The Anti-Inflammatory Effect of Multi-Wavelength Light-Emitting Diode Irradiation Attenuates Dry Eye Symptoms in a Scopolamine-Induced Mouse Model of Dry Eye
Abstract
1. Introduction
2. Results
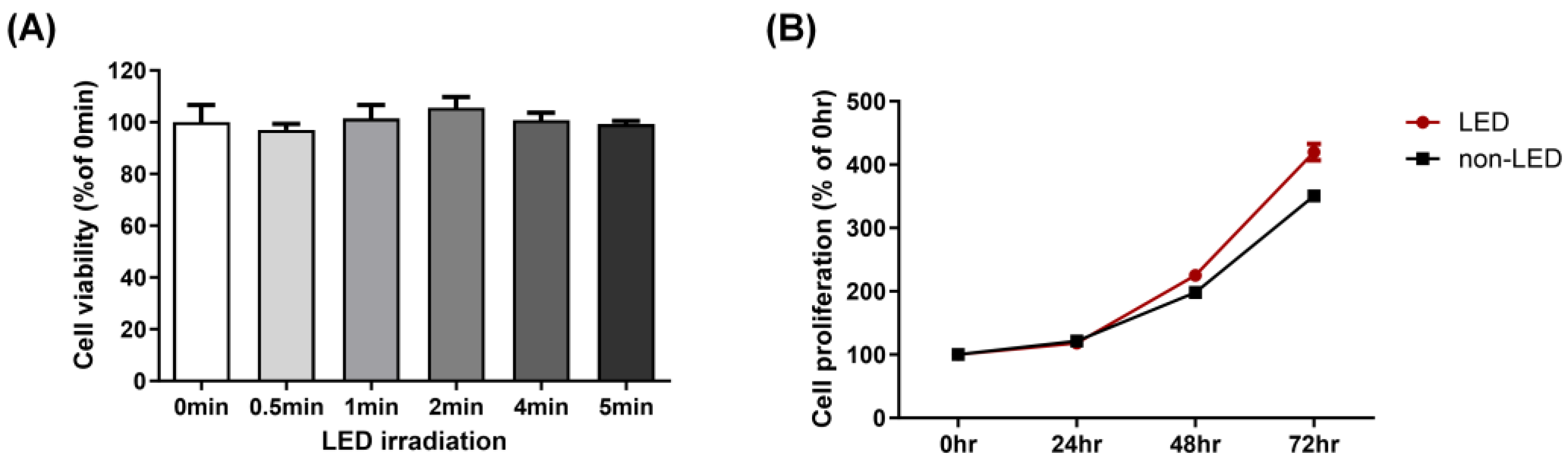
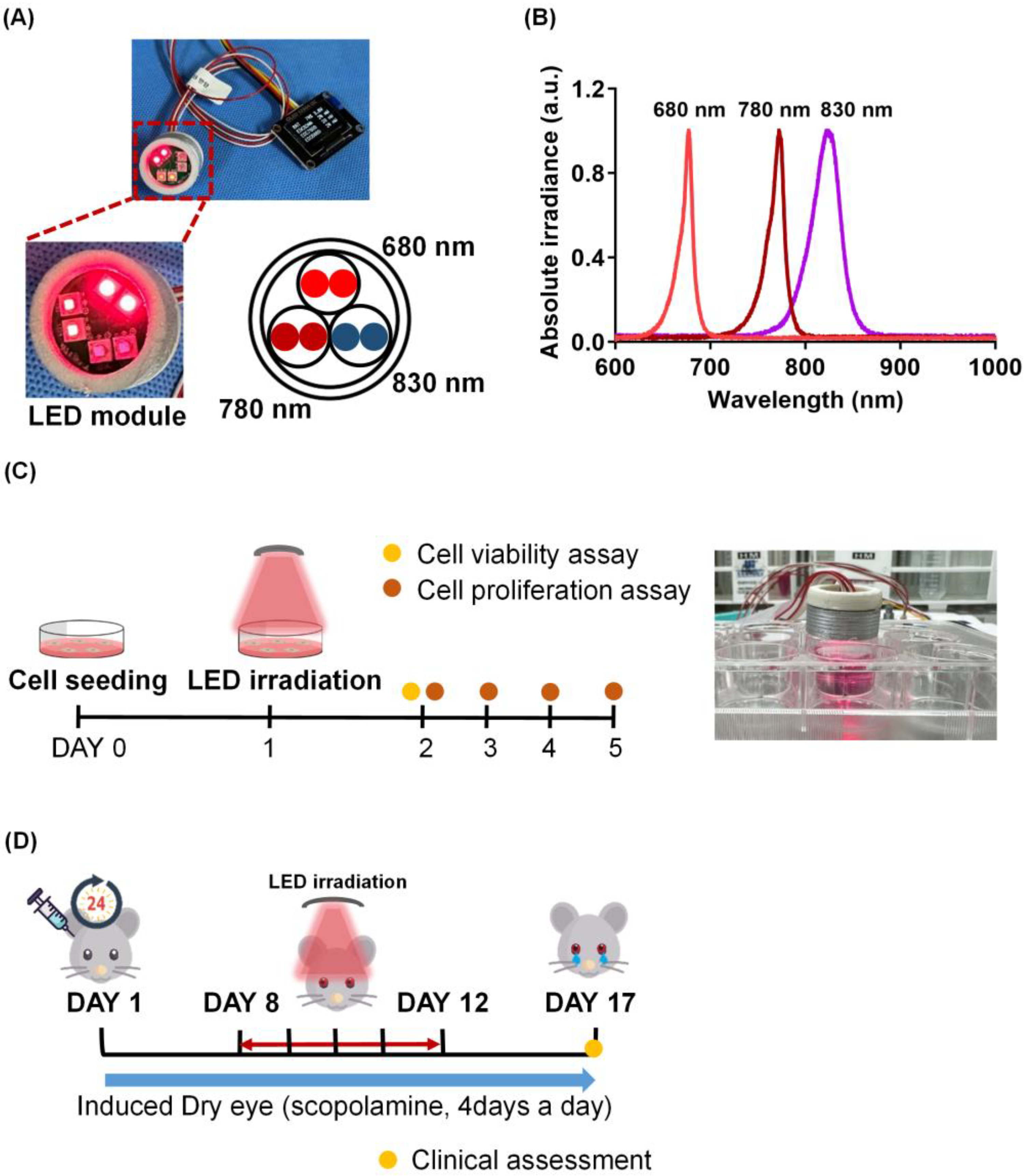
2.1. Multi-Wavelength LED Irradiation Has No Toxicity on Human Corneal Epithelial Cells
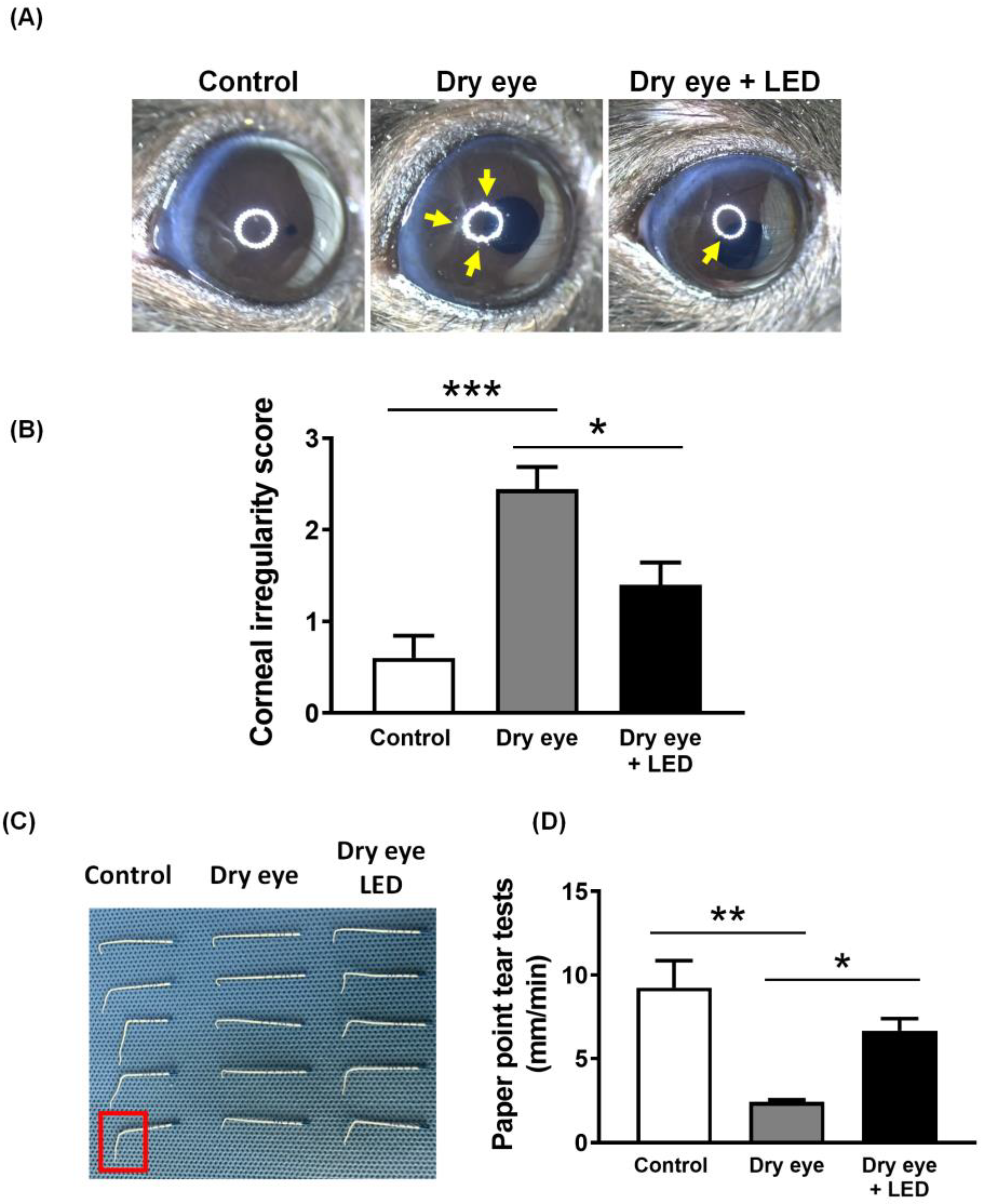
2.2. Effect of Multi-Wavelength LED Irradiation on the Clinical Features of Scopolamine-Induced Dry Eye
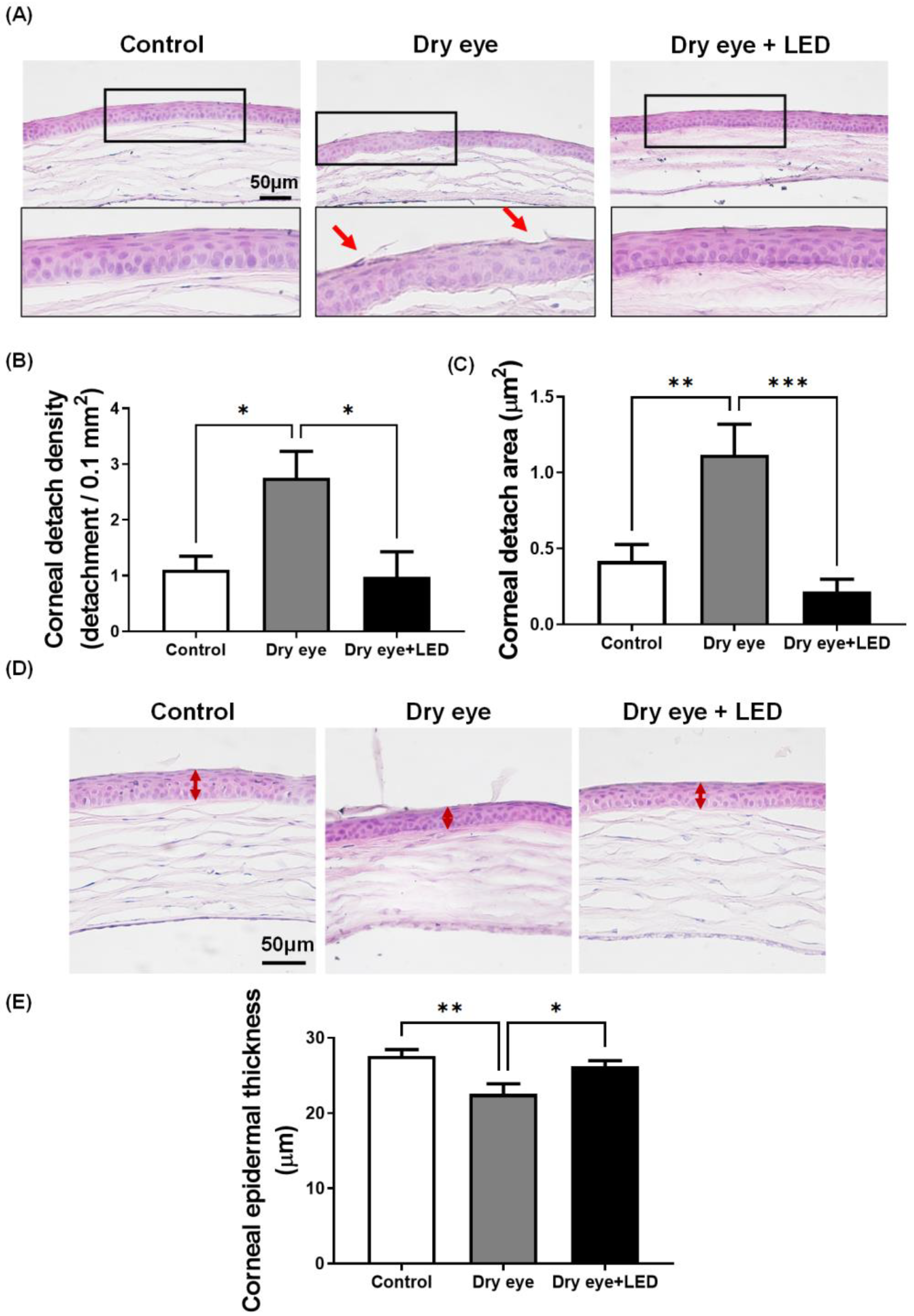
2.3. Effect of Multi-Wavelength LED Irradiation on Corneal Epithelia
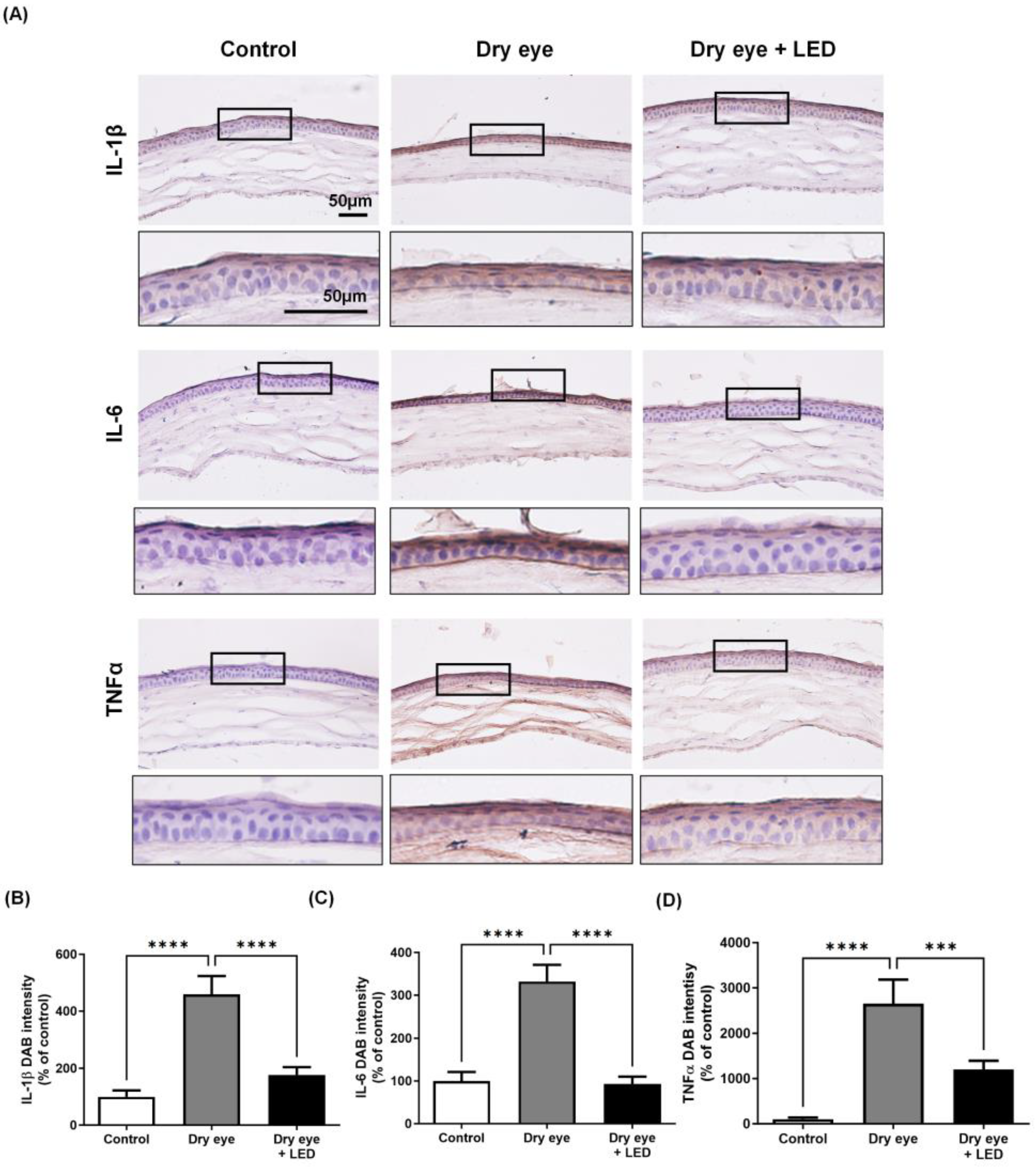
2.4. Effect of Multi-Wavelength LED Irradiation on Pro-Inflammatory Cytokine Levels
3. Discussion
4. Materials and Methods
4.1. LED Treatment
4.2. Cell Viability and Proliferation Assays
4.3. Mouse Model of Dry Eye and the Experimental Procedure
4.4. Tear Volume Test
4.5. Evaluation of Corneal Damage
4.6. Histology
4.7. Immunohistochemistry
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gayton, J.L. Etiology, prevalence, and treatment of dry eye disease. Clin. Ophthalmol. 2009, 3, 405–412. [Google Scholar] [CrossRef]
- Han, S.B.; Hyon, J.Y.; Woo, S.J.; Lee, J.J.; Kim, T.H.; Kim, K.W. Prevalence of dry eye disease in an elderly Korean population. Arch. Ophthalmol. 2011, 129, 633–638. [Google Scholar] [CrossRef]
- de Paiva, C.S.; Lindsey, J.L.; Pflugfelder, S.C. Assessing the severity of keratitis sicca with videokeratoscopic indices. Ophthalmology 2003, 110, 1102–1109. [Google Scholar] [CrossRef]
