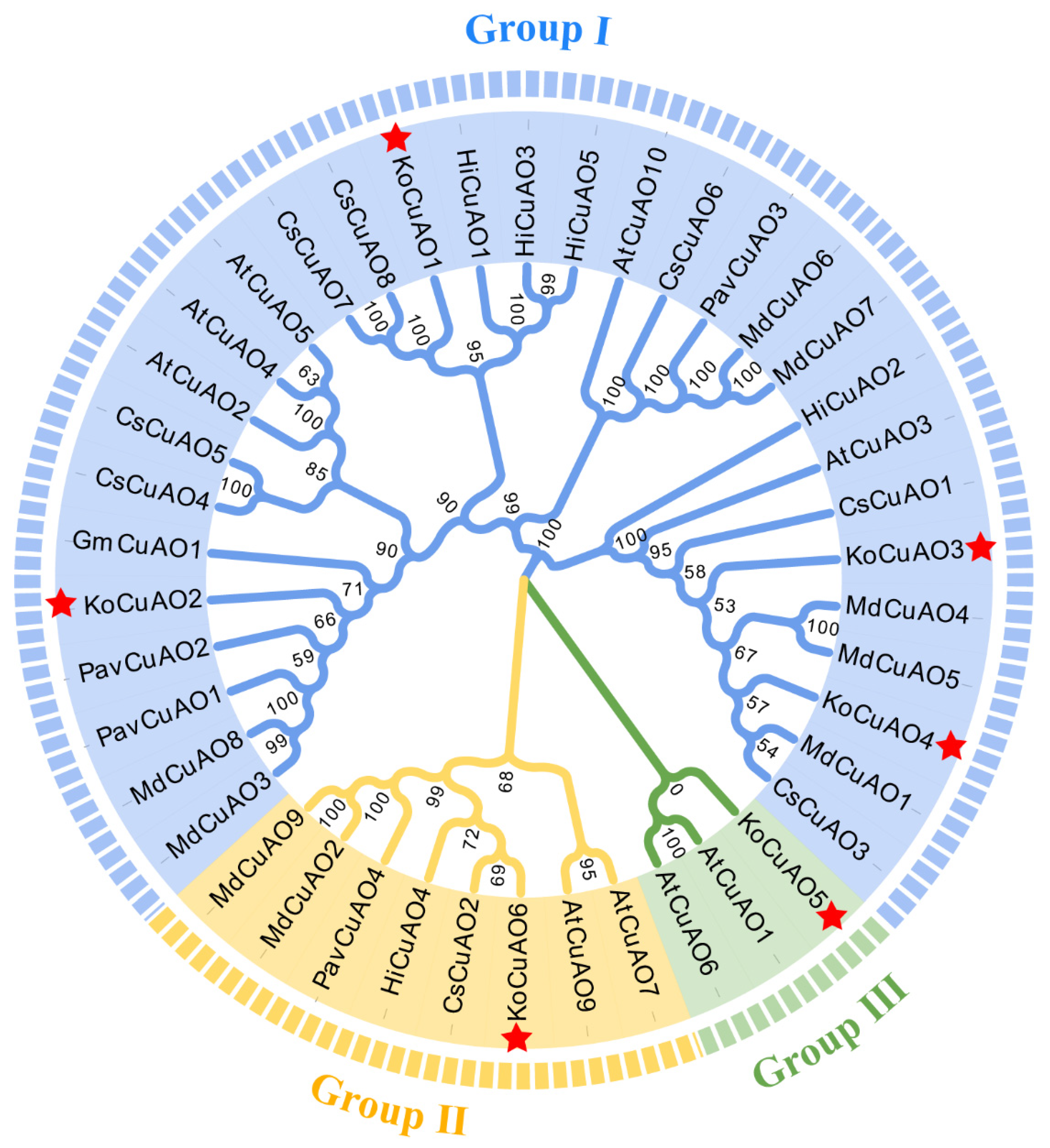
2.3. CuAO Protein Phylogenetic Relationships
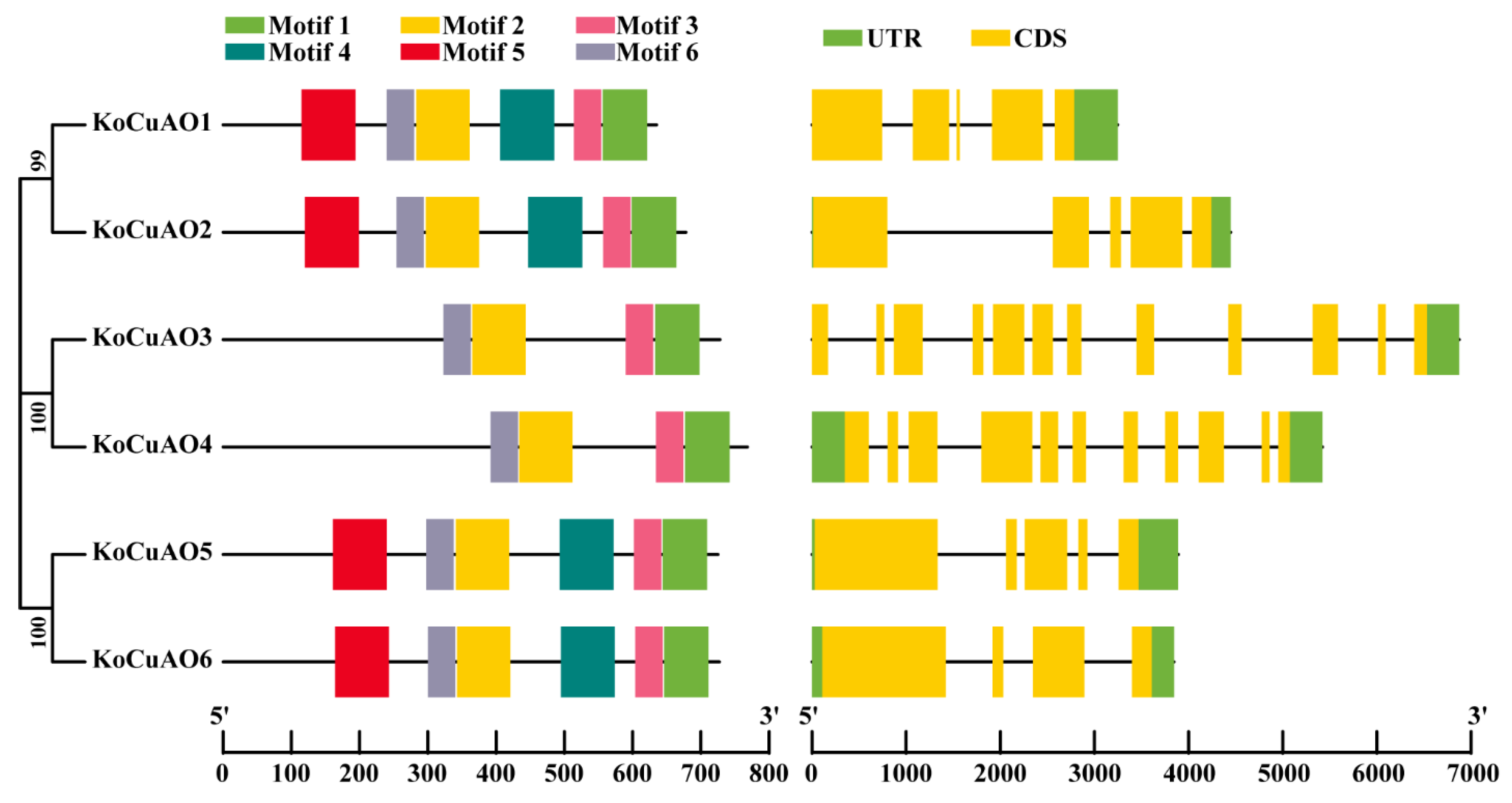
2.4. Investigation of the Genetic Structure and Conserved Features of CuAO Genes
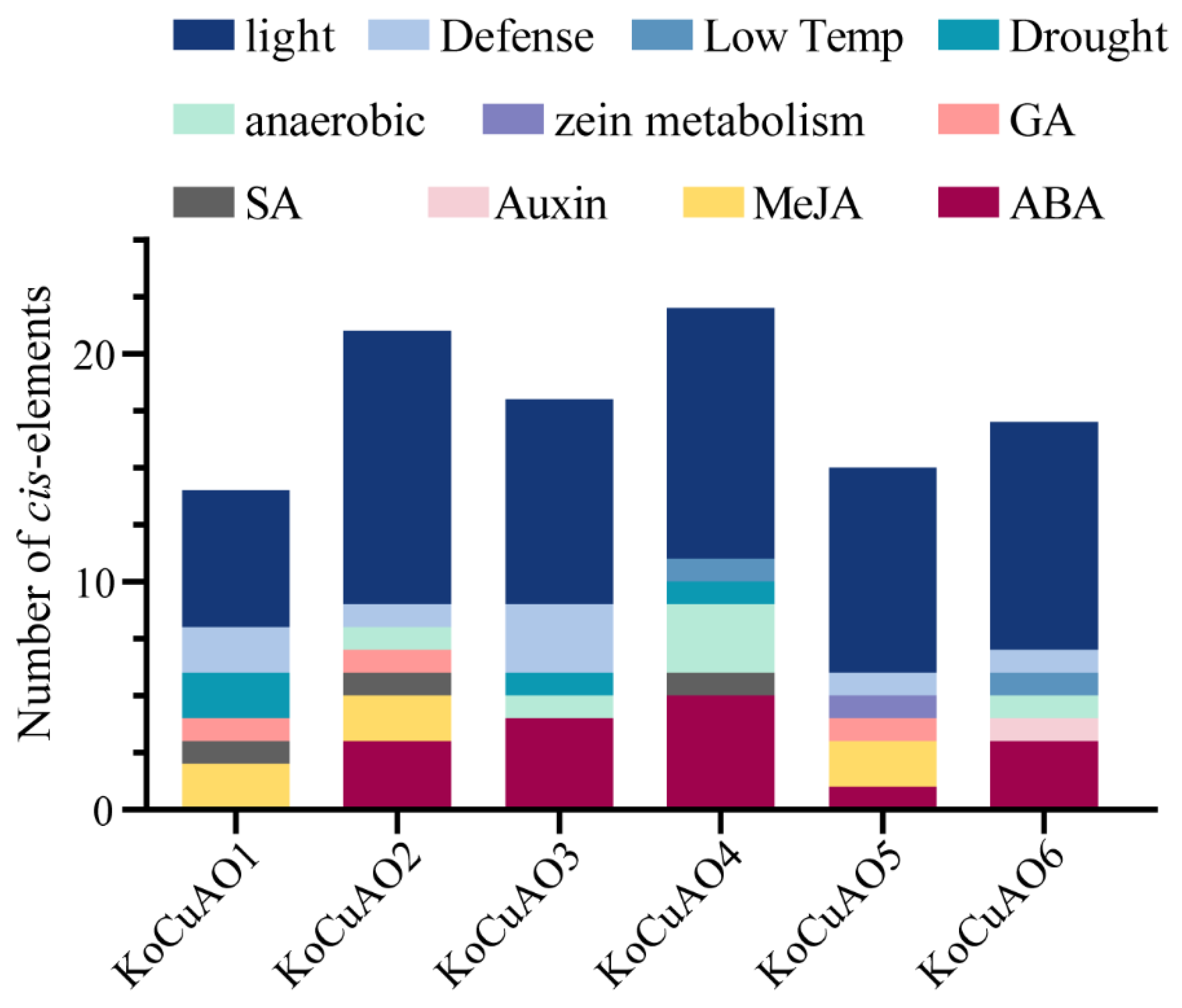
2.5. Cis-Regulatory Elements in the Promoters of Six CuAO Genes
2.6. Duplication Analysis of CuAO Gene Family
2.7. Expression Analysis of KoCuAO Genes under Copper Treatment
3. Discussion
4. Materials and Methods
4.1. Characterization and Identification of the CuAO Genes in Kandelia obovata
4.2. Chromosomal Distribution
4.3. Phylogenetic Tree Construction
4.4. Gene Structure and Significant Motif Analyses
4.5. Duplication Analysis
4.6. Cis-Regulatory Elements (CREs)
4.7. Three-Dimensional Structure and Subcellular Localization
4.8. Plant Material and Environmental Conditions
4.9. Quantitative Real-Time PCR Assays
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zakaria, M.M.; Stegemann, T.; Sievert, C.; Kruse, L.H.; Kaltenegger, E.; Girreser, U.; Serhat, S.C. Insights into Polyamine Metabolism: Homospermidine Is Double-Oxidized in Two Discrete Steps by a Single Copper-Containing Amine Oxidase in Pyrrolizidine Alkaloid Biosynthesis. Plant Cell 2022, 34, 2364–2382. [Google Scholar] [CrossRef]
- Galston, A.W.; Sawhney, R.K. Polyamines in Plant Physiology. Plant Physiol. 1990, 94, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of Amine Oxidases in Plant Development and Defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Wen, Z.; Shang, C.; Cai, X.; Hou, Q.; Qiao, G. Copper Amine Oxidase (CuAO)-Mediated Polyamine Catabolism Plays Potential Roles in Sweet Cherry (Prunus Avium L.) Fruit Development and Ripening. Int. J. Mol. Sci. 2022, 23, 12112. [Google Scholar] [CrossRef]
- Wang, W.; Wu, H.; Liu, J.H. Genome-Wide Identification and Expression Profiling of Copper-Containing Amine Oxidase Genes in Sweet Orange (Citrus Sinensis). Tree Genet. Genomes 2017, 13, 31. [Google Scholar] [CrossRef]
