Tartary Buckwheat (Fagopyrum tataricum) FtTT8 Inhibits Anthocyanin Biosynthesis and Promotes Proanthocyanidin Biosynthesis
Abstract
:1. Introduction
2. Results
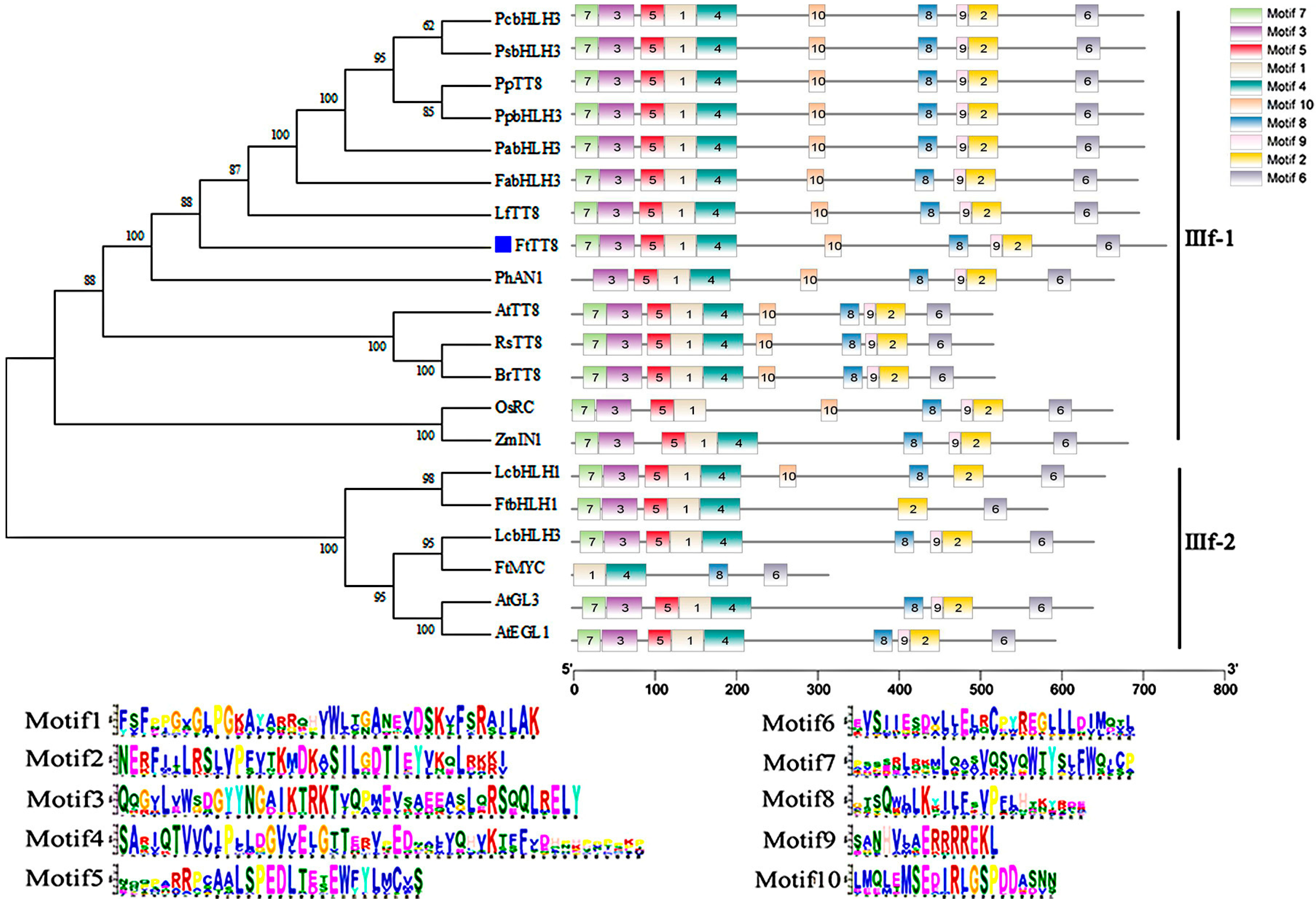
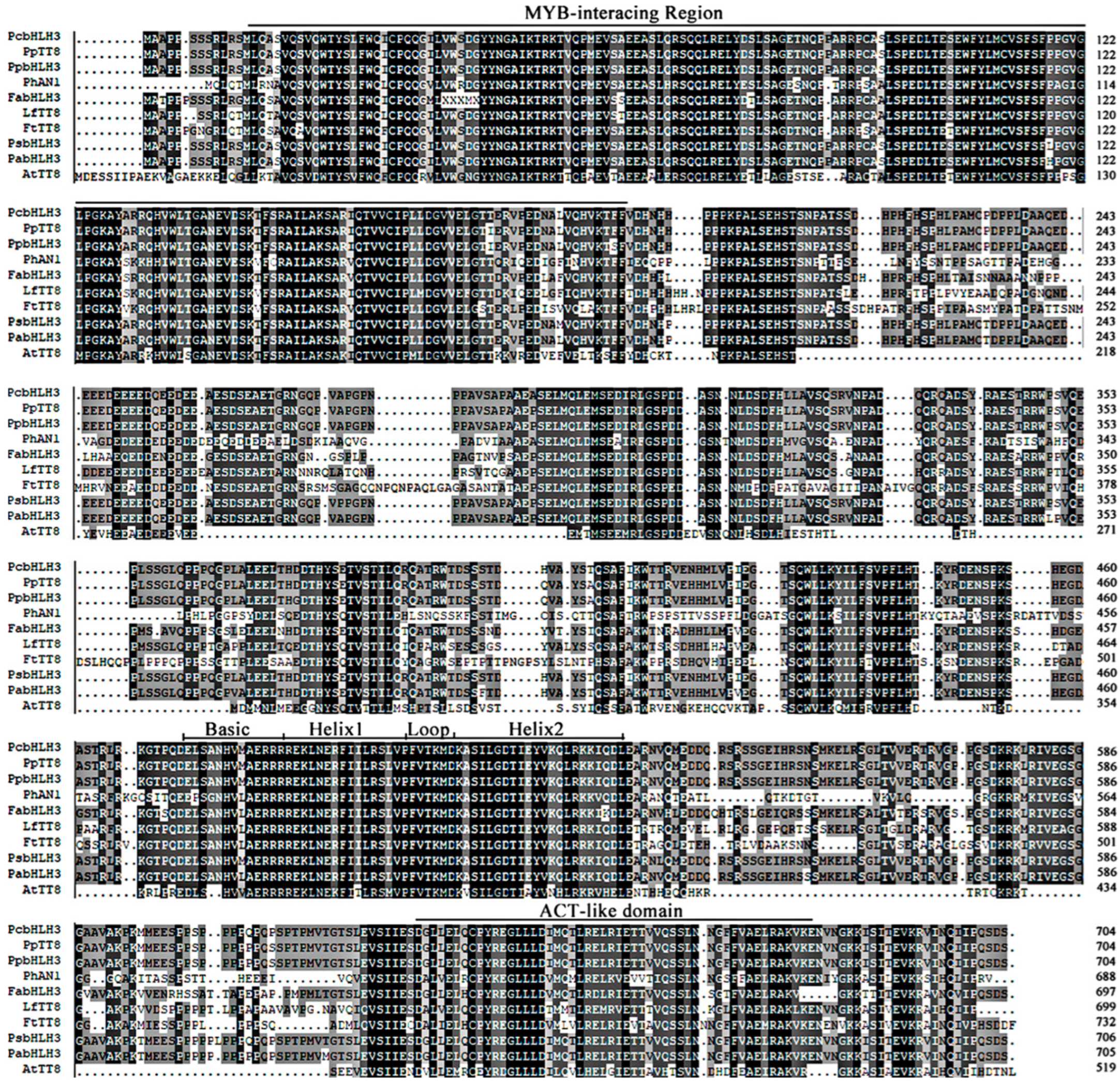
2.1. Cloning and Molecular Characterization of FtTT8
2.2. Analysis of Subcellular Location and Transcription Activation of FtTT8
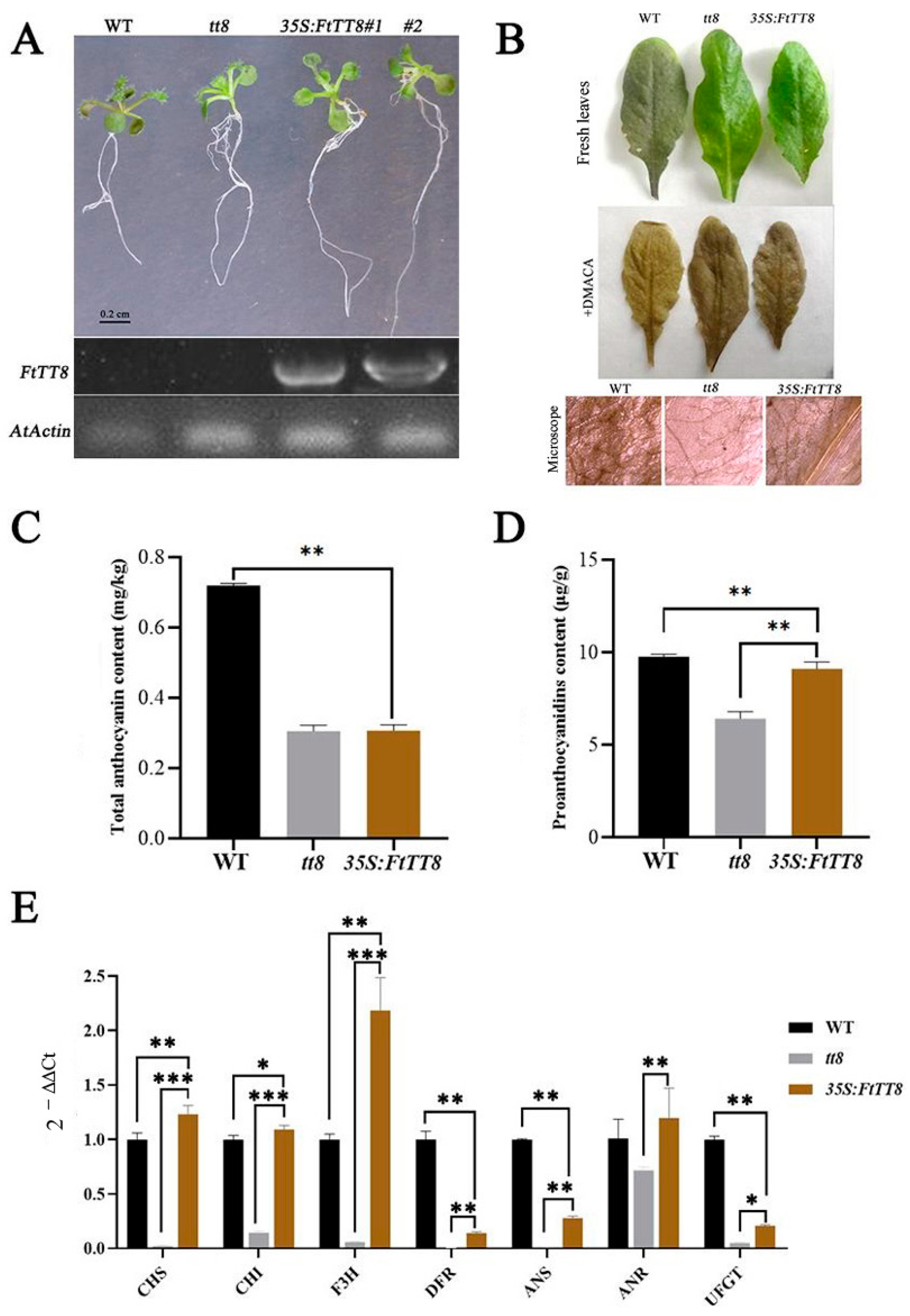
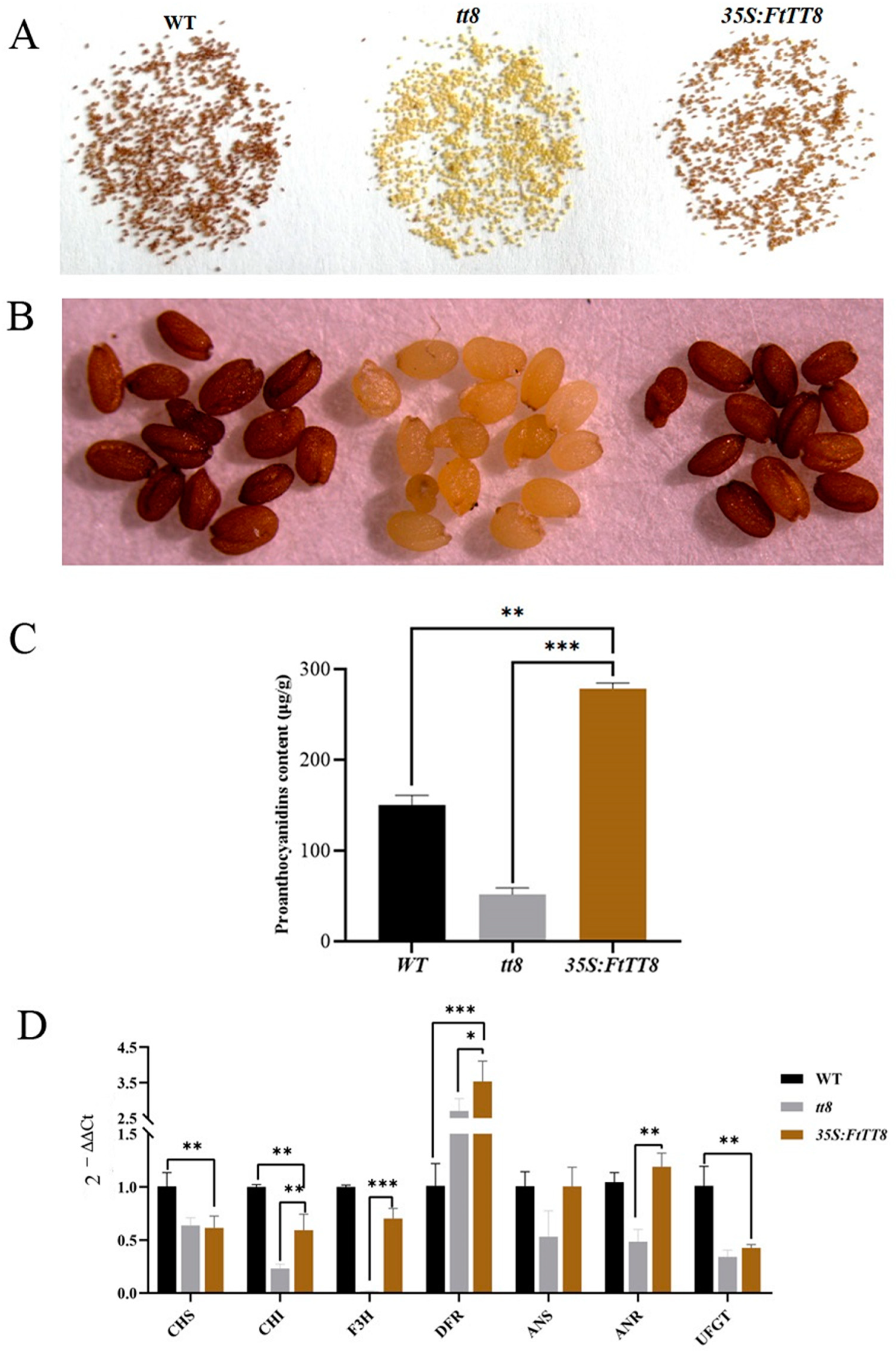
2.3. Complementation of the Arabidopsis tt8 Mutant by FtTT8
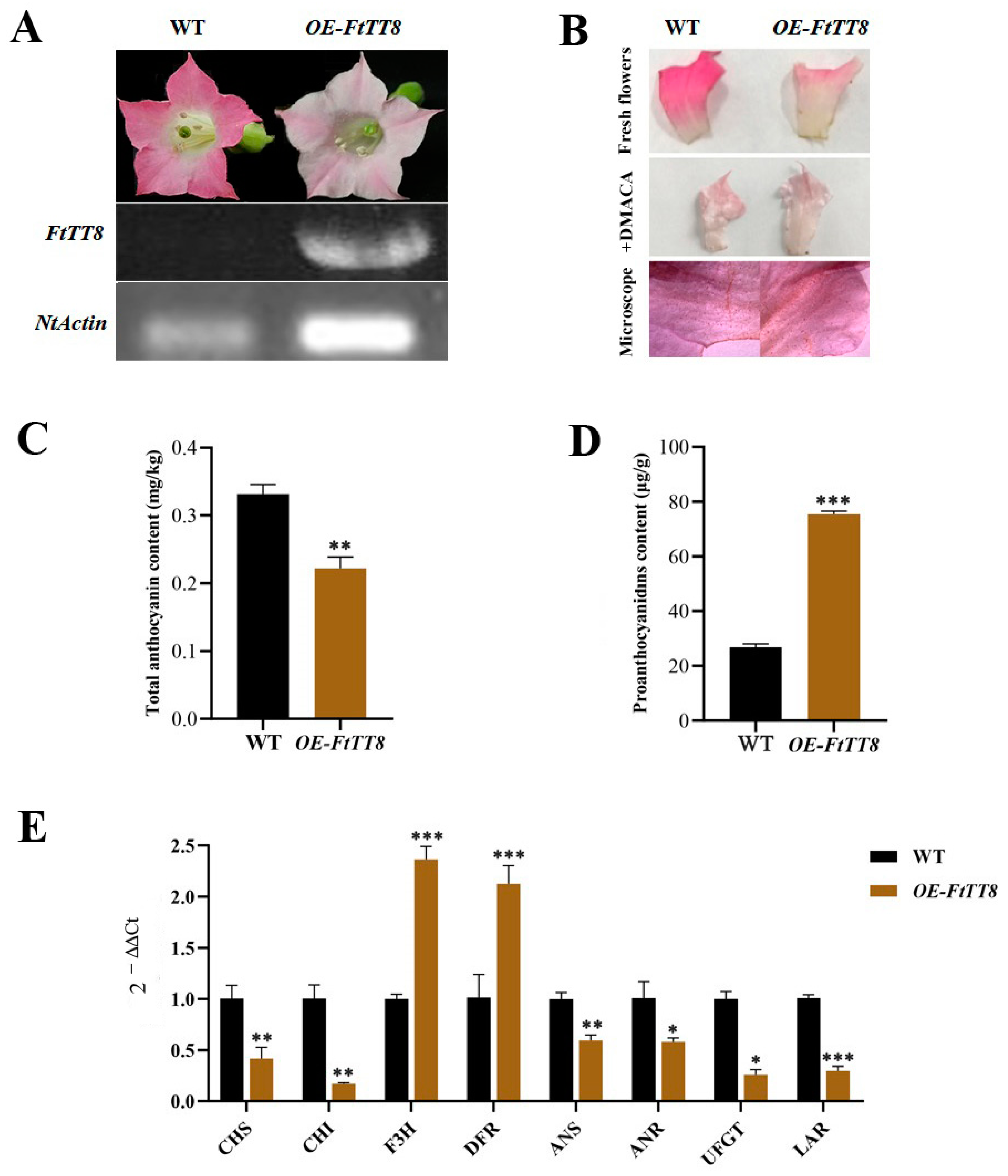
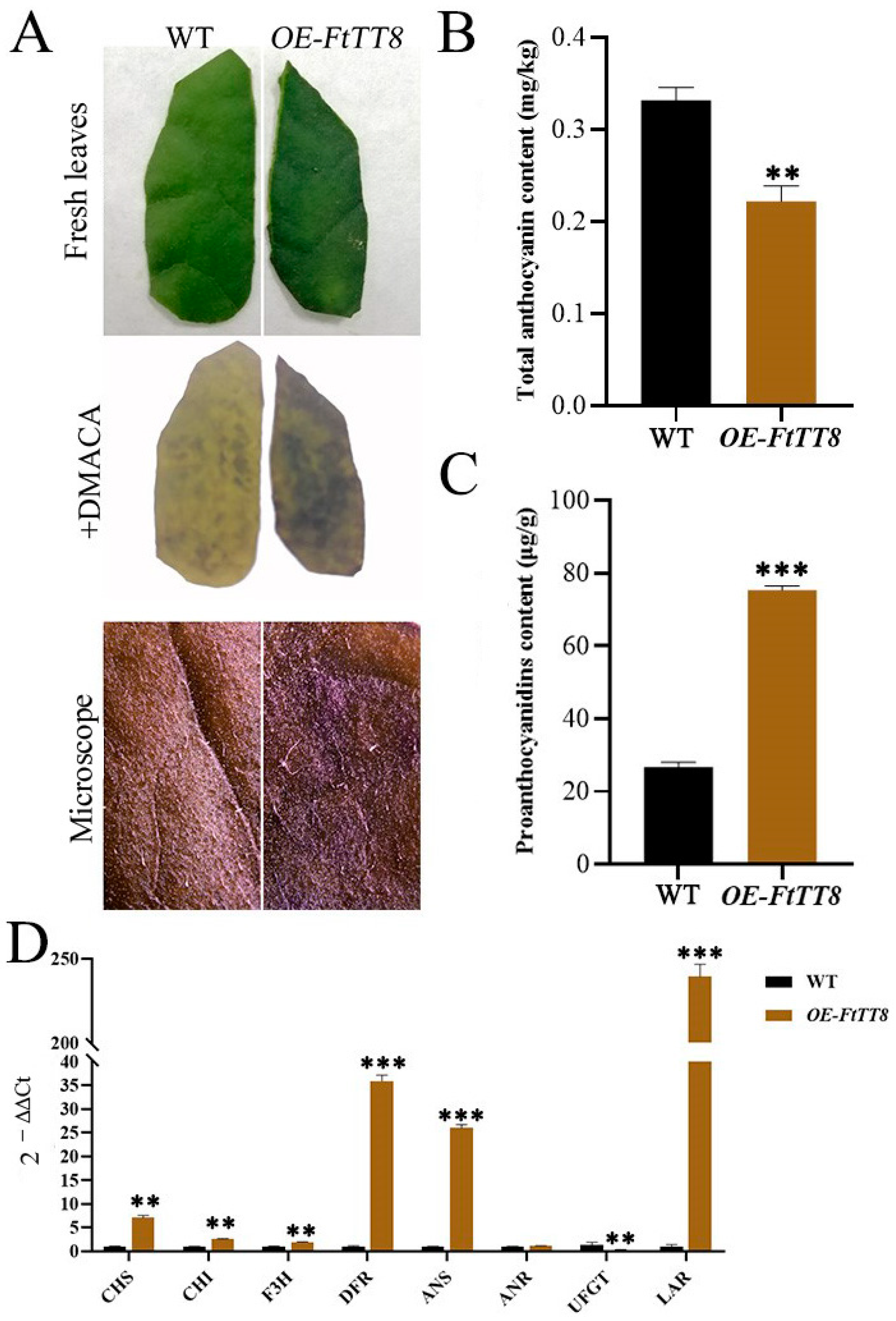
2.4. Overexpression of FtTT8 in K326 Tobacco
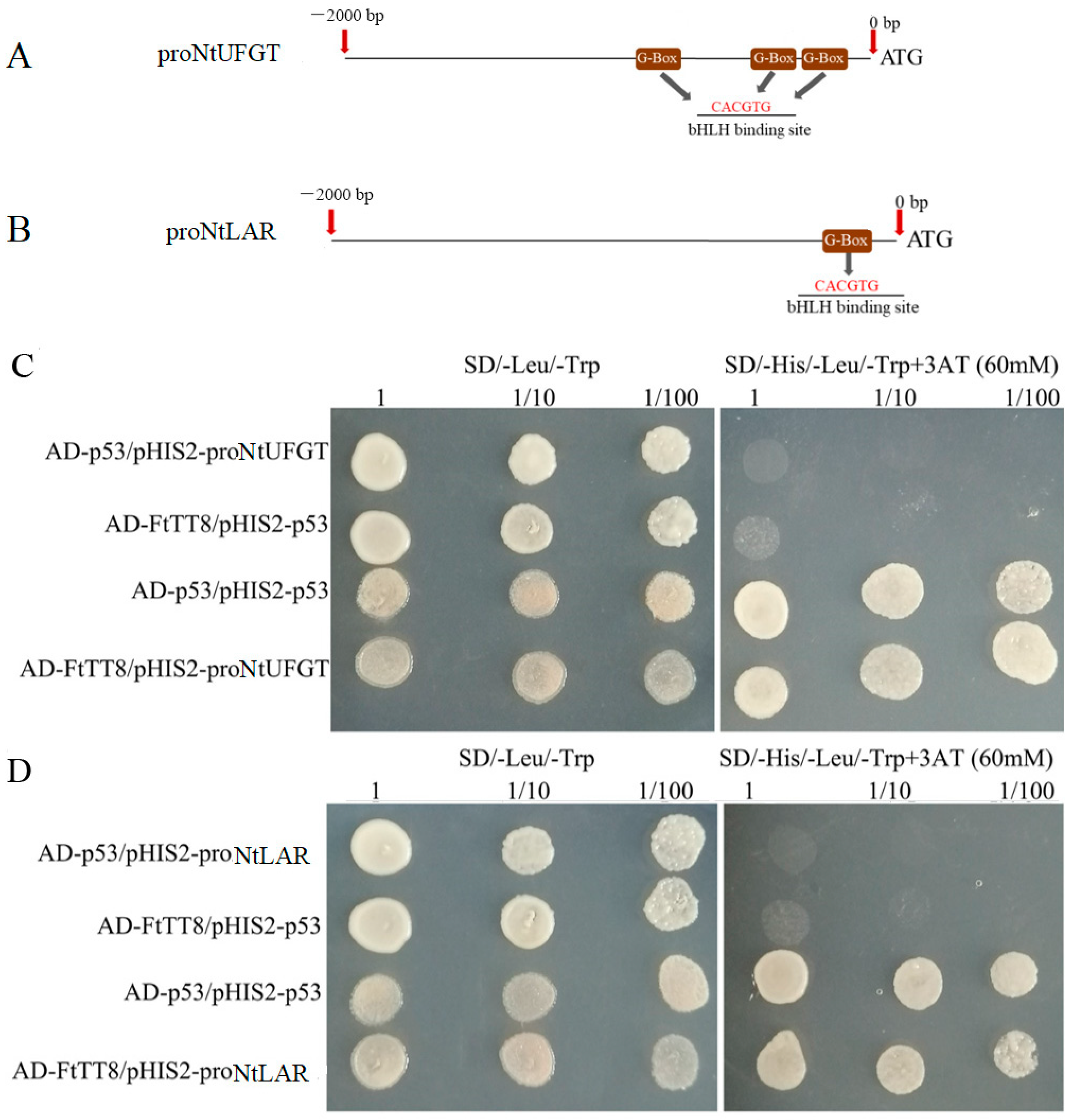
2.5. FtTT8 Combined to the Promoters of NtUFGT and NtLAR
3. Discussion
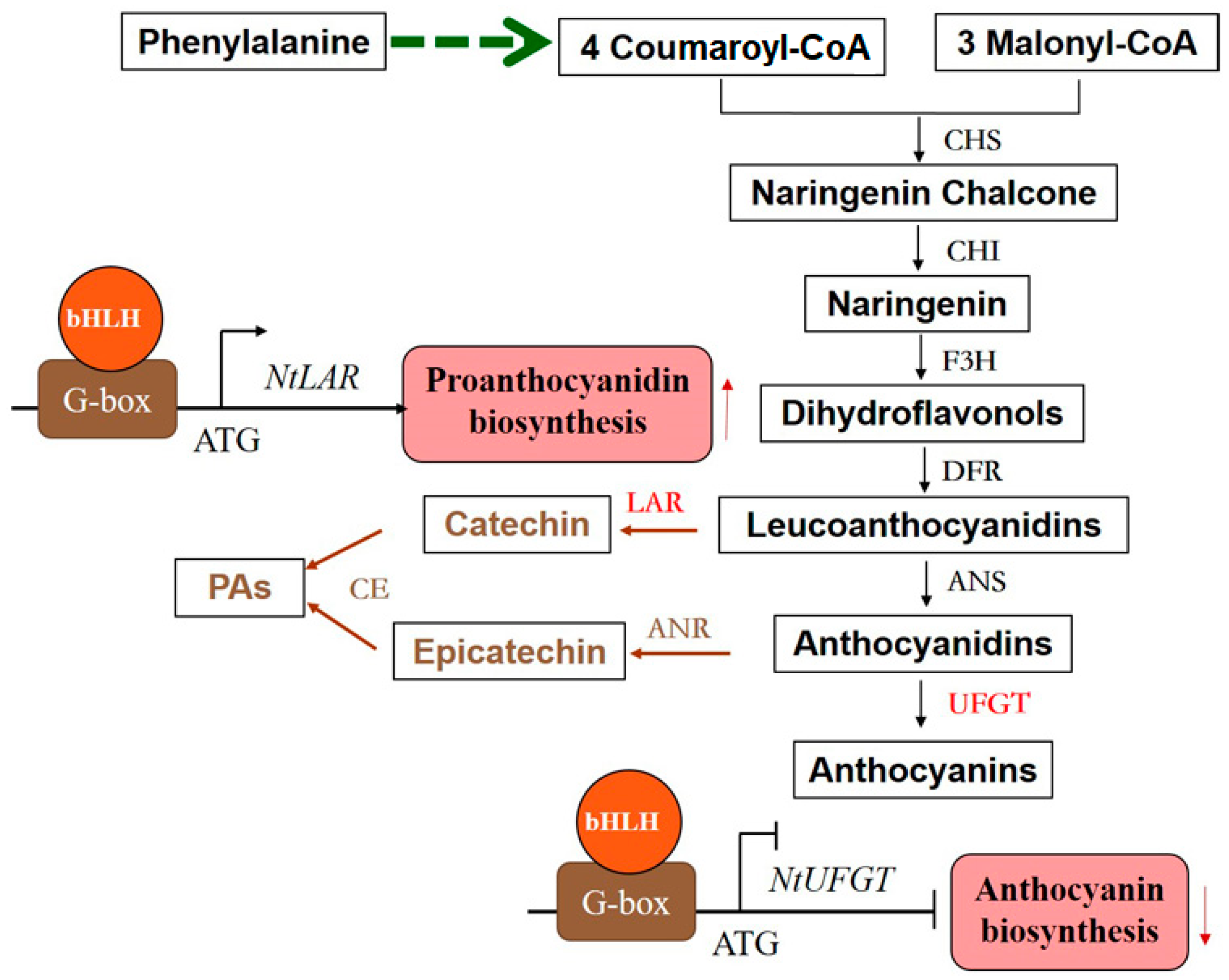
3.1. The Different Functions of FtTT8 in the Regulation Anthocyanin and Proanthocyanidin Biosynthesis
3.2. FtTT8 Interacted with the Promoter of NtUFGT and NtLAR to Play Roles in the Regulation of Anthocyanin and Proanthocyanidin Biosynthesis
4. Materials and Methods
4.1. Plant Materials
4.2. Cloning and Characterization of FtTT8
4.3. Arabidopsis Protoplast Transient Expression Assay
4.4. Yeast Two-Hybrid Assay
4.5. Transformation of Arabidopsis tt8 Mutant and Tobacco by FtTT8 Gene
4.6. Detection of Anthocyanins and Proanthocyanidins (PAs)
4.7. qRT-PCR Analysis
4.8. Yeast One-Hybrid Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huda, M.N.; Lu, S.; Jahan, T.; Ding, M.; Jha, R.; Zhang, K.; Zhang, W.; Georgiev, M.I.; Park, S.U.; Zhou, M. Treasure from garden: Bioactive compounds of buckwheat. Food Chem. 2021, 335, 127653. [Google Scholar] [CrossRef]
- Ohsako, T.; Ohnishi, O. Intra-and interspecific phylogeny of wild Fagopyrum (Polygonaceae) species based on nucleotide sequences of noncoding regions in chloroplast DNA. Am. J. Bot. 2000, 87, 573–582. [Google Scholar] [CrossRef]
- Tang, Y.; Ding, M.Q.; Tang, Y.X.; Wu, Y.M.; Shao, J.R.; Zhou, M.L. Germplasm resources of buckwheat in germplasm resources of buckwheat in China. In Molecular Breeding and Nutritional Aspects of Buckwheat; Zhou, M., Kreft, I., Woo, S.-H., Chrungoo, N., Wieslander, G., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 13–20. [Google Scholar]