- Goto, E.; Yagi, Y.; Matsumoto, Y.; Tsubota, K. Impaired functional visual acuity of dry eye patients. Arch. Ophthalmol. 2002, 133, 181–186. [Google Scholar] [CrossRef]
- Musch, D.C.; Sugar, A.; Meyer, R.F. Demographic and predisposing factors in corneal ulceration. Arch. Ophthalmol. 1983, 101, 1545–1548. [Google Scholar] [CrossRef]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry eye disease: An immune-mediated ocular surface disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef]
- Stern, M.E.; Pflugfelder, S.C. Inflammation in dry eye. Ocul. Surf. 2004, 2, 124–130. [Google Scholar] [CrossRef]
- De Paiva, C.S.; Corrales, R.M.; Villarreal, A.L.; Farley, W.J.; Li, D.-Q.; Stern, M.E.; Pflugfelder, S.C. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression, MAPK activation in the corneal epithelium in experimental dry eye. Exp. Eye Res. 2006, 83, 526–535. [Google Scholar] [CrossRef]
- Phadatare, S.P.; Momin, M.; Nighojkar, P.; Askarkar, S.; Singh, K.K. A comprehensive review on dry eye disease: Diagnosis, medical management, recent developments, and future challenges. Adv. Pharm. 2015, 2015, 704946. [Google Scholar] [CrossRef]
- Noecker, R. Effects of common ophthalmic preservatives on ocular health. Adv. Ther. 2001, 18, 205–215. [Google Scholar] [CrossRef]
- Merry, G.F.; Munk, M.R.; Dotson, R.S.; Walker, M.G.; Devenyi, R.G. Photobiomodulation reduces drusen volume and improves visual acuity and contrast sensitivity in dry age-related macular degeneration. Acta Ophthalmol. 2017, 95, e270–e277. [Google Scholar] [CrossRef]
