Local Effects of Steroid Hormones within the Bone Microenvironment
Abstract
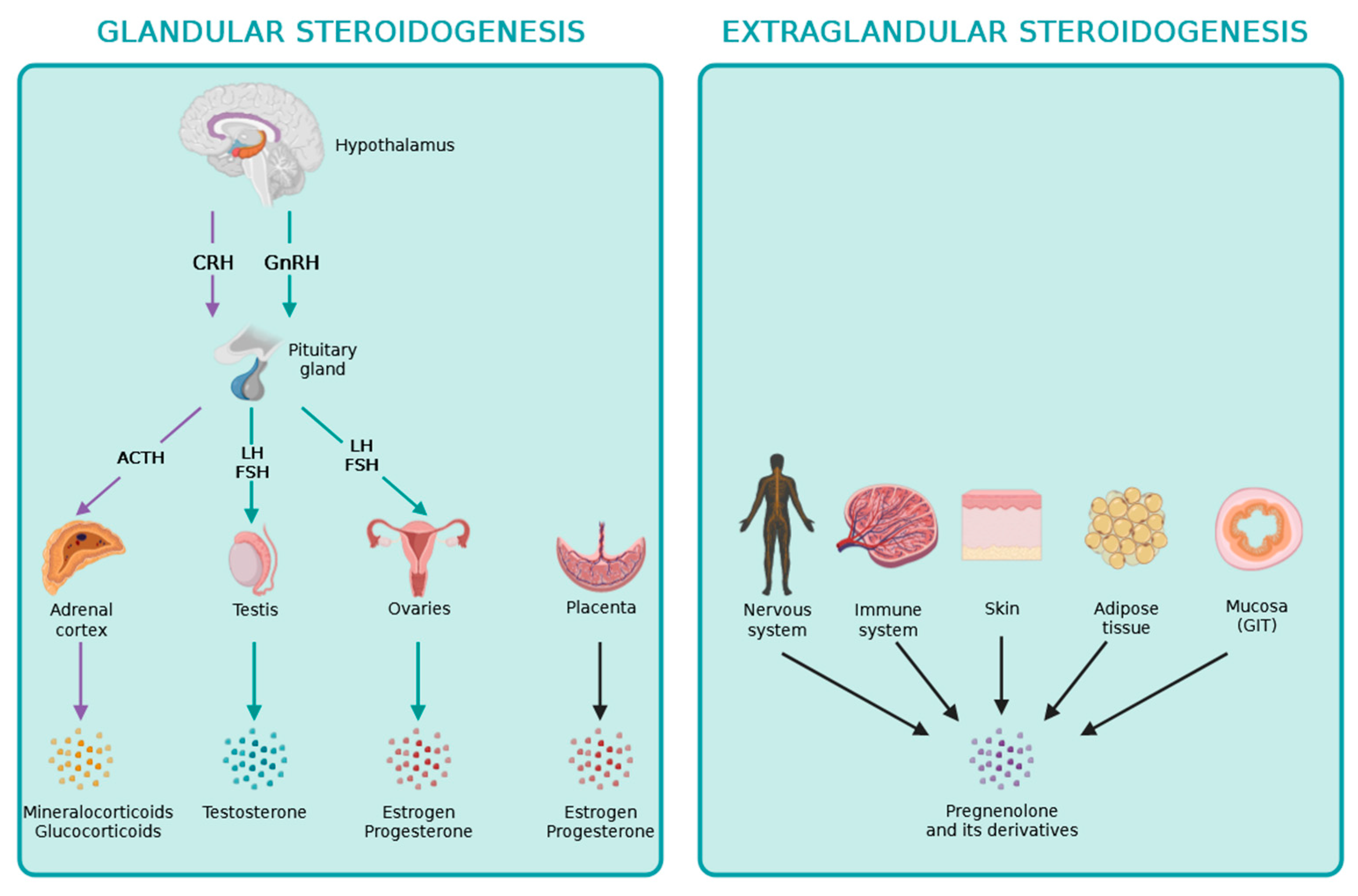
:1. Introduction
2. Glandular Steroidogenesis
3. Extraglandular Steroidogenesis
3.1. Local Steroidogenesis within the Thymus
3.2. Local Steroidogenesis within the Nervous System
3.3. Local Steroidogenesis within the Immune System
3.4. Local Steroidogenesis within the Skin
3.5. Local Steroidogenesis within the Adipose Tissue
3.6. Local Steroidogenesis within the Intestinal Mucosa
4. Local Effects of Steroid Hormones on Bone Cells
4.1. Role of Steroid Hormones on Osteoblasts
4.1.1. Effects of Glucocorticoids on Osteoblasts
4.1.2. Effects of Sex Steroids on Osteoblasts
4.2. Role of Steroid Hormones on Osteocytes
Effects of Glucocorticoids and Sex Steroids on Osteocytes
4.3. Role of Steroid Hormones on Osteoclasts
Effects of Glucocorticoids and Sex Steroids on Osteoclasts
4.4. Role of Extraglandular Steroidogenesis in the Bone Microenvironment
4.5. Role of Secosteroids in the Modulation of Bone Homeostasis
5. Clinical Relevance
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miller, W.L.; Auchus, R.J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 2011, 32, 81–151. [Google Scholar] [CrossRef]
- Miller, W.L. Steroid Hormone Synthesis in Mitochondria. Mol. Cell Endocrinol. 2013, 379, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Strushkevich, N.; MacKenzie, F.; Cherkesova, T.; Grabovec, I.; Usanov, S.; Park, H.W. Structural Basis for Pregnenolone Biosynthesis by the Mitochondrial Monooxygenase System. Proc. Natl. Acad. Sci. USA 2011, 108, 10139–10143. [Google Scholar] [CrossRef] [PubMed]
- Arakane, F.; Sugawara, T.; Nishino, H.; Liu, Z.; Holt, J.A.; Pain, D.; Stocco, D.M.; Miller, W.L.; Strauss, J.F. Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial import sequence: Implications for the mechanism of StAR action. Proc. Natl. Acad. Sci. USA 1996, 93, 13731–13736. [Google Scholar] [CrossRef]
- Hall, P.F. Cytochromes P450 and the regulation of steroid synthesis. Steroids 1986, 48, 133–196. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.K.; Auchus, R.J. Cellular redox state regulates hydroxysteroid dehydrogenase activity and intracellular hormone potency. Endocrinology 2005, 146, 2531–2538. [Google Scholar] [CrossRef] [PubMed]
- Mesiano, S.; Coulter, C.L.; Jaffe, R.B. Localization of cytochrome P450 cholesterol side-chain cleavage, cytochrome P450 17α-hydroxylase/17,20 lyase, and 3β-hydroxysteroid dehydrogenase-isomerase steroidogenic enzymes in human and rhesus monkey fetal adrenal glands: Reappraisal of functional zonation. J. Clin. Endocrinol. Metab. 1993, 77, 1184–1189. [Google Scholar] [PubMed]
- Suzuki, T.; Sasano, H.; Takeyama, J.; Kaneko, C.; Freije, W.A.; Carr, B.R.; Rainey, W.E. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: Immunohistochemical studies. Clin. Endocrinol. 2000, 53, 739–747. [Google Scholar] [CrossRef]
- Endoh, A.; Kristiansen, S.B.; Casson, P.R.; Buster, J.E.; Hornsby, P.J. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3β-hydroxysteroid dehydrogenase. J. Clin. Endocrinol. Metab. 1996, 81, 3558–3565. [Google Scholar]
- Mapes, S.; Corbin, C.J.; Tarantal, A.; Conley, A. The primate adrenal zona reticularis is defined by expression of cytochrome b5, 17α-hydroxylase/17,20-lyase cytochrome P450 (P450c17) and NADPH-cytochrome P450 reductase (reductase) but not 3β-hydroxysteroid dehydrogenase/Δ5-4 isomerase (3β-HSD). J. Clin. Endocrinol. Metab. 1999, 84, 3382–3385. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Mapes, S.M.; Corbin, C.J.; Conley, A.J. Morphological adrenarche in rhesus macaques: Development of the zona reticularis is concurrent with fetal zone regression in the early neonatal period. J. Endocrinol. 2008, 199, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.G.; Akahira, J.; Suzuki, T.; Nio, M.; Nakamura, Y.; Suzuki, H.; Rainey, W.E.; Sasano, H. Development of the human adrenal zona reticularis: Morphometric and immunocytochemical studies from birth to adolescence. J. Endocrinol. 