WGA-M001, a Mixture of Total Extracts of Tagetes erecta and Ocimum basilicum, Synergistically Alleviates Cartilage Destruction by Inhibiting ERK and NF-κB Signaling
Abstract
:1. Introduction
2. Results
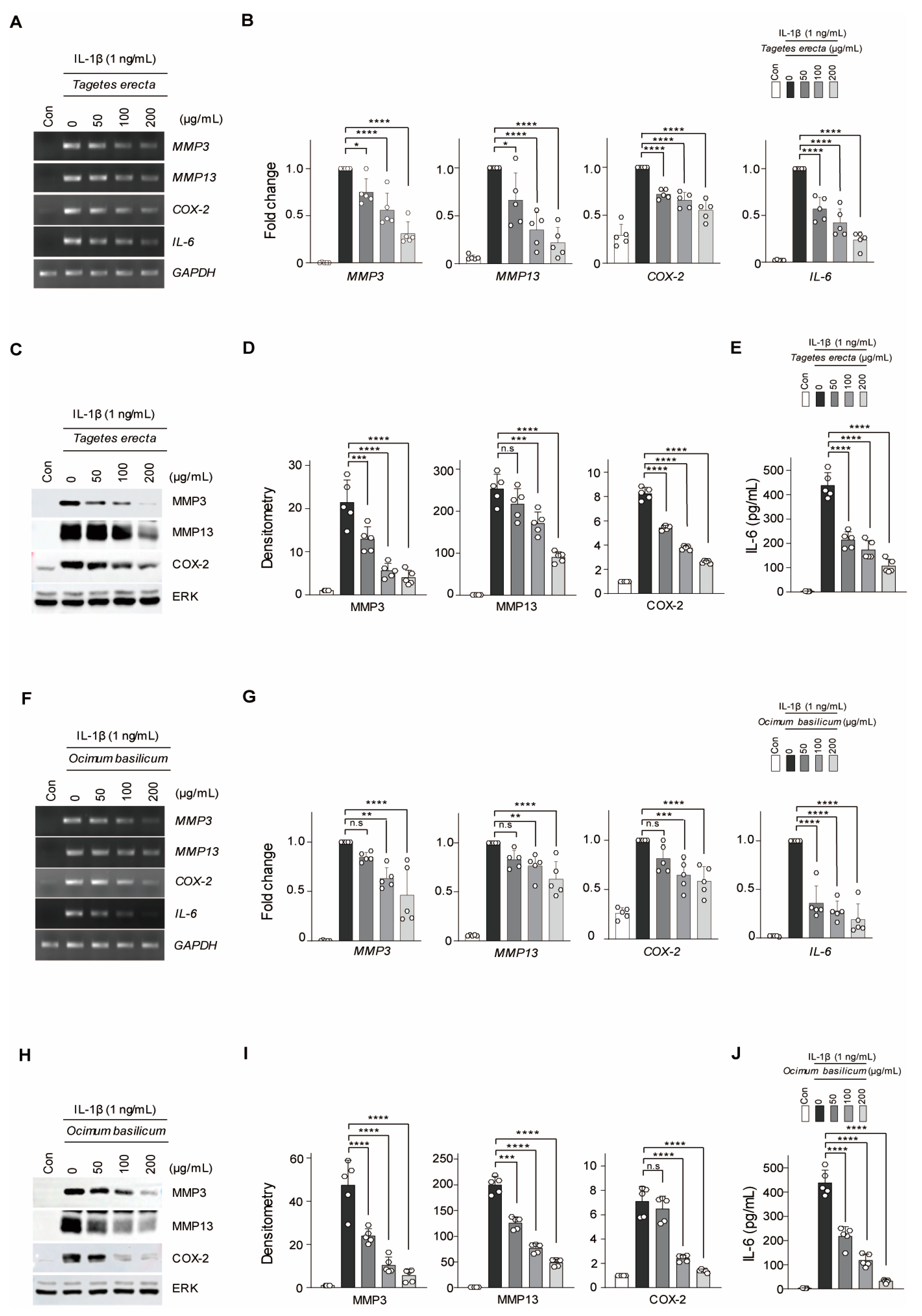
2.1. Therapeutic Effects of T. erecta and O. basilicum under In Vitro Conditions Mimicking OA
2.2. WGA-M001 Effectively Attenuates Catabolic Factor Expression
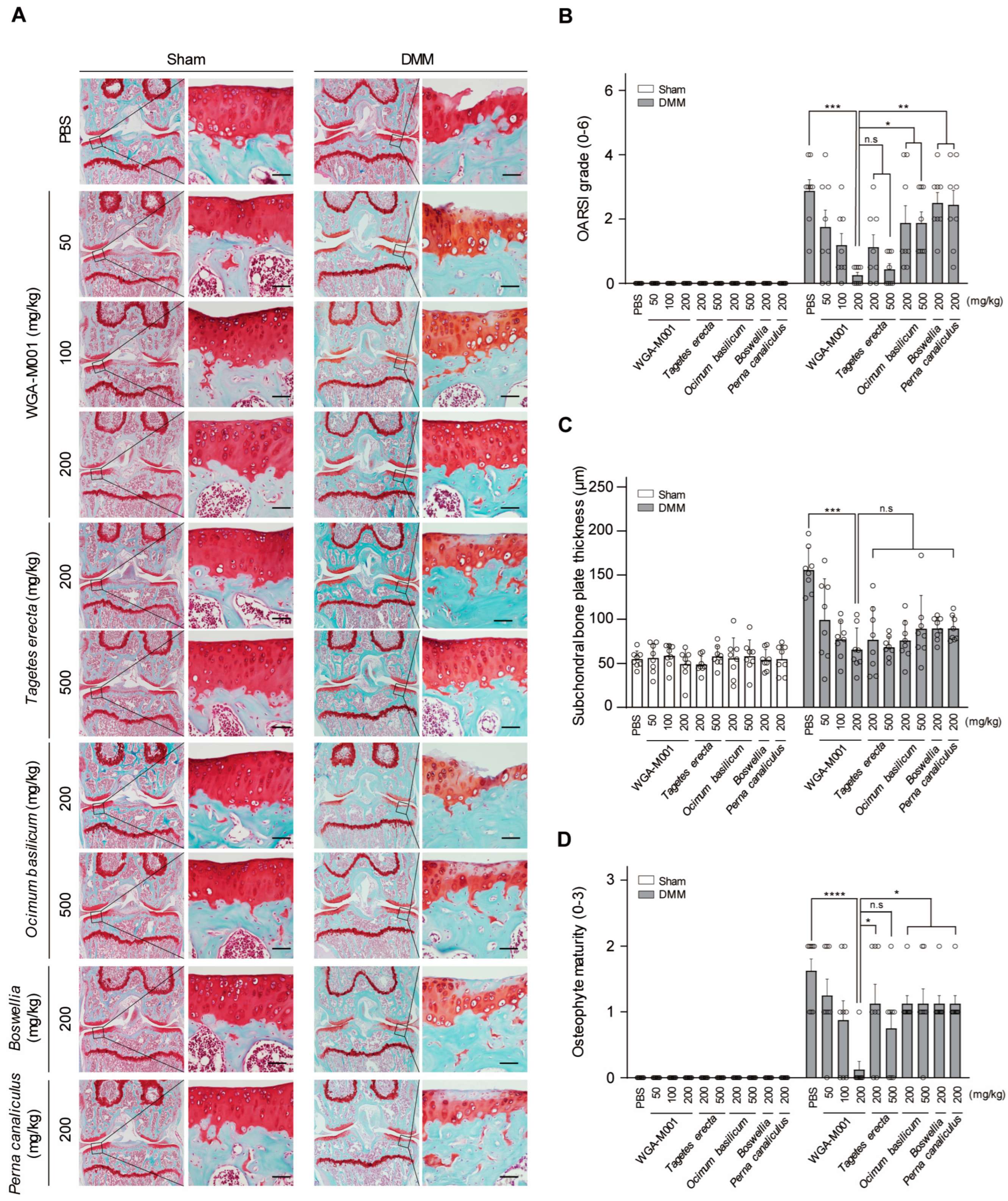
2.3. T. erecta and O. basilicum Mixtures Show a Synergistical Effect in the DMM-Induced OA Mouse Model
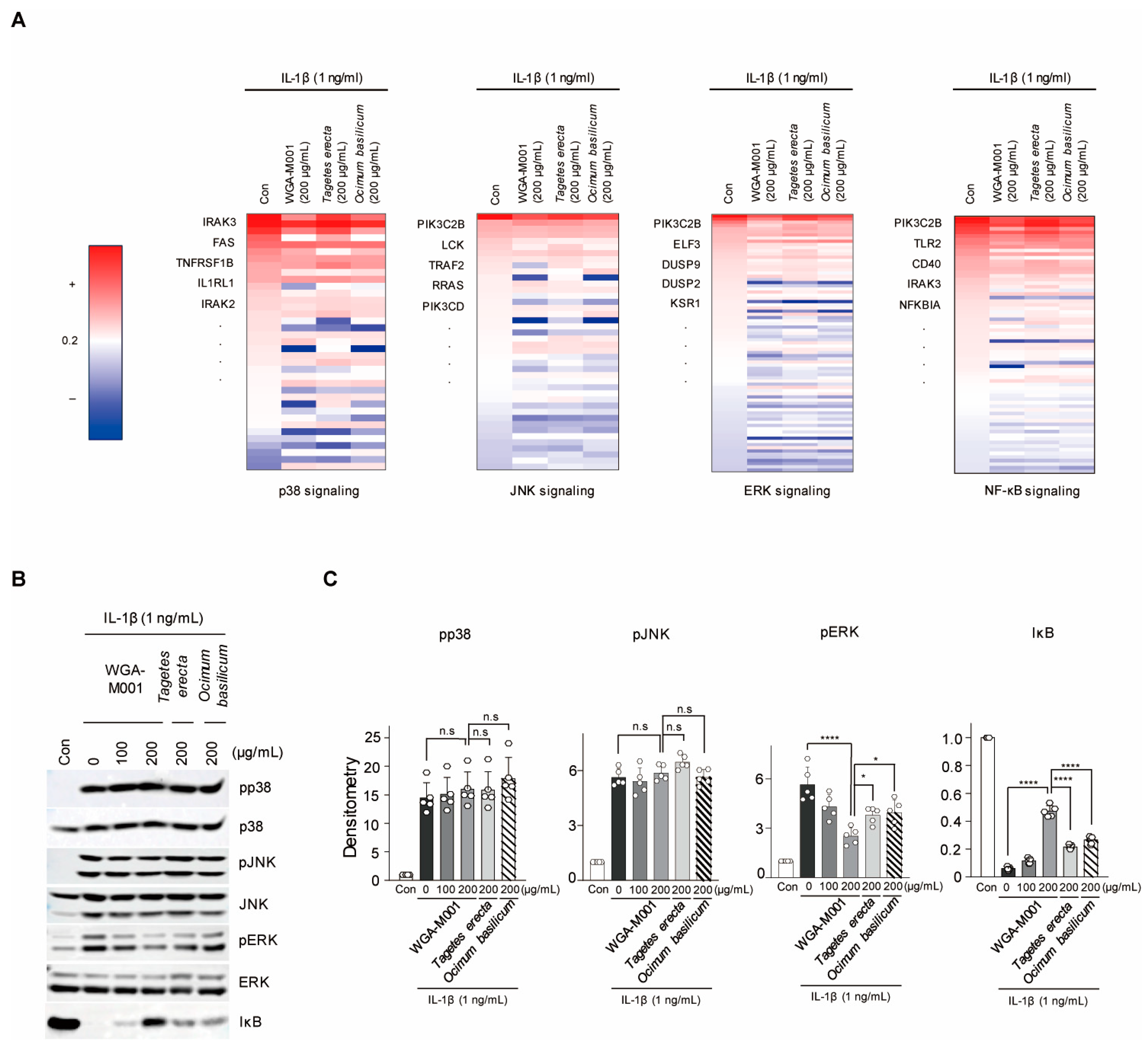
2.4. WGA-M001 Attenuates OA Pathogenesis through Effective Gene Regulation with Respect to ERK and NF-κB Signaling
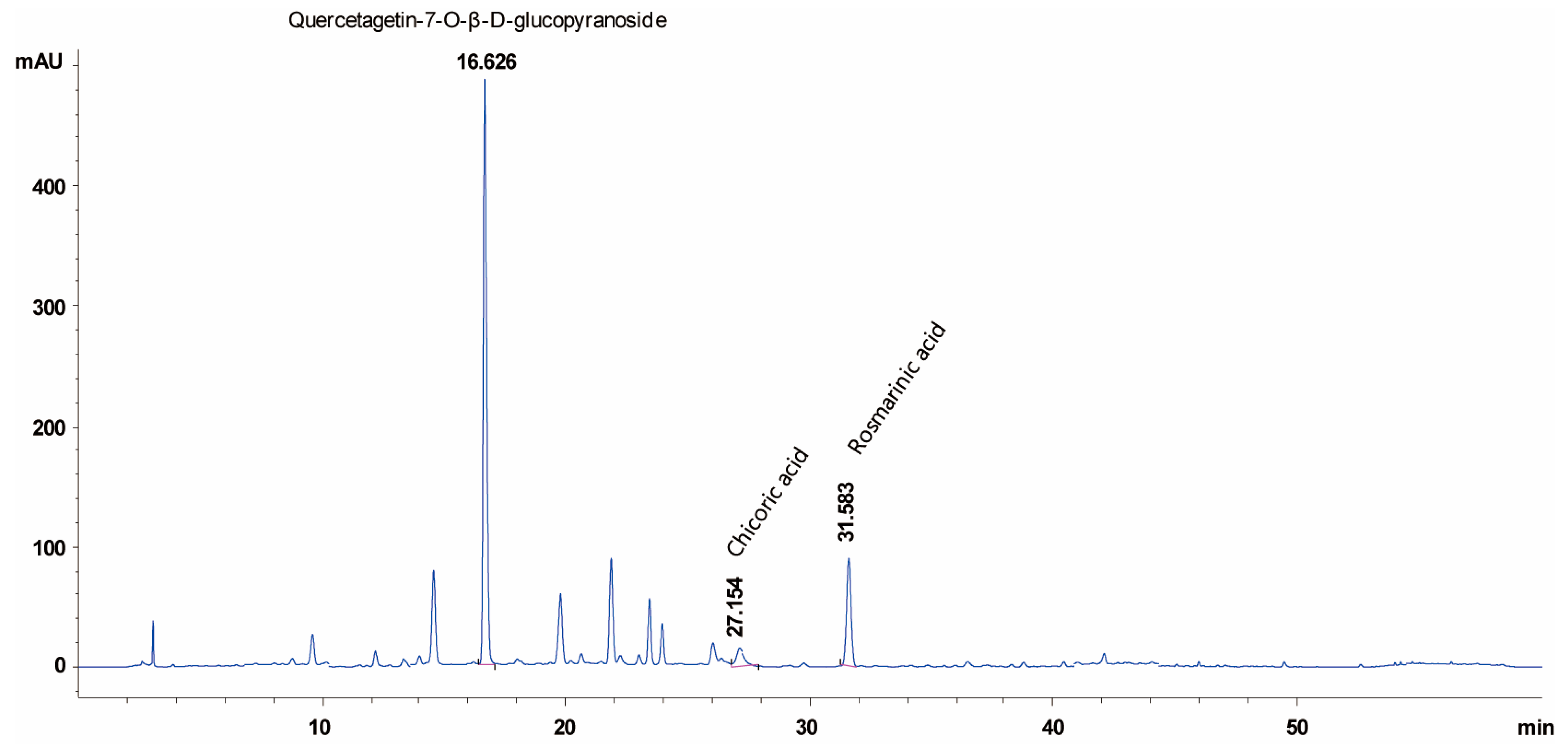
2.5. WGA-M001 Contains Quercetagetin-7-O-β-D-Glucopyranoside, Chicoric Acid, and Rosmarinic Acid
3. Discussion
4. Materials and Methods
4.1. Sample Treatment and Reagents
4.2. Primary Culture of Mouse Chondrocytes and Experimental Animals
4.3. LDH Assay
4.4. RT-PCR and Quantitative RT-PCR (qRT-PCR)
4.5. Protein Extraction, Western Blotting, and IL-6 ELISA Assay
4.6. In Silico Analysis
4.7. DMM-Induced OA Mouse Model and Oral Administration
4.8. Histological Analysis of Cartilage Destruction
4.9. HPLC Analysis of WGA-M001
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peng, Z.; Sun, H.; Bunpetch, V.; Koh, Y.; Wen, Y.; Wu, D.; Ouyang, H. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials 2021, 268, 120555. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeon, J.; Shin, M.; Won, Y.; Lee, M.; Kwak, J.S.; Lee, G.; Rhee, J.; Ryu, J.H.; Chun, C.H. Regulation of the catabolic cascade in osteoarthritis by the zinc-ZIP8-MTF1 axis. Cell 2014, 156, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.A.; Loeser, R.F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1β signaling in osteoarthritis—Chondrocytes in focus. Cell Signal. 