- Marques, E.C.P.; Lopes, F.P.; Nascimento, I.C.; Morelli, J.; Pereira, M.V.; Meiken, V.M.M.; Pinheiro, S.L. Photobiomodulation and photodynamic therapy for the treatment of oral mucositis in patients with cancer. Photodiagnosis Photodyn. Ther. 2020, 29, 101621. [Google Scholar] [CrossRef]
- Ryu, J.H.; Park, J.; Kim, B.-Y.; Kim, Y.; Kim, N.G.; Shin, Y.-I. Photobiomodulation ameliorates inflammatory parameters in fibroblast-like synoviocytes and experimental animal models of rheumatoid arthritis. Front. Immunol. 2023, 14, 1122581. [Google Scholar] [CrossRef]
- Hong, N.; Kim, H.J.; Kang, K.; Park, J.O.; Mun, S.; Kim, H.-G.; Kang, B.H.; Chung, P.-S.; Lee, M.Y.; Ahn, J.-C. Photobiomodulation improves the synapses and cognitive function and ameliorates epileptic seizure by inhibiting downregulation of Nlgn3. Cell Biosci. 2023, 13, 8. [Google Scholar] [CrossRef]
- Shen, W.; Teo, K.Y.C.; Wood, J.P.M.; Vaze, A.; Chidlow, G.; Ao, J.; Lee, S.-R.; Yam, M.X.; Cornish, E.E.; Fraser-Bell, S.; et al. Preclinical and clinical studies of photobiomodulation therapy for macular oedema. Diabetologia 2020, 63, 1900–1915. [Google Scholar] [CrossRef]
- Anders, J.J.; Lanzafame, R.J.; Arany, P.R. Low-level light/laser therapy versus photobiomodulation therapy. Photomed. Laser Surg. 2015, 33, 183–184. [Google Scholar] [CrossRef]
- Hawkins, D.; Houreld, N.; Abrahamse, H. Low level laser therapy (LLLT) as an effective therapeutic modality for delayed wound healing. Ann. N. Y. Acad. Sci. 2005, 1056, 486–493. [Google Scholar] [CrossRef]
