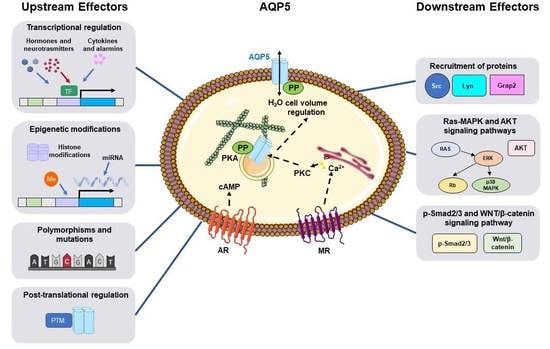
Aquaporin-5 Dynamic Regulation
Abstract
:1. Introduction
2. Functional Impact of Single Nucleotide Polymorphism and Mutation of Aqp5 Gene
3. Transcriptional Regulation of Aqp5 Expression
3.1. Hormonal, Neuronal, and Inflammatory Stimuli
3.1.1. Steroid Hormones
3.1.2. Thyroid and Parathyroid Hormones
3.1.3. Neurotransmitters
3.1.4. Inflammatory Stimuli
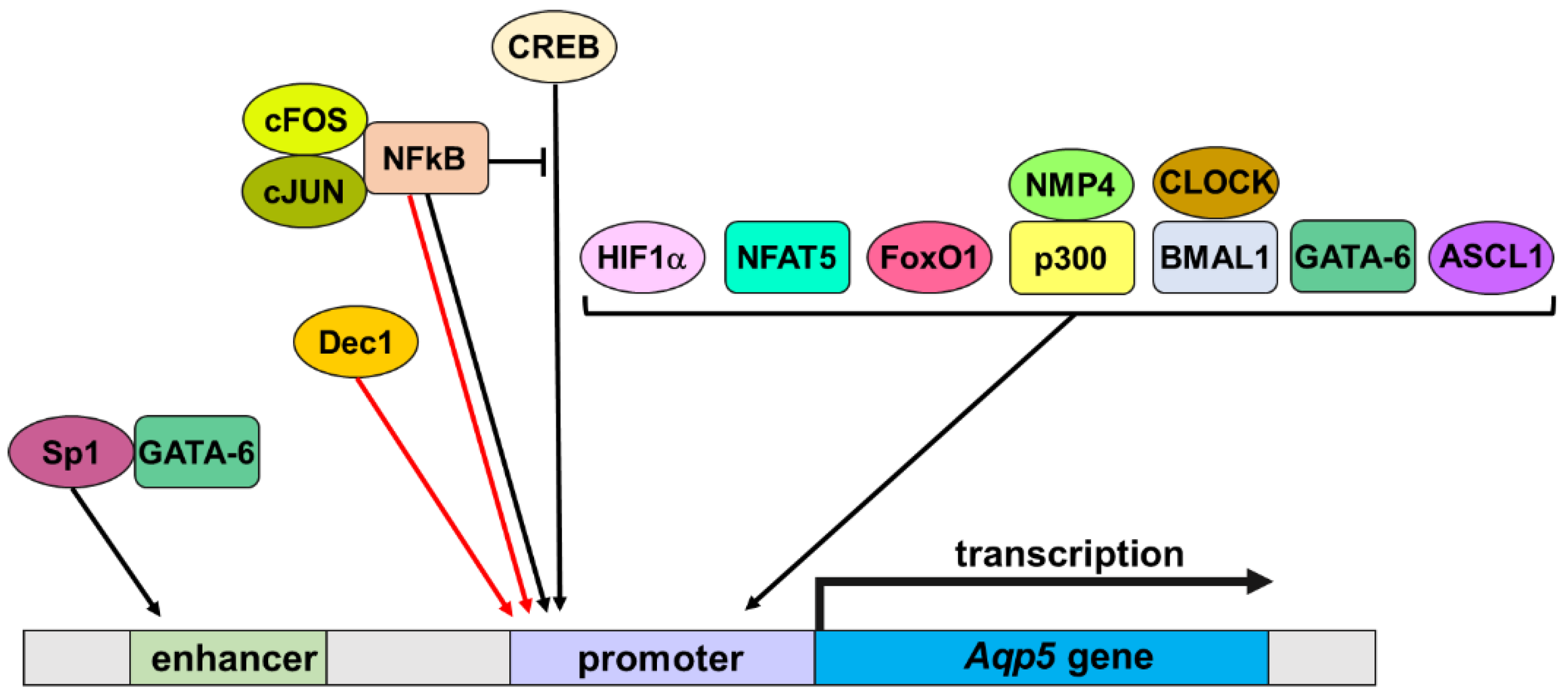
3.2. Transcription Factors
3.2.1. CREB
3.2.2. NFkB
3.2.3. HIF1α
3.2.4. NFAT5
3.2.5. FoxO1
3.2.6. NMP4 and p300
3.2.7. BMAL1 and CLOCK
3.2.8. GATA-6
3.2.9. Dec1 and Achaete-Scute Family BHLH Transcription Factor 1
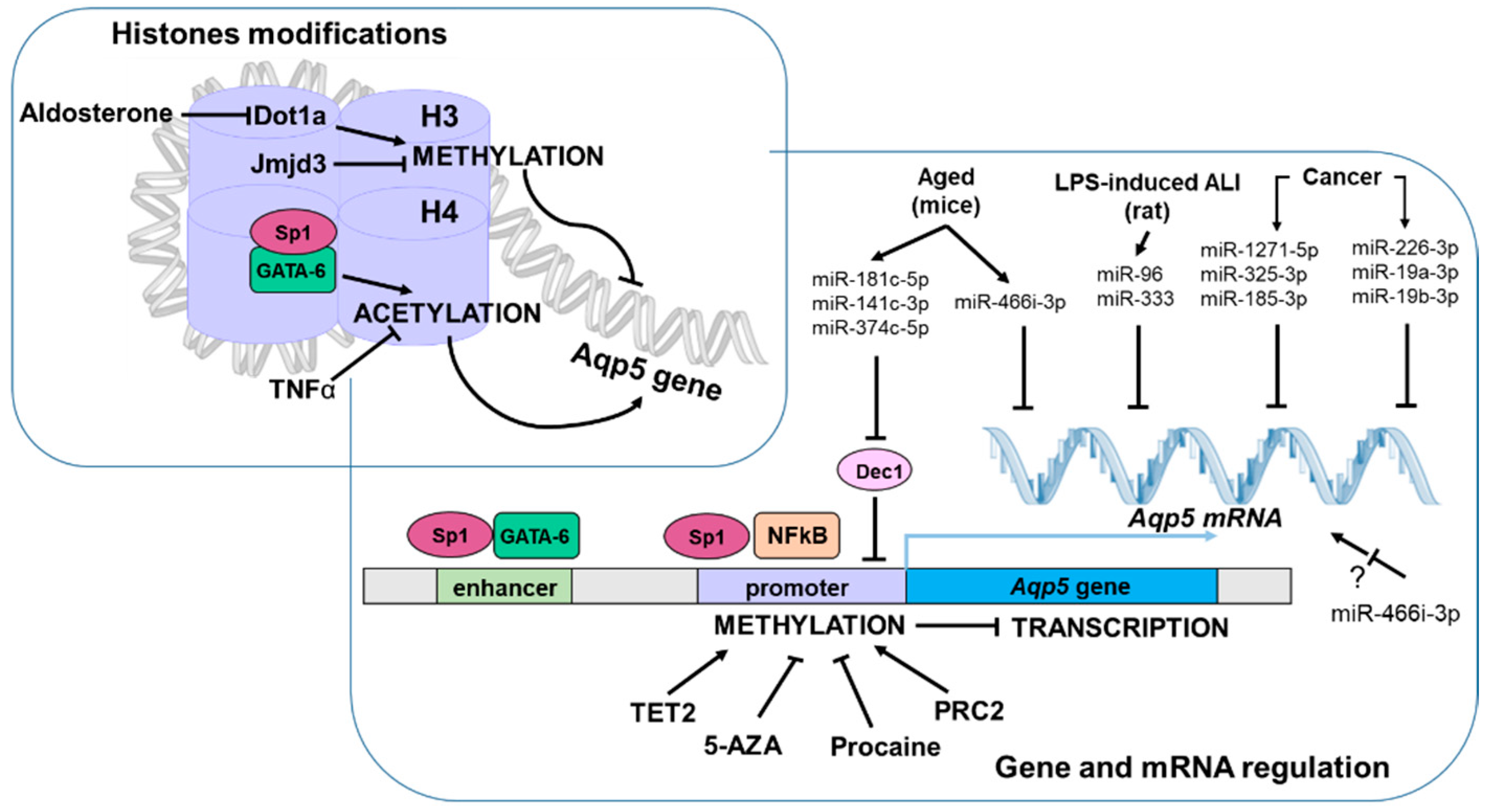
3.3. Epigenetic Modifications
3.3.1. DNA Methylation
3.3.2. Histone Modifications
3.3.3. miRNA
4. Post-Translational Regulation of AQP5
4.1. Phosphorylation
4.2. Lipidation
4.3. Glycosylation
4.4. Ubiquitination
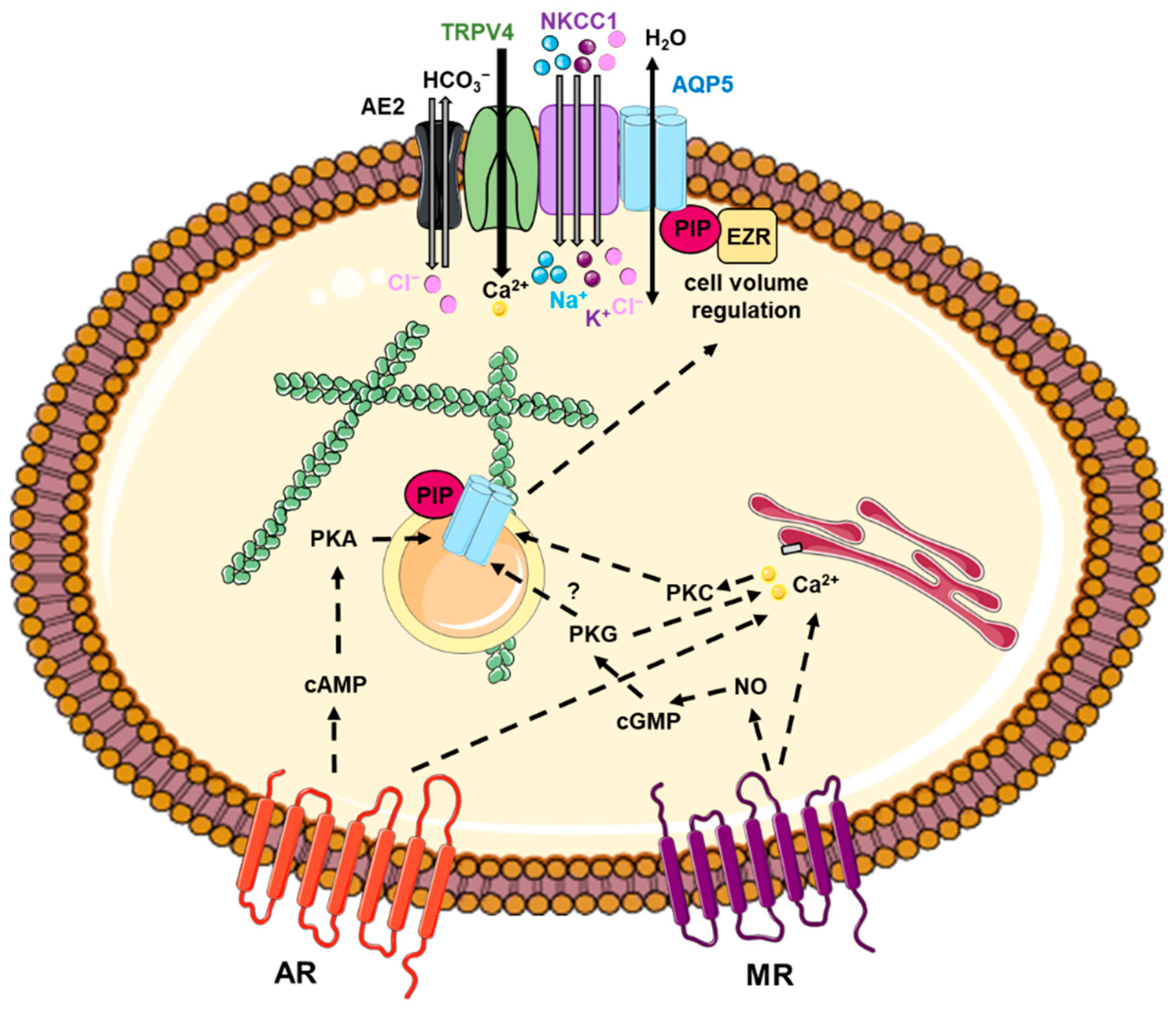
5. AQP5 Trafficking
5.1. Involvement of Intracellular Calcium Increase
5.2. Involvement of cAMP Increase
5.3. Interactions between AQP5 and Protein Partners
5.4. Involvement of Cytoskeleton
5.5. Involvement of Other Mechanisms
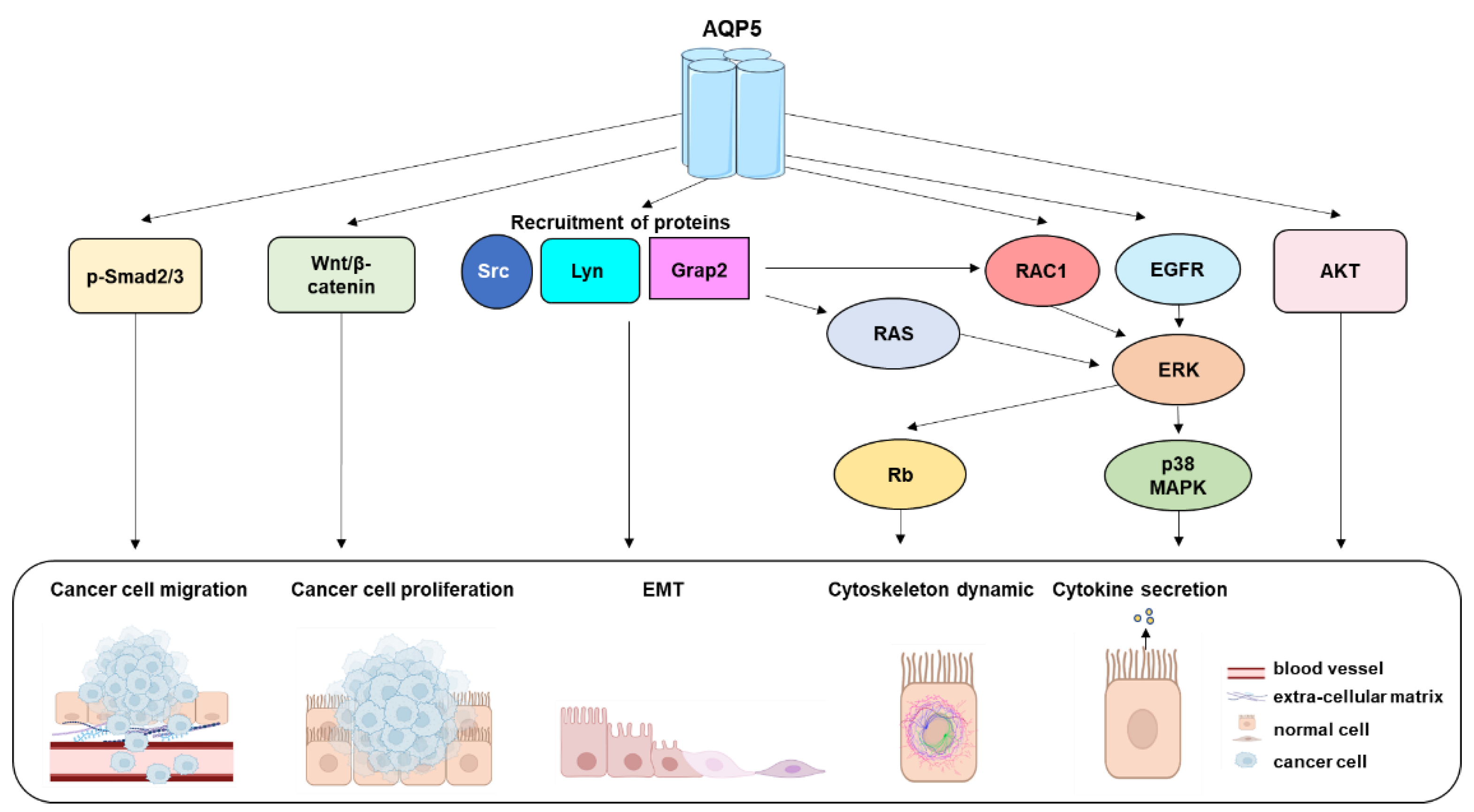
6. Downstream Effectors of AQP5
6.1. Ras-MAPK Signaling Pathway
6.2. Wingless/Integrated (WNT)/β-Catenin Signaling Pathway
6.3. p-Smad2/3 Pathway
6.4. p-Akt Pathway
6.5. Recruitment of Proteins
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agre, P. Aquaporin Water Channels (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 2004, 43, 4278–4290. [Google Scholar] [CrossRef]
- Laloux, T.; Junqueira, B.; Maistriaux, L.C.; Ahmed, J.; Jurkiewicz, A.; Chaumont, F. Plant and Mammal Aquaporins: Same but Different. Int. J. Mol. Sci. 2018, 19, 521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horsefield, R.; Nordén, K.; Fellert, M.; Backmark, A.; Törnroth-Horsefield, S.; Terwisscha van Scheltinga, A.C.; Kvassman, J.; Kjellbom, P.; Johanson, U.; Neutze, R. High-Resolution X-ray Structure of Human Aquaporin 5. Proc. Natl. Acad. Sci. USA 2008, 105, 13327–13332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.D.; Bhakta, K.Y.; Raina, S.; Yonescu, R.; Griffin, C.A.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Preston, G.M.; Agre, P. The Human Aquaporin-5 Gene. Molecular Characterization and Chromosomal Localization. J. Biol. Chem. 1996, 271, 8599–8604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raina, S.; Preston, G.M.; Guggino, W.B.; Agre, P. Molecular Cloning and Characterization of an Aquaporin CDNA from Salivary, Lacrimal, and Respiratory Tissues. J. Biol. Chem. 1995, 270, 1908–1912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, C.; Pimpão, C.; Mósca, A.F.; Coxixo, A.S.; Lopes, D.; da Silva, I.V.; Pedersen, P.A.; Antunes, F.; Soveral, G. Human Aquaporin-5 Facilitates Hydrogen Peroxide Permeation Affecting Adaption to Oxidative Stress and Cancer Cell Migration. Cancers 2019, 11, 932. [Google Scholar] [CrossRef] [Green Version]
- Geyer, R.R.; Musa-Aziz, R.; Qin, X.; Boron, W.F. Relative CO(2)/NH(3) Selectivities of Mammalian Aquaporins 0-9. Am. J. Physiol. Cell Physiol. 2013, 304, C985–C994. [Google Scholar] [CrossRef] [Green Version]
- Alishahi, M.; Kamali, R. A Novel Molecular Dynamics Study of CO2 Permeation through Aquaporin-5. Eur. Phys. J. E 2019, 42, 151. [Google Scholar] [CrossRef]
- Kourghi, M.; Pei, J.V.; De Ieso, M.L.; Nourmohammadi, S.; Chow, P.H.; Yool, A.J. Fundamental Structural and Functional Properties of Aquaporin Ion Channels Found across the Kingdoms of Life. Clin. Exp. Pharmacol. Physiol. 2018, 45, 401–409. [Google Scholar] [CrossRef] [Green Version]