- Joshi, D.C.; Zhang, K.; Wang, C.; Chandora, R.; Khurshid, M.; Li, J.; He, M.; Georgiev, M.I.; Zhou, M. Strategic enhancement of genetic gain for nutraceutical development in buckwheat: A genomics-driven perspective. Biotechnol. Adv. 2020, 39, 107479. [Google Scholar] [CrossRef]
- Christa, K.; Soral-Śmietana, M. Buckwheat grains and buckwheat, products-nutritional and prophylactic value of their components—A review. Czech J. Food Sci. 2003, 26, 153–162. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Zhou, M.L.; Tang, Y.; Li, F.L.; Tang, Y.X.; Shao, J.R.; Xue, W.T.; Wu, Y.M. Bioactive compounds in functional buckwheat food. Food Res. Int. 2012, 49, 389–395. [Google Scholar] [CrossRef]
- Li, H.Y.; Lv, Q.Y.; Ma, C.; Qu, J.; Cai, F.; Deng, J.; Huang, J.; Ran, P.; Shi, T.; Chen, Q. Metabolite profiling and transcriptome analyses provide insights into the flavonoid biosynthesis in the developing seed of Tartary buckwheat (Fagopyrum tataricum). J. Agric. Food Chem. 2019, 67, 11262–11276. [Google Scholar] [CrossRef]
- Deng, J.; Dong, F.; Wu, C.; Zhao, J.; Li, H.Y.; Huang, J.; Shi, T.X.; Meng, Z.Y.; Cai, F.; Chen, Q.F. Comparative metabolomics analysis between red- and white-flowered common buckwheat cultivars. Phyton-Int. J. Exp. Bot. 2021, 90, 859. [Google Scholar] [CrossRef]
- Kreft, S.; Strukelj, B.; Gaberscik, A.; Kreft, I. Rutin in buckwheat herbs grown at different UV-B radiation levels: Comparison of two UV spectrophotometric and an HPLC method. J. Exp. Bot. 2002, 53, 1801–1804. [Google Scholar] [CrossRef]
- Wang, H.; Liu, S.; Cui, Y.; Wang, Y.; Guo, Y.; Wang, X.; Liu, J.; Piao, C. Hepatoprotective effects of flavonoids from common buckwheat hulls in type 2 diabetic rats and HepG2 cells. Food Sci. Nutr. 2021, 9, 4793–4802. [Google Scholar] [CrossRef]
- Javed, H.; Khan, M.M.; Ahmad, A.; Vaibhav, K.; Ahmad, M.E.; Khan, A.; Ashafaq, M.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of Alzheimer type. Neuroscience 2012, 210, 340–352. [Google Scholar] [CrossRef]
