Shear Stress and Endothelial Mechanotransduction in Trauma Patients with Hemorrhagic Shock: Hidden Coagulopathy Pathways and Novel Therapeutic Strategies
Abstract
1. Introduction
2. Trauma-Induced Coagulopathy
3. Hemodynamic Perturbations in Massive Trauma
3.1. Macro- and Microcirculation
3.2. Sympathoadrenal Activation
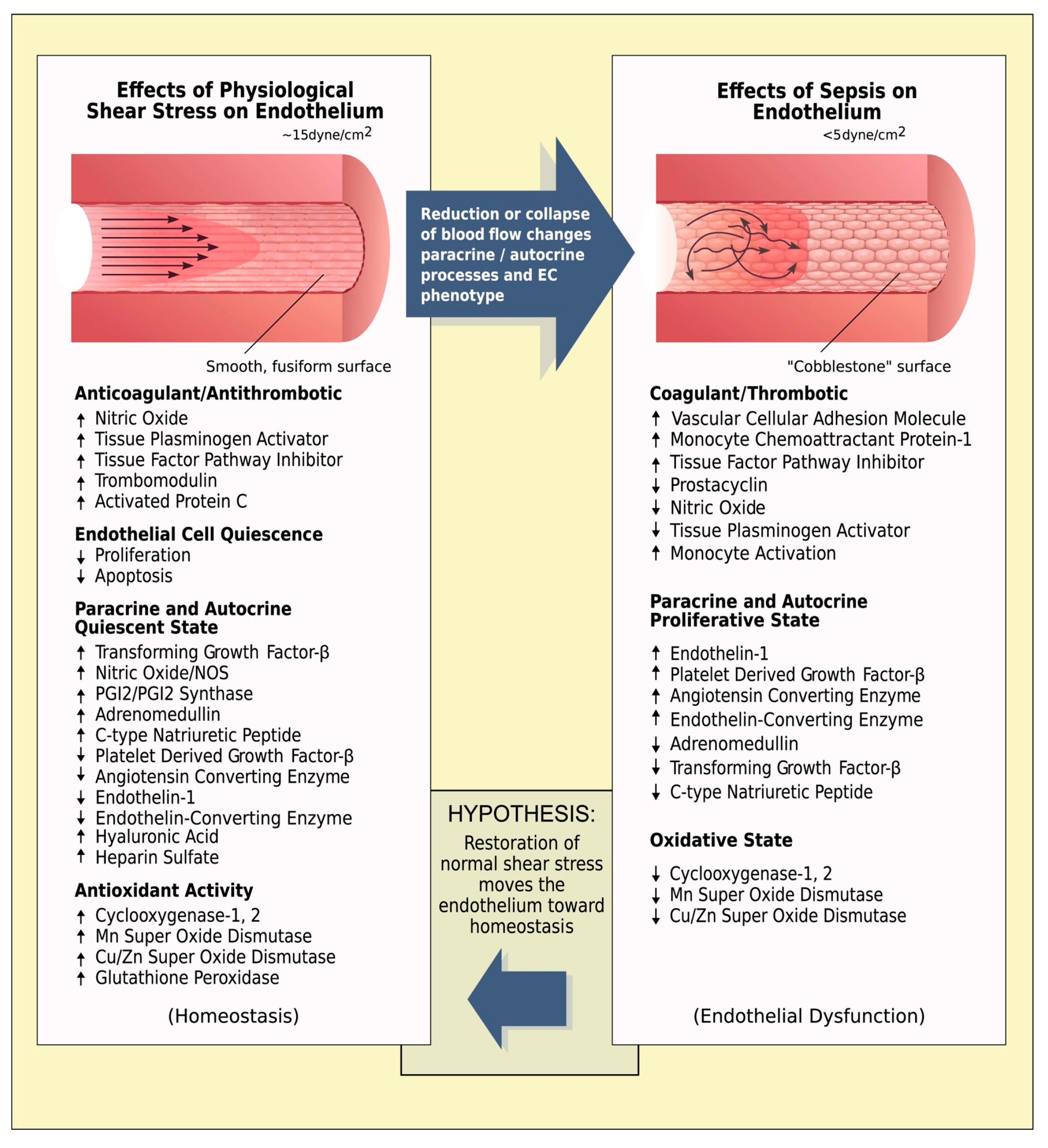
3.3. Endotheliopathy of Trauma
4. Mechanical Forces Exerted on the Vasculature
4.1. Endothelial Surface Mechanosensing
4.2. Endothelial Nuclear Mechanosensing
4.3. Effects of Shear Stress on Endothelial Cell Junctions
4.4. Effect of Arterial Stiffening on Endothelial Mechanosensing
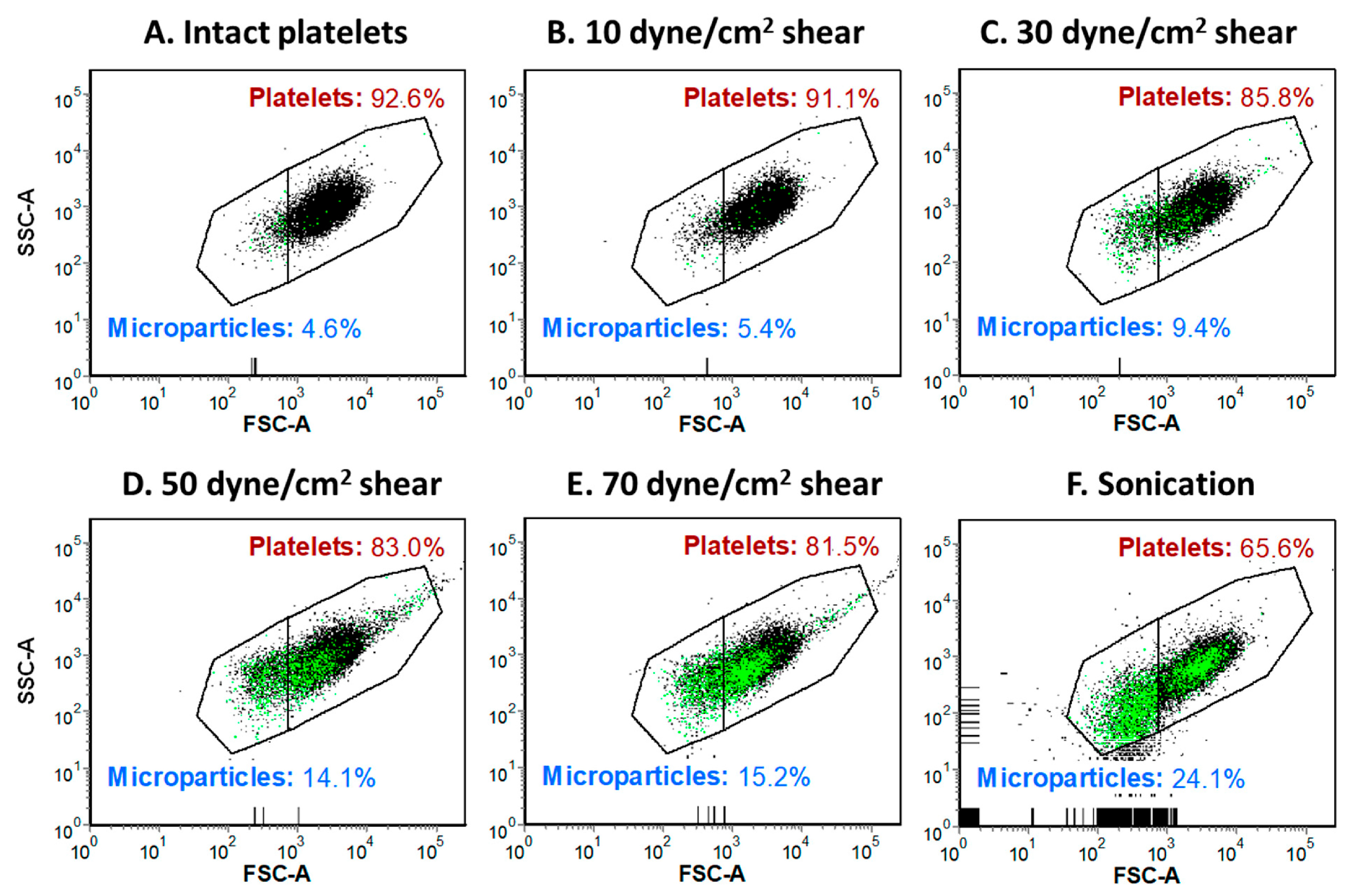
4.5. Effects of Shear Stress on Blood Cells
5. Low Endothelial Shear Stress in Patients with Hemorrhagic Shock
6. High Endothelial Shear Stress in Patients with Hemorrhagic Shock
7. Novel Pharmacologic Approaches Targeting Shear-Mediated Endothelial Dysfunction
7.1. Shear-Responsive Drug Delivery Systems
7.2. Antioxidants
7.3. Vasopressor Therapy
7.3.1. Non-Hemodynamic Effects of Catecholamines
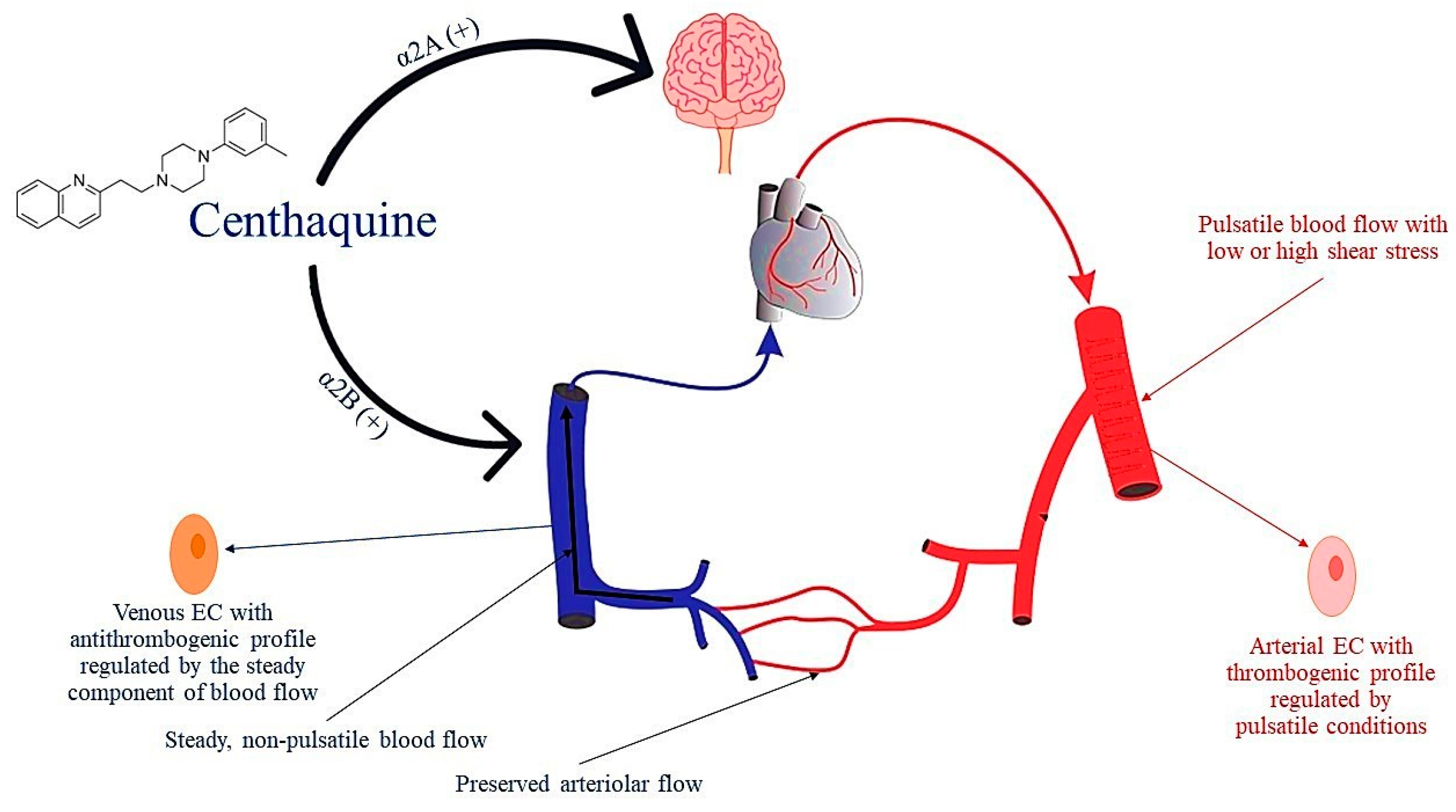
7.3.2. Targeting the Venous Side of the Circulation
7.3.3. Centhaquine
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossaint, R.; Afshari, A.; Bouillon, B.; Cerny, V.; Cimpoesu, D.; Curry, N.; Duranteau, J.; Filipescu, D.; Grottke, O.; Grønlykke, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Sixth edition. Crit. Care 2023, 27, 80. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, C.M.; Wagener, B.M.; Chalkias, A.; Siddiqui, S.; Douin, D.J. Massive Trauma and Resuscitation Strategies. Anesthesiol. Clin. 2023, 41, 283–301. [Google Scholar] [CrossRef] [PubMed]
- Mira, J.C.; Brakenridge, S.C.; Moldawer, L.L.; Moore, F.A. Persistent Inflammation, Immunosuppression and Catabolism Syndrome. Crit. Care Clin. 2017, 33, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Brohi, K.; Gruen, R.L.; Holcomb, J.B. Why are bleeding trauma patients still dying? Intensive Care Med. 2019, 45, 709–711. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, J.B.; Lynn, M.; McKenney, M.G.; Cohn, S.M.; Murtha, M. Early coagulopathy predicts mortality in trauma. J. Trauma 2003, 55, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.E.; Moore, H.B.; Kornblith, L.Z.; Neal, M.D.; Hoffman, M.; Mutch, N.J.; Schöchl, H.; Hunt, B.J.; Sauaia, A. Trauma-induced coagulopathy. Nat. Rev. Dis. Primers 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Brohi, K.; Singh, J.; Heron, M.; Coats, T. Acute traumatic coagulopathy. J. Trauma 2003, 54, 1127–1130. [Google Scholar] [CrossRef]
- Barrett, L.; Curry, N.; Abu-Hanna, J. Experimental Models of Traumatic Injuries: Do They Capture the Coagulopathy and Underlying Endotheliopathy Induced by Human Trauma? Int. J. Mol. Sci. 2023, 24, 11174. [Google Scholar] [CrossRef]
- Moore, H.B.; Moore, E.E.; Chapman, M.P.; Gonzalez, E.; Slaughter, A.L.; Morton, A.P.; D’Alessandro, A.; Hansen, K.C.; Sauaia, A.; Banerjee, A.; et al. Viscoelastic measurements of platelet function, not fibrinogen function, predicts sensitivity to tissue-type plasminogen activator in trauma patients. J. Thromb. Haemost. 2015, 13, 1878–1887. [Google Scholar] [CrossRef]
- Frith, D.; Goslings, J.C.; Gaarder, C.; Maegele, M.; Cohen, M.J.; Allard, S.; Johansson, P.I.; Stanworth, S.; Thiemermann, C.; Brohi, K. Definition and drivers of acute traumatic coagulopathy: Clinical and experimental investigations. J. Thromb. Haemost. 2010, 8, 1919–1925. [Google Scholar] [CrossRef]
- Moore, E.E.; Thomas, G. Orr Memorial Lecture. Staged laparotomy for the hypothermia, acidosis, and coagulopathy syndrome. Am. J. Surg. 1996, 172, 405–410. [Google Scholar] [CrossRef]
- Martini, W.Z. Coagulation complications following trauma. Mil. Med. Res. 2016, 3, 35. [Google Scholar] [CrossRef]
- Shaz, B.H.; Winkler, A.M.; James, A.B.; Hillyer, C.D.; MacLeod, J.B. Pathophysiology of early trauma-induced coagulopathy: Emerging evidence for hemodilution and coagulation factor depletion. J. Trauma 2011, 70, 1401–1407. [Google Scholar] [CrossRef]
- Martini, W.Z.; Holcomb, J.B. Acidosis and coagulopathy: The differential effects on fibrinogen synthesis and breakdown in pigs. Ann. Surg. 2007, 246, 831–835. [Google Scholar] [CrossRef]
- Martini, W.Z.; Dubick, M.A.; Pusateri, A.E.; Park, M.S.; Ryan, K.L.; Holcomb, J.B. Does bicarbonate correct coagulation function impaired by acidosis in swine? J. Trauma 2006, 61, 99–106. [Google Scholar] [CrossRef]
- Meng, Z.H.; Wolberg, A.S.; Monroe, D.M., 3rd; Hoffman, M. The effect of temperature and pH on the activity of factor VIIa: Implications for the efficacy of high-dose factor VIIa in hypothermic and acidotic patients. J. Trauma 2003, 55, 886–891. [Google Scholar] [CrossRef]
- Martini, W.Z.; Pusateri, A.E.; Uscilowicz, J.M.; Delgado, A.V.; Holcomb, J.B. Independent contributions of hypothermia and acidosis to coagulopathy in swine. J. Trauma 2005, 58, 1002–1009; discussion 1009–1010. [Google Scholar] [CrossRef]
- Martini, W.Z. The effects of hypothermia on fibrinogen metabolism and coagulation function in swine. Metabolism 2007, 56, 214–221. [Google Scholar] [CrossRef]
- Bozza, F.A.; Shah, A.M.; Weyrich, A.S.; Zimmerman, G.A. Amicus or adversary: Platelets in lung biology, acute injury, and inflammation. Am. J. Respir Cell Mol. Biol. 2009, 40, 123–134. [Google Scholar] [CrossRef]
- Freedman, J.E. CD40-CD40L and platelet function: Beyond hemostasis. Circ. Res. 2003, 92, 944–946. [Google Scholar] [CrossRef]
- Windeløv, N.A.; Ostrowski, S.R.; Johansson, P.I.; Wanscher, M.; Larsen, C.F.; Sørensen, A.M.; Rasmussen, L.S. Circulating levels of platelet α-granule cytokines in trauma patients. Inflamm. Res. 2015, 64, 235–241. [Google Scholar] [CrossRef]
- Kutcher, M.E.; Howard, B.M.; Sperry, J.L.; Hubbard, A.E.; Decker, A.L.; Cuschieri, J.; Minei, J.P.; Moore, E.E.; Brownstein, B.H.; Maier, R.V.; et al. Evolving beyond the vicious triad: Differential mediation of traumatic coagulopathy by injury, shock, and resuscitation. J. Trauma Acute Care Surg. 2015, 78, 516–523. [Google Scholar] [CrossRef]
