The Fibrinolytic System and Its Measurement: History, Current Uses and Future Directions for Diagnosis and Treatment
Abstract
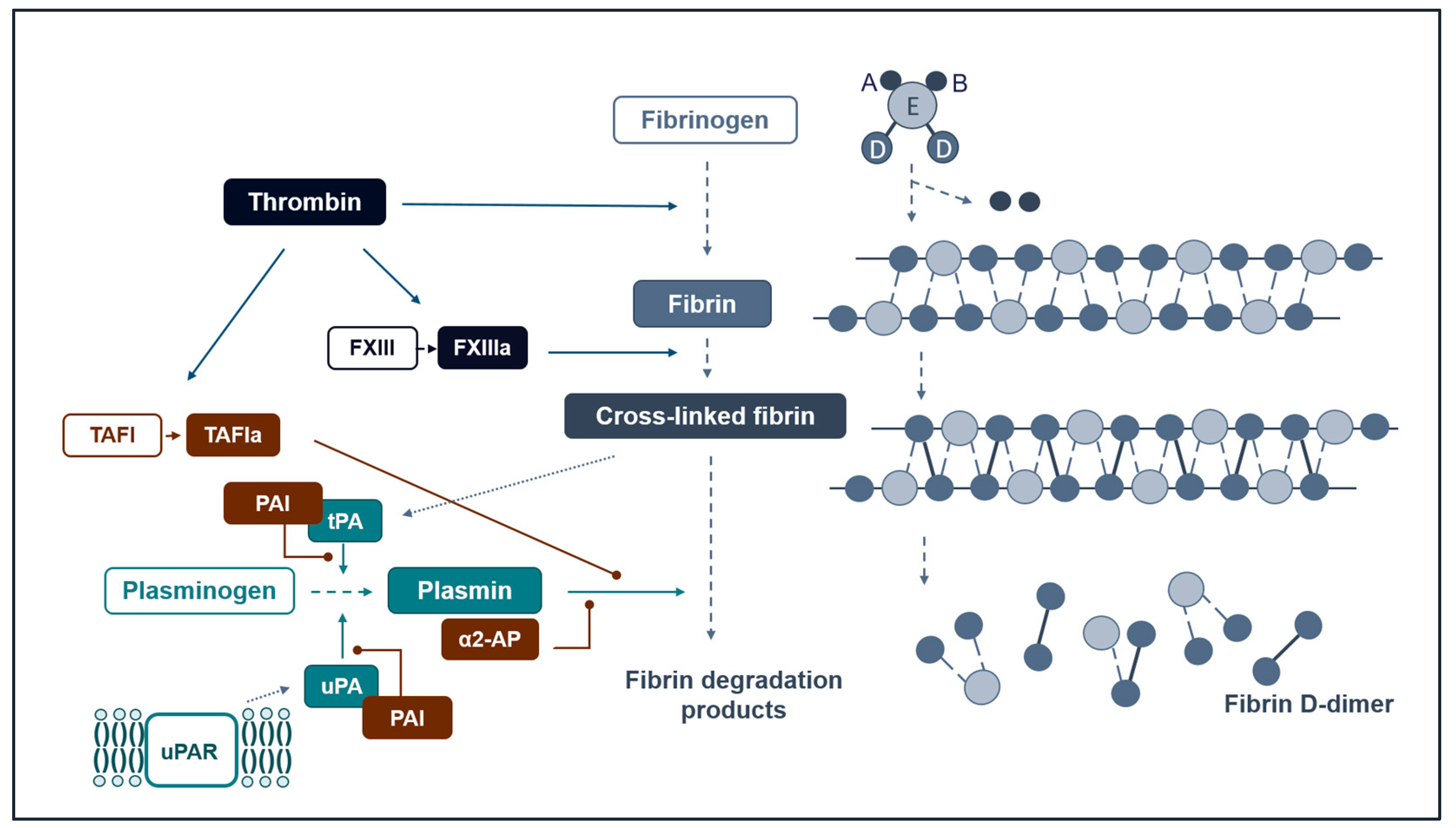
:1. Introduction
2. Historical Discovery of the Fibrinolytic System and Current Concepts
Our Current Understanding of Fibrinolysis
3. Measuring Fibrinolysis
3.1. Early Observations and Methods
3.2. Dynamic Assays
3.2.1. Euglobulin Lysis Time and Plasma-Based Clot Formation and Lysis Assays
3.2.2. Plasmin Generation
3.2.3. Fibrin Clot Structure
3.2.4. Viscoelastic Tests
3.3. Measurement of Circulating Factors
3.3.1. Circulating Pro- and Antifibrinolytic Proteins
3.3.2. Fibrin Degradation Products
4. Fibrinolysis in Specific Clinical Settings
4.1. Fibrinolysis in Trauma
4.1.1. Major Trauma
4.1.2. Brain Trauma
4.2. Fibrinolysis in Coronary Artery Disease
4.3. Fibrinolysis in Sepsis
4.4. Venous Thromboembolism
Pulmonary Embolism
4.5. Fibrinolysis in Liver Disease
4.5.1. Cirrhosis
4.5.2. Acute Liver Failure and Liver Transplantation
4.6. Beyond Bleeding and Thrombosis Risk: Hereditary Angioedema
5. The Fibrinolytic System as a Treatment Target
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwaan, H.C. From fibrinolysis to the plasminogen-plasmin system and beyond: A remarkable growth of knowledge, with personal observations on the history of fibrinolysis. Semin. Thromb. Hemost. 2014, 40, 585–591. [Google Scholar] [CrossRef]
- Ismail, A.A.; Shaker, B.T.; Bajou, K. The Plasminogen-Activator Plasmin System in Physiological and Pathophysiological Angiogenesis. Int. J. Mol. Sci. 2021, 23, 337. [Google Scholar] [CrossRef]
- Syrovets, T.; Lunov, O.; Simmet, T. Plasmin as a proinflammatory cell activator. J. Leukoc. Biol. 2012, 92, 509–519. [Google Scholar] [CrossRef]
- Tillett, W.S.; Garner, R.L. The fibrinolytic activity of hemolytic streptococci. J. Exp. Med. 1933, 58, 485–502. [Google Scholar] [CrossRef]
- Collaborators, C.-T.; Shakur, H.; Roberts, I.; Bautista, R.; Caballero, J.; Coats, T.; Dewan, Y.; El-Sayed, H.; Gogichaishvili, T.; Gupta, S.; et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): A randomised, placebo-controlled trial. Lancet 2010, 376, 23–32. [Google Scholar] [CrossRef]
- Collaborators, W.T. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): An international, randomised, double-blind, placebo-controlled trial. Lancet 2017, 389, 2105–2116. [Google Scholar] [CrossRef]
- Longstaff, C. Measuring fibrinolysis: From research to routine diagnostic assays. J. Thromb. Haemost. 2018, 16, 652–662. [Google Scholar] [CrossRef]
- Green, J.R. Note on the Action of Sodium Chloride in dissolving Fibrin. J. Physiol. 1887, 8, 372–377. [Google Scholar] [CrossRef]
- Macfarlane, R.G.; Biggs, R. Fibrinolysis; its mechanism and significance. Blood 1948, 3, 1167–1187. [Google Scholar] [CrossRef]
- Christensen, L.R.; Macleod, C.M. A proteolytic enzyme of serum: Characterization, activation, and reaction with inhibitors. J. Gen. Physiol. 1945, 28, 559–583. [Google Scholar] [CrossRef]
- Astrup, T.; Stage, A. Isolation of a soluble fibrinolytic activator from animal tissue. Nature 1952, 170, 929. [Google Scholar] [CrossRef] [PubMed]
- Kwaan, H.C.; Lo, R.; McFadzean, A.J. On the production of plasma fibrinolytic activity within veins. Clin. Sci. 1957, 16, 241–253. [Google Scholar] [PubMed]
- Todd, A.S. The histological localisation of fibrinolysin activator. J. Pathol. Bacteriol. 1959, 78, 281–283. [Google Scholar] [CrossRef] [PubMed]
- Tillett, W.S.; Sherry, S. The effect in patients of streptococcal fibrinolysin (streptokinase) and streptococcal desoxyribonuclease on fibrinous, purulent, and sanguinous pleural exudations. J. Clin. Investig. 1949, 28, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Konstantinides, S.V.; Meyer, G.; Becattini, C.; Bueno, H.; Geersing, G.J.; Harjola, V.P.; Huisman, M.V.; Humbert, M.; Jennings, C.S.; Jiménez, D.; et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur. Heart J. 2020, 41, 543–603. [Google Scholar] [CrossRef]
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 2021, 6, I–LXII. [Google Scholar] [CrossRef]
- Nielsen, L.S.; Kellerman, G.M.; Behrendt, N.; Picone, R.; Danø, K.; Blasi, F. A 55,000–60,000 Mr receptor protein for urokinase-type plasminogen activator. Identification in human tumor cell lines and partial purification. J. Biol. Chem. 1988, 263, 2358–2363. [Google Scholar] [CrossRef] [PubMed]
- Ploug, M.; Rønne, E.; Behrendt, N.; Jensen, A.L.; Blasi, F.; Danø, K. Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J. Biol. Chem. 1991, 266, 1926–1933. [Google Scholar] [CrossRef]
