Comprehensive Analysis and Functional Verification of the Pinus massoniana NBS-LRR Gene Family Involved in the Resistance to Bursaphelenchus xylophilus
Abstract
:1. Introduction
2. Results
2.1. Identification of NBS Genes in P. massoniana
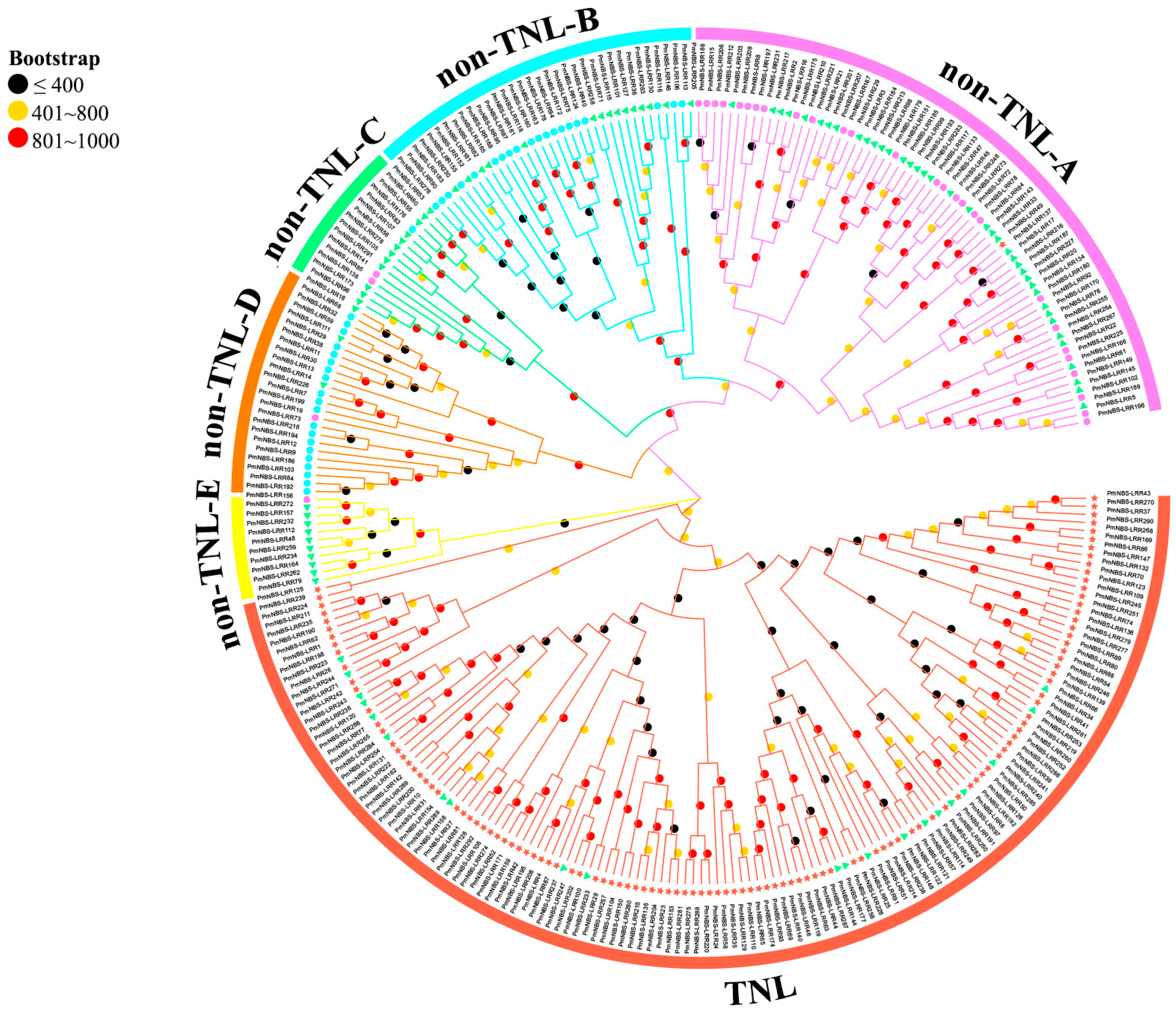
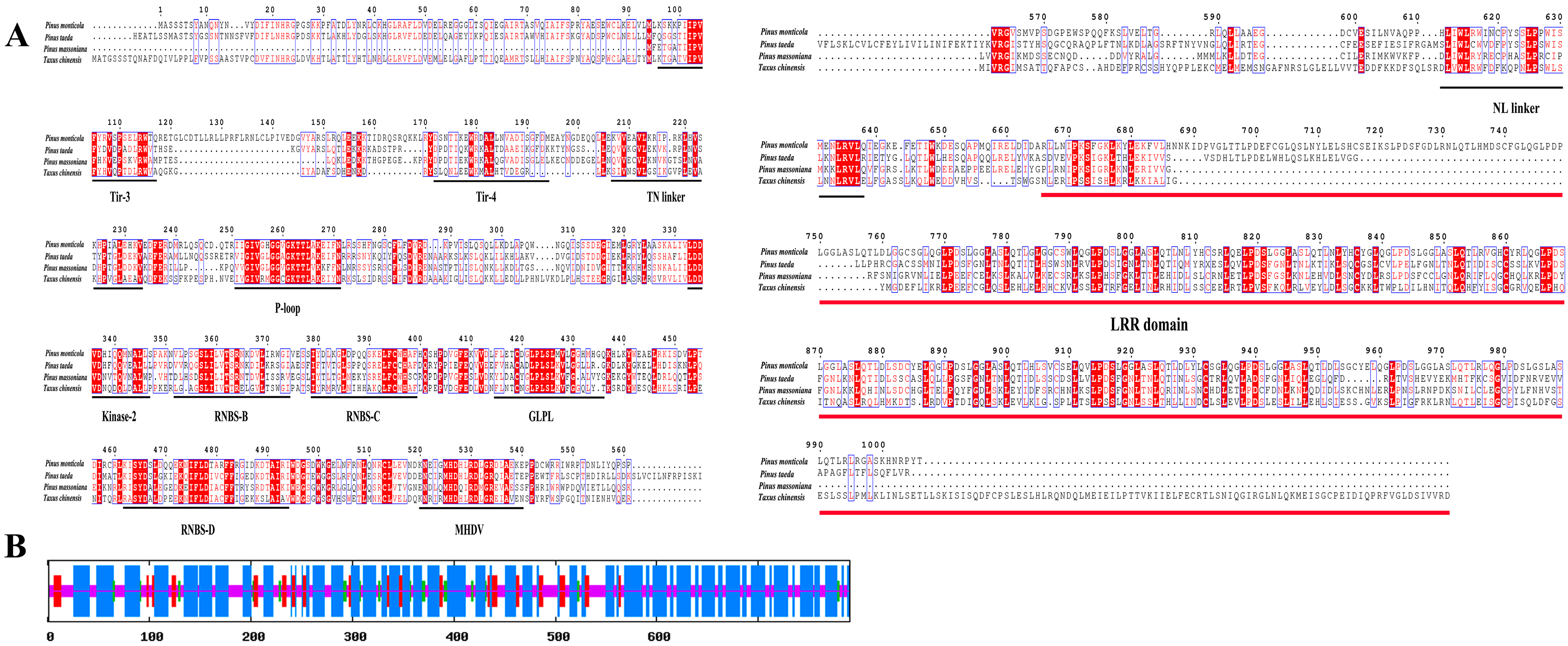
2.2. Sequence, Phylogenetic, and Conserved Motif Analyses of NBS-LRR Resistance Genes in P. massoniana
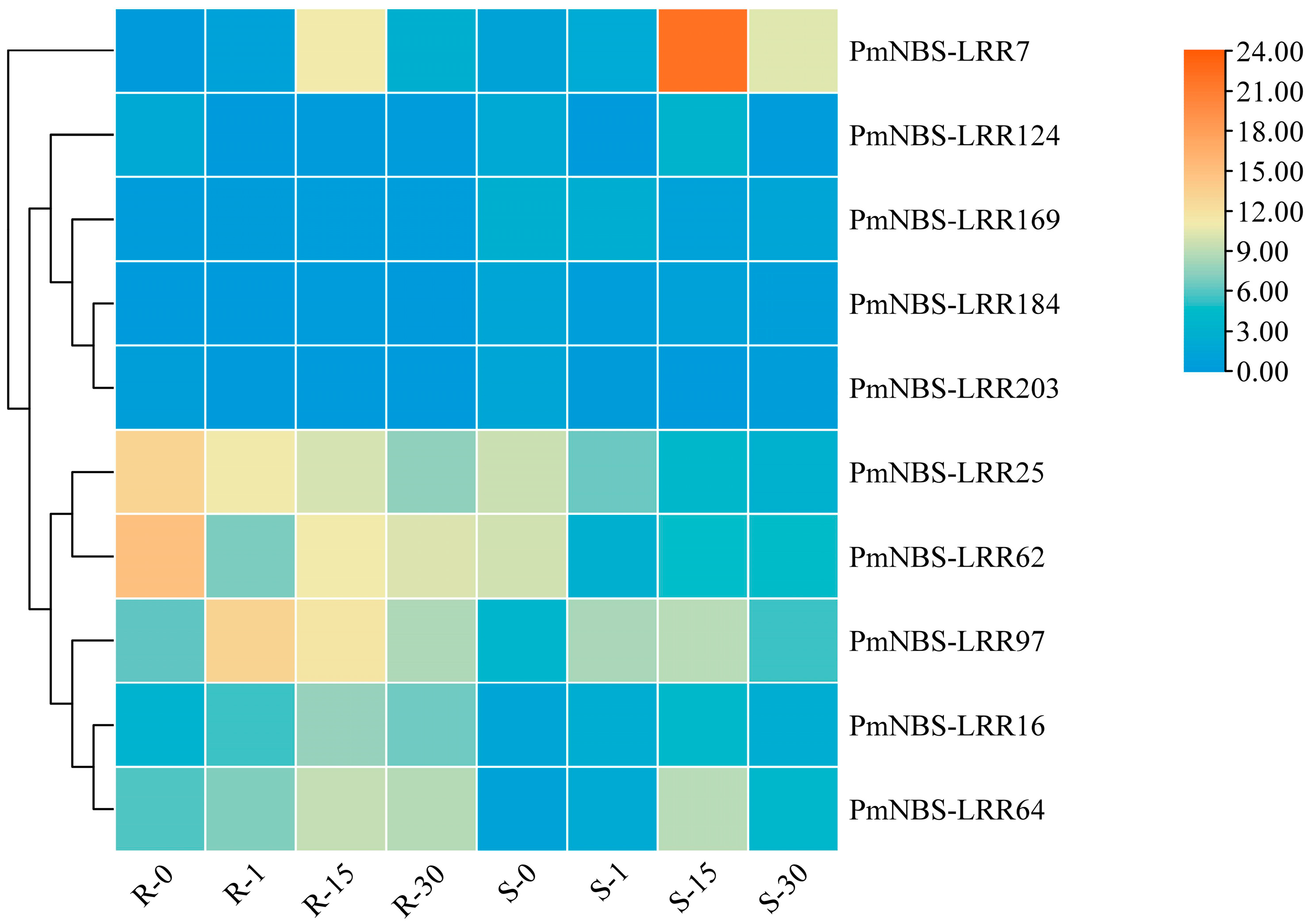
2.3. Differential Gene Expression Pattern of NBS-LRR and Cloning of PmNBS-LRR97 in P. massoniana
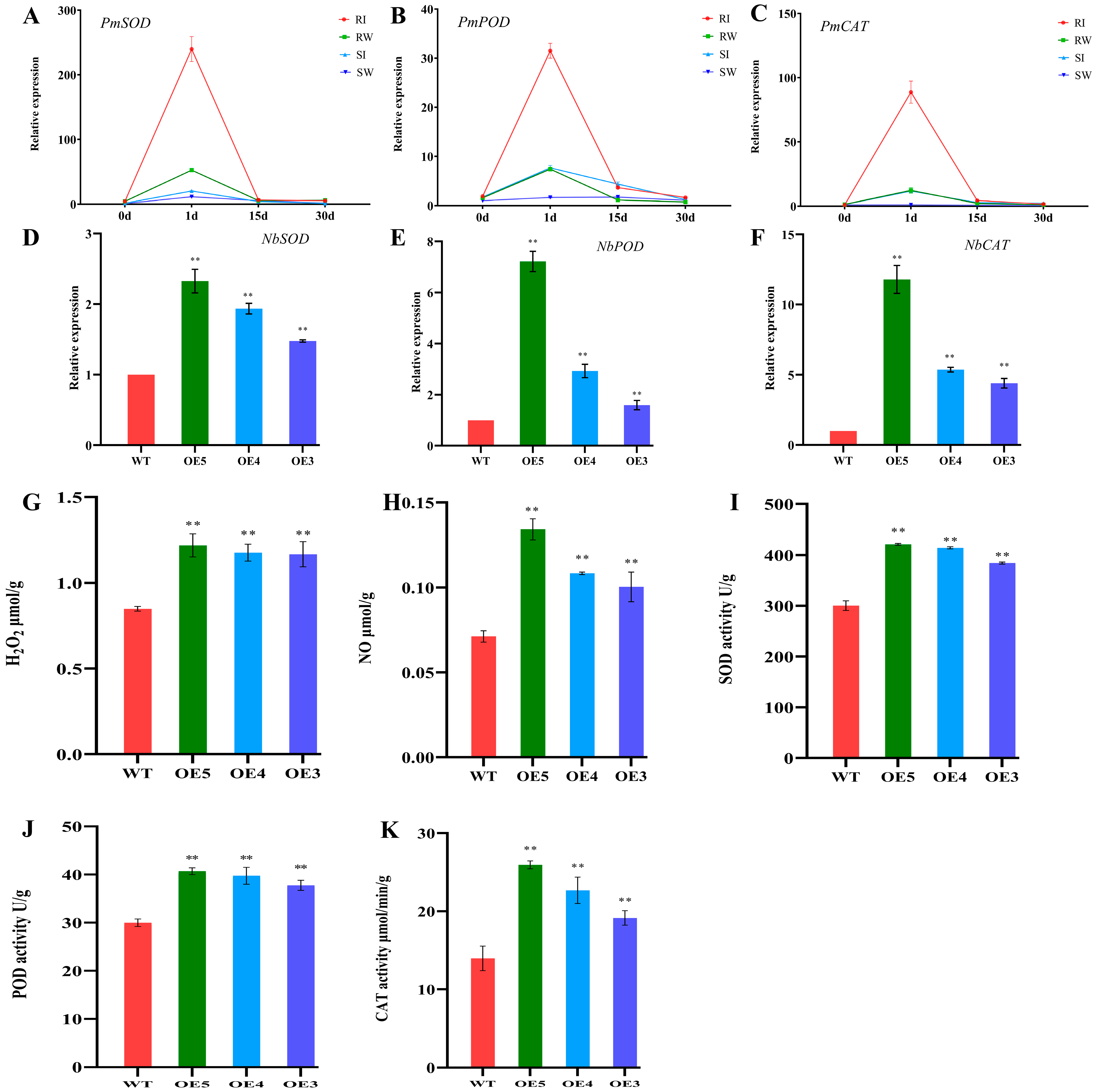
2.4. Expression Pattern of PmNBS-LRR97 in Resistant and Susceptible P. massoniana
2.5. Overexpression of PmNBS-LRR97 Enhances Plant Growth and Increases the Content of Defense Signaling Molecules
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Treatment
4.2. Identification and Analysis of NBS-LRR Genes in P. massoniana
4.3. Phylogenetic and Conserved Motif Analysis of NBS-LRR Genes
4.4. Heatmap of Differentially Expressed NBS-LRR Genes
4.5. Gene Cloning and Sequence Analysis
4.6. Real-Time Quantitative PCR (qRT-PCR) Analysis
4.7. Subcellular Localization of PmNBS-LRR97
4.8. Generation of PmNBS-LRR97 Transgenic Lines in Tobacco
4.9. Determination of H2O2, NO, SOD, POD, and CAT in Tobacco
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boller, T.; He, S.Y. Innate immunity in plants: An arms race between pattern recognition receptors in plants and effectors in microbial pathogens. Science 2009, 324, 742–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.D.G.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.F. PTI-ETI crosstalk: An integrative view of plant immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef] [PubMed]
- Panstruga, R.; Parker, J.E.; Schulze-Lefert, P. SnapShot: Plant immune response pathways. Cell 2009, 136, 978.e1–978.e3. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, T.; Sato, Y.; Takahashi, H. Microarray analysis of R-gene-mediated resistance to viruses. In Plant Virology Protocols; Uyeda, I., Masuta, C., Eds.; Humana Press: New York, NY, USA, 2015; pp. 197–218. [Google Scholar] [CrossRef]
- Kourelis, J.; Van, D. Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell 2018, 30, 285–299. [Google Scholar] [CrossRef] [Green Version]
- Kourelis, J.; Sakai, T.; Adachi, H.; Kamoun, S. RefPlantNLR: A comprehensive collection of experimentally validated plant NLRs. PLoS Biol. 2021, 19, e3001124. [Google Scholar] [CrossRef]