- Mitrophanov, A.Y.; Szlam, F.; Sniecinski, R.M.; Levy, J.H.; Reifman, J. Controlled Multifactorial Coagulopathy: Effects of Dilution, Hypothermia, and Acidosis on Thrombin Generation In Vitro. Anesth. Analg. 2020, 130, 1063–1076. [Google Scholar] [CrossRef]
- Schwarz, G.; Droogmans, G.; Nilius, B. Shear stress induced membrane currents and calcium transients in human vascular endothelial cells. Pflugers. Arch. 1992, 421, 394–396. [Google Scholar] [CrossRef]
- Kawahara, K.; Matsuzaki, K. Activation of calcium channel by shear-stress in cultured renal distal tubule cells. Biochem. Biophys. Res. Commun. 1992, 184, 198–205. [Google Scholar] [CrossRef]
- Gerhold, K.A.; Schwartz, M.A. Ion Channels in Endothelial Responses to Fluid Shear Stress. Physiology 2016, 31, 359–369. [Google Scholar] [CrossRef]
- Hayakawa, M. Pathophysiology of trauma-induced coagulopathy: Disseminated intravascular coagulation with the fibrinolytic phenotype. J. Intensive Care 2017, 5, 14. [Google Scholar] [CrossRef]
- Huber, D.; Cramer, E.M.; Kaufmann, J.E.; Meda, P.; Massé, J.M.; Kruithof, E.K.; Vischer, U.M. Tissue-type plasminogen activator (t-PA) is stored in Weibel-Palade bodies in human endothelial cells both in vitro and in vivo. Blood 2002, 99, 3637–3645. [Google Scholar] [CrossRef]
- Ditzel, R.M., Jr.; Anderson, J.L.; Eisenhart, W.J.; Rankin, C.J.; DeFeo, D.R.; Oak, S.; Siegler, J. A review of transfusion- and trauma-induced hypocalcemia: Is it time to change the lethal triad to the lethal diamond? J. Trauma Acute Care Surg. 2020, 88, 434–439. [Google Scholar] [CrossRef]
- Paszkowiak, J.J.; Dardik, A. Arterial wall shear stress: Observations from the bench to the bedside. Vasc. Endovasc. Surg. 2003, 37, 47–57. [Google Scholar] [CrossRef]
- White, N.J.; Wang, Y.; Fu, X.; Cardenas, J.C.; Martin, E.J.; Brophy, D.F.; Wade, C.E.; Wang, X.; St John, A.E.; Lim, E.B.; et al. Post-translational oxidative modification of fibrinogen is associated with coagulopathy after traumatic injury. Free Radic. Biol. Med. 2016, 96, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.B.; Moore, E.E.; Gonzalez, E.; Chapman, M.P.; Chin, T.L.; Silliman, C.C.; Banerjee, A.; Sauaia, A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: The spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J. Trauma Acute Care Surg. 2014, 77, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Latimer, A.J.; Counts, C.R.; Van Dyke, M.; Bulger, N.; Maynard, C.; Rea, T.D.; Kudenchuk, P.J.; Utarnachitt, R.B.; Blackwood, J.; Poel, A.J.; et al. The compensatory reserve index for predicting hemorrhagic shock in prehospital trauma. Shock 2023, 60, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Kirkman, E.; Watts, S. Haemodynamic changes in trauma. Br. J. Anaesth. 2014, 113, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Barcroft, H.; Edholm, O.G.; McMichael, J.; Sharpey-Schafer, E.P. Posthaemorrhagic fainting: Study by cardiac output and forearm blood flow. Lancet 1944, 1, 489–491. [Google Scholar] [CrossRef]
- Little, R.A.; Marshall, H.W.; Kirkman, E. Attenuation of the acute cardiovascular responses to haemorrhage by tissue injury in the conscious rat. Q. J. Exp. Physiol. 1989, 74, 825–833. [Google Scholar] [CrossRef]
- Evans, R.G.; Ludbrook, J. Chemosensitive cardiopulmonary afferents and the haemodynamic response to simulated haemorrhage in conscious rabbits. Br. J. Pharmacol. 1991, 102, 533–539. [Google Scholar] [CrossRef]
- Dinnar, U. Metabolic and mechanical control of the microcirculation. Adv. Exp. Med. Biol. 1993, 346, 243–254. [Google Scholar]
- Schubert, R.; Mulvany, M.J. The myogenic response: Established facts and attractive hypotheses. Clin. Sci. 1999, 96, 313–326. [Google Scholar] [CrossRef]
- Farina, A.; Fasano, A.; Rosso, F. A theoretical model for the Fåhræus effect in medium-large microvessels. J. Theor. Biol. 2023, 558, 111355. [Google Scholar] [CrossRef]
- Bunch, C.M.; Chang, E.; Moore, E.E.; Moore, H.B.; Kwaan, H.C.; Miller, J.B.; Al-Fadhl, M.D.; Thomas, A.V.; Zackariya, N.; Patel, S.S.; et al. SHock-INduced Endotheliopathy (SHINE): A mechanistic justification for viscoelastography-guided resuscitation of traumatic and non-traumatic shock. Front. Physiol. 2023, 14, 1094845. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.P.; Rizoli, S.B.; Lejnieks, B.; Min, A.; Shiu, M.Y.; Peng, H.T.; Baker, A.J.; Hutchison, M.G.; Churchill, N.; Inaba, K.; et al. Sympathoadrenal Activation is Associated with Acute Traumatic Coagulopathy and Endotheliopathy in Isolated Brain Injury. Shock 2016, 46, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Stensballe, J.; Ostrowski, S.R. Shock induced endotheliopathy (SHINE) in acute critical illness—A unifying pathophysiologic mechanism. Crit. Care 2017, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.V.; Liberati, D.M.; Diebel, L.N. Disparate effects of catecholamines under stress conditions on endothelial glycocalyx injury: An in vitro model. Am. J. Surg. 2017, 214, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, S.R.; Henriksen, H.H.; Stensballe, J.; Gybel-Brask, M.; Cardenas, J.C.; Baer, L.A.; Cotton, B.A.; Holcomb, J.B.; Wade, C.E.; Johansson, P.I. Sympathoadrenal activation and endotheliopathy are drivers of hypocoagulability and hyperfibrinolysis in trauma: A prospective observational study of 404 severely injured patients. J. Trauma Acute Care Surg. 2017, 82, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yu, W.K.; Lin, Z.L.; Tan, S.J.; Bai, X.W.; Ding, K.; Li, N. Chemical sympathectomy attenuates inflammation, glycocalyx shedding and coagulation disorders in rats with acute traumatic coagulopathy. Blood Coagul. Fibrinolysis 2015, 26, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Di Battista, A.P.; Rhind, S.G.; Hutchison, M.G.; Hassan, S.; Shiu, M.Y.; Inaba, K.; Topolovec-Vranic, J.; Neto, A.C.; Rizoli, S.B.; Baker, A.J. Inflammatory cytokine and chemokine profiles are associated with patient outcome and the hyperadrenergic state following acute brain injury. J. Neuroinflamm. 2016, 13, 40. [Google Scholar] [CrossRef]