2019, 53, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, M.P.; Brinckerhoff, C.E. Early response genes induced in chondrocytes stimulated with the inflammatory cytokine interleukin-1beta. Arthritis Res. Ther. 2001, 3, 381–388. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, S.; Su, H.; Cheng, J. Isoliquiritigenin Inhibits IL-1β-Induced Production of Matrix Metalloproteinase in Articular Chondrocytes. Mol. Ther. Methods Clin. Dev. 2018, 9, 153–159. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, Y.; Li, L.; Li, X.; Qiu, B.; Hu, Y. Cardamonin alleviates chondrocytes inflammation and cartilage degradation of osteoarthritis by inhibiting ferroptosis via p53 pathway. Food Chem. Toxicol. 2023, 174, 113644. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Cherifi, C.; Maillet, J.; Ea, H.K.; Bouaziz, W.; Funck-Brentano, T.; Cohen-Solal, M.; Hay, E.; Richette, P. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann. Rheum. Dis. 2017, 76, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Tchetina, E.V.; Di Battista, J.A.; Zukor, D.J.; Antoniou, J.; Poole, A.R. Prostaglandin PGE2 at very low concentrations suppresses collagen cleavage in cultured human osteoarthritic articular cartilage: This involves a decrease in expression of proinflammatory genes, collagenases and COL10A1, a gene linked to chondrocyte hypertrophy. Arthritis Res. Ther. 2007, 9, R75. [Google Scholar] [PubMed]
- Zhang, W.; Ouyang, H.; Dass, C.R.; Xu, J. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef]
- Richard, M.J.; Driban, J.B.; McAlindon, T.E. Pharmaceutical treatment of osteoarthritis. Osteoarthr. Cartil. 2023, 31, 458–466. [Google Scholar] [CrossRef]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef]
- Zhang, W.; Moskowitz, R.W.; Nuki, G.; Abramson, S.; Altman, R.D.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M. OARSI recommendations for the management of hip and knee osteoarthritis, Part II: OARSI evidence-based, expert consensus guidelines. Osteoarthr. Cartil. 2008, 16, 137–162. [Google Scholar] [CrossRef]