- Eugen-Olsen, J.; Andersen, O.; Linneberg, A.; Ladelund, S.; Hansen, T.W.; Langkilde, A.; Petersen, J.; Pielak, T.; Møller, L.N.; Jeppesen, J.; et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J. Intern. Med. 2010, 268, 296–308. [Google Scholar] [CrossRef]
- Napolitano, F.; Giudice, V.; Selleri, C.; Montuori, N. Plasminogen System in the Pathophysiology of Sepsis: Upcoming Biomarkers. Int. J. Mol. Sci. 2023, 24, 12376. [Google Scholar] [CrossRef] [PubMed]
- Sudhini, Y.R.; Wei, C.; Reiser, J. suPAR: An Inflammatory Mediator for Kidneys. Kidney Dis. 2022, 8, 265–274. [Google Scholar] [CrossRef]
- Thunø, M.; Macho, B.; Eugen-Olsen, J. suPAR: The molecular crystal ball. Dis. Markers 2009, 27, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, V.; Jayaraman, P.S.; Zaitsev, S.V.; Lebedeva, T.; Bdeir, K.; Kershaw, R.; Holman, K.R.; Parfyonova, Y.V.; Semina, E.V.; Beloglazova, I.B.; et al. Urokinase-type Plasminogen Activator (uPA) Promotes Angiogenesis by Attenuating Proline-rich Homeodomain Protein (PRH) Transcription Factor Activity and De-repressing Vascular Endothelial Growth Factor (VEGF) Receptor Expression. J. Biol. Chem. 2016, 291, 15029–15045. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, S.; Chen, J.; Lee, A.; Akassoglou, K.; Norman, J.; Goligorsky, M.S. Plasmin-dependent and -independent effects of plasminogen activators and inhibitor-1 on ex vivo angiogenesis. Am. J. Physiol.-Heart Circ. Physiol. 2001, 281, H1784–H1792. [Google Scholar] [CrossRef] [PubMed]
- Strickland, S.; Reich, E.; Sherman, M.I. Plasminogen activator in early embryogenesis: Enzyme production by trophoblast and parietal endoderm. Cell 1976, 9, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Syrovets, T.; Jendrach, M.; Rohwedder, A.; Schüle, A.; Simmet, T. Plasmin-induced expression of cytokines and tissue factor in human monocytes involves AP-1 and IKKbeta-mediated NF-kappaB activation. Blood 2001, 97, 3941–3950. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.C.; Seeds, N.W. Tissue plasminogen activator expression in the embryonic nervous system. Brain Res. Dev. Brain Res. 1994, 81, 41–49. [Google Scholar] [CrossRef]
- Rao, J.S.; Gujrati, M.; Chetty, C. Tumor-associated soluble uPAR-directed endothelial cell motility and tumor angiogenesis. Oncogenesis 2013, 2, e53. [Google Scholar] [CrossRef]
- Rakic, J.M.; Lambert, V.; Munaut, C.; Bajou, K.; Peyrollier, K.; Alvarez-Gonzalez, M.L.; Carmeliet, P.; Foidart, J.M.; Noël, A. Mice without uPA, tPA, or plasminogen genes are resistant to experimental choroidal neovascularization. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, F.; Montuori, N. The Role of the Plasminogen Activation System in Angioedema: Novel Insights on the Pathogenesis. J. Clin. Med. 2021, 10, 518. [Google Scholar] [CrossRef]
- Hethershaw, E.L.; Cilia La Corte, A.L.; Duval, C.; Ali, M.; Grant, P.J.; Ariëns, R.A.; Philippou, H. The effect of blood coagulation factor XIII on fibrin clot structure and fibrinolysis. J. Thromb. Haemost. 2014, 12, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Horrevoets, A.J.; Pannekoek, H.; Nesheim, M.E. A steady-state template model that describes the kinetics of fibrin-stimulated [Glu1]- and [Lys78]plasminogen activation by native tissue-type plasminogen activator and variants that lack either the finger or kringle-2 domain. J. Biol. Chem. 1997, 272, 2183–2191. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N. Discovery of alpha2-plasmin inhibitor and its congenital deficiency. J. Thromb. Haemost. 2005, 3, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Binder, B.R.; Christ, G.; Gruber, F.; Grubic, N.; Hufnagl, P.; Krebs, M.; Mihaly, J.; Prager, G.W. Plasminogen activator inhibitor 1: Physiological and pathophysiological roles. News Physiol. Sci. 2002, 17, 56–61. [Google Scholar] [CrossRef]
- Bajzar, L.; Morser, J.; Nesheim, M. TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex. J. Biol. Chem. 1996, 271, 16603–16608. [Google Scholar] [CrossRef] [PubMed]
- Wolberg, A.S.; Monroe, D.M.; Roberts, H.R.; Hoffman, M. Elevated prothrombin results in clots with an altered fiber structure: A possible mechanism of the increased thrombotic risk. Blood 2003, 101, 3008–3013. [Google Scholar] [CrossRef]
- Gabriel, D.A.; Muga, K.; Boothroyd, E.M. The effect of fibrin structure on fibrinolysis. J. Biol. Chem. 1992, 267, 24259–24263. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Park, D.; Lesty, C.; Soria, J.; Soria, C.; Montalescot, G.; Weisel, J.W. Influence of fibrin network conformation and fibrin fiber diameter on fibrinolysis speed: Dynamic and structural approaches by confocal microscopy. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1354–1361. [Google Scholar] [CrossRef] [PubMed]