- Naumann, D.N.; Hazeldine, J.; Davies, D.J.; Bishop, J.; Midwinter, M.J.; Belli, A.; Harrison, P.; Lord, J.M. Endotheliopathy of Trauma is an on-Scene Phenomenon, and is Associated with Multiple Organ Dysfunction Syndrome: A Prospective Observational Study. Shock 2018, 49, 420–428. [Google Scholar] [CrossRef]
- Jenkins, D.H.; Rappold, J.F.; Badloe, J.F.; Berséus, O.; Blackbourne, L.; Brohi, K.H.; Butler, F.K.; Cap, A.P.; Cohen, M.J.; Davenport, R.; et al. Trauma hemostasis and oxygenation research position paper on remote damage control resuscitation: Definitions, current practice, and knowledge gaps. Shock 2014, 41 (Suppl. S1), 3–12. [Google Scholar] [CrossRef]
- Nair, A.B.; Schreiber, M.A.; Pati, S. Defining and Assessing the Endotheliopathy of Trauma and Its Implications on Trauma-Induced Coagulopathy and Trauma-Related Outcomes. In Trauma Induced Coagulopathy; Moore, H.B., Neal, M.D., Moore, E.E., Eds.; Springer: Cham, Switzerland, 2021; pp. 117–133. [Google Scholar]
- Kozar, R.A.; Pati, S. Syndecan-1 restitution by plasma after hemorrhagic shock. J. Trauma Acute Care Surg. 2015, 78, S83–S86. [Google Scholar] [CrossRef]
- Wu, F.; Chipman, A.; Pati, S.; Miyasawa, B.; Corash, L.; Kozar, R.A. Resuscitative Strategies to Modulate the Endotheliopathy of Trauma: From Cell to Patient. Shock 2020, 53, 575–584. [Google Scholar] [CrossRef]
- Torres, L.N.; Sondeen, J.L.; Ji, L.; Dubick, M.A.; Torres Filho, I. Evaluation of resuscitation fluids on endothelial glycocalyx, venular blood flow, and coagulation function after hemorrhagic shock in rats. J. Trauma Acute Care Surg. 2013, 75, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Torres Filho, I.P.; Torres, L.N.; Salgado, C.; Dubick, M.A. Plasma syndecan-1 and heparan sulfate correlate with microvascular glycocalyx degradation in hemorrhaged rats after different resuscitation fluids. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1468–H1478. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Calderon, A.S.; Ryan, K.L.; Klemcke, H.G.; Mdaki, K.S.; Hudson, I.L.; Meledeo, M.A. Can polyethylene glycol-20k replace albumin for prehospital treatment of hemorrhagic shock when full resuscitation is unavailable? Shock 2023, 59, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Kozar, R.A.; Peng, Z.; Zhang, R.; Holcomb, J.B.; Pati, S.; Park, P.; Ko, T.C.; Paredes, A. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth. Analg. 2011, 112, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.; Statkevicius, S.; Schött, U.; Johansson, P.I.; Bentzer, P. Effects of fresh frozen plasma, Ringer’s acetate and albumin on plasma volume and on circulating glycocalyx components following haemorrhagic shock in rats. Intensive Care Med. Exp. 2016, 4, 6. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Kozar, R. Plasma activates a novel Syndecan1-Pak1 pathway to enhance endothelial barrier integrity. Shock 2016, 45, 87. [Google Scholar]
- Gröger, M.; Pasteiner, W.; Ignatyev, G.; Matt, U.; Knapp, S.; Atrasheuskaya, A.; Bukin, E.; Friedl, P.; Zinkl, D.; Hofer-Warbinek, R.; et al. Peptide Bβ(15-42) preserves endothelial barrier function in shock. PLoS ONE 2009, 4, e5391. [Google Scholar] [CrossRef]
- Jennewein, C.; Mehring, M.; Tran, N.; Paulus, P.; Ockelmann, P.A.; Habeck, K.; Latsch, K.; Scheller, B.; Zacharowski, K.; Mutlak, H. The fibrinopeptide bβ15-42 reduces inflammation in mice subjected to polymicrobial sepsis. Shock 2012, 38, 275–280. [Google Scholar] [CrossRef]
- Chatterjee, S. Endothelial Mechanotransduction, Redox Signaling and the Regulation of Vascular Inflammatory Pathways. Front. Physiol. 2018, 9, 524. [Google Scholar] [CrossRef]
- van Hinsbergh, V.W. Endothelium--role in regulation of coagulation and inflammation. Semin Immunopathol 2012, 34, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Yau, J.W.; Teoh, H.; Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015, 15, 130. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.Q.; Schwarz, U.S.; Riveline, D.; Goichberg, P.; Tzur, G.; Sabanay, I.; Mahalu, D.; Safran, S.; Bershadsky, A.; Addadi, L.; et al. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001, 3, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, J.D.; Dufresne, E.R.; Schwartz, M.A. Mechanotransduction and extracellular matrix homeostasis. Nat. Rev. Mol. Cell Biol. 2014, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, N.; Bandyopadhyay, C.; Coon, B.G.; Yun, S.; Schwartz, M.A. Endothelial fluid shear stress sensing in vascular health and disease. J. Clin. Investig. 2016, 126, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Tarbell, J.M. The adaptive remodeling of endothelial glycocalyx in response to fluid shear stress. PLoS ONE 2014, 9, e86249. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.M.; Tarbell, J.M. Mechano-sensing and transduction by endothelial surface glycocalyx: Composition, structure, and function. Wiley Interdiscip. Rev. Syst. Biol. Med. 2013, 5, 381–390. [Google Scholar] [CrossRef]
- Tarbell, J.M.; Ebong, E.E. The endothelial glycocalyx: A mechano-sensor and -transducer. Sci. Signal. 2008, 1, pt8. [Google Scholar]
- Magder, S. Volume and its relationship to cardiac output and venous return. Crit. Care 2016, 20, 271. [Google Scholar] [CrossRef]
- Laou, E.; Papagiannakis, N.; Papadopoulou, A.; Choratta, T.; Sakellakis, M.; Ippolito, M.; Pantazopoulos, I.; Cortegiani, A.; Chalkias, A. Effects of Vasopressin Receptor Agonists during the Resuscitation of Hemorrhagic Shock: A Systematic Review and Meta-Analysis of Experimental and Clinical Studies. J. Pers. Med. 2023, 13, 1143. [Google Scholar] [CrossRef]
- Chalkias, A. The interplay between ‘rest volume’, mean circulatory filling pressure, and cardiac output that drives venous return. Eur. J. Anaesthesiol. 2023, 40, 146–147. [Google Scholar] [CrossRef]