- Planas-Portell, J.; Gallart, M.; Tiburcio, A.F.; Altabella, T. Copper-Containing Amine Oxidases Contribute to Terminal Polyamine Oxidation in Peroxisomes and Apoplast of Arabidopsis Thaliana. BMC Plant Biol. 2013, 13, 109. [Google Scholar] [CrossRef]
- Zarei, A.; Trobacher, C.P.; Cooke, A.R.; Meyers, A.J.; Hall, J.C.; Shelp, B.J. Apple Fruit Copper Amine Oxidase Isoforms: Peroxisomal MdAO1 Prefers Diamines as Substrates, Whereas Extracellular MdAO2 Exclusively Utilizes Monoamines. Plant Cell Physiol. 2015, 56, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Medda, R.; Padiglia, A.; Floris, G. Plant Copper-Amine Oxidases. Phytochemistry 1995, 39, 1–9. [Google Scholar] [CrossRef]
- Di Paolo, M.L.; Lunelli, M.; Fuxreiter, M.; Rigo, A.; Simon, I.; Scarpa, M. Active Site Residue Involvement in Monoamine or Diamine Oxidation Catalysed by Pea Seedling Amine Oxidase. FEBS J. 2011, 278, 1232–1243. [Google Scholar] [CrossRef]
- Nizam, A.; Meera, S.P.; Kumar, A. Genetic and Molecular Mechanisms Underlying Mangrove Adaptations to Intertidal Environments. iScience 2022, 25, 103547. [Google Scholar] [CrossRef]
- Krauss, K.W.; Mckee, K.L.; Lovelock, C.E.; Cahoon, D.R.; Saintilan, N.; Reef, R.; Chen, L. How Mangrove Forests Adjust to Rising Sea Level. New Phytol. 2014, 202, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.J.; Sun, W.H.; Tsai, W.C.; Xiang, S.; Lai, X.K.; Chen, D.Q.; Liu, X.D.; Wang, Y.F.; Le, Y.X.; Chen, S.M.; et al. Chromosome-Scale Assembly of the Kandelia Obovata Genome. Hortic. Res. 2020, 7, 75. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.M.; Liu, X.; Huang, X.J.; Yang, J.J.; Qin, P.T.; Zhou, H.; Jiang, M.G.; Liao, H.Z. Genome-Wide Identification and Expression Analysis of the NAC Gene Family in Kandelia Obovata, a Typical Mangrove Plant. Curr. Issues Mol. Biol. 2022, 44, 5622–5637. [Google Scholar] [CrossRef] [PubMed]
- Sheue, C.R.; Liu, H.Y.; Yong, J.W.H. Kandelia Obovata (Rhizophoraceae), a New Mangrove Species from Eastern Asia. Taxon 2003, 52, 287–294. [Google Scholar] [CrossRef]