- Bezerra-Neto, J.P.; Araújo, F.C.; Ferreira-Neto, J.R.C.; Silva, R.L.O.; Borges, A.N.C.; Matos, M.K.S.; Silva, J.B.; Silva, M.D.; Kido, E.A.; Benko-Iseppon, A.M. NBS-LRR genes—Plant health sentinels: Structure, roles, evolution and biotechnological applications. In Applied Plant Biotechnology for Improving Resistance to Biotic Stress; Poltronieri, P., Hong, Y., Eds.; Academic Press: Cambridge, UK, 2020; pp. 63–120. [Google Scholar] [CrossRef]
- Tameling, W.I.L.; Vossen, J.H.; Albrecht, M.; Lengauer, T.; Berden, J.A.; Haring, M.A.; Cornelissen, B.J.C.; Takken, F.L.W. Mutations in the NB-ARC domain of I-2 that impair ATP hydrolysis cause autoactivation. Plant Physiol. 2006, 140, 1233–1245. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J.G.; Dodds, P.N.; Lawrence, G.J. Flax rust resistance gene specificity is based on direct resistance-avirulence protein interactions. Annu. Rev. Phytopathol. 2007, 45, 289–306. [Google Scholar] [CrossRef]
- Meyers, B.C.; Kozik, A.; Griego, A.; Kuang, H.; Michelmore, R.W. Genome-wide analysis of NBS-LRR-encoding genes in Arabidopsis. Plant Cell 2003, 15, 809–834. [Google Scholar] [CrossRef] [Green Version]
- Kohler, A.; Rinaldi, C.; Duplessis, S.; Baucher, M.; Geelen, D.; Duchaussoy, F.; Meyers, B.C.; Boerjan, W.; Martin, F. Genome-wide identification of NBS resistance genes in Populus trichocarpa. Plant Mol. Biol. 2008, 66, 619–636. [Google Scholar] [CrossRef] [PubMed]
- Christie, N.; Tobias, P.A.; Naidoo, S.; Külheim, C. The Eucalyptus grandis NBS-LRR gene family: Physical clustering and expression hotspots. Front. Plant Sci. 2016, 6, 1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, T.; Wang, Y.; Chen, J.Q.; Araki, H.; Jing, Z.; Jiang, K.; Shen, J.; Tian, D. Genome-wide identification of NBS genes in japonica rice reveals significant expansion of divergent non-TIR NBS-LRR genes. Mol. Genet. Genom. 2004, 271, 402–415. [Google Scholar] [CrossRef]
- Kang, Y.J.; Kim, K.H.; Shim, S.; Yoon, M.Y.; Sun, S.; Kim, M.Y.; Van, K.; Lee, A.H. Genome-wide mapping of NBS-LRR genes and their association with disease resistance in soybean. BMC Plant Biol. 2012, 12, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, R.; Ponce, O.; Ramirez, M.; Mostajo, N.; Orjeda, G. Genome-wide identification and mapping of NBS-encoding resistance genes in Solanum tuberosum group phureja. PLoS ONE 2012, 7, e34775. [Google Scholar] [CrossRef]
- Cai, D.; Kleine, M.; Kifle, S.; Harloff, H.J.; Sandal, N.N.; Marcker, K.A.; Klein-Lankhorst, R.M.; Salentijn, E.M.J.; Lange, W.; Stiekema, W.J.; et al. Positional cloning of a gene for nematode resistance in sugar beet. Science 1997, 275, 832–834. [Google Scholar] [CrossRef]