- Baeyens, N. Fluid shear stress sensing in vascular homeostasis and remodeling: Towards the development of innovative pharmacological approaches to treat vascular dysfunction. Biochem. Pharmacol. 2018, 158, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Gosgnach, W.; Challah, M.; Coulet, F.; Michel, J.B.; Battle, T. Shear stress induces angiotensin converting enzyme expression in cultured smooth muscle cells: Possible involvement of bFGF. Cardiovasc. Res. 2000, 45, 486–492. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyakawa, A.A.; de Lourdes Junqueira, M.; Krieger, J.E. Identification of two novel shear stress responsive elements in rat angiotensin I converting enzyme promoter. Physiol. Genom. 2004, 17, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Dekker, R.J.; van Thienen, J.V.; Rohlena, J.; de Jager, S.C.; Elderkamp, Y.W.; Seppen, J.; de Vries, C.J.; Biessen, E.A.; van Berkel, T.J.; Pannekoek, H.; et al. Endothelial KLF2 links local arterial shear stress levels to the expression of vascular tone-regulating genes. Am. J. Pathol. 2005, 167, 609–618. [Google Scholar] [CrossRef]
- Barauna, V.G.; Campos, L.C.; Miyakawa, A.A.; Krieger, J.E. ACE as a mechanosensor to shear stress influences the control of its own regulation via phosphorylation of cytoplasmic Ser(1270). PLoS ONE 2011, 6, e22803. [Google Scholar] [CrossRef] [PubMed]
- Hrenak, J.; Simko, F. Renin-Angiotensin System: An Important Player in the Pathogenesis of Acute Respiratory Distress Syndrome. Int. J. Mol. Sci. 2020, 21, 8038. [Google Scholar] [CrossRef] [PubMed]
- Lupu, F.; Kinasewitz, G.; Dormer, K. The role of endothelial shear stress on haemodynamics, inflammation, coagulation and glycocalyx during sepsis. J. Cell Mol. Med. 2020, 24, 12258–12271. [Google Scholar] [CrossRef]
- Samet, M.M.; Lelkes, P.L. The hemodynamic environment of the endothelium in vivo and its simulation in vitro. In Mechanical Forces and the Endothelium; Lelkes, P.L., Ed.; Harwood Academic Publishers: Amsterdam, The Netherland, 1999; pp. 1–32. [Google Scholar]
- Waite, L.; Fine, J. Applied Biofluid Mechanics; McGraw-Hill: New York, NY, USA, 2007. [Google Scholar]
- Ballermann, B.J.; Dardik, A.; Eng, E.; Liu, A. Shear stress and the endothelium. Kidney Int. Suppl. 1998, 67, S100–S108. [Google Scholar] [CrossRef]
- Jones, S.A.; Giddens, D.P.; Loth, F.; Zarins, C.K.; Kajiya, F.; Morita, I.; Hiramatsu, O.; Ogasawara, Y.; Tsujioka, K. In-vivo measurements of blood flow velocity profiles in canine ilio-femoral anastomotic bypass grafts. J. Biomech. Eng. 1997, 119, 30–38. [Google Scholar] [CrossRef]
- Oyre, S.; Pedersen, E.M.; Ringgaard, S.; Boesiger, P.; Paaske, W.P. In vivo wall shear stress measured by magnetic resonance velocity mapping in the normal human abdominal aorta. Eur. J. Vasc. Endovasc. Surg. 1997, 13, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, Y.S.; Chien, S. Shear stress-initiated signaling and its regulation of endothelial function. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2191–2198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lee, T.S.; Kolb, E.M.; Sun, K.; Lu, X.; Sladek, F.M.; Kassab, G.S.; Garland, T., Jr.; Shyy, J.Y. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Kadohama, T.; Nishimura, K.; Hoshino, Y.; Sasajima, T.; Sumpio, B.E. Effects of different types of fluid shear stress on endothelial cell proliferation and survival. J. Cell Physiol. 2007, 212, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Rennier, K.; Ji, J.Y. The role of death-associated protein kinase (DAPK) in endothelial apoptosis under fluid shear stress. Life Sci. 2013, 93, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Orr, A.W.; Sanders, J.M.; Bevard, M.; Coleman, E.; Sarembock, I.J.; Schwartz, M.A. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: A potential role in atherosclerosis. J. Cell Biol. 2005, 169, 191–202. [Google Scholar] [CrossRef]
- Fleming, I.; Fisslthaler, B.; Dixit, M.; Busse, R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J. Cell Sci. 2005, 118, 4103–4111. [Google Scholar] [CrossRef]
- Otte, L.A.; Bell, K.S.; Loufrani, L.; Yeh, J.C.; Melchior, B.; Dao, D.N.; Stevens, H.Y.; White, C.R.; Frangos, J.A. Rapid changes in shear stress induce dissociation of a G alpha(q/11)-platelet endothelial cell adhesion molecule-1 complex. J. Physiol. 2009, 587, 2365–2373. [Google Scholar] [CrossRef]
- Funk, S.D.; Yurdagul, A., Jr.; Green, J.M.; Jhaveri, K.A.; Schwartz, M.A.; Orr, A.W. Matrix-specific protein kinase A signaling regulates p21-activated kinase activation by flow in endothelial cells. Circ. Res. 2010, 106, 1394–1403. [Google Scholar] [CrossRef]
- Coon, B.G.; Baeyens, N.; Han, J.; Budatha, M.; Ross, T.D.; Fang, J.S.; Yun, S.; Thomas, J.L.; Schwartz, M.A. Intramembrane binding of VE-cadherin to VEGFR2 and VEGFR3 assembles the endothelial mechanosensory complex. J. Cell Biol. 2015, 208, 975–986. [Google Scholar] [CrossRef]
- Chien, S. Mechanotransduction and endothelial cell homeostasis: The wisdom of the cell. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1209–H1224. [Google Scholar] [CrossRef]
- Frueh, J.; Maimari, N.; Homma, T.; Bovens, S.M.; Pedrigi, R.M.; Towhidi, L.; Krams, R. Systems biology of the functional and dysfunctional endothelium. Cardiovasc. Res. 2013, 99, 334–341. [Google Scholar] [CrossRef]
- Mannion, A.J.; Holmgren, L. Nuclear mechanosensing of the aortic endothelium in health and disease. Dis. Models Mech. 2023, 16, dmm050361. [Google Scholar] [CrossRef]
- Tkachenko, E.; Gutierrez, E.; Saikin, S.K.; Fogelstrand, P.; Kim, C.; Groisman, A.; Ginsberg, M.H. The nucleus of endothelial cell as a sensor of blood flow direction. Biol. Open 2013, 2, 1007–1012. [Google Scholar] [CrossRef] [PubMed]
- Nava, M.M.; Miroshnikova, Y.A.; Biggs, L.C.; Whitefield, D.B.; Metge, F.; Boucas, J.; Vihinen, H.; Jokitalo, E.; Li, X.; García Arcos, J.M.; et al. Heterochromatin-Driven Nuclear Softening Protects the Genome against Mechanical Stress-Induced Damage. Cell 2020, 181, 800–817. [Google Scholar] [CrossRef]