2009, 203, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Flück, C.E.; Miller, W.L.; Auchus, R.J. The 17,20 lyase activity of cytochrome P450c17 from human fetal testis favors the Δ5 steroidogenic pathway. J. Clin. Endocrinol. Metab. 2003, 88, 3762–3766. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, R.; Tapanainen, J.; Chung, B.C.; Matteson, K.J.; Miller, W.L. Hormonal regulation of P450scc (20,22-desmolase) and P450c17 (17α-hydroxylase/17,20-lyase) in cultured human granulosa cells. J. Clin. Endocrinol. Metab. 1986, 63, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.C.; Polanco, J.R.; Min, S.; Michael, S.D. Mitochondrial 3β-hydroxysteroid dehydrogenase (HSD) is essential for the synthesis of progesterone by corpora lutea: An hypothesis. Reprod. Biol. Endocrinol. 2005, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.C.; Matteson, K.J.; Voutilainen, R.; Mohandas, T.K.; Miller, W.L. Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta. Proc. Natl. Acad. Sci. USA 1986, 83, 8962–8966. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Steroidogenesis: Unanswered Questions. Trends Endocrinol. Metab. 2017, 28, 771–793. [Google Scholar] [CrossRef]
- Chakraborty, S.; Pramanik, J.; Mahata, B. Revisiting steroidogenesis and its role in immune regulation with the advanced tools and technologies. Genes. Immun. 2021, 22, 125–140. [Google Scholar] [CrossRef]
- Vacchio, M.S.; Papadopoulos, V.; Ashwell, J.D. Steroid production in the thymus: Implications for thymocyte selection. J. Exp. Med. 1994, 179, 1835–1846. [Google Scholar] [CrossRef]
- King, S.R.; Manna, P.R.; Ishii, T.; Syapin, P.J.; Ginsberg, S.D.; Wilson, K.; Walsh, L.P.; Parker, K.L.; Stocco, D.M.; Smith, R.G.; et al. An essential component in steroid synthesis, the steroidogenic acute regulatory protein, is expressed in discrete regions of the brain. J. Neurosci. 2002, 22, 10613–10620. [Google Scholar] [CrossRef]
- Belelli, D.; Lambert, J.J. Neurosteroids: Endogenous regulators of the GABA(A) receptor. Nat. Rev. Neurosci. 2005, 6, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Rubinow, K.B. An intracrine view of sex steroids, immunity, and metabolic regulation. Mol. Metab. 2018, 15, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Milewich, L.; Kaimal, V.; Toews, G.B. Androstenedione metabolism in human alveolar macrophages. J. Clin. Endocrinol. Metab. 1983, 56, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Cutolo, M.; Accardo, S.; Villaggio, B.; Barone, A.; Sulli, A.; Balleari, E.; Bason, C.; Felli, L.; Granata, O.M.; Amodio, R.; et al. Androgen metabolism and inhibition of interleukin-1 synthesis in primary cultured human synovial macrophages. Mediators Inflamm. 1995, 4, 138–143. [Google Scholar] [CrossRef]
- Schmidt, M.; Kreutz, M.; Loffler, G.; Scholmerich, J.; Straub, R.H. Conversion of dehydroepiandrosterone to downstream steroid hormones in macrophages. J. Endocrinol. 2000, 164, 161–169. [Google Scholar] [CrossRef]
- Krukowski, K.; Eddy, J.; Kosik, K.L.; Konley, T.; Janusek, L.W.; Mathews, H.L. Glucocorticoid dysregulation of natural killer cell function through epigenetic modification. Brain Behav. Immun. 2011, 25, 239–249. [Google Scholar] [CrossRef]
- Eddy, J.L.; Krukowski, K.; Janusek, L.; Mathews, H.L. Glucocorticoids regulate natural killer cell function epigenetically. Cell Immunol. 2014, 290, 120–130. [Google Scholar] [CrossRef]
- Morgan, D.J.; Davis, D.M. Distinct effects of dexamethasone on human natural killer cell responses dependent on cytokines. Front. Immunol. 2017, 8, 432. [Google Scholar] [CrossRef]
- Vitale, C.; Chiossone, L.; Cantoni, C.; Morreale, G.; Cottalasso, F.; Moretti, S.; Pistorio, A.; Haupt, R.; Lanino, E.; Dini, G.; et al. The corticosteroid-induced inhibitory effect on NK cell function reflects down-regulation and/or dysfunction of triggering receptors involved in natural cytotoxicity. Eur. J. Immunol. 2004, 34, 3028–3038. [Google Scholar] [CrossRef]