- Ameye, L.G.; Chee, W.S. Osteoarthritis and nutrition. From nutraceuticals to functional foods: A systematic review of the scientific evidence. Arthritis Res. Ther. 2006, 8, R127. [Google Scholar] [CrossRef]
- Shan, W.; Cheng, C.; Huang, W.; Ding, Z.; Luo, S.; Cui, G.; Lu, W.; Liu, F.; Xu, J.; He, W. Angiopoietin-like 2 upregulation promotes human chondrocyte injury via NF-κB and p38/MAPK signaling pathway. J. Bone Miner. Metab. 2019, 37, 976–986. [Google Scholar] [CrossRef]
- Li, Z.; Dai, A.; Yang, M.; Chen, S.; Deng, Z.; Li, L. p38MAPK Signaling Pathway in Osteoarthritis: Pathological and Therapeutic Aspects. J. Inflamm. Res. 2022, 15, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Erickson, E.A.; Long, D.L. Mitogen-activated protein kinases as therapeutic targets in osteoarthritis. Curr. Opin. Rheumatol. 2008, 20, 581–586. [Google Scholar] [CrossRef]
- Studer, R.K.; Chu, C.R. p38 MAPK and COX2 inhibition modulate human chondrocyte response to TGF-beta. J. Orthop. Res. 2005, 23, 454–461. [Google Scholar] [CrossRef]
- Rasheed, Z.; Akhtar, N.; Haqqi, T.M. Advanced glycation end products induce the expression of interleukin-6 and interleukin-8 by receptor for advanced glycation end product-mediated activation of mitogen-activated protein kinases and nuclear factor-κB in human osteoarthritis chondrocytes. Rheumatology 2010, 50, 838–851. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Li, S.; Wu, Y.; Yu, C.; Ni, L.; Xiao, J.; Shao, Z.; Zhu, H.; Wang, J. Tangeretin suppresses osteoarthritis progression via the Nrf2/NF-κB and MAPK/NF-κB signaling pathways. Phytomedicine 2022, 98, 153928. [Google Scholar] [CrossRef] [PubMed]
- Roman-Blas, J.A.; Jimenez, S.A. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthr. Cartil. 2006, 14, 839–848. [Google Scholar] [CrossRef]