- Danielsson, B.E.; Tieu, K.V.; Spagnol, S.T.; Vu, K.K.; Cabe, J.I.; Raisch, T.B.; Dahl, K.N.; Conway, D.E. Chromatin condensation regulates endothelial cell adaptation to shear stress. Mol. Biol. Cell 2022, 33, ar101. [Google Scholar] [CrossRef]
- Buglak, D.B.; Bougaran, P.; Kulikauskas, M.R.; Liu, Z.; Monaghan-Benson, E.; Gold, A.L.; Marvin, A.P.; Burciu, A.; Tanke, N.T.; Oatley, M.; et al. Nuclear SUN1 stabilizes endothelial cell junctions via microtubules to regulate blood vessel formation. Elife 2023, 12, e83652. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Hutterer, E.; Hultin, S.; Bergman, O.; Kolbeinsdottir, S.; Jin, H.; Forteza, M.J.; Ketelhuth, D.F.J.; Roy, J.; et al. The VE-cadherin/AmotL2 mechanosensory pathway suppresses aortic inflammation and the formation of abdominal aortic aneurysms. Nat. Cardiovasc. Res. 2023, 2, 629–644. [Google Scholar] [CrossRef]
- Chuntharpursat-Bon, E.; Povstyan, O.V.; Ludlow, M.J.; Carrier, D.J.; Debant, M.; Shi, J.; Gaunt, H.J.; Bauer, C.C.; Curd, A.; Simon Futers, T.; et al. PIEZO1 and PECAM1 interact at cell-cell junctions and partner in endothelial force sensing. Commun. Biol. 2023, 6, 358. [Google Scholar] [CrossRef] [PubMed]
- Albarrán-Juárez, J.; Iring, A.; Wang, S.; Joseph, S.; Grimm, M.; Strilic, B.; Wettschureck, N.; Althoff, T.F.; Offermanns, S. Piezo1 and Gq/G11 promote endothelial inflammation depending on flow pattern and integrin activation. J. Exp. Med. 2018, 215, 2655–2672. [Google Scholar] [CrossRef] [PubMed]
- Luik, A.L.; Hannocks, M.J.; Loismann, S.; Kapupara, K.; Cerina, M.; van der Stoel, M.; Tsytsyura, Y.; Glyvuk, N.; Nordenvall, C.; Klingauf, J.; et al. Endothelial basement membrane laminins—New players in mouse and human myoendothelial junctions and shear stress communication. Matrix Biol. 2023, 121, 56–73. [Google Scholar] [CrossRef] [PubMed]
- Chalkias, A.; Xenos, M. Relationship of Effective Circulating Volume with Sublingual Red Blood Cell Velocity and Microvessel Pressure Difference: A Clinical Investigation and Computational Fluid Dynamics Modeling. J. Clin. Med. 2022, 11, 4885. [Google Scholar] [CrossRef] [PubMed]
- Noria, S.; Cowan, D.B.; Gotlieb, A.I.; Langille, B.L. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ. Res. 1999, 85, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Baeyens, N.; Nicoli, S.; Coon, B.G.; Ross, T.D.; Van den Dries, K.; Han, J.; Lauridsen, H.M.; Mejean, C.O.; Eichmann, A.; Thomas, J.L.; et al. Vascular remodeling is governed by a VEGFR3-dependent fluid shear stress set point. elife 2015, 4, e04645. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Pang, K.L.; Rozbesky, D.; Nather, K.; Keen, A.; Lachowski, D.; Kong, Y.; Karia, D.; Ameismeier, M.; Huang, J.; et al. The guidance receptor plexin D1 is a mechanosensor in endothelial cells. Nature 2020, 578, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Bosseboeuf, E.; Chikh, A.; Chaker, A.B.; Mitchell, T.P.; Vignaraja, D.; Rajendrakumar, R.; Khambata, R.S.; Nightingale, T.D.; Mason, J.C.; Randi, A.M.; et al. Neuropilin-1 interacts with VE-cadherin and TGFBR2 to stabilize adherens junctions and prevent activation of endothelium under flow. Sci. Signal. 2023, 16, eabo4863. [Google Scholar] [CrossRef]
- Cheng, L.; Yue, H.; Zhang, H.; Liu, Q.; Du, L.; Liu, X.; Xie, J.; Shen, Y. The influence of microenvironment stiffness on endothelial cell fate: Implication for occurrence and progression of atherosclerosis. Life Sci. 2023, 334, 122233. [Google Scholar] [CrossRef]
- Hogan, B.; Shen, Z.; Zhang, H.; Misbah, C.; Barakat, A.I. Shear stress in the microvasculature: Influence of red blood cell morphology and endothelial wall undulation. Biomech. Model. Mechanobiol. 2019, 18, 1095–1109. [Google Scholar] [CrossRef]
- Atwell, S.; Badens, C.; Charrier, A.; Helfer, E.; Viallat, A. Dynamics of Individual Red Blood Cells Under Shear Flow: A Way to Discriminate Deformability Alterations. Front. Physiol. 2022, 12, 775584. [Google Scholar] [CrossRef]
- Meram, E.; Yilmaz, B.D.; Bas, C.; Atac, N.; Yalcin, O.; Meiselman, H.J.; Baskurt, O.K. Shear stress-induced improvement of red blood cell deformability. Biorheology 2013, 50, 165–176. [Google Scholar] [CrossRef]
- McNamee, A.P.; Simmonds, M.J.; Inoue, M.; Horobin, J.T.; Hakozaki, M.; Fraser, J.F.; Watanabe, N. Erythrocyte morphological symmetry analysis to detect sublethal trauma in shear flow. Sci. Rep. 2021, 11, 23566. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, H.; Lee, B.K.; Shin, S. Influence of shear stress on erythrocyte aggregation. Clin. Hemorheol. Microcirc. 2016, 62, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Moazzam, F.; DeLano, F.A.; Zweifach, B.W.; Schmid-Schönbein, G.W. The leukocyte response to fluid stress. Proc. Natl. Acad. Sci. USA 1997, 94, 5338–5343. [Google Scholar] [CrossRef]
- Wurzinger, L.J.; Opitz, R.; Blasberg, P.; Schmid-Schönbein, H. Platelet and coagulation parameters following millisecond exposure to laminar shear stress. Thromb. Haemost. 1985, 54, 381–386. [Google Scholar] [CrossRef]
- Fogelson, A.L.; Neeves, K.B. Fluid Mechanics of Blood Clot Formation. Annu. Rev. Fluid Mech. 2015, 47, 377–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mondal, N.K.; Zheng, S.; Koenig, S.C.; Slaughter, M.S.; Griffith, B.P.; Wu, Z.J. High shear induces platelet dysfunction leading to enhanced thrombotic propensity and diminished hemostatic capacity. Platelets 2019, 30, 112–119. [Google Scholar] [CrossRef]
- Li, L.; Wang, S.; Han, K.; Qi, X.; Ma, S.; Li, L.; Yin, J.; Li, D.; Li, X.; Qian, J. Quantifying Shear-induced Margination and Adhesion of Platelets in Microvascular Blood Flow. J. Mol. Biol. 2023, 435, 167824. [Google Scholar] [CrossRef]
- Davies, P.F. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 2009, 6, 16–26. [Google Scholar] [CrossRef]