- Quatrini, L.; Wieduwild, E.; Escaliere, B.; Filtjens, J.; Chasson, L.; Laprie, C.; Vivier, E.; Ugolini, S. Endogenous glucocorticoids control host resistance to viral infection through the tissue-specific regulation of PD-1 expression on NK cells. Nat. Immunol. 2018, 19, 954–962. [Google Scholar] [CrossRef]
- Quatrini, L.; Vacca, P.; Tumino, N.; Besi, F.; Di Pace, A.L.; Scordamaglia, F.; Martini, S.; Munari, E.; Mingari, M.C.; Ugolini, S.; et al. Glucocorticoids and the cytokines IL-12, IL-15, and IL-18 present in the tumor microenvironment induce PD-1 expression on human natural killer cells. J. Allergy Clin. Immunol. 2021, 147, 349–360. [Google Scholar] [CrossRef]
- Curran, E.M.; Berghaus, L.J.; Vernetti, N.J.; Saporita, A.J.; Lubahn, D.B.; Estes, D.M. Natural killer cells express estrogen receptor-alpha and estrogen receptor-beta and can respond to estrogen via a non-estrogen receptor-alpha-mediated pathway. Cell Immunol. 2001, 214, 12–20. [Google Scholar] [CrossRef]
- Chantakru, S.; Wang, W.C.; van den Heuvel, M.; Bashar, S.; Simpson, A.; Chen, Q.; Croy, B.A.; Evans, S.S. Coordinate regulation of lymphocyte-endothelial interactions by pregnancy-associated hormones. J. Immunol. 2003, 171, 4011–4019. [Google Scholar] [CrossRef]
- Gibson, D.A.; Greaves, E.; Critchley, H.O.; Saunders, P.T. Estrogen-dependent regulation of human uterine natural killer cells promotes vascular remodelling via secretion of CCL2. Hum. Reprod. 2015, 30, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Sentman, C.L.; Meadows, S.K.; Wira, C.R.; Eriksson, M. Recruitment of uterine NK cells: Induction of CXC chemokine ligands 10 and 11 in human endometrium by estradiol and progesterone. J. Immunol. 2004, 173, 6760–6766. [Google Scholar] [CrossRef] [PubMed]
- Piemonti, L.; Monti, P.; Allavena, P.; Sironi, M.; Soldini, L.; Leone, B.E.; Socci, C.; Di Carlo, V. Glucocorticoids affect human dendritic cell differentiation and maturation. J. Immunol. 1999, 162, 6473–6481. [Google Scholar] [CrossRef]
- Xia, C.Q.; Peng, R.; Beato, F.; Clare-Salzler, M.J. Dexamethasone induces IL-10-producing monocyte-derived dendritic cells with durable immaturity. Scand. J. Immunol. 2005, 62, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Matyszak, M.K.; Citterio, S.; Rescigno, M.; Ricciardi-Castagnoli, P. Differential effects of corticosteroids during different stages of dendritic cell maturation. Eur. J. Immunol. 2000, 30, 1233–1242. [Google Scholar] [CrossRef]
- Larange, A.; Antonios, D.; Pallardy, M.; Kerdine-Romer, S. Glucocorticoids inhibit dendritic cell maturation induced by Toll-like receptor 7 and Toll-like receptor 8. J. Leukoc. Biol. 2012, 91, 105–117. [Google Scholar] [CrossRef]
- Boor, P.P.; Metselaar, H.J.; Mancham, S.; Tilanus, H.W.; Kusters, J.G.; Kwekkeboom, J. Prednisolone suppresses the function and promotes apoptosis of plasmacytoid dendritic cells. Am. J. Transpl. 2006, 6, 2332–2341. [Google Scholar] [CrossRef]
- Shodell, M.; Siegal, F.P. Corticosteroids depress IFN-alpha-producing plasmacytoid dendritic cells in human blood. J. Allergy Clin. Immunol. 2001, 108, 446–448. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Thomas, J.A. Steroids inhibit uptake and/or processing but not presentation of antigen by airway dendritic cells. Immunology 1997, 91, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ju, D.; Wang, Q.; Zhang, M.; Xia, D.; Zhang, L.; Yu, H.; Cao, X. Dexamethasone inhibits the antigen presentation of dendritic cells in MHC class II pathway. Immunol. Lett. 2001, 76, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Munitic, I.; Mittelstadt, P.R.; Castro, E.; Ashwell, J.D. Suppression of dendritic cell-derived IL-12 by endogenous glucocorticoids is protective in LPS-induced sepsis. PLoS Biol. 2015, 13, e1002269. [Google Scholar] [CrossRef] [PubMed]