- Törnroth-Horsefield, S.; Chivasso, C.; Strandberg, H.; D’Agostino, C.; O’Neale, C.V.T.; Schey, K.L.; Delporte, C. Insight into the Mammalian Aquaporin Interactome. Int. J. Mol. Sci. 2022, 23, 9615. [Google Scholar] [CrossRef]
- Henderson, S.W.; Nourmohammadi, S.; Ramesh, S.A.; Yool, A.J. Aquaporin Ion Conductance Properties Defined by Membrane Environment, Protein Structure, and Cell Physiology. Biophys. Rev. 2022, 14, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, P.; Conner, M.T.; Bill, R.M.; Conner, A.C. Structural Determinants of Oligomerization of the Aquaporin-4 Channel. J. Biol. Chem. 2016, 291, 6858–6871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takata, K.; Matsuzaki, T.; Tajika, Y. Aquaporins: Water Channel Proteins of the Cell Membrane. Prog. Histochem. Cytochem. 2004, 39, 1–83. [Google Scholar] [CrossRef]
- Agre, P.; King, L.S.; Yasui, M.; Guggino, W.B.; Ottersen, O.P.; Fujiyoshi, Y.; Engel, A.; Nielsen, S. Aquaporin Water Channels--from Atomic Structure to Clinical Medicine. J. Physiol. (Lond.) 2002, 542, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Verkman, A.S.; Mitra, A.K. Structure and Function of Aquaporin Water Channels. Am. J. Physiol. Renal. Physiol. 2000, 278, F13–F28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delporte, C. Aquaporins and Gland Secretion. Adv. Exp. Med. Biol. 2017, 969, 63–79. [Google Scholar] [CrossRef]
- Delporte, C.; Soyfoo, M. Aquaporins: Unexpected Actors in Autoimmune Diseases. Autoimmun. Rev. 2022, 21, 103131. [Google Scholar] [CrossRef]
- D’Agostino, C.; Elkashty, O.A.; Chivasso, C.; Perret, J.; Tran, S.D.; Delporte, C. Insight into Salivary Gland Aquaporins. Cells 2020, 9, 1547. [Google Scholar] [CrossRef]
- Mobasheri, A.; Barrett-Jolley, R. Aquaporin Water Channels in the Mammary Gland: From Physiology to Pathophysiology and Neoplasia. J. Mammary Gland. Biol. Neoplasia 2014, 19, 91–102. [Google Scholar] [CrossRef] [Green Version]
- Yadav, E.; Yadav, N.; Hus, A.; Yadav, J.S. Aquaporins in Lung Health and Disease: Emerging Roles, Regulation, and Clinical Implications. Respir. Med. 2020, 174, 106193. [Google Scholar] [CrossRef]
- Song, Y.; Verkman, A.S. Aquaporin-5 Dependent Fluid Secretion in Airway Submucosal Glands. J. Biol. Chem. 2001, 276, 41288–41292. [Google Scholar] [CrossRef] [Green Version]
- Grey, A.C.; Walker, K.L.; Petrova, R.S.; Han, J.; Wilmarth, P.A.; David, L.L.; Donaldson, P.J.; Schey, K.L. Verification and Spatial Localization of Aquaporin-5 in the Ocular Lens. Exp. Eye Res. 2013, 108, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrova, R.S.; Schey, K.L.; Donaldson, P.J.; Grey, A.C. Spatial Distributions of AQP5 and AQP0 in Embryonic and Postnatal Mouse Lens Development. Exp. Eye Res. 2015, 132, 124–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schey, K.L.; Petrova, R.S.; Gletten, R.B.; Donaldson, P.J. The Role of Aquaporins in Ocular Lens Homeostasis. Int. J. Mol. Sci. 2017, 18, 2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Kang, B.W.; Kim, J.G.; Jung, J.H.; Lee, J.; Kim, W.W.; Park, H.Y.; Jeong, J.-H.; Jeong, J.Y.; Park, J.-Y.; et al. AQP5 Variants Affect Tumoral Expression of AQP5 and Survival in Patients with Early Breast Cancer. Oncology 2017, 92, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Adamzik, M.; Frey, U.H.; Bitzer, K.; Jakob, H.; Baba, H.A.; Schmieder, R.E.; Schneider, M.P.; Heusch, G.; Peters, J.; Siffert, W. A Novel-1364A/C Aquaporin 5 Gene Promoter Polymorphism Influences the Responses to Salt Loading of the Renin-Angiotensin-Aldosterone System and of Blood Pressure in Young Healthy Men. Basic Res. Cardiol. 2008, 103, 598–610. [Google Scholar] [CrossRef]