- Hong, N.; Kang, G.W.; Park, J.O.; Chung, P.-S.; Lee, M.Y.; Ahn, J.-C. Photobiomodulation regulates adult neurogenesis in the hippocampus in a status epilepticus animal model. Sci. Rep. 2022, 12, 15246. [Google Scholar] [CrossRef]
- de Castro, M.S.; Miyazawa, M.; Nogueira, E.S.C.; Chavasco, J.K.; Brancaglion, G.A.; Cerdeira, C.D.; Nogueira, D.A.; Ionta, M.; Hanemann, J.A.C.; Brigagão, M.R.P.L.; et al. Photobiomodulation enhances the Th1 immune response of human monocytes. Lasers Med. Sci. 2022, 37, 135–148. [Google Scholar] [CrossRef]
- Yoon, S.-R.; Hong, N.; Lee, M.-Y.; Ahn, J.-C. Photobiomodulation with a 660-Nanometer Light-Emitting Diode Promotes Cell Proliferation in Astrocyte Culture. Cells 2021, 10, 1664. [Google Scholar] [CrossRef]
- Kim, U.-J.; Hong, N.; Ahn, J.-C. Photobiomodulation Attenuated Cognitive Dysfunction and Neuroinflammation in a Prenatal Valproic Acid-Induced Autism Spectrum Disorder Mouse Model. Int. J. Mol. Sci. 2022, 23, 16099. [Google Scholar] [CrossRef]
- Pereira, E.d.S.B.M.; Basting, R.T.; Abdalla, H.B.; Garcez, A.S.; Napimoga, M.H.; Clemente-Napimoga, J.T. Photobiomodulation inhibits inflammation in the temporomandibular joint of rats. J. Photochem. Photobiol. B Biol. 2021, 222, 112281. [Google Scholar] [CrossRef]
- García, V.C.; Cadiz, B.; Herrera, P.; Díaz, A.; Schmachtenberg, O. Evaluation of Photobiomodulation and Boldine as Alternative Treatment Options in Two Diabetic Retinopathy Models. Int. J. Mol. Sci. 2023, 24, 7918. [Google Scholar] [CrossRef]