- Paharkova-Vatchkova, V.; Maldonado, R.; Kovats, S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J. Immunol. 2004, 172, 1426–1436. [Google Scholar] [CrossRef]
- Xiu, F.; Anipindi, V.C.; Nguyen, P.V.; Boudreau, J.; Liang, H.; Wan, Y.; Snider, D.P.; Kaushic, C. High physiological concentrations of progesterone reverse estradiol-mediated changes in differentiation and functions of bone marrow derived dendritic cells. PLoS ONE 2016, 11, e0153304. [Google Scholar] [CrossRef] [PubMed]
- Shimba, A.; Cui, G.; Tani-Ichi, S.; Ogawa, M.; Abe, S.; Okazaki, F.; Kitano, S.; Miyachi, H.; Yamada, H.; Hara, T.; et al. Glucocorticoids drive diurnal oscillations in T cell distribution and responses by inducing interleukin-7 receptor and CXCR4. Immunity 2018, 48, 286–298.e6. [Google Scholar] [CrossRef]
- Franchimont, D.; Galon, J.; Gadina, M.; Visconti, R.; Zhou, Y.; Aringer, M.; Frucht, D.M.; Chrousos, G.P.; O’Shea, J.J. Inhibition of Th1 immune response by glucocorticoids: Dexamethasone selectively inhibits IL-12-induced Stat4 phosphorylation in T lymphocytes. J. Immunol. 2000, 164, 1768–1774. [Google Scholar] [CrossRef]
- Liberman, A.C.; Druker, J.; Refojo, D.; Holsboer, F.; Arzt, E. Glucocorticoids inhibit GATA-3 phosphorylation and activity in T cells. FASEB J. 2009, 23, 1558–1571. [Google Scholar] [CrossRef]
- Maeda, N.; Maruhashi, T.; Sugiura, D.; Shimizu, K.; Okazaki, I.M.; Okazaki, T. Glucocorticoids potentiate the inhibitory capacity of programmed cell death 1 by up-regulating its expression on T cells. J. Biol. Chem. 2019, 294, 19896–19906. [Google Scholar] [CrossRef]
- Ugor, E.; Prenek, L.; Pap, R.; Berta, G.; Ernszt, D.; Najbauer, J.; Németh, P.; Boldizsár, F.; Berki, T. Glucocorticoid hormone treatment enhances the cytokine production of regulatory T cells by upregulation of Foxp3 expression. Immunobiology 2018, 223, 422–431. [Google Scholar] [CrossRef]
- Kim, D.; Nguyen, Q.T.; Lee, J.; Lee, S.H.; Janocha, A.; Kim, S.; Le, H.T.; Dvorina, N.; Weiss, K.; Cameron, M.J.; et al. Anti-inflammatory roles of glucocorticoids are mediated by Foxp3(+) regulatory T cells via a miR-342-dependent mechanism. Immunity 2020, 53, 581–596.e5. [Google Scholar] [CrossRef] [PubMed]
- Pandolfi, J.; Baz, P.; Fernández, P.; Discianni Lupi, A.; Payaslián, F.; Billordo, L.A.; Fainboim, L.; Arruvito, L. Regulatory and effector T-cells are differentially modulated by Dexamethasone. Clin. Immunol. 2013, 149, 400–410. [Google Scholar] [CrossRef]
- Engler, J.B.; Kursawe, N.; Solano, M.E.; Patas, K.; Wehrmann, S.; Heckmann, N.; Lühder, F.; Reichardt, H.M.; Arck, P.C.; Gold, S.M.; et al. Glucocorticoid receptor in T cells mediates protection from autoimmunity in pregnancy. Proc. Natl. Acad. Sci. USA 2017, 114, E181–E190. [Google Scholar] [CrossRef]
- Lélu, K.; Laffont, S.; Delpy, L.; Paulet, P.E.; Périnat, T.; Tschanz, S.A.; Pelletier, L.; Engelhardt, B.; Guéry, J.C. Estrogen receptor alpha signaling in T lymphocytes is required for estradiol-mediated inhibition of Th1 and Th17 cell differentiation and protection against experimental autoimmune encephalomyelitis. J. Immunol. 2011, 187, 2386–2393. [Google Scholar] [CrossRef]
- Maret, A.; Coudert, J.D.; Garidou, L.; Foucras, G.; Gourdy, P.; Krust, A.; Dupont, S.; Chambon, P.; Druet, P.; Bayard, F.; et al. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo. Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur. J. Immunol. 2003, 33, 512–521. [Google Scholar] [CrossRef]
- Chen, R.Y.; Fan, Y.M.; Zhang, Q.; Liu, S.; Li, Q.; Ke, G.L.; Li, C.; You, Z. Estradiol inhibits Th17 cell differentiation through inhibition of RORgammaT transcription by recruiting the ERalpha/REA complex to estrogen response elements of the RORgammaT promoter. J. Immunol. 2015, 194, 4019–4028. [Google Scholar] [CrossRef] [PubMed]