- He, L.; Pan, Y.; Yu, J.; Wang, B.; Dai, G.; Ying, X. Decursin alleviates the aggravation of osteoarthritis via inhibiting PI3K-Akt and NF-kB signal pathway. Int. Immunopharmacol. 2021, 97, 107657. [Google Scholar] [CrossRef]
- Rojas-Sandoval, J. Tagetes erecta (Mexican Marigold); CABI: Wallingford, UK, 2022; Volume CABI Compendium. [Google Scholar]
- Meurer, M.C.; Mees, M.; Mariano, L.N.B.; Boeing, T.; Somensi, L.B.; Mariott, M.; da Silva, R.; Dos Santos, A.C.; Longo, B.; Santos França, T.C. Hydroalcoholic extract of Tagetes erecta L. flowers, rich in the carotenoid lutein, attenuates inflammatory cytokine secretion and improves the oxidative stress in an animal model of ulcerative colitis. Nutr. Res. 2019, 66, 95–106. [Google Scholar] [CrossRef]
- Calderón Bravo, H.; Vera Céspedes, N.; Zura-Bravo, L.; Muñoz, L.A. Basil Seeds as a Novel Food, Source of Nutrients and Functional Ingredients with Beneficial Properties: A Review. Foods 2021, 10, 1467. [Google Scholar] [CrossRef]
- Bensaid, A.; Boudard, F.; Servent, A.; Morel, S.; Portet, K.; Guzman, C.; Vitou, M.; Bichon, F.; Poucheret, P. Differential Nutrition-Health Properties of Ocimum basilicum Leaf and Stem Extracts. Foods 2022, 11, 1699. [Google Scholar] [CrossRef]
- Moliner, C.; Barros, L.; Dias, M.I.; López, V.; Langa, E.; Ferreira, I.; Gómez-Rincón, C. Edible Flowers of Tagetes erecta L. as Functional Ingredients: Phenolic Composition, Antioxidant and Protective Effects on Caenorhabditis elegans. Nutrients 2018, 10, 2002. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.; Lee, J.S.; Oh, H.; Kang, L.-J.; Hwang, Y.; Chae, S.; Lee, I.-J.; Kim, S.J.; Woo, H.; Eyun, S.-I. Fructose-Derived Levan Nanoparticles Protect Against Osteoarthritis by Directly Blocking CD44 Activation. Small 2022, 18, 2202146. [Google Scholar] [CrossRef]