- Rump, K.; Spellenberg, T.; von Busch, A.; Wolf, A.; Ziehe, D.; Thon, P.; Rahmel, T.; Adamzik, M.; Koos, B.; Unterberg, M. AQP5-1364A/C Polymorphism Affects AQP5 Promoter Methylation. Int. J. Mol. Sci. 2022, 23, 11813. [Google Scholar] [CrossRef]
- Adamzik, M.; Frey, U.H.; Möhlenkamp, S.; Scherag, A.; Waydhas, C.; Marggraf, G.; Dammann, M.; Steinmann, J.; Siffert, W.; Peters, J. Aquaporin 5 Gene Promoter--1364A/C Polymorphism Associated with 30-Day Survival in Severe Sepsis. Anesthesiology 2011, 114, 912–917. [Google Scholar] [CrossRef] [Green Version]
- Bergmann, L.; Nowak, H.; Siffert, W.; Peters, J.; Adamzik, M.; Koos, B.; Rahmel, T. Major Adverse Kidney Events Are Associated with the Aquaporin 5 -1364A/C Promoter Polymorphism in Sepsis: A Prospective Validation Study. Cells 2020, 9, 904. [Google Scholar] [CrossRef] [Green Version]
- Rahmel, T.; Rump, K.; Peters, J.; Adamzik, M. Aquaporin 5 -1364A/C Promoter Polymorphism Is Associated with Pulmonary Inflammation and Survival in Acute Respiratory Distress Syndrome. Anesthesiology 2019, 130, 404–413. [Google Scholar] [CrossRef]
- Rahmel, T.; Nowak, H.; Rump, K.; Siffert, W.; Peters, J.; Adamzik, M. The Aquaporin 5 -1364A/C Promoter Polymorphism Impacts on Resolution of Acute Kidney Injury in Pneumonia Evoked ARDS. PLoS ONE 2018, 13, e0208582. [Google Scholar] [CrossRef] [Green Version]
- Rahmel, T.; Nowak, H.; Rump, K.; Koos, B.; Schenker, P.; Viebahn, R.; Adamzik, M.; Bergmann, L. The Aquaporin 5 -1364A/C Promoter Polymorphism Is Associated with Cytomegalovirus Infection Risk in Kidney Transplant Recipients. Front. Immunol. 2019, 10, 2871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambertz, N.; Hindy, N.E.; Adler, C.; Rump, K.; Adamzik, M.; Keyvani, K.; Bankfalvi, A.; Siffert, W.; Erol Sandalcioglu, I.; Bachmann, H.S. Expression of Aquaporin 5 and the AQP5 Polymorphism A(-1364)C in Association with Peritumoral Brain Edema in Meningioma Patients. J. Neurooncol. 2013, 112, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Blaydon, D.C.; Lind, L.K.; Plagnol, V.; Linton, K.J.; Smith, F.J.D.; Wilson, N.J.; McLean, W.H.I.; Munro, C.S.; South, A.P.; Leigh, I.M.; et al. Mutations in AQP5, Encoding a Water-Channel Protein, Cause Autosomal-Dominant Diffuse Nonepidermolytic Palmoplantar Keratoderma. Am. J. Hum. Genet. 2013, 93, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Krøigård, A.B.; Hetland, L.E.; Clemmensen, O.; Blaydon, D.C.; Hertz, J.M.; Bygum, A. The First Danish Family Reported with an AQP5 Mutation Presenting Diffuse Non-Epidermolytic Palmoplantar Keratoderma of Bothnian Type, Hyperhidrosis and Frequent Corynebacterium Infections: A Case Report. BMC Dermatol. 2016, 16, 7. [Google Scholar] [CrossRef] [Green Version]
- Cao, X.; Yin, J.; Wang, H.; Zhao, J.; Zhang, J.; Dai, L.; Zhang, J.; Jiang, H.; Lin, Z.; Yang, Y. Mutation in AQP5, Encoding Aquaporin 5, Causes Palmoplantar Keratoderma Bothnia Type. J. Investig. Dermatol. 2014, 134, 284–287. [Google Scholar] [CrossRef] [Green Version]
- Wada, Y.; Kusakabe, M.; Nagai, M.; Imai, Y.; Yamanishi, K. Japanese Case of Bothnian-Type Palmoplantar Keratoderma with a Novel Missense Mutation of p.Trp35Ser in Extracellular Loop A of Aquaporin-5. J. Dermatol. 2019, 46, e104–e106. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, H.; Kamali, R. Molecular Dynamics Study of Water Transport through AQP5-R188C Mutant Causing Palmoplantar Keratoderma (PPK) Using the Gating Mechanism Concept. Biophys. Chem. 2021, 277, 106655. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Di, G.; Hu, S.; Liu, Y.; Dai, Y.; Chen, P. AQP5 Regulates Vimentin Expression via MiR-124–3p.1 to Protect Lens Transparency. Exp. Eye Res. 2021, 205, 108485. [Google Scholar] [CrossRef]