- Ariëns, R.A. Fibrin(ogen) and thrombotic disease. J. Thromb. Haemost. 2013, 11 (Suppl. S1), 294–305. [Google Scholar] [CrossRef]
- Dunn, E.J.; Ariens, R.A.; Grant, P.J. The influence of type 2 diabetes on fibrin structure and function. Diabetologia 2005, 48, 1198–1206. [Google Scholar] [CrossRef]
- Fan, N.K.; Keegan, P.M.; Platt, M.O.; Averett, R.D. Experimental and imaging techniques for examining fibrin clot structures in normal and diseased states. J. Vis. Exp. 2015, 1, e52019. [Google Scholar] [CrossRef]
- Astrup, T.; Mullertz, S. The fibrin plate method for estimating fibrinolytic activity. Arch. Biochem. Biophys. 1952, 40, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.P.; Aronson, D.L. An automated fibrinolytic assay performed in microtiter plates. Thromb. Res. 1987, 47, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.A.; Jacobson, L.J.; Miller, B.I.; Hathaway, W.E.; Manco-Johnson, M.J. A new euglobulin clot lysis assay for global fibrinolysis. Thromb. Res. 2003, 112, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Sakakibara, K.; Rydzewski, A.; Urano, S.; Takada, Y.; Takada, A. Relationships between euglobulin clot lysis time and the plasma levels of tissue plasminogen activator and plasminogen activator inhibitor 1. Thromb. Haemost. 1990, 63, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Pieters, M.; Philippou, H.; Undas, A.; de Lange, Z.; Rijken, D.C.; Mutch, N.J. An international study on the feasibility of a standardized combined plasma clot turbidity and lysis assay: Communication from the SSC of the ISTH. J. Thromb. Haemost. 2018, 16, 1007–1012. [Google Scholar] [CrossRef]
- Miszta, A.; Huskens, D.; Donkervoort, D.; Roberts, M.J.M.; Wolberg, A.S.; de Laat, B. Assessing Plasmin Generation in Health and Disease. Int. J. Mol. Sci. 2021, 22, 2758. [Google Scholar] [CrossRef]
- Pieters, M.; Undas, A.; Marchi, R.; De Maat, M.P.; Weisel, J.; Ariëns, R.A. An international study on the standardization of fibrin clot permeability measurement: Methodological considerations and implications for healthy control values. J. Thromb. Haemost. 2012, 10, 2179–2181. [Google Scholar] [CrossRef]
- Rossaint, R.; Afshari, A.; Bouillon, B.; Cerny, V.; Cimpoesu, D.; Curry, N.; Duranteau, J.; Filipescu, D.; Grottke, O.; Gronlykke, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Sixth edition. Crit. Care 2023, 27, 80. [Google Scholar] [CrossRef]
- Chapman, M.P.; Moore, E.E.; Moore, H.B.; Gonzalez, E.; Morton, A.P.; Chandler, J.; Fleming, C.D.; Ghasabyan, A.; Silliman, C.C.; Banerjee, A.; et al. The “Death Diamond”: Rapid thrombelastography identifies lethal hyperfibrinolysis. J. Trauma Acute Care Surg. 2015, 79, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Raza, I.; Davenport, R.; Rourke, C.; Platton, S.; Manson, J.; Spoors, C.; Khan, S.; De’Ath, H.D.; Allard, S.; Hart, D.P.; et al. The incidence and magnitude of fibrinolytic activation in trauma patients. J. Thromb. Haemost. 2013, 11, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.J.; Kleinegris, M.C.; van Oerle, R.; Spronk, H.M.; Lance, M.D.; Ten Cate, H.; Henskens, Y.M. Validation of a modified thromboelastometry approach to detect changes in fibrinolytic activity. Thromb. J. 2016, 14, 1. [Google Scholar] [CrossRef]
- Larsen, J.B.; Hvas, C.L.; Hvas, A.M. Modified Rotational Thromboelastometry Protocol Using Tissue Plasminogen Activator for Detection of Hypofibrinolysis and Hyperfibrinolysis. Methods Mol. Biol. 2023, 2663, 763–773. [Google Scholar] [CrossRef]
- Panigada, M.; Zacchetti, L.; L’Acqua, C.; Cressoni, M.; Anzoletti, M.B.; Bader, R.; Protti, A.; Consonni, D.; D’Angelo, A.; Gattinoni, L. Assessment of Fibrinolysis in Sepsis Patients with Urokinase Modified Thromboelastography. PLoS ONE 2015, 10, e0136463. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Shapiro, A.D. Plasminogen activator inhibitor type 1 deficiency. Haemophilia 2008, 14, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- Schuster, V.; Zeitler, P.; Seregard, S.; Ozcelik, U.; Anadol, D.; Luchtman-Jones, L.; Meire, F.; Mingers, A.M.; Schambeck, C.; Kreth, H.W. Homozygous and compound-heterozygous type I plasminogen deficiency is a common cause of ligneous conjunctivitis. Thromb. Haemost. 2001, 85, 1004–1010. [Google Scholar]
- Declerck, P.J.; Moreau, H.; Jespersen, J.; Gram, J.; Kluft, C. Multicenter evaluation of commercially available methods for the immunological determination of plasminogen activator inhibitor-1 (PAI-1). Thromb. Haemost. 1993, 70, 858–863. [Google Scholar] [CrossRef]