- Nigro, P.; Abe, J.; Berk, B.C. Flow shear stress and atherosclerosis: A matter of site specificity. Antioxid. Redox Signal. 2011, 15, 1405–1414. [Google Scholar] [CrossRef]
- Roy, T.K.; Secomb, T.W. Effects of impaired microvascular flow regulation on metabolism-perfusion matching and organ function. Microcirculation 2021, 28, e12673. [Google Scholar] [CrossRef]
- Chappell, D.; Jacob, M.; Rehm, M.; Stoeckelhuber, M.; Welsch, U.; Conzen, P.; Becker, B.F. Heparinase selectively sheds heparan sulphate from the endothelial glycocalyx. Biol. Chem. 2008, 389, 79–82. [Google Scholar] [CrossRef]
- Hutchings, S.D.; Naumann, D.N.; Hopkins, P.; Mellis, C.; Riozzi, P.; Sartini, S.; Mamuza, J.; Harris, T.; Midwinter, M.J.; Wendon, J. Microcirculatory Impairment Is Associated With Multiple Organ Dysfunction Following Traumatic Hemorrhagic Shock: The MICROSHOCK Study. Crit. Care Med. 2018, 46, e889–e896. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, D.; Birukov, K.G. Mechanosensing and Mechanoregulation of Endothelial Cell Functions. Compr. Physiol. 2019, 9, 873–904. [Google Scholar]
- Hernandez, G.; Bruhn, A.; Ince, C. Microcirculation in sepsis: New perspectives. Curr. Vasc. Pharmacol. 2013, 11, 161–169. [Google Scholar] [PubMed]
- Ebong, E.E.; Lopez-Quintero, S.V.; Rizzo, V.; Spray, D.C.; Tarbell, J.M. Shear-induced endothelial NOS activation and remodeling via heparan sulfate, glypican-1, and syndecan-1. Integr. Biol. 2014, 6, 338–347. [Google Scholar] [CrossRef]
- De Backer, D.; Creteur, J.; Dubois, M.J.; Sakr, Y.; Vincent, J.L. Microvascular alterations in patients with acute severe heart failure and cardiogenic shock. Am. Heart J. 2004, 147, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Merdji, H.; Levy, B.; Jung, C.; Ince, C.; Siegemund, M.; Meziani, F. Microcirculatory dysfunction in cardiogenic shock. Ann. Intensive Care 2023, 13, 38. [Google Scholar] [CrossRef]
- Cai, B.; Deitch, E.A.; Ulloa, L. Novel insights for systemic inflammation in sepsis and hemorrhage. Mediat. Inflamm. 2010, 2010, 642462. [Google Scholar] [CrossRef] [PubMed]
- Munley, J.A.; Kelly, L.S.; Gillies, G.S.; Pons, E.E.; Coldwell, P.S.; Kannan, K.B.; Whitley, E.M.; Bible, L.E.; Efron, P.A.; Mohr, A.M. Narrowing the gap: Preclinical trauma with postinjury sepsis model with increased clinical relevance. Shock 2023, 60, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Yakusheva, A.A.; Butov, K.R.; Bykov, G.A.; Závodszky, G.; Eckly, A.; Ataullakhanov, F.I.; Gachet, C.; Panteleev, M.A.; Mangin, P.H. Traumatic vessel injuries initiating hemostasis generate high shear conditions. Blood Adv. 2022, 6, 4834–4846. [Google Scholar] [CrossRef]
- Tsiklidis, E.J.; Sinno, T.; Diamond, S.L. Coagulopathy implications using a multiscale model of traumatic bleeding matching macro- and microcirculation. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H73–H86. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.I.; Stensballe, J.; Rasmussen, L.S.; Ostrowski, S.R. High circulating adrenaline levels at admission predict increased mortality after trauma. J. Trauma Acute Care Surg. 2012, 72, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Gouverneur, M.; Berg, B.; Nieuwdorp, M.; Stroes, E.; Vink, H. Vasculoprotective properties of the endothelial glycocalyx: Effects of fluid shear stress. J. Intern. Med. 2006, 259, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Roka-Moiia, Y.; Ammann, K.R.; Miller-Gutierrez, S.; Sheriff, J.; Bluestein, D.; Italiano, J.E.; Flaumenhaft, R.C.; Slepian, M.J. Shear-Mediated Platelet Microparticles Demonstrate Phenotypic Heterogeneity as to Morphology, Receptor Distribution, and Hemostatic Function. Int. J. Mol. Sci. 2023, 24, 7386. [Google Scholar] [CrossRef] [PubMed]
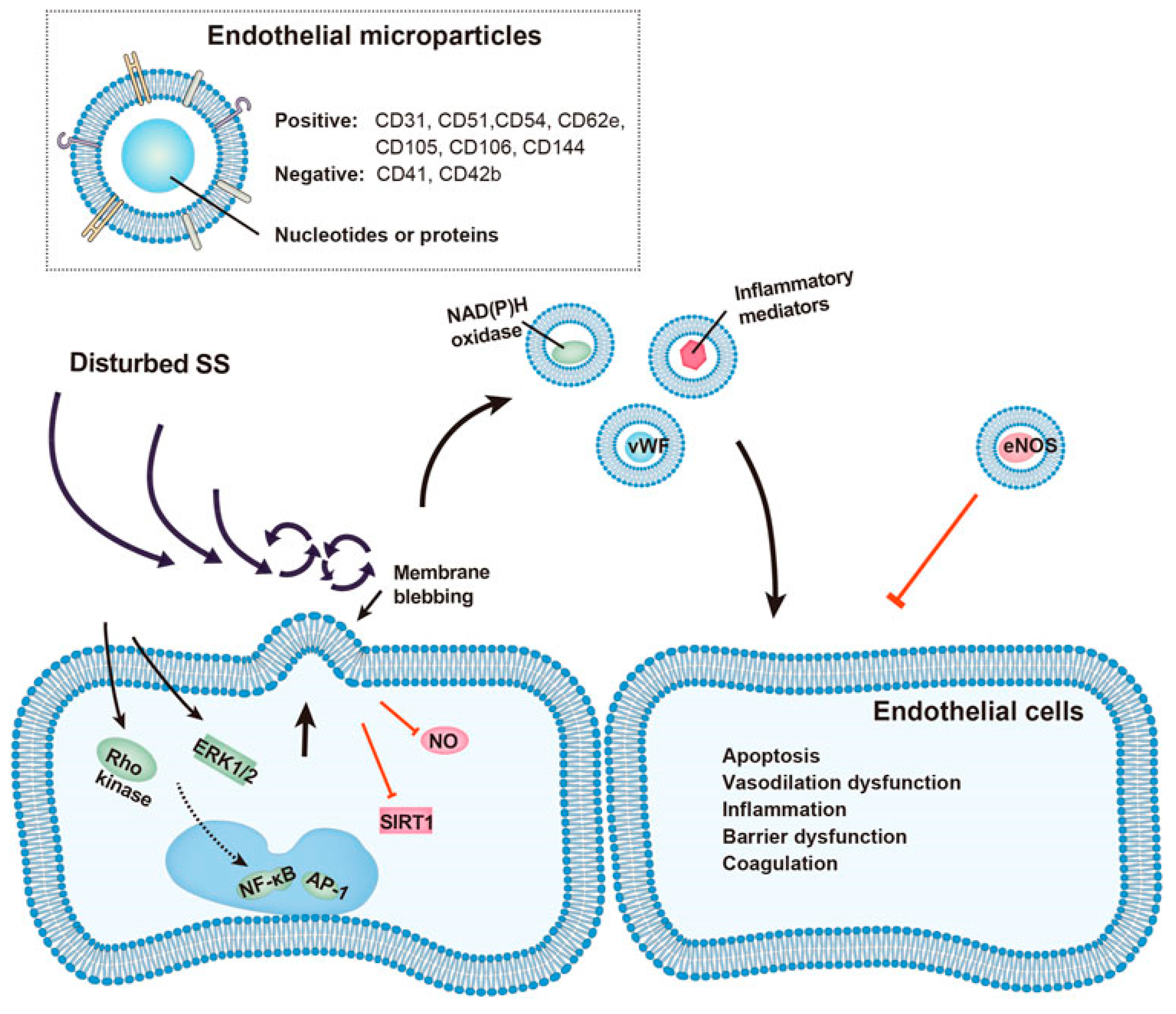
- Feng, S.; Chen, J.W.; Shu, X.Y.; Aihemaiti, M.; Quan, J.W.; Lu, L.; Zhang, R.Y.; Yang, C.D.; Wang, X.Q. Endothelial microparticles: A mechanosensitive regulator of vascular homeostasis and injury under shear stress. Front. Cell Dev. Biol. 2022, 10, 980112. [Google Scholar] [CrossRef]
- Jy, W.; Jimenez, J.J.; Mauro, L.M.; Horstman, L.L.; Cheng, P.; Ahn, E.R.; Bidot, C.J.; Ahn, Y.S. Endothelial microparticles induce formation of platelet aggregates via a von Willebrand factor/ristocetin dependent pathway, rendering them resistant to dissociation. J. Thromb. Haemost. 2005, 3, 1301–1308. [Google Scholar] [CrossRef]
- Densmore, J.C.; Signorino, P.R.; Ou, J.; Hatoum, O.A.; Rowe, J.J.; Shi, Y.; Kaul, S.; Jones, D.W.; Sabina, R.E.; Pritchard, K.A., Jr.; et al. Endothelium-derived microparticles induce endothelial dysfunction and acute lung injury. Shock 2006, 26, 464–471. [Google Scholar] [CrossRef]
- Abbas, M.; Jesel, L.; Auger, C.; Amoura, L.; Messas, N.; Manin, G.; Rumig, C.; León-González, A.J.; Ribeiro, T.P.; Silva, G.C.; et al. Endothelial Microparticles From Acute Coronary Syndrome Patients Induce Premature Coronary Artery Endothelial Cell Aging and Thrombogenicity: Role of the Ang II/AT1 Receptor/NADPH Oxidase-Mediated Activation of MAPKs and PI3-Kinase Pathways. Circulation 2017, 135, 280–296. [Google Scholar] [CrossRef]
- Barak, O.F.; Mladinov, S.; Hoiland, R.L.; Tremblay, J.C.; Thom, S.R.; Yang, M.; Mijacika, T.; Dujic, Z. Disturbed blood flow worsens endothelial dysfunction in moderate-severe chronic obstructive pulmonary disease. Sci. Rep. 2017, 7, 16929. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, J.; Li, C.; Song, G.; Shan, Y. Mild therapeutic hypothermia reduces ischemia-reperfusion injury after zone 1 reboa in a swine hemorrhagic shock model. Shock 2023, 60, 434–442. [Google Scholar] [CrossRef]
- Schanche, T.; Kondratiev, T.; Tveita, T. Extracorporeal rewarming from experimental hypothermia: Effects of hydroxyethyl starch versus saline priming on fluid balance and blood flow distribution. Exp. Physiol. 2019, 104, 1353–1362. [Google Scholar] [CrossRef]