- Song, W.Y.; Wang, G.L.; Chen, L.L.; Kim, H.S.; Pi, L.Y.; Holsten, T.; Gardner, J.; Wang, B.; Zhai, W.X.; Zhu, L.H.; et al. A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 1995, 270, 1804–1806. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Cao, Y.; Yang, Z.; Xu, C.; Li, X.; Wang, S.; Zhang, Q. Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J. 2004, 37, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Xun, H.; Yang, X.; He, H.; Wang, M.; Guo, P.; Wang, Y.; Pang, J.; Dong, Y.; Feng, X.; Wang, S.; et al. Over-expression of GmKR3, a TIR-NBS-LRR type R gene, confers resistance to multiple viruses in soybean. Plant Mol. Biol. 2019, 99, 95–111. [Google Scholar] [CrossRef]
- Zhao, L.; Sun, J. Pinewood nematode Bursaphelenchus xylophilus (Steiner and Buhrer) Nickel. In Biological Invasions and Its Management in China; Wan, F., Jiang, M., Zhan, A., Eds.; Springer: Singapore, 2017; pp. 3–21. [Google Scholar] [CrossRef]
- Ryss, A.Y.; Kulinich, O.A.; Sutherland, J.R. Pine wilt disease: A short review of worldwide research. For. Stud. China 2011, 13, 132–138. [Google Scholar] [CrossRef]
- Zhao, B.G.; Futai, K.; Sutherland, J.R.; Takeuchi, Y. Pine Wilt Disease; Springer: Tokyo, Japan, 2008; p. 459. [Google Scholar] [CrossRef]
- Ye, J.R. Epidemic status of pine wilt disease in China and its prevention and control techniques and counter measures. Sci. Silvae Sin. 2019, 55, 1–10. [Google Scholar] [CrossRef]
- Yi, C.K.; Byun, B.H.; Park, J.D.; Yang, S.I.; Chang, K.H. First finding of the pine wood nematode, Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle and its insect vector in Korea. Res. Rep. For. Res. Inst. 1989, 38, 141–149. [Google Scholar]
- Valadas, V.; Oliveira, S.; Espada, M.; Laranjo, M.; Barbosa, P. The pine wood nematode, Bursaphelenchus xylophilus, in Portugal: Possible introductions and spread routes of a serious biological invasion revealed by molecular methods. Nematology 2012, 14, 899–911. [Google Scholar] [CrossRef]
- Vicente, C.; Espada, M.; Vieira, P.; Mota, M. Pine wilt disease: A threat to European forestry. Eur. J. Plant Pathol. 2012, 133, 89–99. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus xylophilus, causal agent of pine wilt disease on Pinus pinaster in Northwestern Spain. Plant Dis. 2011, 95, 776. [Google Scholar] [CrossRef]
- Mota, M.M.; Futai, K.; Vieira, P. Pine wilt disease and the pinewood nematode, Bursaphelenchus xylophilus. In Integrated Management of Fruit Crops Nematodes; Ciancio, A., Mukerji, K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 253–274. [Google Scholar] [CrossRef]