- Olson, J.D. D-dimer: An Overview of Hemostasis and Fibrinolysis, Assays, and Clinical Applications. Adv. Clin. Chem. 2015, 69, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Donati, M.B. Assays for fibrinogen-fibrin degradation products in biological fluids: Some methodological aspects. Thromb. Et Diath. Haemorrh. 1975, 34, 652–660. [Google Scholar] [CrossRef]
- Ratky, S.M.; Martin, M.J.; Gordon, Y.B.; Baker, L.R.; Chard, T.; Leslie, J. A comparison between radioimmunoassay and other immunological techniques for the measurement of fibrinogen/fibrin degradation products in serum. Br. J. Haematol. 1975, 30, 145–149. [Google Scholar] [CrossRef]
- Gaffney, P.J. Subunit relationships between fibrinogen and fibrin degradation products. Thromb. Res. 1973, 2, 201–217. [Google Scholar] [CrossRef]
- Greenberg, C.S.; Devine, D.V.; McCrae, K.M. Measurement of plasma fibrin D-dimer levels with the use of a monoclonal antibody coupled to latex beads. Am. J. Clin. Pathol. 1987, 87, 94–100. [Google Scholar] [CrossRef]
- Franchini, M.; Mannucci, P.M. Primary hyperfibrinolysis: Facts and fancies. Thromb. Res. 2018, 166, 71–75. [Google Scholar] [CrossRef]
- Frischmuth, T.; Hindberg, K.; Aukrust, P.; Ueland, T.; Braekkan, S.K.; Hansen, J.B.; Morelli, V.M. Elevated plasma levels of plasminogen activator inhibitor-1 are associated with risk of future incident venous thromboembolism. J. Thromb. Haemost. 2022, 20, 1618–1626. [Google Scholar] [CrossRef] [PubMed]
- Hvas, C.L.; Larsen, J.B.; Adelborg, K.; Christensen, S.; Hvas, A.M. Dynamic Hemostasis and Fibrinolysis Assays in Intensive Care COVID-19 Patients and Association with Thrombosis and Bleeding-A Systematic Review and a Cohort Study. Semin. Thromb. Hemost. 2021, 48, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.B.; Hvas, A.M. Fibrinolytic Alterations in Sepsis: Biomarkers and Future Treatment Targets. Semin. Thromb. Hemost. 2021, 47, 589–600. [Google Scholar] [CrossRef] [PubMed]
- von Meijenfeldt, F.A.; Lisman, T. Fibrinolysis in Patients with Liver Disease. Semin. Thromb. Hemost. 2021, 47, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Ząbczyk, M.; Natorska, J.; Undas, A. Factor XIII and Fibrin Clot Properties in Acute Venous Thromboembolism. Int. J. Mol. Sci. 2021, 22, 1607. [Google Scholar] [CrossRef]
- Undas, A. Fibrinolysis in Venous Thromboembolism. Semin. Thromb. Hemost. 2021, 47, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Kashuk, J.L.; Moore, E.E.; Millikan, J.S.; Moore, J.B. Major abdominal vascular trauma--a unified approach. J. Trauma 1982, 22, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Kornblith, L.Z.; Moore, H.B.; Cohen, M.J. Trauma-induced coagulopathy: The past, present, and future. J. Thromb. Haemost. 2019, 17, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Knudson, M.M.; Moore, H.B.; Moore, E.E.; Kornblith, L.Z.; Kiraly, L.N.; McNutt, M.K.; Wade, C.E.; Bruns, B.R.; Sauaia, A. Tissue plasminogen activator resistance is an early predictor of posttraumatic venous thromboembolism: A prospective study from the CLOTT research group. J. Trauma Acute Care Surg. 2022, 93, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.B.; Moore, E.E. Temporal Changes in Fibrinolysis following Injury. Semin. Thromb. Hemost. 2020, 46, 189–198. [Google Scholar] [CrossRef]
- Cardenas, J.C.; Wade, C.E.; Cotton, B.A.; George, M.J.; Holcomb, J.B.; Schreiber, M.A.; White, N.J.; Group, P.S. TEG Lysis Shutdown Represents Coagulopathy in Bleeding Trauma Patients: Analysis of the PROPPR Cohort. Shock 2019, 51, 273–283. [Google Scholar] [CrossRef]
- Moore, H.B.; Moore, E.E.; Huebner, B.R.; Dzieciatkowska, M.; Stettler, G.R.; Nunns, G.R.; Lawson, P.J.; Ghasabyan, A.; Chandler, J.; Banerjee, A.; et al. Fibrinolysis shutdown is associated with a fivefold increase in mortality in trauma patients lacking hypersensitivity to tissue plasminogen activator. J. Trauma Acute Care Surg. 2017, 83, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Rowell, S.E.; Meier, E.N.; McKnight, B.; Kannas, D.; May, S.; Sheehan, K.; Bulger, E.M.; Idris, A.H.; Christenson, J.; Morrison, L.J.; et al. Effect of Out-of-Hospital Tranexamic Acid vs. Placebo on 6-Month Functional Neurologic Outcomes in Patients With Moderate or Severe Traumatic Brain Injury. JAMA J. Am. Med. Assoc. 2020, 324, 961–974. [Google Scholar] [CrossRef]
- PATCH-Trauma Investigators; The ANZICS Clinical Trials Group; Gruen, R.L.; Mitra, B.; Bernard, S.A.; McArthur, C.J.; Burns, B.; Gantner, D.C.; Maegele, M.; Cameron, P.A. Prehospital Tranexamic Acid for Severe Trauma. N. Engl. J. Med. 2023, 389, 127–136. [Google Scholar] [CrossRef]