- Qin, X.; Boron, W.F. Mutation of a Single Amino Acid Converts the Human Water Channel Aquaporin 5 into an Anion Channel. Am. J. Physiol.-Cell Physiol. 2013, 305, C663–C672. [Google Scholar] [CrossRef]
- Karabasil, M.R.; Hasegawa, T.; Azlina, A.; Purwanti, N.; Yao, C.; Akamatsu, T.; Tomioka, S.; Hosoi, K. Effects of Naturally Occurring G103D Point Mutation of AQP5 on Its Water Permeability, Trafficking and Cellular Localization in the Submandibular Gland of Rats. Biol. Cell 2011, 103, 69–86. [Google Scholar] [CrossRef]
- Satoh, K.; Seo, Y.; Matsuo, S.; Karabasil, M.R.; Matsuki-Fukushima, M.; Nakahari, T.; Hosoi, K. Roles of AQP5/AQP5-G103D in Carbamylcholine-Induced Volume Decrease and in Reduction of the Activation Energy for Water Transport by Rat Parotid Acinar Cells. Pflugers Arch.-Eur. J. Physiol. 2012, 464, 375–389. [Google Scholar] [CrossRef] [PubMed]
- Tanski, D.; Skowronska, A.; Tanska, M.; Lepiarczyk, E.; Skowronski, M.T. The In Vitro Effect of Steroid Hormones, Arachidonic Acid, and Kinases Inhibitors on Aquaporin 1, 2, 5, and 7 Gene Expression in the Porcine Uterine Luminal Epithelial Cells during the Estrous Cycle. Cells 2021, 10, 832. [Google Scholar] [CrossRef]
- de Oliveira, V.; Schaefer, J.; Abu-Rafea, B.; Vilos, G.A.; Vilos, A.G.; Bhattacharya, M.; Radovick, S.; Babwah, A.V. Uterine Aquaporin Expression Is Dynamically Regulated by Estradiol and Progesterone and Ovarian Stimulation Disrupts Embryo Implantation without Affecting Luminal Closure. Mol. Hum. Reprod. 2020, 26, 154–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Q.; Zhang, H.; Wang, Q.; Li, R.; Jin, Y.; Wang, H.; Ma, T.; Qiao, J.; Duan, E. Aquaporin-Dependent Excessive Intrauterine Fluid Accumulation Is a Major Contributor in Hyper-Estrogen Induced Aberrant Embryo Implantation. Cell Res. 2015, 25, 139–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaihoko, Y.; Tsugami, Y.; Suzuki, N.; Suzuki, T.; Nishimura, T.; Kobayashi, K. Distinct Expression Patterns of Aquaporin 3 and 5 in Ductal and Alveolar Epithelial Cells in Mouse Mammary Glands before and after Parturition. Cell Tissue Res. 2020, 380, 513–526. [Google Scholar] [CrossRef]
- Salleh, N.; Mokhtar, H.M.; Kassim, N.M.; Giribabu, N. Testosterone Induces Increase in Aquaporin (AQP)-1, 5, and 7 Expressions in the Uteri of Ovariectomized Rats. J. Membr. Biol. 2015, 248, 1097–1105. [Google Scholar] [CrossRef]
- Ducza, E.; Seres, A.B.; Hajagos-Tóth, J.; Falkay, G.; Gáspár, R. Oxytocin Regulates the Expression of Aquaporin 5 in the Late-Pregnant Rat Uterus. Mol. Reprod. Dev. 2014, 81, 524–530. [Google Scholar] [CrossRef] [Green Version]
- Skowronska, A.; Mlotkowska, P.; Okrasa, S.; Nielsen, S.; Skowronski, M.T. Modulatory Effects of Steroid Hormones, Oxytocin, Arachidonic Acid, Forskolin and Cyclic AMP on the Expression of Aquaporin 1 and Aquaporin 5 in the Porcine Uterus during Placentation. J. Physiol. Pharmacol. 2016, 67, 311–319. [Google Scholar]
- Skowronski, M.T.; Mlotkowska, P.; Tanski, D.; Lepiarczyk, E.; Kempisty, B.; Jaskiewicz, L.; Pareek, C.S.; Skowronska, A. Pituitary Hormones (FSH, LH, PRL, and GH) Differentially Regulate AQP5 Expression in Porcine Ovarian Follicular Cells. Int. J. Mol. Sci. 2019, 20, 4914. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.X.; Fei, X.W.; Zhao, L.; Ye, X.L.; Xin, L.B.; Qu, Y.; Xu, K.H.; Wu, R.J.; Lin, J. Aquaporin 5 Plays a Role in Estrogen-Induced Ectopic Implantation of Endometrial Stromal Cells in Endometriosis. PLoS ONE 2015, 10, e0145290. [Google Scholar] [CrossRef] [PubMed]