- Schleimer, R.P.; Jacques, A.; Shin, H.S.; Lichtenstein, L.M.; Plaut, M. Inhibition of T cell-mediated cytotoxicity by anti-inflammatory steroids. J. Immunol. 1984, 132, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Gruver-Yates, A.L.; Quinn, M.A.; Cidlowski, J.A. Analysis of glucocorticoid receptors and their apoptotic response to dexamethasone in male murine B cells during development. Endocrinology 2014, 155, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmson, A.S.; Lantero Rodriguez, M.; Stubelius, A.; Fogelstrand, P.; Johansson, I.; Buechler, M.B.; Lianoglou, S.; Kapoor, V.N.; Johansson, M.E.; Fagman, J.B.; et al. Testosterone is an endogenous regulator of BAFF and splenic B cell number. Nat. Commun. 2018, 9, 2067. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Li, L.; Liu, X.; Ma, C.; Zhang, J.; Jiao, Y.; You, L.; Chen, Z.J.; Zhao, Y. Estrogen promotes B cell activation in vitro through down-regulating CD80 molecule expression. Gynecol. Endocrinol. 2011, 27, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Mahata, B.; Zhang, X.; Kolodziejczyk, A.A.; Proserpio, V.; Haim-Vilmovsky, L.; Taylor, A.E.; Hebenstreit, D.; Dingler, F.A.; Moignard, V.; Göttgens, B.; et al. Single-cell RNA sequencing reveals T helper cells synthesizing steroids de novo to contribute to immune homeostasis. Cell Rep. 2014, 22, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Mahata, B.; Pramanik, J.; van der Weyden, L.; Polanski, K.; Kar, G.; Riedel, A.; Chen, X.; Fonseca, N.A.; Kundu, K.; Campos, L.S.; et al. Tumors induce de novo steroid biosynthesis in T cells to evade immunity. Nat. Commun. 2020, 11, 3588. [Google Scholar] [CrossRef]
- Hannen, R.F.; Michael, A.E.; Jaulim, A.; Bhogal, R.; Burrin, J.M.; Philpott, M.P. Steroid synthesis by primary human keratinocytes; implications for skin disease. Biochem. Biophys. Res. Commun. 2011, 404, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.; Zbytek, B.; Nikolakis, G.; Manna, P.R.; Skobowiat, C.; Zmijewski, M.; Li, W.; Janjetovic, Z.; Postlethwaite, A.; Zouboulis, C.C.; et al. Steroidogenesis in the skin: Implications for local immune functions. J. Steroid Biochem. Mol. Biol. 2013, 137, 107–123. [Google Scholar] [CrossRef]
- Byeon, H.R.; Lee, S.H. Expression of steroidogenesis-related genes in rat adipose tissues. Dev. Reprod. 2016, 20, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Papadopoulos, V.; Vihma, V. Steroid biosynthesis in adipose tissue. Steroids 2015, 103, 89–104. [Google Scholar] [CrossRef]
- Cima, I.; Corazza, N.; Dick, B.; Fuhrer, A.; Herren, S.; Jakob, S.; Ayuni, E.; Mueller, C.; Brunner, T. Intestinal epithelial cells synthesize glucocorticoids and regulate T cell activation. J. Exp. Med. 2004, 200, 1635–1646. [Google Scholar] [CrossRef]
- Ahmed, A.; Schmidt, C.; Brunner, T. Extra-adrenal glucocorticoid synthesis in the intestinal mucosa: Between immune homeostasis and immune escape. Front. Immunol. 2019, 10, 1438. [Google Scholar] [CrossRef]
- Sirianni, R.; Seely, J.B.; Attia, G.; Stocco, D.M.; Carr, B.R.; Pezzi, V.; Rainey, W.E. Liver receptor homologue-1 is expressed in human steroidogenic tissues and activates transcription of genes encoding steroidogenic enzymes. J. Endocrinol. 2002, 174, R13–R17. [Google Scholar] [CrossRef]
- Bayrer, J.R.; Wang, H.; Nattiv, R.; Suzawa, M.; Escusa, H.S.; Fletterick, R.J.; Klein, O.D.; Moore, D.D.; Ingraham, H.A. LRH-1 mitigates intestinal inflammatory disease by maintaining epithelial homeostasis and cell survival. Nat. Commun. 2018, 9, 4055. [Google Scholar] [CrossRef] [PubMed]
- Almeida, M.; Laurent, M.R.; Dubois, V.; Claessens, F.; O’Brien, C.A.; Bouillon, R.; Vanderschueren, D.; Manolagas, S.C. Estrogens and androgens in skeletal physiology and pathophysiology. Physiol. Rev. 2017, 97, 135–187. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Monroe, D.G. Regulation of bone metabolism by sex steroids. Cold Spring Harb. Perspect. Med. 2018, 8, a031211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Mak, W.; Zheng, Y.; Dunstan, C.R.; Seibel, M.J. Osteoblasts Directly Control Lineage Commitment of Mesenchymal Progenitor Cells Through Wnt Signaling. J. Biol. Chem. 2008, 283, 1936–1945. [Google Scholar] [CrossRef]