- Giri, C.; Ochieng, E.; Tieszen, L.L.; Zhu, Z.; Singh, A.; Loveland, T.; Masek, J.; Duke, N. Status and Distribution of Mangrove Forests of the World Using Earth Observation Satellite Data. Glob. Ecol. Biogeogr. 2011, 20, 154–159. [Google Scholar] [CrossRef]
- Wardiatno, Y.; Mardiansyah; Prartono, T.; Tsuchiya, M. Possible Food Sources of Macrozoobenthos in the Manko Mangrove Ecosystem, Okinawa (Japan): A Stable Isotope Analysis Approach. Trop. Life Sci. Res. 2015, 26, 53–65. [Google Scholar] [PubMed]
- Zhou, Q.; Tu, C.; Fu, C.; Li, Y.; Zhang, H.; Xiong, K.; Zhao, X.; Li, L.; Waniek, J.J.; Luo, Y. Characteristics and Distribution of Microplastics in the Coastal Mangrove Sediments of China. Sci. Total Environ. 2020, 703, 134807. [Google Scholar] [CrossRef]
- Li, T.; Ye, Y. Dynamics of Decomposition and Nutrient Release of Leaf Litter in Kandelia Obovata Mangrove Forests with Different Ages in Jiulongjiang Estuary, China. Ecol. Eng. 2014, 73, 454–460. [Google Scholar] [CrossRef]
- Wang, C.W.; Wong, S.L.; Liao, T.S.; Weng, J.H.; Chen, M.N.; Huang, M.Y.; Chen, C.I. Photosynthesis in Response to Salinity and Submergence in Two Rhizophoraceae Mangroves Adapted to Different Tidal Elevations. Tree Physiol. 2022, 42, 1016–1028. [Google Scholar] [CrossRef]
- Shen, X.; Li, R.; Chai, M.; Cheng, S.; Tam, N.F.Y.; Han, J. Does Combined Heavy Metal Stress Enhance Iron Plaque Formation and Heavy Metal Bioaccumulation in Kandelia Obovata? Environ. Exp. Bot. 2021, 186, 104463. [Google Scholar] [CrossRef]
- Bodin, N.; N’Gom-Kâ, R.; Kâ, S.; Thiaw, O.T.; Tito de Morais, L.; Le Loc’h, F.; Rozuel-Chartier, E.; Auger, D.; Chiffoleau, J.F. Assessment of Trace Metal Contamination in Mangrove Ecosystems from Senegal, West Africa. Chemosphere 2013, 90, 150–157. [Google Scholar] [CrossRef]