- Zhong, L.; Lin, Y.; Wang, C.; Niu, B.; Xu, Y.; Zhao, G.; Zhao, J. Chemical Profile, Antimicrobial and antioxidant activity assessment of the crude extract and its main flavonoids from Tartary buckwheat sprouts. Molecules 2022, 27, 374. [Google Scholar] [CrossRef]
- Koyama, M.; Nakamura, C.; Nakamura, K. Changes in phenols contents from buckwheat sprouts during growth stage. J. Food Sci. Technol. 2013, 50, 86–93. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, N.; Wang, S.; Wang, J.; Peng, W. A review: The nutrition components, active substances and flavonoid accumulation of Tartary buckwheat sprouts and innovative physical technology for seeds germinating. Front. Nutr. 2023, 10, 1168361. [Google Scholar] [CrossRef]
- Kim, S.J.; Maeda, T.; Sarker, M.Z.; Takigawa, S.; Matsuura-Endo, C.; Yamauchi, H.; Mukasa, Y.; Saito, K.; Hashimoto, N.; Noda, T.; et al. Identification of anthocyanins in the sprouts of buckwheat. J. Agric. Food Chem. 2007, 55, 6314–6318. [Google Scholar] [CrossRef]
- Deng, J.; Wang, L.; Damaris, R.N.; Zhao, J.; Zhang, L.; Wang, T.; Yang, C.; Huang, J.; Shi, T.; Zhu, L.; et al. Genome-wide identification of R2R3-MYB transcription factor family in Tartary buckwheat (Fagopyrum tataricum) identifies a member involved in anthocyanin biosynthesis. Agronomy 2023, 13, 2117. [Google Scholar] [CrossRef]
- Li, Z.; Ahammed, G.J. Hormonal regulation of anthocyanin biosynthesis for improved stress tolerance in plants. Plant Physiol. Biochem. 2023, 201, 107835. [Google Scholar] [CrossRef]
- Kaur, S.; Tiwari, V.; Kumari, A.; Chaudhary, E.; Sharma, A.; Ali, U.; Garg, M. Protective and defensive role of anthocyanins under plant abiotic and biotic stresses: An emerging application in sustainable agriculture. J. Biotechnol. 2023, 361, 12–29. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Zhao, P.; Chu, L.; Wang, K.; Zhao, B.; Li, Y.; Yang, K.; Wan, P. Analyses on the pigment composition of different seed coat colors in adzuki bean. Food Sci. Nutr. 2022, 10, 2611–2626. [Google Scholar] [CrossRef]