- Cho, C.; Oh, H.; Lee, J.S.; Kang, L.J.; Oh, E.J.; Hwang, Y.; Kim, S.J.; Bae, Y.S.; Kim, E.J.; Kang, H.C. Prussian blue nanozymes coated with Pluronic attenuate inflammatory osteoarthritis by blocking c-Jun N-terminal kinase phosphorylation. Biomaterials 2023, 297, 122131. [Google Scholar] [CrossRef]
- Wei, Y.; Luo, L.; Gui, T.; Yu, F.; Yan, L.; Yao, L.; Zhong, L.; Yu, W.; Han, B.; Patel, J.M.; et al. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci. Transl. Med. 2021, 13, eabb3946. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; van Dijk, C.N.; Mueller-Gerbl, M. The basic science of the subchondral bone. Knee Surg. Sports Traumatol. Arthrosc. 2010, 18, 419–433. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, P.M.; van den Berg, W.B. Osteophytes: Relevance and biology. Osteoarthr. Cartil. 2007, 15, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Noh, H.J.; Lee, H.; Park, H.H.; Ha, Y.J.; Park, S.H.; Lee, H.; Kim, S.J.; Kang, H.C.; Eyun, S.I. TRIM24-RIP3 axis perturbation accelerates osteoarthritis pathogenesis. Ann. Rheum. Dis. 2020, 79, 1635–1643. [Google Scholar] [CrossRef]
- Zapata, A.; Fernández-Parra, R. Management of Osteoarthritis and Joint Support Using Feed Supplements: A Scoping Review of Undenatured Type II Collagen and Boswellia serrata. Animals 2023, 13, 870. [Google Scholar] [CrossRef]
- Blain, E.J.; Ali, A.Y.; Duance, V.C. Boswellia frereana (frankincense) suppresses cytokine-induced matrix metalloproteinase expression and production of pro-inflammatory molecules in articular cartilage. Phytother. Res. 2010, 24, 905–912. [Google Scholar] [CrossRef]
- Brien, S.; Prescott, P.; Coghlan, B.; Bashir, N.; Lewith, G. Systematic review of the nutritional supplement Perna Canaliculus (green-lipped mussel) in the treatment of osteoarthritis. Qjm Int. J. Med. 2008, 101, 167–179. [Google Scholar] [CrossRef]