- Maas, A.I.R.; Steyerberg, E.W.; Citerio, G. Tranexamic acid in traumatic brain injury: Systematic review and meta-analysis trumps a large clinical trial? Intensive Care Med. 2021, 47, 74–76. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.B.; Moore, E.E.; Gonzalez, E.; Chapman, M.P.; Chin, T.L.; Silliman, C.C.; Banerjee, A.; Sauaia, A. Hyperfibrinolysis, physiologic fibrinolysis, and fibrinolysis shutdown: The spectrum of postinjury fibrinolysis and relevance to antifibrinolytic therapy. J. Trauma Acute Care Surg. 2014, 77, 811–817. [Google Scholar] [CrossRef]
- Samuels, J.M.; Moore, E.E.; Silliman, C.C.; Banerjee, A.; Cohen, M.J.; Ghasabyan, A.; Chandler, J.; Coleman, J.R.; Sauaia, A. Severe traumatic brain injury is associated with a unique coagulopathy phenotype. J. Trauma Acute Care Surg. 2019, 86, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.N.; Farrell, D.H.; Rowell, S.E. Fibrinolysis in Traumatic Brain Injury: Diagnosis, Management, and Clinical Considerations. Semin. Thromb. Hemost. 2021, 47, 527–537. [Google Scholar] [CrossRef]
- Nakae, R.; Murai, Y.; Wada, T.; Fujiki, Y.; Kanaya, T.; Takayama, Y.; Suzuki, G.; Naoe, Y.; Yokota, H.; Yokobori, S. Hyperfibrinolysis and fibrinolysis shutdown in patients with traumatic brain injury. Sci. Rep. 2022, 12, 19107. [Google Scholar] [CrossRef]
- Collaborators, C.-T. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): A randomised, placebo-controlled trial. Lancet 2019, 394, 1713–1723. [Google Scholar] [CrossRef]
- Honeybul, S.; Ho, K.M.; Rosenfeld, J.V. The role of tranexamic acid in traumatic brain injury. J. Clin. Neurosci. 2022, 99, 1–4. [Google Scholar] [CrossRef]
- Fletcher, A.P.; Alkjaersig, N.; Smyrniotis, F.E.; Sherry, S. The treatment of patients suffering from early myocardial infarction with massive and prolonged streptokinase therapy. Trans. Assoc. Am. Physicians 1958, 71, 287–296. [Google Scholar]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, ehad191. [Google Scholar] [CrossRef]
- Undas, A.; Plicner, D.; Stepień, E.; Drwiła, R.; Sadowski, J. Altered fibrin clot structure in patients with advanced coronary artery disease: A role of C-reactive protein, lipoprotein(a) and homocysteine. J. Thromb. Haemost. 2007, 5, 1988–1990. [Google Scholar] [CrossRef]
- Reddel, C.J.; Curnow, J.L.; Voitl, J.; Rosenov, A.; Pennings, G.J.; Morel-Kopp, M.C.; Brieger, D.B. Detection of hypofibrinolysis in stable coronary artery disease using the overall haemostatic potential assay. Thromb. Res. 2013, 131, 457–462. [Google Scholar] [CrossRef]
- Ramanathan, R.; Gram, J.B.; Sidelmann, J.J.; Dey, D.; Kusk, M.W.; Nørgaard, B.L.; Sand, N.P.R. Sex difference in fibrin clot lysability: Association with coronary plaque composition. Thromb. Res. 2019, 174, 129–136. [Google Scholar] [CrossRef]
- Undas, A.; Szułdrzynski, K.; Stepien, E.; Zalewski, J.; Godlewski, J.; Tracz, W.; Pasowicz, M.; Zmudka, K. Reduced clot permeability and susceptibility to lysis in patients with acute coronary syndrome: Effects of inflammation and oxidative stress. Atherosclerosis 2008, 196, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Fatah, K.; Silveira, A.; Tornvall, P.; Karpe, F.; Blombäck, M.; Hamsten, A. Proneness to formation of tight and rigid fibrin gel structures in men with myocardial infarction at a young age. Thromb. Haemost. 1996, 76, 535–540. [Google Scholar] [CrossRef]
- Ząbczyk, M.; Natorska, J.; Undas, A. Fibrin Clot Properties in Atherosclerotic Vascular Disease: From Pathophysiology to Clinical Outcomes. J. Clin. Med. 2021, 10, 2999. [Google Scholar] [CrossRef] [PubMed]
- Neergaard-Petersen, S.; Ajjan, R.; Hvas, A.M.; Hess, K.; Larsen, S.B.; Kristensen, S.D.; Grove, E.L. Fibrin clot structure and platelet aggregation in patients with aspirin treatment failure. PLoS ONE 2013, 8, e71150. [Google Scholar] [CrossRef]
- Gram, J.; Jespersen, J.; Kluft, C.; Rijken, D.C. On the usefulness of fibrinolysis variables in the characterization of a risk group for myocardial reinfarction. Acta Medica Scand. 1987, 221, 149–153. [Google Scholar] [CrossRef]
- Sumaya, W.; Wallentin, L.; James, S.K.; Siegbahn, A.; Gabrysch, K.; Bertilsson, M.; Himmelmann, A.; Ajjan, R.A.; Storey, R.F. Fibrin clot properties independently predict adverse clinical outcome following acute coronary syndrome: A PLATO substudy. Eur. Heart J. 2018, 39, 1078–1085. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Raith, E.P.; Udy, A.A.; Bailey, M.; McGloughlin, S.; MacIsaac, C.; Bellomo, R.; Pilcher, D.V.; Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcomes and Resource Evaluation (CORE). Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults With Suspected Infection Admitted to the Intensive Care Unit. JAMA J. Am. Med. Assoc. 2017, 317, 290–300. [Google Scholar] [CrossRef]