- Di Marino, M.; Conigliaro, P.; Aiello, F.; Valeri, C.; Giannini, C.; Mancino, R.; Modica, S.; Nucci, C.; Perricone, R.; Cesareo, M. Combined Low-Level Light Therapy and Intense Pulsed Light Therapy for the Treatment of Dry Eye in Patients with Sjögren’s Syndrome. J. Ophthalmol. 2021, 2021, 2023246. [Google Scholar] [CrossRef]
- Eells, J.T.; Gopalakrishnan, S.; Valter, K. Near-Infrared Photobiomodulation in Retinal Injury and Disease. Adv. Exp. Med. Biol. 2016, 854, 437–441. [Google Scholar]
- Hamblin, M.R. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017, 4, 337–361. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.B.; Seo, J.H.; Kim, H.; Cho, K.J. Effect of 808-nm Laser Photobiomodulation Treatment in Blepharitis Rat Model. Cornea 2021, 40, 358–363. [Google Scholar] [CrossRef]
- Stonecipher, K.; Abell, T.G.; Chotiner, B.; Chotiner, E.; Potvin, R. Combined low level light therapy and intense pulsed light therapy for the treatment of meibomian gland dysfunction. Clin. Ophthalmol. 2019, 13, 993–999. [Google Scholar] [CrossRef]
- Lemp, M.A.; Foulks, G.N. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul. Surf. 2007, 5, 75–92. [Google Scholar]
- Colligris, B.; Alkozi, H.A.; Pintor, J. Recent developments on dry eye disease treatment compounds. Saudi J. Ophthalmol. Off. J. Saudi Ophthalmol. Soc. 2014, 28, 19–30. [Google Scholar] [CrossRef]
- Chung, H.; Dai, T.; Sharma, S.K.; Huang, Y.Y.; Carroll, J.D.; Hamblin, M.R. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012, 40, 516–533. [Google Scholar] [CrossRef]
- Hong, N. Photobiomodulation as a treatment for neurodegenerative disorders: Current and future trends. Biomed. Eng. Lett. 2019, 9, 359–366. [Google Scholar] [CrossRef]