- Webster, J.; Mota, M. Pine wilt disease: Global issues, trade and economic impact. In Pine Wilt Disease: A Worldwide Threat to Forest Ecosystems; Mota, M., Vieira, P., Eds.; Springer: Heidelberg, Germany, 2008; pp. 1–3. [Google Scholar]
- Liu, B.; Liu, Q.; Zhou, Z.; Yin, H.; Xie, Y. Overexpression of geranyl diphosphate synthase (PmGPPS1) boosts monoterpene and diterpene production involved in the response to pine wood nematode invasion. Tree Physiol. 2022, 42, 411–424. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Q.; Zhou, Z.; Yin, H.; Xie, Y.; Wei, Y. Two terpene synthases in resistant Pinus massoniana contribute to defence against Bursaphelenchus xylophilus. Plant Cell Environ. 2021, 44, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xie, Y.; Yin, H.; Zhou, Z.; Liu, Q. Identification and defensive characterization of PmCYP720B11v2 from Pinus massoniana. Int. J. Mol. Sci. 2022, 23, 6640. [Google Scholar] [CrossRef]
- Custers, J.H.; Harrison, S.J.; Sela-Buurlage, M.B.; Van Deventer, E.; Lageweg, W.; Howe, P.W.; Van Der Meijs, P.J.; Ponstein, A.S.; Simons, B.H.; Melchers, L.S.; et al. Isolation and characterisation of a class of carbohydrate oxidases from higher plants, with a role in active defence. Plant J. 2004, 39, 147–160. [Google Scholar] [CrossRef]
- Fernandez-Gutierrez, A.; Gutierrez-Gonzalez, J.J. Bioinformatic-based approaches for disease-resistance gene discovery in plants. Agronomy 2021, 11, 2259. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Shao, Z.Q.; Wang, Q.; Hang, Y.Y.; Xue, J.Y.; Wang, B.; Chen, J.Q. Uncovering the dynamic evolution of nucleotide-binding site-leucine-rich repeat (NBS-LRR) genes in Brassicaceae. J. Integr. Plant Biol. 2016, 58, 165–177. [Google Scholar] [CrossRef]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe interactions: Shaping the evolution of the plant immune response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arya, P.; Kumar, G.; Acharya, V.; Singh, A.K. Genome-wide identification and expression analysis of NBS-encoding genes in Malus x domestica and expansion of NBS genes family in Rosaceae. PLoS ONE 2014, 9, e107987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Yang, C.; Wu, X.; Wang, Y.; Wang, B.; Wu, X.; Lu, Z.; Li, G. Genome-wide characterization of NBS-LRR family genes and expression analysis under powdery mildew stress in Lagenaria siceraria. Physiol. Mol. Plant Pathol. 2022, 118, 101798. [Google Scholar] [CrossRef]
- Alamery, S.; Tirnaz, S.; Bayer, P.; Tollenaere, R.; Chaloub, B.; Edwards, D.; Batley, J. Genome-wide identification and comparative analysis of NBS-LRR resistance genes in Brassica napus. Crop. Pasture Sci. 2017, 69, 79–93. [Google Scholar] [CrossRef]