- Levi, M.; van der Poll, T. Coagulation and sepsis. Thromb. Res. 2017, 149, 38–44. [Google Scholar] [CrossRef]
- Lisman, T.; Arefaine, B.; Adelmeijer, J.; Zamalloa, A.; Corcoran, E.; Smith, J.G.; Bernal, W.; Patel, V.C. Global hemostatic status in patients with acute-on-chronic liver failure and septics without underlying liver disease. J. Thromb. Haemost. 2021, 19, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.B.; Aggerbeck, M.A.; Larsen, K.M.; Hvas, C.L.; Hvas, A.M. Fibrin Network Formation and Lysis in Septic Shock Patients. Int. J. Mol. Sci. 2021, 22, 9540. [Google Scholar] [CrossRef] [PubMed]
- Semeraro, F.; Colucci, M.; Caironi, P.; Masson, S.; Ammollo, C.T.; Teli, R.; Semeraro, N.; Magnoli, M.; Salati, G.; Isetta, M.; et al. Platelet Drop and Fibrinolytic Shutdown in Patients With Sepsis. Crit. Care Med. 2018, 46, e221–e228. [Google Scholar] [CrossRef] [PubMed]
- Pregernig, A.; Müller, M.; Held, U.; Beck-Schimmer, B. Prediction of mortality in adult patients with sepsis using six biomarkers: A systematic review and meta-analysis. Ann. Intensive Care 2019, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Xiong, H.; Yan, P.; Shuai, T.; Liu, J.; Zhu, L.; Lu, J.; Yang, K.; Liu, J. The Diagnostic and Prognostic Value of Supar in Patients With Sepsis: A Systematic Review and Meta-Analysis. Shock 2019, 53, 416. [Google Scholar] [CrossRef] [PubMed]
- Brailovsky, Y.; Lakhter, V.; Newman, J.; Allen, S.; Elkaryoni, A.; Desai, P.; Masic, D.; Bechara, C.F.; Bontekoe, E.; Hoppensteadt, D.; et al. Fibrinolytic Status and Risk of Death After Acute Pulmonary Embolism. Clin. Appl. Thromb. Hemost. 2023, 29, 10760296231162079. [Google Scholar] [CrossRef]
- Chopard, R.; Meneveau, N.; Ecarnot, F. Catheter-based therapy for acute pulmonary embolism: An overview of current evidence. Arch. Cardiovasc. Dis. 2022, 115, 397–405. [Google Scholar] [CrossRef]
- Leebeek, F.W.; Kluft, C.; Knot, E.A.; de Maat, M.P.; Wilson, J.H. A shift in balance between profibrinolytic and antifibrinolytic factors causes enhanced fibrinolysis in cirrhosis. Gastroenterology 1991, 101, 1382–1390. [Google Scholar] [CrossRef]
- Fisher, C.; Patel, V.C.; Stoy, S.H.; Singanayagam, A.; Adelmeijer, J.; Wendon, J.; Shawcross, D.L.; Lisman, T.; Bernal, W. Balanced haemostasis with both hypo- and hyper-coagulable features in critically ill patients with acute-on-chronic-liver failure. J. Crit. Care 2018, 43, 54–60. [Google Scholar] [CrossRef]
- Tornai, I.; Harsfalvi, J.; Boda, Z.; Udvardy, M.; Pfliegler, G.; Rak, K. Endothelium releases more von Willebrand factor and tissue-type plasminogen activator upon venous occlusion in patients with liver cirrhosis than in normals. Haemostasis 1993, 23, 58–64. [Google Scholar] [CrossRef]
- Fletcher, A.P.; Biederman, O.; Moore, D.; Alkjaersig, N.; Sherry, S. Abnormal Plasminogen-Plasmin System Activity (Fibrinolysis) in Patients with Hepatic Cirrhosis: Its Cause and Consequences. J. Clin. Investig. 1964, 43, 681–695. [Google Scholar] [CrossRef]
- Kwaan, H.C.; McFadzean, A.J.; Cook, J. On plasma fibrinolytic activity in cryptogenetic splenomegaly. Scott. Med. J. 1957, 2, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Das, P.C.; Cash, J.D. Fibrinolysis at rest and after exercise in hepatic cirrhosis. Br. J. Haematol. 1969, 17, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Potze, W.; Siddiqui, M.S.; Boyett, S.L.; Adelmeijer, J.; Daita, K.; Sanyal, A.J.; Lisman, T. Preserved hemostatic status in patients with non-alcoholic fatty liver disease. J. Hepatol. 2016, 65, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, P.L.; Thomsen, K.L.; Sorensen, M.; Vilstrup, H.; Hvas, A.M. Impaired fibrinolysis without hypercoagulability characterises patients with non-alcoholic fatty liver disease. Thromb. Res. 2022, 213, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Bos, S.; van den Boom, B.; Kamphuisen, P.W.; Adelmeijer, J.; Blokzijl, H.; Schreuder, T.; Lisman, T. Haemostatic Profiles are Similar across All Aetiologies of Cirrhosis. Thromb. Haemost. 2019, 119, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Stine, J.G.; Niccum, B.A.; Zimmet, A.N.; Intagliata, N.; Caldwell, S.H.; Argo, C.K.; Northup, P.G. Increased risk of venous thromboembolism in hospitalized patients with cirrhosis due to non-alcoholic steatohepatitis. Clin. Transl. Gastroenterol. 2018, 9, 140. [Google Scholar] [CrossRef]
- Zanetto, A.; Campello, E.; Pelizzaro, F.; Farinati, F.; Burra, P.; Simioni, P.; Senzolo, M. Haemostatic alterations in patients with cirrhosis and hepatocellular carcinoma: Laboratory evidence and clinical implications. Liver Int. 2022, 42, 1229–1240. [Google Scholar] [CrossRef]