- Goo, H.; Kim, H.; Ahn, J.-C.; Cho, K.J. Effects of low-level light therapy at 740 nm on dry eye disease in vivo. Med. Lasers 2019, 8, 50–58. [Google Scholar] [CrossRef]
- Park, Y.; Kim, H.; Kim, S.; Cho, K.J. Effect of low-level light therapy in patients with dry eye: A prospective, randomized, observer-masked trial. Sci. Rep. 2022, 12, 3575. [Google Scholar] [CrossRef]
- Findlay, Q.; Reid, K. Dry eye disease: When to treat and when to refer. Aust. Prescr. 2018, 41, 160–163. [Google Scholar] [CrossRef]
- Rhee, Y.-H.; Cho, K.J.; Ahn, J.-C.; Chung, P.-S. Effect of photobiomodulation on wound healing of the corneal epithelium through Rho-GTPase. Med. Lasers 2017, 6, 67–76. [Google Scholar] [CrossRef]
- Zhang, J.; Yan, X.; Li, H. Analysis of the correlations of mucins, inflammatory markers, and clinical tests in dry eye. Cornea 2013, 32, 928–932. [Google Scholar] [CrossRef]
- Choi, W.; Noh, H.; Yeo, A.; Jang, H.; Ahn, H.K.; Song, Y.J.; Lee, H.K. The Effect of TNF-α Blocker HL036337 and Its Best Concentration to Inhibit Dry Eye Inflammation. Korean J. Ophthalmol. 2016, 30, 302–308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ji, Y.W.; Byun, Y.J.; Choi, W.; Jeong, E.; Kim, J.S.; Noh, H.; Kim, E.S.; Song, Y.J.; Park, S.K.; Lee, H.K. Neutralization of ocular surface TNF-α reduces ocular surface and lacrimal gland inflammation induced by in vivo dry eye. Investig. Opthalmol. Vis. Sci. 2013, 54, 7557–7566. [Google Scholar] [CrossRef]