- Abubakar, U.S.; Zulkifli, S.Z.; Ismail, A. Heavy Metals Bioavailability and Pollution Indices Evaluation in the Mangrove Surface Sediment of Sungai Puloh, Malaysia. Environ. Earth Sci. 2018, 77, 225. [Google Scholar] [CrossRef]
- Cheng, S.; Tam, N.F.Y.; Li, R.; Shen, X.; Niu, Z.; Chai, M.; Qiu, G.Y. Temporal Variations in Physiological Responses of Kandelia Obovata Seedlings Exposed to Multiple Heavy Metals. Mar. Pollut. Bull. 2017, 124, 1089–1095. [Google Scholar] [CrossRef]
- Hu, Z.; Wenjiao, Z. Effects of Zinc Stress on Growth and Antioxidant Enzyme Responses of Kandelia Obovata Seedlings. Toxicol. Environ. Chem. 2015, 97, 1190–1201. [Google Scholar] [CrossRef]
- Shen, X.; Li, R.; Chai, M.; Cheng, S.; Niu, Z.; Qiu, G.Y. Interactive Effects of Single, Binary and Trinary Trace Metals (Lead, Zinc and Copper) on the Physiological Responses of Kandelia Obovata Seedlings. Environ. Geochem. Health 2019, 41, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.Y.; Wang, Y.S. Physiological and Biochemical Responses in the Leaves of Two Mangrove Plant Seedlings (Kandelia Candel and Bruguiera Gymnorrhiza) Exposed to Multiple Heavy Metals. J. Hazard. Mater. 2010, 182, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Li, X.; Xiao, J.; Wang, S. Molecular and Functional Analyses of COPT/Ctr-Type Copper Transporter-like Gene Family in Rice. BMC Plant Biol. 2011, 11, 69. [Google Scholar] [CrossRef]
- Hu, Z.; Fu, Q.; Zheng, J.; Zhang, A.; Wang, H. Transcriptomic and Metabolomic Analyses Reveal That Melatonin Promotes Melon Root Development under Copper Stress by Inhibiting Jasmonic Acid Biosynthesis. Hortic. Res. 2020, 7, 79. [Google Scholar] [CrossRef]
- Cano-Gauci, D.F.; Sarkar, B. Reversible Zinc Exchange between Metallothionein and the Estrogen Receptor Zinc Finger. FEBS Lett. 1996, 386, 1–4. [Google Scholar] [CrossRef]
- Hussain, Q.; Ye, T.; Nkoh, N.J.; Zhou, Q.; Shang, C. Genome-Wide Identification and Expression Analysis of the Copper Transporter (COPT/Ctr) Gene Family in Kandelia Obovata, a Typical Mangrove Plant. Int. J. Mol. Sci. 2023, 24, 15579. [Google Scholar] [CrossRef]