- Schepis, F.; Turco, L.; Bianchini, M.; Villa, E. Prevention and Management of Bleeding Risk Related to Invasive Procedures in Cirrhosis. Semin. Liver Dis. 2018, 38, 215–229. [Google Scholar] [CrossRef]
- Turon, F.; Casu, S.; Hernandez-Gea, V.; Garcia-Pagan, J.C. Variceal and other portal hypertension related bleeding. Best Pract. Res. Clin. Gastroenterol. 2013, 27, 649–664. [Google Scholar] [CrossRef]
- Blasi, A.; Patel, V.C.; Adelmeijer, J.; Azarian, S.; Hernandez Tejero, M.; Calvo, A.; Fernandez, J.; Bernal, W.; Lisman, T. Mixed Fibrinolytic Phenotypes in Decompensated Cirrhosis and Acute-on-Chronic Liver Failure with Hypofibrinolysis in Those With Complications and Poor Survival. Hepatology 2020, 71, 1381–1390. [Google Scholar] [CrossRef]
- Thaler, J.; Lisman, T.; Quehenberger, P.; Hell, L.; Schwabl, P.; Scheiner, B.; Bucsics, T.; Nieuwland, R.; Ay, C.; Trauner, M.; et al. Intraperitoneal Activation of Coagulation and Fibrinolysis in Patients with Cirrhosis and Ascites. Thromb. Haemost. 2022, 122, 353–362. [Google Scholar] [CrossRef]
- Lisman, T.; Bakhtiari, K.; Adelmeijer, J.; Meijers, J.C.; Porte, R.J.; Stravitz, R.T. Intact thrombin generation and decreased fibrinolytic capacity in patients with acute liver injury or acute liver failure. J. Thromb. Haemost. 2012, 10, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Driever, E.G.; Stravitz, R.T.; Zhang, J.; Adelmeijer, J.; Durkalski, V.; Lee, W.M.; Lisman, T. VWF/ADAMTS13 Imbalance, But Not Global Coagulation or Fibrinolysis, Is Associated With Outcome and Bleeding in Acute Liver Failure. Hepatology 2021, 73, 1882–1891. [Google Scholar] [CrossRef]
- Dzik, W.H.; Arkin, C.F.; Jenkins, R.L.; Stump, D.C. Fibrinolysis during liver transplantation in humans: Role of tissue-type plasminogen activator. Blood 1988, 71, 1090–1095. [Google Scholar] [CrossRef]
- Porte, R.J.; Bontempo, F.A.; Knot, E.A.; Lewis, J.H.; Kang, Y.G.; Starzl, T.E. Systemic effects of tissue plasminogen activator-associated fibrinolysis and its relation to thrombin generation in orthotopic liver transplantation. Transplantation 1989, 47, 978–984. [Google Scholar] [CrossRef] [PubMed]
- Porte, R.J.; Molenaar, I.Q.; Begliomini, B.; Groenland, T.H.; Januszkiewicz, A.; Lindgren, L.; Palareti, G.; Hermans, J.; Terpstra, O.T. Aprotinin and transfusion requirements in orthotopic liver transplantation: A multicentre randomised double-blind study. EMSALT Study Group. Lancet 2000, 355, 1303–1309. [Google Scholar] [CrossRef]
- Molenaar, I.Q.; Warnaar, N.; Groen, H.; Tenvergert, E.M.; Slooff, M.J.; Porte, R.J. Efficacy and safety of antifibrinolytic drugs in liver transplantation: A systematic review and meta-analysis. Am. J. Transplant. 2007, 7, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Zatroch, I.; Dinya, E.; Fazakas, J. New under the sun: ClotPro’s ECA-test detects hyperfibrinolysis in a higher number of patients, more frequently and 9 min earlier. Blood Coagul. Fibrinolysis 2023, 34, 99–104. [Google Scholar] [CrossRef]
- Cicardi, M.; Suffritti, C.; Perego, F.; Caccia, S. Novelties in the Diagnosis and Treatment of Angioedema. J. Investig. Allergol. Clin. Immunol. 2016, 26, 212–221. [Google Scholar] [CrossRef]
- Bork, K.; Wulff, K.; Steinmüller-Magin, L.; Braenne, I.; Staubach-Renz, P.; Witzke, G.; Hardt, J. Hereditary angioedema with a mutation in the plasminogen gene. Allergy 2018, 73, 442–450. [Google Scholar] [CrossRef]
- Nilsson, T.; Bäck, O. Elevated plasmin-alpha 2-antiplasmin complex levels in hereditary angioedema: Evidence for the in vivo efficiency of the intrinsic fibrinolytic system. Thromb. Res. 1985, 40, 817–821. [Google Scholar] [CrossRef] [PubMed]
- van Geffen, M.; Cugno, M.; Lap, P.; Loof, A.; Cicardi, M.; van Heerde, W. Alterations of coagulation and fibrinolysis in patients with angioedema due to C1-inhibitor deficiency. Clin. Exp. Immunol. 2012, 167, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Csuka, D.; Veszeli, N.; Imreh, É.; Zotter, Z.; Skopál, J.; Prohászka, Z.; Varga, L.; Farkas, H. Comprehensive study into the activation of the plasma enzyme systems during attacks of hereditary angioedema due to C1-inhibitor deficiency. Orphanet J. Rare Dis. 2015, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Castellano, G.; Divella, C.; Sallustio, F.; Montinaro, V.; Curci, C.; Zanichelli, A.; Bonanni, E.; Suffritti, C.; Caccia, S.; Bossi, F.; et al. A transcriptomics study of hereditary angioedema attacks. J. Allergy Clin. Immunol. 2018, 142, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, T.; Hide, M.; Yamashita, K.; Ohsawa, I. The use of tranexamic acid for on-demand and prophylactic treatment of hereditary angioedema—A systematic review. J. Cutan. Immunol. Allergy 2018, 1, 126–138. [Google Scholar] [CrossRef]