- Ben, Y.; Chen, J.; Zhu, R.; Gao, L.; Bai, C. Upregulation of AQP3 and AQP5 Induced by Dexamethasone and Ambroxol in A549 Cells. Respir. Physiol. Neurobiol. 2008, 161, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Pajouhi, N.; Owji, M.; Naghibalhossaini, F.; Omrani, G.H.R.; Varedi, M. Modulation by Thyroid Hormone of Myosin Light Chain Phosphorylation and Aquaporin 5 Protein Expression in Intact Lung. J. Physiol. Biochem. 2015, 71, 99–106. [Google Scholar] [CrossRef]
- Ramirez, M.I.; Chung, U.I.; Williams, M.C. Aquaporin-5 Expression, but Not Other Peripheral Lung Marker Genes, Is Reduced in PTH/PTHrP Receptor Null Mutant Fetal Mice. Am. J. Respir. Cell Mol. Biol. 2000, 22, 367–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.-B.; Kang, M.-Q.; Huang, L.-P.; Zhuo, Y.; Li, X.; Lai, F.-C. CHRNA1 Promotes the Pathogenesis of Primary Focal Hyperhidrosis. Mol. Cell. Neurosci. 2021, 111, 103598. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-B.; Lin, N.-L.; Li, X.; Kang, M.-Q. Antagonist of Chrna1 Prevents the Pathogenesis of Primary Focal Hyperhidrosis. Ann. Clin. Transl. Neurol. 2022, 9, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Lin, M.; Yang, J.-H.; Chen, J.-F.; Tu, Y.-R. Overexpression of AQP5 Was Detected in Axillary Sweat Glands of Primary Focal Hyperhidrosis Patients. Dermatology 2016, 232, 150–155. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Jian, K.R.; Lin, C.-S.; Wang, H.-W.; Liu, S.-C. Dexamethasone Attenuates Methacholine-Mediated Aquaporin 5 Downregulation in Human Nasal Epithelial Cells via Suppression of NF-ΚB Activation. Int. Forum Allergy Rhinol. 2018, 8, 64–71. [Google Scholar] [CrossRef]
- Zhou, B.; Ann, D.K.; Flodby, P.; Minoo, P.; Liebler, J.M.; Crandall, E.D.; Borok, Z. Rat Aquaporin-5 4.3-Kb 5’-Flanking Region Differentially Regulates Expression in Salivary Gland and Lung in Vivo. Am. J. Physiol. Cell Physiol. 2008, 295, C111–C120. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Kawedia, J.D.; Menon, A.G. Cyclic AMP Regulates Aquaporin 5 Expression at Both Transcriptional and Post-Transcriptional Levels through a Protein Kinase A Pathway. J. Biol. Chem. 2003, 278, 32173–32180. [Google Scholar] [CrossRef] [Green Version]
- Cammalleri, M.; Amato, R.; Olivieri, M.; Pezzino, S.; Bagnoli, P.; Dal Monte, M.; Rusciano, D. Effects of Topical Gabapentin on Ocular Pain and Tear Secretion. Front. Pharmacol. 2021, 12, 671238. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-L.; Lin, C.-S.; Wang, H.-W.; Jian, K.R.; Liu, S.-C. Chlorpheniramine Attenuates Histamine-Mediated Aquaporin 5 Downregulation in Human Nasal Epithelial Cells via Suppression of NF-ΚB Activation. Int. J. Med. Sci. 2017, 14, 1268–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wang, X.; Ma, L.; Zhang, R. Histamine Downregulates Aquaporin 5 in Human Nasal Epithelial Cells. Am. J. Rhinol. Allergy 2015, 29, 188–192. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-B.; Chen, J.-F.; Lai, F.-C.; Li, X.; Xie, J.-B.; Tu, Y.-R.; Kang, M.-Q. Involvement of Activin a Receptor Type 1 (ACVR1) in the Pathogenesis of Primary Focal Hyperhidrosis. Biochem. Biophys. Res. Commun. 2020, 528, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.; Shi, Y.; Yang, J. Roles of P38 MAPK and JNK in TGF-Β1-Induced Human Alveolar Epithelial to Mesenchymal Transition. Arch. Med. Res. 2013, 44, 93–98. [Google Scholar] [CrossRef]