- Coombs, G.B.; Tremblay, J.C.; Shkredova, D.A.; Carr, J.M.J.R.; Wakeham, D.J.; Patrician, A.; Ainslie, P.N. Distinct contributions of skin and core temperatures to flow-mediated dilation of the brachial artery following passive heating. J. Appl. Physiol. 2021, 130, 149–159. [Google Scholar] [CrossRef]
- Brunt, V.E.; Weidenfeld-Needham, K.M.; Comrada, L.N.; Francisco, M.A.; Eymann, T.M.; Minson, C.T. Serum from young, sedentary adults who underwent passive heat therapy improves endothelial cell angiogenesis via improved nitric oxide bioavailability. Temperature 2019, 6, 169–178. [Google Scholar] [CrossRef]
- Dietrichs, E.S.; Håheim, B.; Kondratiev, T.; Traasdahl, E.; Tveita, T. Effects of hypothermia and rewarming on cardiovascular autonomic control in vivo. J. Appl. Physiol. 2018, 124, 850–859. [Google Scholar] [CrossRef]
- Hemingway, H.W.; Richey, R.E.; Moore, A.M.; Shokraeifard, A.M.; Thomas, G.C.; Olivencia-Yurvati, A.H.; Romero, S.A. Shear stress induced by acute heat exposure is not obligatory to protect against endothelial ischemia-reperfusion injury in humans. J. Appl. Physiol. 2022, 132, 199–208. [Google Scholar] [CrossRef]
- Cheng, J.L.; Au, J.S.; MacDonald, M.J. Peripheral artery endothelial function responses to altered shear stress patterns in humans. Exp. Physiol. 2019, 104, 1126–1135. [Google Scholar] [CrossRef]
- Imamura, M.; Biro, S.; Kihara, T.; Yoshifuku, S.; Takasaki, K.; Otsuji, Y.; Minagoe, S.; Toyama, Y.; Tei, C. Repeated thermal therapy improves impaired vascular endothelial function in patients with coronary risk factors. J. Am. Coll. Cardiol. 2001, 38, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Holme, M.N.; Fedotenko, I.A.; Abegg, D.; Althaus, J.; Babel, L.; Favarger, F.; Reiter, R.; Tanasescu, R.; Zaffalon, P.L.; Ziegler, A.; et al. Shear-stress sensitive lenticular vesicles for targeted drug delivery. Nat. Nanotechnol. 2012, 7, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Korin, N.; Kanapathipillai, M.; Matthews, B.D.; Crescente, M.; Brill, A.; Mammoto, T.; Ghosh, K.; Jurek, S.; Bencherif, S.A.; Bhatta, D.; et al. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science 2012, 337, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Zeibi Shirejini, S.; Carberry, J.; Alt, K.; Gregory, S.D.; Hagemeyer, C.E. Shear-Responsive Drug Delivery Systems in Medical Devices: Focus on Thrombosis and Bleeding. Adv. Funct. Mater. 2023, 33, 2303717. [Google Scholar] [CrossRef]
- Saxer, T.; Zumbuehl, A.; Müller, B. The use of shear stress for targeted drug delivery. Cardiovasc. Res. 2013, 99, 328–333. [Google Scholar] [CrossRef]
- Abdullah, S.; Karim, M.; Legendre, M.; Rodriguez, L.; Friedman, J.; Cotton-Betteridge, A.; Drury, R.; Packer, J.; Guidry, C.; Duchesne, J.; et al. Hemorrhagic Shock and Resuscitation Causes Glycocalyx Shedding and Endothelial Oxidative Stress Preferentially in the Lung and Intestinal Vasculature. Shock 2021, 56, 803–812. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Li, B.; Gao, X.; Liu, Y.; Mao, W.; Chen, S.L. Resveratrol ameliorates low shear stress-induced oxidative stress by suppressing ERK/eNOS-Thr495 in endothelial cells. Mol. Med. Rep. 2014, 10, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.K.; Luo, J.Y.; Lau, C.W.; Chen, Z.Y.; Tian, X.Y.; Huang, Y. Pharmacological basis and new insights of resveratrol action in the cardiovascular system. Br. J. Pharmacol. 2020, 177, 1258–1277. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.P.; Hwang, T.L.; Hwang, T.L.; Yen, C.H.; Lau, Y.T. Resveratrol prevents endothelial dysfunction and aortic superoxide production after trauma hemorrhage through estrogen receptor-dependent hemeoxygenase-1 pathway. Crit. Care Med. 2010, 38, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, P.; Xu, S.; Koroleva, M.; Zhang, S.; Si, S.; Jin, Z.G. Tannic acid as a plant-derived polyphenol exerts vasoprotection via enhancing KLF2 expression in endothelial cells. Sci. Rep. 2017, 7, 6686. [Google Scholar] [CrossRef]
- Parmar, K.M.; Nambudiri, V.; Dai, G.; Larman, H.B.; Gimbrone, M.A., Jr.; García-Cardeña, G. Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J. Biol. Chem. 2005, 280, 26714–26719. [Google Scholar] [CrossRef]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef]
- de Nigris, F.; Lerman, L.O.; Ignarro, S.W.; Sica, G.; Lerman, A.; Palinski, W.; Ignarro, L.J.; Napoli, C. Beneficial effects of antioxidants and L-arginine on oxidation-sensitive gene expression and endothelial NO synthase activity at sites of disturbed shear stress. Proc. Natl. Acad. Sci. USA 2003, 100, 1420–1425. [Google Scholar] [CrossRef]
- Carter, S.E.; Faulkner, A.; Rakobowchuk, M. The role of prostaglandin and antioxidant availability in recovery from forearm ischemia-reperfusion injury in humans. J. Hypertens. 2014, 32, 339–351. [Google Scholar] [CrossRef]
- Sena, C.M.; Leandro, A.; Azul, L.; Seiça, R.; Perry, G. Vascular Oxidative Stress: Impact and Therapeutic Approaches. Front. Physiol. 2018, 9, 1668. [Google Scholar] [CrossRef]
- He, P.; Talukder, M.A.H.; Gao, F. Oxidative Stress and Microvessel Barrier Dysfunction. Front. Physiol. 2020, 11, 472. [Google Scholar] [CrossRef]
- Rosnoblet, C.; Vischer, U.M.; Gerard, R.D.; Irminger, J.C.; Halban, P.A.; Kruithof, E.K. Storage of tissue-type plasminogen activator in Weibel-Palade bodies of human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1796–1803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rosenberg, J.B.; Foster, P.A.; Kaufman, R.J.; Vokac, E.A.; Moussalli, M.; Kroner, P.A.; Montgomery, R.R. Intracellular trafficking of factor VIII to von Willebrand factor storage granules. J. Clin. Investig. 1998, 101, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Emeis, J.J.; van den Eijnden-Schrauwen, Y.; van den Hoogen, C.M.; de Priester, W.; Westmuckett, A.; Lupu, F. An endothelial storage granule for tissue-type plasminogen activator. J. Cell Biol. 1997, 139, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Wall, R.T.; Counts, R.B.; Harker, L.A.; Striker, G.E. Binding and release of factor VIII/von Willebrand’s factor by human endothelial cells. Br. J. Haematol. 1980, 46, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Vischer, U.M.; Wollheim, C.B. Epinephrine induces von Willebrand factor release from cultured endothelial cells: Involvement of cyclic AMP-dependent signalling in exocytosis. Thromb. Haemost. 1997, 77, 1182–1188. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, R.J.; Pipe, S.W. Regulation of factor VIII expression and activity by von Willebrand factor. Thromb. Haemost. 1999, 82, 201–208. [Google Scholar] [PubMed]