- Peñarrubia, L.; Andrés-Colás, N.; Moreno, J.; Puig, S. Regulation of Copper Transport in Arabidopsis Thaliana: A Biochemical Oscillator? J. Biol. Inorg. Chem. 2010, 15, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Burkhead, J.L.; Gogolin Reynolds, K.A.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper Homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.W.; Yu, K.F.; Zhang, G.; Wang, W.X. Accumulation and Partitioning of Seven Trace Metals in Mangroves and Sediment Cores from Three Estuarine Wetlands of Hainan Island, China. J. Hazard. Mater. 2011, 190, 631–638. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, R.; Chai, M.; Shen, X.; Xu, H.; Qiu, G. Heavy Metal Contamination and Ecological Risk in Futian Mangrove Forest Sediment in Shenzhen Bay, South China. Mar. Pollut. Bull. 2015, 101, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.; Li, R.; Shen, X.; Yu, L.; Han, J. Multiple Heavy Metals Affect Root Response, Iron Plaque Formation, and Metal Bioaccumulation of Kandelia Obovata. Sci. Rep. 2022, 12, 14389. [Google Scholar] [CrossRef]
- Fraudentali, I.; Rodrigues-pousada, R.A.; Angelini, R.; Ghuge, S.A.; Cona, A. Plant Copper Amine Oxidases: Key Players in Hormone Signaling Leading to Stress-Induced Phenotypic Plasticity. Int. J. Mol. Sci. 2021, 22, 5136. [Google Scholar] [CrossRef] [PubMed]
- Delis, C.; Dimou, M.; Flemetakis, E.; Aivalakis, G.; Katinakis, P. A Root- and Hypocotyl-Specific Gene Coding for Copper-Containing Amine Oxidase Is Related to Cell Expansion in Soybean Seedlings. J. Exp. Bot. 2006, 57, 101–111. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Wang, W.; Li, D.; Hu, X.; Wang, H.; Wei, M.; Liu, Q.; Wang, Z.; Li, C. Genome-Wide Identification and Expression Profiling of the Copper Transporter Gene Family in Populus Trichocarpa. Plant Physiol. Biochem. 2015, 97, 451–460. [Google Scholar] [CrossRef]