- Willis, B.C.; Liebler, J.M.; Luby-Phelps, K.; Nicholson, A.G.; Crandall, E.D.; du Bois, R.M.; Borok, Z. Induction of Epithelial-Mesenchymal Transition in Alveolar Epithelial Cells by Transforming Growth Factor-Beta1: Potential Role in Idiopathic Pulmonary Fibrosis. Am. J. Pathol. 2005, 166, 1321–1332. [Google Scholar] [CrossRef]
- Yamamura, Y.; Motegi, K.; Kani, K.; Takano, H.; Momota, Y.; Aota, K.; Yamanoi, T.; Azuma, M. TNF-α Inhibits Aquaporin 5 Expression in Human Salivary Gland Acinar Cells via Suppression of Histone H4 Acetylation. J. Cell. Mol. Med. 2012, 16, 1766–1775. [Google Scholar] [CrossRef]
- Towne, J.E.; Krane, C.M.; Bachurski, C.J.; Menon, A.G. Tumor Necrosis Factor-Alpha Inhibits Aquaporin 5 Expression in Mouse Lung Epithelial Cells. J. Biol. Chem. 2001, 276, 18657–18664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mezzasoma, L.; Cagini, L.; Antognelli, C.; Puma, F.; Pacifico, E.; Talesa, V.N. TNF-α Regulates Natriuretic Peptides and Aquaporins in Human Bronchial Epithelial Cells BEAS-2B. Mediat. Inflamm. 2013, 2013, 159349. [Google Scholar] [CrossRef] [Green Version]
- Limaye, A.; Hall, B.E.; Zhang, L.; Cho, A.; Prochazkova, M.; Zheng, C.; Walker, M.; Adewusi, F.; Burbelo, P.D.; Sun, Z.J.; et al. Targeted TNF-α Overexpression Drives Salivary Gland Inflammation. J. Dent. Res. 2019, 98, 713–719. [Google Scholar] [CrossRef]
- Shi, Z.; Ye, W.; Zhang, J.; Zhang, F.; Yu, D.; Yu, H.; Chen, B.; Zhou, M.; Sun, H. LipoxinA4 Attenuates Acute Pancreatitis-Associated Acute Lung Injury by Regulating AQP-5 and MMP-9 Expression, Anti-Apoptosis and PKC/SSeCKS-Mediated F-Actin Activation. Mol. Immunol. 2018, 103, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.K.; Siddiqui, A.A.; Modica, L.A.; Dykes, R.; Simmons, C.; Schmidt, J.; Krishnaswamy, G.A.; Berk, S.L. Interferon-Alpha Upregulates Gene Expression of Aquaporin-5 in Human Parotid Glands. J. Interferon Cytokine Res. 1999, 19, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Skowron-zwarg, M.; Boland, S.; Caruso, N.; Coraux, C.; Marano, F.; Tournier, F. Interleukin-13 Interferes with CFTR and AQP5 Expression and Localization during Human Airway Epithelial Cell Differentiation. Exp. Cell Res. 2007, 313, 2695–2702. [Google Scholar] [CrossRef]
- Krane, C.M.; Deng, B.; Mutyam, V.; McDonald, C.A.; Pazdziorko, S.; Mason, L.; Goldman, S.; Kasaian, M.; Chaudhary, D.; Williams, C.; et al. Altered Regulation of Aquaporin Gene Expression in Allergen and IL-13-Induced Mouse Models of Asthma. Cytokine 2009, 46, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Lotze, M.T.; Tracey, K.J. High-Mobility Group Box 1 Protein (HMGB1): Nuclear Weapon in the Immune Arsenal. Nat. Rev. Immunol. 2005, 5, 331–342. [Google Scholar] [CrossRef]
- Müller, S.; Scaffidi, P.; Degryse, B.; Bonaldi, T.; Ronfani, L.; Agresti, A.; Beltrame, M.; Bianchi, M.E. New EMBO Members’ Review: The Double Life of HMGB1 Chromatin Protein: Architectural Factor and Extracellular Signal. EMBO J. 2001, 20, 4337–4340. [Google Scholar] [CrossRef]
- Sarrand, J.; Baglione, L.; Parisis, D.; Soyfoo, M. The Involvement of Alarmins in the Pathogenesis of Sjögren’s Syndrome. Int. J. Mol. Sci. 2022, 23, 5671. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, M.; Wang, Y.; Sun, S. Suppression of High-Mobility Group Box 1 Ameliorates Xerostomia in a Sjögren Syndrome-Triggered Mouse Model. Can. J. Physiol. Pharmacol. 2020, 98, 351–356. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, D.H.; Jeong, H.J.; Ryu, J.S.; Kim, Y.J.; Oh, J.Y.; Kim, M.K.; Wee, W.R. Effects of Subconjunctival Administration of Anti-High Mobility Group Box 1 on Dry Eye in a Mouse Model of Sjögren’s Syndrome. PLoS ONE 2017, 12, e0183678. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.Y.; Yao, Y.; Hu, R.; Dai, F.F.; Zhang, H.; Mao, Z.F. Cyclic Adenosine Monophosphate-Protein Kinase A Signal Pathway May Be Involved in Pulmonary Aquaporin-5 Expression in Ischemia/Reperfusion Rats Following Deep Hypothermia Cardiac Arrest. Genet. Mol. Res. 2016, 15, 251. [Google Scholar] [CrossRef]