- Aimbire, F.; Albertini, R.; Pacheco, M.T.; Castro-Faria-Neto, H.C.; Leonardo, P.S.; Iversen, V.V.; Lopes-Martins, R.A.; Bjordal, J.M. Low-level laser therapy induces dose-dependent reduction of TNFalpha levels in acute inflammation. Photomed. Laser Surg. 2006, 24, 33–37. [Google Scholar] [CrossRef]
- Mesquita-Ferrari, R.A.; Martins, M.D.; Silva, J.A.; da Silva, T.D.; Piovesan, R.F.; Pavesi, V.C.S.; Bussadori, S.K.; Fernandes, K.P.S. Effects of low-level laser therapy on expression of TNF-α and TGF-β in skeletal muscle during the repair process. Lasers Med. Sci. 2011, 26, 335–340. [Google Scholar] [CrossRef]
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef]
- Dartt, D.A. Signal transduction and control of lacrimal gland protein secretion: A review. Curr. Eye Res. 1989, 8, 619–636. [Google Scholar] [CrossRef]
- Dartt, D.A. Regulation of tear secretion. Adv. Exp. Med. Biol. 1994, 350, 1–9. [Google Scholar]
- Duque, G.A.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Markoulli, M.; Chandramohan, N.; Papas, E.B. Photobiomodulation (low-level light therapy) and dry eye disease. Clin. Exp. Optom. 2021, 104, 561–566. [Google Scholar] [CrossRef]
- Gouws, P.; Barabas, S.; Gouws, A. Efficacy of PorTable 445 nm Laser Versus Intense Pulsed Light Treatment for Dry Eye: A Prospective Randomized Pilot Study. Photobiomodul. Photomed. Laser Surg. 2023, 41, 120–124. [Google Scholar] [CrossRef]
- Lange, R.R.; Lima, L.; Montiani-Ferreira, F. Measurement of tear production in black-tufted marmosets (Callithrix penicillata) using three different methods: Modified Schirmer’s I, phenol red thread and standardized endodontic absorbent paper points. Vet. Ophthalmol. 2012, 15, 376–382. [Google Scholar] [CrossRef]