- Mota, A.P.Z.; Vidigal, B.; Danchin, E.G.J.; Togawa, R.C. Comparative root transcriptome of wild Arachis reveals NBS-LRR genes related to nematode resistance. BMC Plant Biol. 2018, 18, 159. [Google Scholar] [CrossRef]
- Yue, J.X.; Meyers, B.C.; Chen, J.Q.; Tian, D.; Yang, S. Tracing the origin and evolutionary history of plant nucleotide-binding site-leucine-rich repeat (NBSLRR) genes. New Phytol. 2012, 193, 1049–1063. [Google Scholar] [CrossRef]
- Wu, J.; Zhu, J.; Wang, L.; Wang, S. Genome-wide association study identifies NBS-LRR-encoding genes related with anthracnose and common bacterial blight in the common bean. Front. Plant Sci. 2017, 8, 1398. [Google Scholar] [CrossRef] [Green Version]
- Pendleton, A.L.; Smith, K.E.; Feau, N.; Martin, F.M.; Grigoriev, I.V.; Hamelin, R.; Nelson, C.D.; Burleigh, J.G.; Davis, J.M. Duplications and losses in gene families of rust pathogens highlight putative effectors. Front. Plant Sci. 2014, 5, 299. [Google Scholar] [CrossRef]
- Peraza-Echeverria, S.; Dale, J.L.; Harding, R.M.; Smith, M.K.; Collet, C. Characterization of disease resistance gene candidates of the nucleotide binding site (NBS) type from banana and correlation of a transcriptional polymorphism with resistance to Fusarium oxysporum f.sp. cubense race 4. Mol. Breeding 2008, 22, 565–579. [Google Scholar] [CrossRef]
- Radwan, O.; Gandhi, S.; Heesacker, A.; Whitaker, B.; Taylor, C.; Plocik, A.; Kesseli, R.; Kozik, A.; Michelmore, R.W.; Knapp, S.J. Genetic diversity and genomic distribution of homologs encoding NBS-LRR disease resistance proteins in sunflower. Mol. Genet. Genom. 2008, 280, 111–125. [Google Scholar] [CrossRef]
- Veena, M.; Melvin, P.; Prabhu, S.A.; Shailasree, S.; Shetty, H.S.; Kini, K.R. Molecular cloning of a coiled-coil-nucleotide-binding-site-leucine-rich repeat gene from pearl millet and its expression pattern in response to the downy mildew pathogen. Mol. Biol. Reps. 2016, 43, 117–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Ding, F.; Peng, H.; Huang, Y.; Lu, J. Molecular cloning of a CC-NBS-LRR gene from Vitis quinquangularis and its expression pattern in response to downy mildew pathogen infection. Mol. Genet. Genom. 2018, 293, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Liu, J.; Bao, S.; Yang, Y.; Zhuang, Y. Molecular cloning and characterization of a wild eggplant Solanum aculeatissimum NBS-LRR gene, involved in plant resistance to Meloidogyne incognita. Int. J. Mol. Sci. 2018, 19, 583. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.J.; Xiang, Y. Characterization of the western white pine TIR-NBS-LRR (PmTNL2) gene by transcript profiling and promoter analysis. Genome 2019, 62, 477–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, M.S.; Calafell, S.J.; Thomas, D.Y.; Hallett, M.T. Refining protein subcellular localization. PLoS Comput. Biol. 2005, 1, e66. [Google Scholar] [CrossRef]