- Elokdah, H.; Abou-Gharbia, M.; Hennan, J.K.; McFarlane, G.; Mugford, C.P.; Krishnamurthy, G.; Crandall, D.L. Tiplaxtinin, a novel, orally efficacious inhibitor of plasminogen activator inhibitor-1: Design, synthesis, and preclinical characterization. J. Med. Chem. 2004, 47, 3491–3494. [Google Scholar] [CrossRef]
- Hennan, J.K.; Morgan, G.A.; Swillo, R.E.; Antrilli, T.M.; Mugford, C.; Vlasuk, G.P.; Gardell, S.J.; Crandall, D.L. Effect of tiplaxtinin (PAI-039), an orally bioavailable PAI-1 antagonist, in a rat model of thrombosis. J. Thromb. Haemost. 2008, 6, 1558–1564. [Google Scholar] [CrossRef]
- Singh, S.; Houng, A.; Reed, G.L. Releasing the Brakes on the Fibrinolytic System in Pulmonary Emboli: Unique Effects of Plasminogen Activation and α2-Antiplasmin Inactivation. Circulation 2017, 135, 1011–1020. [Google Scholar] [CrossRef]
- Willemse, J.L.; Heylen, E.; Nesheim, M.E.; Hendriks, D.F. Carboxypeptidase U (TAFIa): A new drug target for fibrinolytic therapy? J. Thromb. Haemost. 2009, 7, 1962–1971. [Google Scholar] [CrossRef]
- Coupland, L.A.; Rabbolini, D.J.; Schoenecker, J.G.; Crispin, P.J.; Miller, J.J.; Ghent, T.; Medcalf, R.L.; Aneman, A.E. Point-of-care diagnosis and monitoring of fibrinolysis resistance in the critically ill: Results from a feasibility study. Crit. Care 2023, 27, 55. [Google Scholar] [CrossRef]
| Clinical Setting | Changes in the Fibrinolytic System |
|---|---|
| Liver disease | |
| Stable cirrhosis | Alcoholic cirrhosis: increased fibrinolysis due to release and defective clearance of tPA Non-alcoholic steatohepatitis cirrhosis: hypofibrinolysis due to increased levels of PAI-1 |
| Decompensated cirrhosis and acute-on-chronic liver failure | Highly variable, ranging from severely hypofibrinolytic to hyperfibrinolytic |
| Acute liver failure | Profound hypofibrinolysis, uncertain clinical relevance |
| Liver transplantation | Hyperfibrinolysis due to defective clearance of tPA in the anhepatic state and increased release from the donor liver. Antifibrinolytic therapy is recommended during surgery |
| Trauma | |
| Major trauma | Hyperfibrinolysis, hypofibrinolysis, and fibrinolytic shutdown. Antifibrinolytic therapy increases survival after major trauma with haemorrhagic shock if administered less than 3 h after trauma |
| Brain trauma | Variable, both hyperfibrinolysis and hypofibrinolysis. Early antifibrinolytic therapy may be beneficial in mild-to-moderate brain injury |
| Sepsis | Consistent findings of hypofibrinolysis in correlation with organ failure. Increased levels of PAI-1, TAFIa/TAFIa, and fibrinogen, decreased levels of plasminogen |
| Cardiovascular disease | Both stable atherosclerosis and ACS are associated with decreased fibrin clot permeability and prolonged lysis times. Clot structure and lysis time may be prognostic marker for reinfarction or cardiovascular mortality in ACS patients |
| Venous thromboembolism (VTE) | |
| DVT | A high PAI-1 level predisposes to VTE. Patients with recurrent DVT have higher PAI-levels than patients without recurrence of DVT. |
| PE | Patients with PE may have looser clot structure than patients with isolated DVT. In high-risk PE patients, systemic thrombolysis is indicated. In cases of contraindications, catheter-based thrombo-aspiration is an alternative. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hvas, C.L.; Larsen, J.B. The Fibrinolytic System and Its Measurement: History, Current Uses and Future Directions for Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 14179. https://doi.org/10.3390/ijms241814179
Hvas CL, Larsen JB. The Fibrinolytic System and Its Measurement: History, Current Uses and Future Directions for Diagnosis and Treatment. International Journal of Molecular Sciences. 2023; 24(18):14179. https://doi.org/10.3390/ijms241814179
Chicago/Turabian StyleHvas, Christine Lodberg, and Julie Brogaard Larsen. 2023. "The Fibrinolytic System and Its Measurement: History, Current Uses and Future Directions for Diagnosis and Treatment" International Journal of Molecular Sciences 24, no. 18: 14179. https://doi.org/10.3390/ijms241814179
APA StyleHvas, C. L., & Larsen, J. B. (2023). The Fibrinolytic System and Its Measurement: History, Current Uses and Future Directions for Diagnosis and Treatment. International Journal of Molecular Sciences, 24(18), 14179. https://doi.org/10.3390/ijms241814179