- Vaughan, S.P.; Baker, J.M.; Primavesi, L.F.; Patil, A.; King, R.; Hassani-Pak, K.; Kulasekaran, S.; Coghill, J.; Ward, J.L.; Huttly, A.K.; et al. Proanthocyanidin biosynthesis in the developing wheat seed coat investigated by chemical and RNA-Seq analysis. Plant Direct. 2022, 6, e453. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, Y.; Li, D.; Wang, W.; Gao, X.; Hao, C.; Feng, H.; Wang, Y.; Li, T. Fine mapping and cloning of a novel BrSCC1 gene for seed coat color in Brassica rapa L. Theor. Appl. Genet. 2023, 136, 11. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Yang, J.; Jiang, W.; Pang, Y. Stacking triple genes increased proanthocyanidins level in Arabidopsis thaliana. PLoS ONE 2020, 15, e0234799. [Google Scholar] [CrossRef]
- Shan, X.; Li, Y.; Yang, S.; Gao, R.; Zhou, L.; Bao, T.; Han, T.; Wang, S.; Gao, X.; Wang, L. A functional homologue of Arabidopsis TTG1 from Freesia interacts with bHLH proteins to regulate anthocyanin and proanthocyanidin biosynthesis in both Freesia hybrida and Arabidopsis thaliana. Plant Physiol. Biochem. 2019, 141, 60–72. [Google Scholar] [CrossRef]
- Kleindt, C.K.; Stracke, R.; Mehrtens, F.; Weisshaar, B. Expression analysis of flavonoid biosynthesis genes during Arabidopsis thaliana silique and seed development with a primary focus on the proanthocyanidin biosynthetic pathway. BMC Res. Notes 2010, 3, 255. [Google Scholar] [CrossRef]
- Li, G.; Michaelis, D.F.; Huang, J.; Serek, M.; Gehl, C. New insights into the genetic manipulation of the R2R3-MYB and CHI gene families on anthocyanin pigmentation in Petunia hybrida. Plant Physiol. Biochem. 2023, 203, 108000. [Google Scholar] [CrossRef]
- Zhang, H.; Koes, R.; Shang, H.; Fu, Z.; Wang, L.; Dong, X.; Zhang, J.; Passeri, V.; Li, Y.; Jiang, H.; et al. Identification and functional analysis of three new anthocyanin R2R3-MYB genes in Petunia. Plant Direct. 2019, 3, e00114. [Google Scholar] [CrossRef]
- Chatham, L.A.; Juvik, J.A. Linking anthocyanin diversity, hue, and genetics in purple corn. G3 2021, 11, jkaa062. [Google Scholar] [CrossRef]