- Shen, Q.H.; Saijo, Y.; Mauch, S.; Biskup, C.; Bieri, S.; Keller, B.; Seki, H.; Ulker, B.; Somssich, I.E.; Schulze-Lefert, P. Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science 2007, 315, 1098–1103. [Google Scholar] [CrossRef]
- Deslandes, L.; Rivas, S. The plant cell nucleus: A true arena for the fight between plants and pathogens. Plant Signal. Behav. 2011, 6, 42–48. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.; Liu, J.; Chang, C.; Zhang, L.; Maekawa, T.; Wang, Q.; Xiao, W.; Liu, Y.; Chai, J.; Takken, F.L.W.; et al. Structure-function analysis of barley NLR immune receptor MLA10 reveals its cell compartment specific activity in cell death and disease resistance. PLoS Pathog. 2012, 8, e1002752. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Xu, Y.; Cao, J.; Zhu, Z.; Jiao, Y.; Wang, Y.; Guan, X.; Yang, Y.; Xu, W.; Fu, Z. Subcellular localization and functional analyses of a PR10 protein gene from Vitis pseudoreticulata in response to Plasmoparaviticola infection. Protoplasma 2013, 250, 129–140. [Google Scholar] [CrossRef]
- Qi, D.; DeYoung, B.J.; Innes, R.W. Structure-function analysis of the coiled-coil and leucine-rich repeat domains of the RPS5 disease resistance protein. Plant Physiol. 2012, 158, 1819–1832. [Google Scholar] [CrossRef] [Green Version]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhu, Z.; Chern, M.; Yin, J.; Yang, C.; Ran, L.; Cheng, M.; He, M.; Wang, K.; Wang, J.; et al. A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell 2017, 170, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Gan, Y.; Zhang, L.; Zhang, Z.; Dong, S.; Li, J.; Wang, Y.; Heng, X. The LCB2 subunit of the sphingolip biosynthesis enzyme serine palmitoyltransferase can function as an attenuator of the hypersensitive response and Bax-induced cell death. New Phytol. 2009, 181, 127–146. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.X.; Zhang, S.B.; Hu, J.X.; Sun, W.X.; Padilla, J.; He, Y.L.; Li, Y.; Yin, Z.Y.; Liu, X.Y.; Wang, W.H.; et al. Phosphorylation-guarded light-harvesting complex II contributes to broad-spectrum blast resistance in rice. Proc. Natl. Acad. Sci. USA 2019, 116, 17572–17577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazalt, A.M.; Beligni, M.V.; Lamattina, L. Nitric oxide preserves the level of chlorophyll in potato leaves infected by Phytophthora infestans. Eur. J. Plant Pathol. 1997, 103, 643–651. [Google Scholar] [CrossRef]