- Alluri, V.K.; Kundimi, S.; Sengupta, K.; Golakoti, T.; Kilari, E.K. An Anti-Inflammatory Composition of Boswellia serrata Resin Extracts Alleviates Pain and Protects Cartilage in Monoiodoacetate-Induced Osteoarthritis in Rats. Evid. Based Complement. Altern. Med. 2020, 2020, 7381625. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.Z. Boswellia serrata, a potential antiinflammatory agent: An overview. Indian J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar] [PubMed]
- Lee, H.; Nam, J.; Jang, H.; Park, Y.S.; Son, M.H.; Lee, I.H.; Eyun, S.I.; Jeon, J.; Yang, S. Novel molecule BBC0901 inhibits BRD4 and acts as a catabolic regulator in the pathogenesis of osteoarthritis. Biomed. Pharmacother. 2023, 166, 115426. [Google Scholar] [CrossRef] [PubMed]
- Daheshia, M.; Yao, J.Q. The interleukin 1beta pathway in the pathogenesis of osteoarthritis. J. Rheumatol. 2008, 35, 2306–2312. [Google Scholar] [CrossRef]
- Goh, S.L.; Persson, M.S.M.; Stocks, J.; Hou, Y.; Welton, N.J.; Lin, J.; Hall, M.C.; Doherty, M.; Zhang, W. Relative Efficacy of Different Exercises for Pain, Function, Performance and Quality of Life in Knee and Hip Osteoarthritis: Systematic Review and Network Meta-Analysis. Sports Med. 2019, 49, 743–761. [Google Scholar] [CrossRef]
- Jang, S.; Lee, K.; Ju, J.H. Recent Updates of Diagnosis, Pathophysiology, and Treatment on Osteoarthritis of the Knee. Int. J. Mol. Sci. 2021, 22, 2619. [Google Scholar] [CrossRef]
- Fan, Z.; Söder, S.; Oehler, S.; Fundel, K.; Aigner, T. Activation of interleukin-1 signaling cascades in normal and osteoarthritic articular cartilage. Am. J. Pathol. 2007, 171, 938–946. [Google Scholar] [CrossRef]
- Choi, M.C.; Jo, J.; Park, J.; Kang, H.K.; Park, Y. NF-κB Signaling Pathways in Osteoarthritic Cartilage Destruction. Cells 2019, 8, 734. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Zou, Z.L.; Sun, M.H.; Yin, W.F.; Yang, L.; Kong, L.Y. Avicularin suppresses cartilage extracellular matrix degradation and inflammation via TRAF6/MAPK activation. Phytomedicine 2021, 91, 153657. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374 Pt 1, 1–20. [Google Scholar] [CrossRef]