- Chatham, L.A.; Paulsmeyer, M.; Juvik, J.A. Prospects for economical natural colorants: Insights from maize. Theor. Appl. Genet. 2019, 132, 2927–2946. [Google Scholar] [CrossRef]
- Lim, S.H.; Kim, D.H.; Lee, J.Y. RsTTG1, a WD40 Protein, Interacts with the bHLH Transcription Factor RsTT8 to regulate anthocyanin and proanthocyanidin biosynthesis in Raphanus sativus. Int. J. Mol. Sci. 2022, 23, 11973. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The Flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Ma, B.; Gao, Q.; Du, H.; Han, Y.; Li, Y.; Cao, Y.; Qi, M.; Zhu, Y.; et al. The Tartary buckwheat genome provides insights into rutin biosynthesis and abiotic stress tolerance. Mol. Plant. 2017, 10, 1224–1237. [Google Scholar] [CrossRef]
- Bai, Y.C.; Li, C.L.; Zhang, J.W.; Li, S.J.; Luo, X.P.; Yao, H.P.; Chen, H.; Zhao, H.X.; Park, S.U.; Wu, Q. Characterization of two tartary buckwheat R2R3-MYB transcription factors and their regulation of proanthocyanidin biosynthesis. Physiol. Plant 2014, 152, 431–440. [Google Scholar] [CrossRef]
- Wang, L.; Deng, R.; Bai, Y.; Wu, H.; Li, C.; Wu, Q.; Zhao, H. Tartary buckwheat R2R3-MYB gene FtMYB3 negatively regulates anthocyanin and proanthocyanidin biosynthesis. Int. J. Mol. Sci. 2022, 23, 2775. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, Q.; Wang, S.; Shi, J.; Dong, Q.; Yao, P.; Shi, G.; Xu, S.; Deng, R.; Li, C.; et al. FtMYB8 from Tartary buckwheat inhibits both anthocyanin/proanthocyanidin accumulation and marginal trichome initiation. BMC Plant Biol. 2019, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zhao, H.; Huang, Y.; Chen, Y.; Wan, M.; Zeng, Z.; Yao, P.; Li, C.; Wang, X.; Chen, H.; et al. FtMYB18 acts as a negative regulator of anthocyanin/proanthocyanidin biosynthesis in Tartary buckwheat. Plant Mol. Biol. 2020, 104, 309–325. [Google Scholar] [CrossRef]
- Wen, C.H.; Tsao, N.W.; Wang, S.Y.; Chu, F.H. Color variation in young and senescent leaves of Formosan sweet gum (Liquidambar formosana) by the gene regulation of anthocyanidin biosynthesis. Physiol. Plant. 