- Noritake, T.; Kawakita, K.; Doke, N. Nitric oxide induces phytoalexin accumulation in potato tuber tissues. Plant Cell Physiol. 1996, 37, 113–116. [Google Scholar] [CrossRef]
- Durner, J.; Wendehenne, D.; Klessig, D.F. Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc. Natl. Acad. Sci. USA. 1998, 95, 10328–10333. [Google Scholar] [CrossRef] [Green Version]
- Paradiso, A.; Caretto, S.; Leone, A.; Bove, A.; Nisi, R.; De Gara, L. ROS production and scavenging under anoxia and re-oxygenation in Arabidopsis cells: A balance between redox signaling and impairment. Front. Plant Sci. 2016, 7, 1803. [Google Scholar] [CrossRef] [Green Version]
- Akimoto-Tomiyama, C.; Sakata, K.; Yazaki, J.; Nakamura, K.; Fujii, F.; Shimbo, K.; Yamamoto, K.; Sasaki, T.; Kishimoto, N.; Kikuchi, S.; et al. Rice gene expression in response to N-acetylchitooligosaccharide elicitor: Comprehensive analysis by DNA microarray with randomly selected ESTs. Plant Mol. Biol. 2003, 52, 537–551. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, Y.; Xu, L.; Hao, Y.; Chen, X.; Zhou, Z. Transcriptomic profiling reveals differentially expressed genes associated with pine wood nematode resistance in masson pine (Pinus massoniana Lamb.). Sci. Rep. 2017, 7, 4693. [Google Scholar] [CrossRef]
- Mistry, J.; Chuguransky, S.; Williams, L.; Qureshi, M.; Salazar, G.A.; Sonnhammer, E.L.L.; Tosatto, S.C.E.; Paladin, L.; Raj, S.; Richardson, L.J.; et al. Pfam: The protein families database in 2021. Nucleic Acids Res. 2021, 49, D412–D419. [Google Scholar] [CrossRef] [PubMed]
- Eddy, S.R. Accelerated profile HMM searches. PLoS Comput. Biol. 2011, 7, e1002195. [Google Scholar] [CrossRef] [Green Version]
- Letunic, I.; Khedkar, S.; Bork, P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021, 49, D458–D460. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Duvaud, S.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASyserver. In The Proteomics Protocols Handbook; Walker, J.M., Ed.; Humana Press: New York, NY, USA, 2005; pp. 571–607. [Google Scholar] [CrossRef]
- Chou, K.C.; Shen, H.B. A new method for predicting the subcellular localization of eukaryotic proteins with both single and multiple sites: EukmPLoc 2.0. PLoS ONE 2010, 5, e9931. [Google Scholar] [CrossRef]
- Armenteros, J.J.A.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [Green Version]