- Hanada, K.; Zou, C.; Lehti-Shiu, M.D.; Shinozaki, K.; Shiu, S.H. Importance of Lineage-Specific Expansion of Plant Tandem Duplicates in the Adaptive Response to Environmental Stimuli. Plant Physiol. 2008, 148, 993–1003. [Google Scholar] [CrossRef]
- Yaghobi, M.; Heidari, P. Genome-Wide Analysis of Aquaporin Gene Family in Triticum Turgidum and Its Expression Profile in Response to Salt Stress. Genes. 2023, 14, 202. [Google Scholar] [CrossRef]
- Hashemipetroudi, S.H.; Arab, M.; Heidari, P.; Kuhlmann, M. Genome-Wide Analysis of the Laccase (LAC) Gene Family in Aeluropus Littoralis: A Focus on Identi Fi Cation, Evolution and Expression Patterns in Response to Abiotic Stresses and ABA Treatment. Front. Plant Sci. 2023, 14, 1112354. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Lyu, S.; Hussain, Q.; Ye, H.; Huang, C.; Li, Y.; Huang, J.; Chen, J.; Wang, K. Analysis of Delta(9) Fatty Acid Desaturase Gene Family and Their Role in Oleic Acid Accumulation in Carya Cathayensis Kernel. Front. Plant Sci. 2023, 14, 1193063. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Q.; Zheng, M.; Chang, W.; Ashraf, M.F.; Khan, R.; Asim, M.; Riaz, M.W.; Alwahibi, M.S.; Elshikh, M.S.; Zhang, R.; et al. Genome-Wide Identification and Expression Analysis of SnRK2 Gene Family in Dormant Vegetative Buds of Liriodendron Chinense in Response to Abscisic Acid, Chilling, and Photoperiod. Genes. 2022, 13, 1305. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Ye, T.; Zhou, Q.; Chen, P.; Li, X.; Li, W.; Chen, S.; Hu, Z.; Zhang, W. Genome-Wide Identification and Bioinformatics Analyses of Host Defense Peptides Snakin/GASA in Mangrove Plants. Genes 2023, 14, 923. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Q.; Zheng, M.; Furqan, M.; Khan, R.; Yasir, M.; Farooq, S.; Zhang, R.; Wu, J. Scientia Horticulturae Genome-Wide Identification, Characterization and Expression Analysis of the ABA Receptor PYL Gene Family in Response to ABA, Photoperiod, and Chilling in Vegetative Buds of Liriodendron Chinense. Sci. Hortic. 2022, 303, 111200. [Google Scholar] [CrossRef]