- Susa, T.; Sawai, N.; Aoki, T.; Iizuka-Kogo, A.; Kogo, H.; Negishi, A.; Yokoo, S.; Takata, K.; Matsuzaki, T. Effects of Repeated Administration of Pilocarpine and Isoproterenol on Aquaporin-5 Expression in Rat Salivary Glands. Acta Histochem. Cytochem. 2013, 46, 187–197. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, M. Role of CAMP-PKA/CREB Pathway in Regulation of AQP 5 Production in Rat Nasal Epithelium. Rhinology 2011, 49, 464–469. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, M. Nuclear Factor Kappa B Pathway Down-Regulates Aquaporin 5 in the Nasal Mucosa of Rats with Allergic Rhinitis. Eur. Arch. Otorhinolaryngol. 2011, 268, 73–81. [Google Scholar] [CrossRef]
- Hollborn, M.; Vogler, S.; Reichenbach, A.; Wiedemann, P.; Bringmann, A.; Kohen, L. Regulation of the Hyperosmotic Induction of Aquaporin 5 and VEGF in Retinal Pigment Epithelial Cells: Involvement of NFAT5. Mol. Vis. 2015, 21, 360–377. [Google Scholar] [PubMed]
- Yao, C.; Purwanti, N.; Karabasil, M.R.; Azlina, A.; Javkhlan, P.; Hasegawa, T.; Akamatsu, T.; Hosoi, T.; Ozawa, K.; Hosoi, K. Potential Down-Regulation of Salivary Gland AQP5 by LPS via Cross-Coupling of NF-KappaB and p-c-Jun/c-Fos. Am. J. Pathol. 2010, 177, 724–734. [Google Scholar] [CrossRef] [Green Version]
- Johnson, Z.I.; Gogate, S.S.; Day, R.; Binch, A.; Markova, D.Z.; Chiverton, N.; Cole, A.; Conner, M.; Shapiro, I.M.; Le Maitre, C.L.; et al. Aquaporin 1 and 5 Expression Decreases during Human Intervertebral Disc Degeneration: Novel HIF-1-Mediated Regulation of Aquaporins in NP Cells. Oncotarget 2015, 6, 11945–11958. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Ann, D.K.; Li, X.; Kim, K.-J.; Lin, H.; Minoo, P.; Crandall, E.D.; Borok, Z. Hypertonic Induction of Aquaporin-5: Novel Role of Hypoxia-Inducible Factor-1α. Am. J. Physiol.-Cell Physiol. 2007, 292, C1280–C1290. [Google Scholar] [CrossRef]
- Kawedia, J.D.; Yang, F.; Sartor, M.A.; Gozal, D.; Czyzyk-Krzeska, M.; Menon, A.G. Hypoxia and Hypoxia Mimetics Decrease Aquaporin 5 (AQP5) Expression through Both Hypoxia Inducible Factor-1α and Proteasome-Mediated Pathways. PLoS ONE 2013, 8, e57541. [Google Scholar] [CrossRef] [Green Version]
- Hoffert, J.D.; Leitch, V.; Agre, P.; King, L.S. Hypertonic Induction of Aquaporin-5 Expression through an ERK-Dependent Pathway. J. Biol. Chem. 2000, 275, 9070–9077. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.-M.; Wang, Y.-S.; Zhang, J.; Li, Y.; Xu, J.-F.; Zhu, J.; Zhao, W.; Chu, D.-K.; Wiedemann, P. Role of PI3K/Akt and MEK/ERK in Mediating Hypoxia-Induced Expression of HIF-1alpha and VEGF in Laser-Induced Rat Choroidal Neovascularization. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1873–1879. [Google Scholar] [CrossRef]