2021, 172, 1750–1763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jiang, C.; Huang, C.; Wen, D.; Lu, J.; Chen, S.; Zhang, T.; Shi, Y.; Xue, J.; Ma, W.; et al. The light-induced transcription factor FtMYB116 promotes accumulation of rutin in Fagopyrum tataricum. Plant Cell Environ. 2019, 42, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Tan, G.; Liu, H.; He, S.; Gao, Y.; An, C. Identification of a bHLH-type G-box binding factor and its regulation activity with G-box and Box I elements of the PsCHS1 promoter. Plant Cell Rep. 2007, 26, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Grain, D.; Le Gourrierec, J.; Harscoët, E.; Berger, A.; Jauvion, V.; Scagnelli, A.; Berger, N.; Bidzinski, P.; Kelemen, Z.; et al. Regulation of flavonoid biosynthesis involves an unexpected complex transcriptional regulation of TT8 expression, in Arabidopsis. New Phytol. 2013, 198, 59–70. [Google Scholar] [CrossRef]
- Ni, J.; Bai, S.; Zhao, Y.; Qian, M.; Tao, R.; Yin, L.; Gao, L.; Teng, Y. Ethylene response factors Pp4ERF24 and Pp12ERF96 regulate blue light-induced anthocyanin biosynthesis in ‘Red Zaosu’ pear fruits by interacting with MYB114. Plant Mol. Biol. 2019, 99, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhao, Y.; Tao, R.; Yin, L.; Gao, L.; Strid, Å.; Qian, M.; Li, J.; Li, Y.; Shen, J.; et al. Ethylene mediates the branching of the jasmonate-induced flavonoid biosynthesis pathway by suppressing anthocyanin biosynthesis in red Chinese pear fruits. Plant Biotechnol. J. 2020, 18, 1223–1240. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Li, J.; Su, M.; Lin, Z.; Chen, L.; Yang, P. A bHLH gene NnTT8 of Nelumbo nucifera regulates anthocyanin biosynthesis. Plant Physiol. Biochem. 2021, 158, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Schaart, J.G.; Dubos, C.; Romero De La Fuente, I.; van Houwelingen, A.M.M.L.; de Vos, R.C.H.; Jonker, H.H.; Xu, W.; Routaboul, J.M.; Lepiniec, L.; Bovy, A.G. Identification and characterization of MYB-bHLH-WD40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa) fruits. New Phytol. 2013, 197, 454–467. [Google Scholar] [CrossRef]