- Gado, M.; Baschant, U.; Hofbauer, L.C.; Henneicke, H. Bad to the Bone: The Effects of Therapeutic Glucocorticoids on Osteoblasts and Osteocytes. Front. Endocrinol. 2022, 13, 835720. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.; Shao, X.; Dunstan, C.R.; Seibel, M.J.; Zhou, H. Biphasic Glucocorticoid-Dependent Regulation of Wnt Expression and its Inhibitors in Mature Osteoblastic Cells. Calcified Tissue Int. 2009, 85, 538–545. [Google Scholar] [CrossRef]
- Hildebrandt, S.; Baschant, U.; Thiele, S.; Tuckermann, J.; Hofbauer, L.C.; Rauner, M. Glucocorticoids Suppress Wnt16 Expression in Osteoblasts In Vitro and In Vivo. Sci. Rep. 2018, 8, 8711. [Google Scholar] [CrossRef] [PubMed]
- Luppen, C.A.; Smith, E.; Spevak, L.; Boskey, A.L.; Frenkel, B. Bone Morphogenetic Protein-2 Restores Mineralization in Glucocorticoid-Inhibited MC3T3-E1 Osteoblast Cultures. J. Bone Mineral Res. 2003, 18, 1186–1197. [Google Scholar] [CrossRef]
- Delany, A.M.; Durant, D.; Canalis, E. Glucocorticoid Suppression of IGF I Transcription in Osteoblasts. Mol. Endocrinol. 2001, 15, 1781–1789. [Google Scholar] [CrossRef]
- Pereira, R.M.R.; Delany, A.M.; Canalis, E. Cortisol Inhibits the Differentiation and Apoptosis of Osteoblasts in Culture. Bone 2001, 28, 484–490. [Google Scholar] [CrossRef]
- Rauch, A.; Seitz, S.; Baschant, U.; Schilling, A.F.; Illing, A.; Stride, B.; Kirilov, M.; Mandic, V.; Takacz, A.; Schmidt-Ullrich, R.; et al. Glucocorticoids Suppress Bone Formation by Attenuating Osteoblast Differentiation via the Monomeric Glucocorticoid Receptor. Cell Metab. 2010, 11, 517–531. [Google Scholar] [CrossRef]
- Sims, N.A.; Clément-Lacroix, P.; Minet, D.; Fraslon-Vanhulle, C.; Gaillard-Kelly, M.; Resche-Rigon, M.; Baron, R. A functional androgen receptor is not sufficient to allow estradiol to protect bone after gonadectomy in estradiol receptor-deficient mice. J. Clin. Investig. 2003, 111, 1319–1327. [Google Scholar] [CrossRef]
- Jilka, R.L.; Takahashi, K.; Munshi, M.; Williams, D.C.; Roberson, P.K.; Manolagas, S.C. Loss of estrogen upregulates osteoblastogenesis in the murine bone marrow: Evidence for autonomy from factors released during bone resorption. J. Clin. Investig. 1998, 101, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Manolagas, S.C. Steroids and osteoporosis: The quest for mechanisms. J. Clin. Investig. 2013, 123, 1919–1921. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the Skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Ferlin, A.; Selice, R.; Carraro, U.; Foresta, C. Testicular function and bone metabolism—Beyond testosterone. Nat. Rev. Endocrinol. 2013, 9, 548–554. [Google Scholar] [CrossRef]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Xiong, J.; Onal, M.; Jilka, R.L.; Weinstein, R.S.; Manolagas, S.C.; O’Brien, C.A. Matrix-embedded cells control osteoclast formation. Nat. Med. 2011, 17, 1235–1241. [Google Scholar] [CrossRef]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Manolagas, S.C.; Parfitt, A.M. For whom the bell tolls: Distress signals from long-lived osteocytes and the pathogenesis of metabolic bone diseases. Bone 2013, 54, 272–278. [Google Scholar] [CrossRef]
- Manolagas, S.C. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr. Rev. 2010, 31, 266–300. [Google Scholar] [CrossRef]
- Almeida, M.; Han, L.; Ambrogini, E.; Bartell, S.M.; Manolagas, S.C. Oxidative stress stimulates apoptosis and activates NF-kappaB in osteoblastic cells via a PKCbeta/p66shc signaling cascade: Counter regulation by estrogens or androgens. Mol. Endocrinol. 2010, 24, 2030–2037. [Google Scholar] [CrossRef]
- Almeida, M.; Han, L.; Ambrogini, E.; Weinstein, R.S.; Manolagas, S.C. Glucocorticoids and tumor necrosis factor (TNF) alpha increase oxidative stress and suppress WNT signaling in osteoblasts. J. Biol. Chem. 2011, 286, 44326–44335. [Google Scholar] [CrossRef]