- Krogh, A.; Larsson, B.; Heijne, G.V.; Sonnhammer, E.L.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, B.; Gao, S.; Lercher, M.J.; Hu, S.; Chen, W.H. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res. 2019, 47, W270–W275. [Google Scholar] [CrossRef]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.J.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.H.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Untergasser, A.; Cutcutache, I.; Koressaar, T.; Ye, J.; Faircloth, B.C.; Remm, M.; Rozen, S.G. Primer3—New capabilities and interfaces. Nucleic Acids Res. 2012, 40, e115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levin, J.M.; Robson, B.; Garnier, J. An algorithm for secondary structure determination in proteins based on sequence similarity. FEBS Lett. 1986, 205, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.M.; Garnier, J. Improvements in a secondary structure prediction method based on a search for local sequence homologies and its use as a model building tool. BBA-Protein Struct. Mol. 1988, 955, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [Green Version]
- Blom, N.; Gammeltoft, S.; Brunak, S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 1999, 294, 1351–1362. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−△△CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Predicted Protein Domain | Letter Code | P. massoniana | A. thaliana | P. trichocarpa |
|---|---|---|---|---|
| NBS | N | 90 | 1 | 53 |
| NBS-LRR | NL | 100 | 6 | 119 |
| TIR-NBS-LRR | TNL | 120 | 92 | 79 |
| TIR-NBS | TN | 68 | 23 | 13 |
| CC-NBS-LRR | CNL | 31 | 51 | 119 |
| CC-NBS | CN | 6 | 5 | 19 |
| RPW8-NBS-LRR | RNL | 41 | -a | - |
| RPW8-NBS | RN | 51 | - | - |
| Other NBS | - | 31 | - | |
| Total NBS genes | 507 | 209 | 402 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Y.; Liu, B.; Gao, K.; Zhao, Y.; Li, W.; Deng, L.; Zhou, Z.; Liu, Q. Comprehensive Analysis and Functional Verification of the Pinus massoniana NBS-LRR Gene Family Involved in the Resistance to Bursaphelenchus xylophilus. Int. J. Mol. Sci. 2023, 24, 1812. https://doi.org/10.3390/ijms24031812
Xie Y, Liu B, Gao K, Zhao Y, Li W, Deng L, Zhou Z, Liu Q. Comprehensive Analysis and Functional Verification of the Pinus massoniana NBS-LRR Gene Family Involved in the Resistance to Bursaphelenchus xylophilus. International Journal of Molecular Sciences. 2023; 24(3):1812. https://doi.org/10.3390/ijms24031812
Chicago/Turabian StyleXie, Yini, Bin Liu, Kai Gao, Yunxiao Zhao, Wenhua Li, Lili Deng, Zhichun Zhou, and Qinghua Liu. 2023. "Comprehensive Analysis and Functional Verification of the Pinus massoniana NBS-LRR Gene Family Involved in the Resistance to Bursaphelenchus xylophilus" International Journal of Molecular Sciences 24, no. 3: 1812. https://doi.org/10.3390/ijms24031812