- Li, P.; Chen, B.; Zhang, G.; Chen, L.; Dong, Q.; Wen, J.; Mysore, K.S.; Zhao, J. Regulation of anthocyanin and proanthocyanidin biosynthesis by Medicago truncatula bHLH transcription factor MtTT8. New Phytol. 2016, 210, 905–921. [Google Scholar] [CrossRef]
- Hu, R.; Zhu, M.; Chen, S.; Li, C.; Zhang, Q.; Gao, L.; Liu, X.; Shen, S.; Fu, F.; Xu, X.; et al. BnbHLH92a negatively regulates anthocyanin and proanthocyanidin biosynthesis in Brassica napus. Crop J. 2023, 11, 374–385. [Google Scholar] [CrossRef]
- Zhao, P.; Li, X.; Jia, J.; Yuan, G.; Chen, S.; Qi, D.; Cheng, L.; Liu, G. Corrigendum: bHLH92 from sheepgrass acts as a negative regulator of anthocyanin/proanthocyandin accumulation and influences seed dormancy. J. Exp. Bot. 2019, 70, 1989. [Google Scholar] [CrossRef]
- Gordân, R.; Shen, N.; Dror, I.; Zhou, T.; Horton, J.; Rohs, R.; Bulyk, M.L. Genomic regions flanking E-box binding sites influence DNA binding specificity of bHLH transcription factors through DNA shape. Cell Rep. 2013, 3, 1093–1104. [Google Scholar] [CrossRef]
- Ahmad, A.; Niwa, Y.; Goto, S.; Ogawa, T.; Shimizu, M.; Suzuki, A.; Kobayashi, K.; Kobayashi, H. bHLH106 Integrates Functions of Multiple Genes through Their G-Box to Confer Salt Tolerance on Arabidopsis. PLoS ONE 2015, 10, e0126872. [Google Scholar] [CrossRef]
- Zhao, J.; Li, H.; Huang, J.; Shi, T.; Meng, Z.; Chen, Q.; Deng, J. Genome-wide analysis of BBX gene family in Tartary buckwheat (Fagopyrum tataricum). PeerJ 2021, 9, e11939. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Li, C.; Li, H.; Lyu, X.; Zhao, T.; Liu, J.; Zuo, Z.; Liu, B. A transient Expression System in Soybean Mesophyll Protoplasts Reveals the Formation of Cytoplasmic GmCRY1 Photobody-Like Structures. Sci. China Life Sci. 2019, 2, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Park, N.I.; Li, X.; Suzuki, T.; Kim, S.J.; Woo, S.H.; Park, C.H.; Park, S.U. Differential Expression of Anthocyanin Biosynthetic Genes and Anthocyanin Accumulation in Tartary Buckwheat Cultivars ‘Hokkai t8’ and ‘Hokkai t10’. J. Agric. Food Chem. 2011, 59, 2356–2361. [Google Scholar] [CrossRef]