| Treatment Wavelength (nm) | 680 nm | 780 nm | 830 nm |
|---|---|---|---|
| Light type | Light-emitting diode | ||
| Chip dimension | 1000 μm × 1000 μm | ||
| Mode | Continuous wave (CW) | ||
| FWHM | 22 nm | 25 nm | 35 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goo, H.; Lee, Y.-J.; Lee, S.; Hong, N. The Anti-Inflammatory Effect of Multi-Wavelength Light-Emitting Diode Irradiation Attenuates Dry Eye Symptoms in a Scopolamine-Induced Mouse Model of Dry Eye. Int. J. Mol. Sci. 2023, 24, 17493. https://doi.org/10.3390/ijms242417493
Goo H, Lee Y-J, Lee S, Hong N. The Anti-Inflammatory Effect of Multi-Wavelength Light-Emitting Diode Irradiation Attenuates Dry Eye Symptoms in a Scopolamine-Induced Mouse Model of Dry Eye. International Journal of Molecular Sciences. 2023; 24(24):17493. https://doi.org/10.3390/ijms242417493
Chicago/Turabian StyleGoo, Hyeyoon, Yea-Jin Lee, Sangkeun Lee, and Namgue Hong. 2023. "The Anti-Inflammatory Effect of Multi-Wavelength Light-Emitting Diode Irradiation Attenuates Dry Eye Symptoms in a Scopolamine-Induced Mouse Model of Dry Eye" International Journal of Molecular Sciences 24, no. 24: 17493. https://doi.org/10.3390/ijms242417493
APA StyleGoo, H., Lee, Y.-J., Lee, S., & Hong, N. (2023). The Anti-Inflammatory Effect of Multi-Wavelength Light-Emitting Diode Irradiation Attenuates Dry Eye Symptoms in a Scopolamine-Induced Mouse Model of Dry Eye. International Journal of Molecular Sciences, 24(24), 17493. https://doi.org/10.3390/ijms242417493