- Cui, G.; Wei, F.; Wei, M.; Xie, L.; Lin, Z.; Feng, X. Modulatory effect of Tagetes erecta flowers essential oils via Nrf2/HO-1/NF-κB/p65 axis mediated suppression of N-methyl-N′nitro-N-nitroguanidine (MNNG) induced gastric cancer in rats. Mol. Cell. Biochem. 2021, 476, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Takahashi-Muto, C.; Nagase, M.; Kassai, M.; Tanaka-Yachi, R.; Kiyose, C. Anti-inflammatory Effects of Extracts of Sweet Basil (Ocimum basilicum L.) on a Co-culture of 3T3-L1 Adipocytes and RAW264.7 Macrophages. J. Oleo Sci. 2020, 69, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Abshirini, M.; Coad, J.; Wolber, F.M.; von Hurst, P.; Miller, M.R.; Tian, H.S.; Kruger, M.C. Green-lipped (greenshell™) mussel (Perna canaliculus) extract supplementation in treatment of osteoarthritis: A systematic review. Inflammopharmacology 2021, 29, 925–938. [Google Scholar] [CrossRef]
- Yu, G.; Xiang, W.; Zhang, T.; Zeng, L.; Yang, K.; Li, J. Effectiveness of Boswellia and Boswellia extract for osteoarthritis patients: A systematic review and meta-analysis. BMC Complement. Med. Ther. 2020, 20, 225. [Google Scholar] [CrossRef]
- Lee, H.; Jang, D.; Jeon, J.; Cho, C.; Choi, S.; Han, S.J.; Oh, E.; Nam, J.; Park, C.H.; Shin, Y.S. Seomae mugwort and jaceosidin attenuate osteoarthritic cartilage damage by blocking IκB degradation in mice. J. Cell. Mol. Med. 2020, 24, 8126–8137. [Google Scholar] [CrossRef]
- Jeon, J.; Kang, L.J.; Lee, K.M.; Cho, C.; Song, E.K.; Kim, W.; Park, T.J.; Yang, S. 3′-Sialyllactose protects against osteoarthritic development by facilitating cartilage homeostasis. J. Cell. Mol. Med. 2018, 22, 57–66. [Google Scholar] [CrossRef]
- Jeon, J.; Lee, H.; Jeon, M.S.; Kim, S.J.; Choi, C.; Kim, K.W.; Yang, D.J.; Lee, S.; Bae, Y.S.; Choi, W.I. Blockade of Activin Receptor IIB Protects Arthritis Pathogenesis by Non-Amplification of Activin A-ACVR2B-NOX4 Axis Pathway. Adv. Sci. 2023, 10, e2205161. [Google Scholar] [CrossRef]