- Abeynayake, S.W.; Panter, S.; Chapman, R.; Webster, T.; Rochfort, S.; Mouradov, A.; Spangenberg, G. Biosynthesis of proanthocyanidins in white clover flowers: Cross talk within the flavonoid pathway. Plant Physiol. 2012, 158, 666–678. [Google Scholar] [CrossRef]
- An, X.H.; Tian, Y.; Chen, K.Q.; Liu, X.J.; Liu, D.D.; Xie, X.B.; Cheng, C.G.; Cong, P.H.; Hao, Y.J. MdMYB9 and MdMYB11 are Involved In the regulation of the JA-Induced Biosynthesis of Anthocyanin and Proanthocyanidin in Apples. Plant Cell Physiol. 2015, 56, 650–662. [Google Scholar] [CrossRef]
- Zhao, R.; Song, X.; Yang, N.; Chen, L.; Xiang, L.; Liu, X.Q.; Zhao, K. Expression of the subgroup IIIf bHLH transcription factor CpbHLH1 from Chimonanthus praecox (L.) in transgenic model plants inhibits anthocyanin accumulation. Plant Cell Rep. 2020, 39, 891–907. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Wang, L.; Zhang, L.; Yang, C.; Huang, J.; Zhu, L.; Chen, Q.; Meng, Z.; Cai, F.; Shi, T. Tartary Buckwheat (Fagopyrum tataricum) FtTT8 Inhibits Anthocyanin Biosynthesis and Promotes Proanthocyanidin Biosynthesis. Int. J. Mol. Sci. 2023, 24, 17368. https://doi.org/10.3390/ijms242417368
Deng J, Wang L, Zhang L, Yang C, Huang J, Zhu L, Chen Q, Meng Z, Cai F, Shi T. Tartary Buckwheat (Fagopyrum tataricum) FtTT8 Inhibits Anthocyanin Biosynthesis and Promotes Proanthocyanidin Biosynthesis. International Journal of Molecular Sciences. 2023; 24(24):17368. https://doi.org/10.3390/ijms242417368
Chicago/Turabian StyleDeng, Jiao, Lijuan Wang, Lan Zhang, Chaojie Yang, Juan Huang, Liwei Zhu, Qingfu Chen, Ziye Meng, Fang Cai, and Taoxiong Shi. 2023. "Tartary Buckwheat (Fagopyrum tataricum) FtTT8 Inhibits Anthocyanin Biosynthesis and Promotes Proanthocyanidin Biosynthesis" International Journal of Molecular Sciences 24, no. 24: 17368. https://doi.org/10.3390/ijms242417368
APA StyleDeng, J., Wang, L., Zhang, L., Yang, C., Huang, J., Zhu, L., Chen, Q., Meng, Z., Cai, F., & Shi, T. (2023). Tartary Buckwheat (Fagopyrum tataricum) FtTT8 Inhibits Anthocyanin Biosynthesis and Promotes Proanthocyanidin Biosynthesis. International Journal of Molecular Sciences, 24(24), 17368. https://doi.org/10.3390/ijms242417368