- Gyori, D.; Mocsai, A. Osteoclasts in Inflammation. In Compendium of Inflammatory Diseases; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Gyori, D.S.; Mocsai, A. Osteoclast Signal Transduction During Bone Metastasis Formation. Front. Cell Dev. Biol. 2020, 8, 507. [Google Scholar] [CrossRef]
- Kimble, R.B.; Vannice, J.L.; Bloedow, D.C.; Thompson, R.C.; Hopfer, W.; Kung, V.T.; Brownfield, C.; Pacifici, R. Interleukin-1 receptor antagonist decreases bone loss and bone resorption in ovariectomized rats. J. Clin. Investig. 1994, 93, 1959–1967. [Google Scholar] [CrossRef]
- Ammann, P.; Rizzoli, R.; Bonjour, J.; Bourrin, S.; Meyer, J.; Vassalli, P.; Garcia, I. Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. J. Clin. Investig. 1997, 99, 1699–1703. [Google Scholar] [CrossRef]
- Kimble, R.B.; Srivastava, S.; Ross, F.P.; Matayoshi, A.; Pacifici, R. Estrogen deficiency increases the ability of stromal cells to support murine osteoclastogenesis via an interleukin-1-and tumor necrosis factor-mediated stimulation of macrophage colony-stimulating factor production. J. Biol. Chem. 1996, 271, 28890–28897. [Google Scholar] [CrossRef]
- Kitazawa, R.; Kimble, R.B.; Vannice, J.L.; Kung, V.T.; Pacifici, R. Interleukin-1 receptor antagonist and tumor necrosis factor binding protein decrease osteoclast formation and bone resorption in ovariectomized mice. J. Clin. Investig. 1994, 94, 2397–2406. [Google Scholar] [CrossRef] [PubMed]
- Charatcharoenwitthaya, N.; Khosla, S.; Atkinson, E.J.; McCready, L.K.; Riggs, B.L. Effect of blockade of TNF-a and interleukin-1 action on bone resorption in early postmenopausal women. J. Bone Miner. Res. 2007, 22, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Roggia, C.; Gao, Y.; Cenci, S.; Weitzmann, M.N.; Toraldo, G.; Isaia, G.; Pacifici, R. Up-regulation of TNF-producing T cells in the bone marrow: A key mechanism by which estrogen deficiency induces bone loss in vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 13960–13965. [Google Scholar] [CrossRef] [PubMed]
- Cenci, S.; Weitzmann, M.N.; Roggia, C.; Namba, N.; Novack, D.; Woodring, J.; Pacifici, R. Estrogen deficiency induces bone loss by enhancing T-cell production of TNF-a. J. Clin. Investig. 2000, 106, 1229–1327. [Google Scholar] [CrossRef]
- Nakamura, T.; Imai, Y.; Matsumoto, T.; Sato, S.; Takeuchi, K.; Igarashi, K.; Harada, Y.; Azuma, Y.; Krust, A.; Yamamoto, Y.; et al. Estrogen prevents bone loss via estrogen receptor alpha and induction of fas ligand in osteoclasts. Cell 2007, 130, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Martin-Millan, M.; Almeida, M.; Ambrogini, E.; Han, L.; Zhao, H.; Weinstein, R.S.; Jilka, R.L.; O’Brien, C.A.; Manolagas, S.C. The estrogen receptor-alpha in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol. Endocrinol. 2010, 24, 323–334. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Glucocorticoids and the osteoclast. Clin. Exp. Rheumatol. 2015, 33 (Suppl. S92), S37–S39. [Google Scholar] [PubMed]
- Moon, H.H.; Clines, K.L.; O’Day, P.J.; Al-Barghouthi, B.M.; Farber, E.A.; Farber, C.R.; Auchus, R.J.; Clines, G.A. Osteoblasts Generate Testosterone From DHEA and Activate Androgen Signaling in Prostate Cancer Cells. J. Bone Miner. Res. 2021, 36, 1566–1579. [Google Scholar] [CrossRef] [PubMed]
- Hagberg Thulin, M.; Nilsson, M.E.; Thulin, P.; Céraline, J.; Ohlsson, C.; Damber, J.E.; Welén, K. Osteoblasts promote castration-resistant prostate cancer by altering intratumoral steroidogenesis. Mol. Cell Endocrinol. 2016, 422, 182–191. [Google Scholar] [CrossRef]
- Huh, J.B.; Benko, P.; Sandor, L.F.; Hiraga, T.; Poliska, S.; Dobo-Nagy, C.; Simpson, J.P.; Homer, N.Z.M.; Mahata, B.; Gyori, D.S. De Novo Steroidogenesis in Tumor Cells Drives Bone Metastasis and Osteoclastogenesis 2023. Available online: https://ssrn.com/abstract=4523146 (accessed on 1 August 2023).