- Sajjad, M.; Ahmad, A.; Riaz, M.W.; Hussain, Q.; Yasir, M.; Lu, M. Recent Genome Resequencing Paraded COBRA-Like Gene Family Roles in Abiotic Stress and Wood Formation in Poplar. Front. Plant Sci. 2023, 14, 1242836. [Google Scholar] [CrossRef]
- Liao, J.; Xu, Y.; Zhang, Z.; Zeng, L.; Qiao, Y.; Guo, Z.; Chen, J.; Jia, B.; Shang, C.; Chen, S. Effect of Cu Addition on Sedimentary Bacterial Community Structure and Heavy Metal Resistance Gene Abundance in Mangrove Wetlands. Front. Mar. Sci. 2023, 10, 1157905. [Google Scholar] [CrossRef]




| Name | Gene ID | Location | AA 1 | Chains 2 | MW 3/kDd | pI 4 | GRAVY 5 | Subcellular (WoLF PSORT) | Subcellular (Plant-Ploc) |
|---|---|---|---|---|---|---|---|---|---|
| KoCuAO1 | geneMaker00012803 | Chr04 2370575-2373822 | 159 | + | 17.09 | 9.72 | 0.309 | Chloroplast | Cytoplasm |
| KoCuAO2 | geneMaker00012751 | Chr04 2377019-2381467 | 106 | - | 11.18 | 8.71 | 0.498 | Cytoskeleton | Cytoplasm |
| KoCuAO3 | geneMaker00008448 | Chr09 2334407-2341281 | 145 | - | 15.64 | 6.82 | 0.566 | Peroxisome | Chloroplast |
| KoCuAO4 | geneMaker00006577 | Chr13 1070360-1075782 | 133 | - | 14.78 | 8.82 | 0.805 | Peroxisome | Chloroplast |
| KoCuAO5 | geneMaker00006577 | Chr15 1433543-1437433 | 133 | - | 14.78 | 8.82 | 0.805 | Peroxisome | Chloroplast |
| KoCuAO6 | geneMaker00006577 | Chr15 1441486-1445331 | 133 | - | 14.78 | 8.82 | 0.805 | Vacuole | Chloroplast |
| Name | Method | Ka | Ks | Ka/Ks | Divergence-Time (MYA) | Duplicated Type |
|---|---|---|---|---|---|---|
| KoCuAO1 and KoCuAO2 | MS | 0.97 | 1.13 | 0.86 | 37.56 | tandem |
| KoCuAO5 and KoCuAO6 | MS | 0.99 | 1.03 | 0.97 | 34.17 | tandem |
| KoCuAO3 and KoCuAO4 | MS | 1.00 | 1.01 | 0.99 | 33.67 | segmental |
| Gene Name | Primer Name | Sequence (5’-3’) | Length | Tm | GC% | Product Length |
|---|---|---|---|---|---|---|
| KoCuAO1 | 1-F | GTCAACCACAGCCCTACCTC | 20 | 60.04 | 60 | 165 |