- Emeis, J.J. Perfused rat hindlegs. A model to study plasminogen activator release. Thromb. Res. 1983, 30, 195–203. [Google Scholar] [CrossRef]
- Zhu, G.J.; Abbadini, M.; Donati, M.B.; Mussoni, L. Tissue-type plasminogen activator release in response to epinephrine in perfused rat hindlegs. Am. J. Physiol. 1989, 256, H404–H410. [Google Scholar] [CrossRef]
- Klöcking, H.P.; Markwardt, F. Pharmacological stimulation of t-PA release. Pharmazie 1994, 49, 227–230. [Google Scholar] [CrossRef] [PubMed]
- von Känel, R.; Dimsdale, J.E. Effects of sympathetic activation by adrenergic infusions on hemostasis in vivo. Eur. J. Haematol. 2000, 65, 357–369. [Google Scholar] [CrossRef]
- Hartmann, C.; Radermacher, P.; Wepler, M.; Nußbaum, B. Non-Hemodynamic Effects of Catecholamines. Shock 2017, 48, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Badimon, L.; Martínez-González, J.; Royo, T.; Lassila, R.; Badimon, J.J. A sudden increase in plasma epinephrine levels transiently enhances platelet deposition on severely damaged arterial wall--studies in a porcine model. Thromb. Haemost. 1999, 82, 1736–1742. [Google Scholar] [PubMed]
- Goto, S.; Handa, S.; Takahashi, E.; Abe, S.; Handa, M.; Ikeda, Y. Synergistic effect of epinephrine and shearing on platelet activation. Thromb. Res. 1996, 84, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.T.; Kroll, M.H.; Chow, T.W.; Hellums, J.D.; Schafer, A.I. Epinephrine and shear stress synergistically induce platelet aggregation via a mechanism that partially bypasses VWF-GP IB interactions. Biorheology 1996, 33, 209–229. [Google Scholar] [CrossRef] [PubMed]
- Aster, R.H. Pooling of platelets in the spleen: Role in the pathogenesis of “hypersplenic” thrombocytopenia. J. Clin. Investig. 1966, 45, 645–657. [Google Scholar] [CrossRef]
- Libre, E.P.; Cowan, D.H.; Watkins, S.P., Jr.; Shulman, N.R. Relationships between spleen, platelets and factor 8 levels. Blood 1968, 31, 358–368. [Google Scholar] [CrossRef]
- Richards, J.E.; Harris, T.; Dünser, M.W.; Bouzat, P.; Gauss, T. Vasopressors in Trauma: A Never Event? Anesth. Analg. 2021, 133, 68–79. [Google Scholar] [CrossRef]
- MacGregor, D.A.; Prielipp, R.C.; Butterworth, J.F., 4th; James, R.L.; Royster, R.L. Relative efficacy and potency of beta-adrenoceptor agonists for generating cAMP in human lymphocytes. Chest 1996, 109, 194–200. [Google Scholar] [CrossRef]
- Geenen, I.L.; Molin, D.G.; van den Akker, N.M.; Jeukens, F.; Spronk, H.M.; Schurink, G.W.; Post, M.J. Endothelial cells (ECs) for vascular tissue engineering: Venous ECs are less thrombogenic than arterial ECs. J. Tissue Eng. Regen. Med. 2015, 9, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Geevarghese, M., 3rd; Patel, K.; Gulati, A.; Ranjan, A.K. Role of adrenergic receptors in shock. Front. Physiol. 2023, 14, 1094591. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.K.; Gulati, A. Controls of Central and Peripheral Blood Pressure and Hemorrhagic/Hypovolemic Shock. J. Clin. Med. 2023, 12, 1108. [Google Scholar] [CrossRef] [PubMed]
- Pais, G.; Gulati, A. Effect of centhaquin on the coagulation cascade using thromboelastography (TEG). Crit. Care Med. 2012, 40, 39–40. [Google Scholar] [CrossRef]
- Gulati, A.; Mulloy, N.; Zhang, Z.; Pais, G. Resuscitative efficacy of centhaquin in a rabbit model of uncontrolled hemorrhagic shock. Crit. Care Med. 2013, 41, A130–A131. [Google Scholar] [CrossRef]
- Walsh, D.; Cunning, C.; Lee, G.; Boylan, J.; McLoughlin, P. Capillary leak and edema after resuscitation: The potential contribution of reduced endothelial shear stress caused by hemodilution. Shock 2023, 60, 487–495. [Google Scholar] [CrossRef]

| Vessel Type | Diameter (mm) | Shear Stress (Dynes cm−2) | Function |
|---|---|---|---|
| Pulmonary artery | 29 | 5 | Distribution of venous blood |
| Ascending aorta | 25 | 12 | Pulse dampening and distribution |
| Descending aorta | 25 | 5–8 | Pulse dampening and distribution |
| Large arteries | 1.0–4.0 | 10–60 | Distribution of arterial blood |
| Small arteries | 0.2–1.0 | 10–80 | Distribution and resistance |
| Venules | 0.01–0.20 | 20–40 | Exchange, collection, and capacitance |
| Veins | 0.2–5.0 | <5 | Capacitance function (blood volume) |
| Vena Cava | 35 | <1–5 | Collection of venous blood |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chalkias, A. Shear Stress and Endothelial Mechanotransduction in Trauma Patients with Hemorrhagic Shock: Hidden Coagulopathy Pathways and Novel Therapeutic Strategies. Int. J. Mol. Sci. 2023, 24, 17522. https://doi.org/10.3390/ijms242417522
Chalkias A. Shear Stress and Endothelial Mechanotransduction in Trauma Patients with Hemorrhagic Shock: Hidden Coagulopathy Pathways and Novel Therapeutic Strategies. International Journal of Molecular Sciences. 2023; 24(24):17522. https://doi.org/10.3390/ijms242417522
Chicago/Turabian StyleChalkias, Athanasios. 2023. "Shear Stress and Endothelial Mechanotransduction in Trauma Patients with Hemorrhagic Shock: Hidden Coagulopathy Pathways and Novel Therapeutic Strategies" International Journal of Molecular Sciences 24, no. 24: 17522. https://doi.org/10.3390/ijms242417522
APA StyleChalkias, A. (2023). Shear Stress and Endothelial Mechanotransduction in Trauma Patients with Hemorrhagic Shock: Hidden Coagulopathy Pathways and Novel Therapeutic Strategies. International Journal of Molecular Sciences, 24(24), 17522. https://doi.org/10.3390/ijms242417522