- Yenki, P.; Bhasin, S.; Liu, L.; Nabavi, N.; Cheng, C.W.; Tam, K.J.; Peacock, J.W.; Adomat, H.H.; Tombe, T.; Fazli, L.; et al. Semaphorin 3C promotes de novo steroidogenesis in prostate cancer cells. Endocr. Relat. Cancer 2023, 30, e230010. [Google Scholar] [CrossRef]
- Lubik, A.A.; Gunter, J.H.; Hendy, S.C.; Locke, J.A.; Adomat, H.H.; Thompson, V.; Herington, A.; Gleave, M.E.; Pollak, M.; Nelson, C.C. Insulin increases de novo steroidogenesis in prostate cancer cells. Cancer Res. 2011, 71, 5754–5764. [Google Scholar] [CrossRef]
- Lubik, A.A.; Gunter, J.H.; Hollier, B.G.; Ettinger, S.; Fazli, L.; Stylianou, N.; Hendy, S.C.; Adomat, H.H.; Gleave, M.E.; Pollak, M.; et al. IGF2 increases de novo steroidogenesis in prostate cancer cells. Endocr. Relat. Cancer 2013, 20, 173–186. [Google Scholar] [CrossRef]
- MacLaughlin, J.; Holick, M.F. Aging decreases the capacity of human skin to produce vitamin D3. J. Clin. Investig. 1985, 76, 1536–1538. [Google Scholar] [CrossRef]
- Merke, J.; Milde, P.; Lewicka, S.; Hügel, U.; Klaus, G.; Mangelsdorf, D.J.; Haussler, M.R.; Rauterberg, E.W.; Ritz, E. Identification and regulation of 1,25-dihydrox- vitamin D3 receptor activity and biosynthesis of 1,25-dihy- droxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J. Clin. Investig. 1989, 83, 1903–1915. [Google Scholar] [CrossRef] [PubMed]
- Di Monaco, M.; Castiglioni, C.; Tappero, R. Parathyroid hormone response to severe vitamin D deficiency is associated with femoral neck bone mineral density: An observational study of 405 women with hip-fracture. Hormones 2016, 15, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Mitri, J.; Mathieu, C.; Badenhoop, K.; Tamer, G.; Orio, F.; Mezza, T.; Vieth, R.; Colao, A.; Pittas, A. Mechanisms in endocrinology: Vitamin D as a potential contributor in endocrine health and disease. Eur. J. Endocrinol. 2014, 171, R101–R110. [Google Scholar] [CrossRef] [PubMed]
- Romano, F.; Serpico, D.; Cantelli, M.; Di Sarno, A.; Dalia, C.; Arianna, R.; Lavorgna, M.; Colao, A.; Di Somma, C. Osteoporosis and dermatoporosis: A review on the role of vitamin D. Front. Endocrinol. 2023, 14, 1231580. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Mahata, B.; Raman, C.; Bereshchenko, O. Editorial: Steroids and Secosteroids in the Modulation of Inflammation and Immunity. Front. Immunol. 2021, 12, 825577. [Google Scholar] [CrossRef]
- Postlethwaite, A.E.; Tuckey, R.C.; Kim, T.K.; Li, W.; Bhattacharya, S.K.; Myers, L.K.; Brand, D.D.; Slominski, A.T. 20S-Hydroxyvitamin D3, a Secosteroid Produced in Humans, Is Anti-Inflammatory and Inhibits Murine Autoimmune Arthritis. Front. Immunol. 2021, 12, 678487. [Google Scholar] [CrossRef] [PubMed]
- Frara, S.; Allora, A.; di Filippo, L.; Formenti, A.M.; Loli, P.; Polizzi, E.; Tradati, D.; Ulivieri, F.M.; Giustina, A. Osteopathy in mild adrenal Cushing’s syndrome and Cushing disease. Best. Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101515. [Google Scholar] [CrossRef]
- Chen, J.S.; Sambrook, P.N. Antiresorptive therapies for osteoporosis: A clinical overview. Nat. Rev. Endocrinol. 2011, 8, 81–91. [Google Scholar] [CrossRef]
- Roy, S.; Sipthorp, J.; Mahata, B.; Pramanik, J.; Hennrich, M.L.; Gavin, A.C.; Ley, S.V.; Teichmann, S.A. CLICK-enabled analogues reveal pregnenolone interactomes in cancer and immune cells. iScience 2021, 24, 102485. [Google Scholar] [CrossRef]
- O’Hara, L.; York, J.P.; Zhang, P.; Smith, L.B. Targeting of GFP-Cre to the mouse Cyp11a1 locus both drives cre recombinase expression in steroidogenic cells and permits generation of Cyp11a1 knock out mice. PLoS ONE 2014, 9, e84541. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandor, L.F.; Ragacs, R.; Gyori, D.S. Local Effects of Steroid Hormones within the Bone Microenvironment. Int. J. Mol. Sci. 2023, 24, 17482. https://doi.org/10.3390/ijms242417482
Sandor LF, Ragacs R, Gyori DS. Local Effects of Steroid Hormones within the Bone Microenvironment. International Journal of Molecular Sciences. 2023; 24(24):17482. https://doi.org/10.3390/ijms242417482
Chicago/Turabian StyleSandor, Luca F., Reka Ragacs, and David S. Gyori. 2023. "Local Effects of Steroid Hormones within the Bone Microenvironment" International Journal of Molecular Sciences 24, no. 24: 17482. https://doi.org/10.3390/ijms242417482