| 1-R | GTGGCTGCTGTTTGCTCTTC | 20 | 60.04 | 55 | ||
| KoCuAO2 | 2-F | TCAGAACCCACCATCCAAGC | 20 | 59.96 | 55 | 223 |
| 2-R | TGCCTCTGTTTTCAGCCGAT | 20 | 59.96 | 50 | ||
| KoCuAO3 | 3-F | TGTCGCATCTCAACGAAGCA | 20 | 60.32 | 50 | 143 |
| 3-F | CAAGTCCCCAAGGCTGCATA | 20 | 60.03 | 55 | ||
| KoCuAO4 | 4-F | GTGTCTGTTTGCACGAAGAGG | 22 | 59.4 | 54.5 | 193 |
| 4-R | TTCACCTCAACAACTGCCAT | 22 | 59.5 | 59.1 | ||
| KoCuAO5 | 5-F | CACTCGTTGTCACTGGACGA | 20 | 59.97 | 55 | 92 |
| 5-R | CCGATCACTTGGGCTTTCCT | 20 | 60.04 | 55 | ||
| KoCuAO6 | 6-F | TCACACGGACTCGTTCACAG | 20 | 59.97 | 55 | 157 |
| 6-R | AGACAATCGCTGCACTCACA | 20 | 59.97 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, Q.; Ye, T.; Shang, C.; Li, S.; Nkoh, J.N.; Li, W.; Hu, Z. Genome-Wide Identification, Characterization, and Expression Analysis of the Copper-Containing Amine Oxidase Gene Family in Mangrove Kandelia obovata. Int. J. Mol. Sci. 2023, 24, 17312. https://doi.org/10.3390/ijms242417312
Hussain Q, Ye T, Shang C, Li S, Nkoh JN, Li W, Hu Z. Genome-Wide Identification, Characterization, and Expression Analysis of the Copper-Containing Amine Oxidase Gene Family in Mangrove Kandelia obovata. International Journal of Molecular Sciences. 2023; 24(24):17312. https://doi.org/10.3390/ijms242417312
Chicago/Turabian StyleHussain, Quaid, Ting Ye, Chenjing Shang, Sihui Li, Jackson Nkoh Nkoh, Wenyi Li, and Zhangli Hu. 2023. "Genome-Wide Identification, Characterization, and Expression Analysis of the Copper-Containing Amine Oxidase Gene Family in Mangrove Kandelia obovata" International Journal of Molecular Sciences 24, no. 24: 17312. https://doi.org/10.3390/ijms242417312
APA StyleHussain, Q., Ye, T., Shang, C., Li, S., Nkoh, J. N., Li, W., & Hu, Z. (2023). Genome-Wide Identification, Characterization, and Expression Analysis of the Copper-Containing Amine Oxidase Gene Family in Mangrove Kandelia obovata. International Journal of Molecular Sciences, 24(24), 17312. https://doi.org/10.3390/ijms242417312