- Cho, C.; Kang, L.J.; Jang, D.; Jeon, J.; Lee, H.; Choi, S.; Han, S.J.; Oh, E.; Nam, J.; Kim, C.S. Cirsium japonicum var. maackii and apigenin block Hif-2α-induced osteoarthritic cartilage destruction. J. Cell. Mol. Med. 2019, 23, 5369–5379. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, E.; Jang, H.; Ok, S.; Eom, J.; Lee, H.; Kim, S.H.; Kim, J.H.; Jeong, Y.M.; Kim, K.J.; Yun, S.P.; et al. WGA-M001, a Mixture of Total Extracts of Tagetes erecta and Ocimum basilicum, Synergistically Alleviates Cartilage Destruction by Inhibiting ERK and NF-κB Signaling. Int. J. Mol. Sci. 2023, 24, 17459. https://doi.org/10.3390/ijms242417459
Oh E, Jang H, Ok S, Eom J, Lee H, Kim SH, Kim JH, Jeong YM, Kim KJ, Yun SP, et al. WGA-M001, a Mixture of Total Extracts of Tagetes erecta and Ocimum basilicum, Synergistically Alleviates Cartilage Destruction by Inhibiting ERK and NF-κB Signaling. International Journal of Molecular Sciences. 2023; 24(24):17459. https://doi.org/10.3390/ijms242417459
Chicago/Turabian StyleOh, Eunjeong, Hahyeong Jang, Subin Ok, Jiwon Eom, Hyunyong Lee, Sung Hun Kim, Jong Hwa Kim, Yu Mi Jeong, Kyeong Jin Kim, Seung Pil Yun, and et al. 2023. "WGA-M001, a Mixture of Total Extracts of Tagetes erecta and Ocimum basilicum, Synergistically Alleviates Cartilage Destruction by Inhibiting ERK and NF-κB Signaling" International Journal of Molecular Sciences 24, no. 24: 17459. https://doi.org/10.3390/ijms242417459
APA StyleOh, E., Jang, H., Ok, S., Eom, J., Lee, H., Kim, S. H., Kim, J. H., Jeong, Y. M., Kim, K. J., Yun, S. P., Kwon, H.-J., Lee, I.-C., Park, J.-Y., & Yang, S. (2023). WGA-M001, a Mixture of Total Extracts of Tagetes erecta and Ocimum basilicum, Synergistically Alleviates Cartilage Destruction by Inhibiting ERK and NF-κB Signaling. International Journal of Molecular Sciences, 24(24), 17459. https://doi.org/10.3390/ijms242417459