- Sugimoto, N.; Matsuzaki, K.; Ishibashi, H.; Tanaka, M.; Sawaki, T.; Fujita, Y.; Kawanami, T.; Masaki, Y.; Okazaki, T.; Sekine, J.; et al. Upregulation of Aquaporin Expression in the Salivary Glands of Heat-Acclimated Rats. Sci. Rep. 2013, 3, 1763. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.L.; Li, Y.; Xu, S.; Nie, Y.; Zhang, J. NFAT5 Regulated by STUB1, Facilitates Malignant Cell Survival and P38 MAPK Activation by Upregulating AQP5 in Chronic Lymphocytic Leukemia. Biochem. Genet. 2021, 59, 870–883. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Jin, F. NFAT5 Promotes Proliferation and Migration of Lung Adenocarcinoma Cells in Part through Regulating AQP5 Expression. Biochem. Biophys. Res. Commun. 2015, 465, 644–649. [Google Scholar] [CrossRef]
- Ren, Y.; Lu, H.; Reinach, P.S.; Zheng, Q.; Li, J.; Tan, Q.; Zhu, H.; Chen, W. Hyperosmolarity-Induced AQP5 Upregulation Promotes Inflammation and Cell Death via JNK1/2 Activation in Human Corneal Epithelial Cells. Sci. Rep. 2017, 7, 4727. [Google Scholar] [CrossRef] [Green Version]
- Snuggs, J.W.; Tessier, S.; Bunning, R.A.B.; Shapiro, I.M.; Risbud, M.V.; Le Maitre, C.L. TonEBP Regulates the Hyperosmotic Expression of Aquaporin 1 and 5 in the Intervertebral Disc. Sci. Rep. 2021, 11, 3164. [Google Scholar] [CrossRef]
- Lee, S.M.; Lee, S.W.; Kang, M.; Choi, J.K.; Park, K.; Byun, J.S.; Kim, D.Y. FoxO1 as a Regulator of Aquaporin 5 Expression in the Salivary Gland. J. Dent. Res. 2021, 100, 1281–1288. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, M.; Yu, X.; Yan, Y.; Yu, B.; Zhang, D. Targeting Forkhead Box O1-Aquaporin 5 Axis Mitigates Neuropathic Pain in a CCI Rat Model through Inhibiting Astrocytic and Microglial Activation. Bioengineered 2022, 13, 8567–8580. [Google Scholar] [CrossRef]
- Rump, K.; Siffert, W.; Peters, J.; Adamzik, M. The Transcription Factor NMP4 Binds to the AQP5 Promoter and Is a Novel Transcriptional Regulator of the AQP5 Gene. DNA Cell Biol. 2016, 35, 322–327. [Google Scholar] [CrossRef]
- Yang, Z.; Bidwell, J.P.; Young, S.R.; Gerard-O’Riley, R.; Wang, H.; Pavalko, F.M. Nmp4/CIZ Inhibits Mechanically-Induced β-Catenin Signaling Activity in Osteoblasts. J. Cell. Physiol. 2010, 223, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Nakamoto, T.; Yamagata, T.; Sakai, R.; Ogawa, S.; Honda, H.; Ueno, H.; Hirano, N.; Yazaki, Y.; Hirai, H. CIZ, a Zinc Finger Protein That Interacts with P130(Cas) and Activates the Expression of Matrix Metalloproteinases. Mol. Cell. Biol. 2000, 20, 1649–1658. [Google Scholar] [CrossRef] [Green Version]
- Janssen, H.; Marynen, P. Interaction Partners for Human ZNF384/CIZ/NMP4--Zyxin as a Mediator for P130CAS Signaling? Exp. Cell Res. 2006, 312, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Login, F.H.; Palmfeldt, J.; Cheah, J.S.; Yamada, S.; Nejsum, L.N. Aquaporin-5 Regulation of Cell-Cell Adhesion Proteins: An Elusive “Tail” Story. Am. J. Physiol. Cell Physiol. 2021, 320, C282–C292. [Google Scholar] [CrossRef]
- Rieger, M.E.; Zhou, B.; Solomon, N.; Sunohara, M.; Li, C.; Nguyen, C.; Liu, Y.; Pan, J.; Minoo, P.; Crandall, E.D.; et al. P300/β-Catenin Interactions Regulate Adult Progenitor Cell Differentiation Downstream of WNT5a/Protein Kinase C (PKC). J. Biol. Chem. 2016, 291, 6569–6582. [Google Scholar] [CrossRef] [Green Version]
- Nomura, J.; Hisatsune, A.; Miyata, T.; Isohama, Y. The Role of CpG Methylation in Cell Type-Specific Expression of the Aquaporin-5 Gene. Biochem. Biophys. Res. Commun. 2007, 353, 1017–1022. [Google Scholar] [CrossRef]
- Zheng, L.; Seon, Y.; McHugh, J.; Papagerakis, S. Clock Genes Show Circadian Rhythms in Salivary Glands. J. Dent. Res. 2012, 91, 783–788. [Google Scholar] [CrossRef] [Green Version]
- Satou, R.; Sato, M.; Kimura, M.; Ishizuka, Y.; Tazaki, M.; Sugihara, N.; Shibukawa, Y. Temporal Expression Patterns of Clock Genes and Aquaporin 5/Anoctamin 1 in Rat Submandibular Gland Cells. Front Physiol. 2017, 8, 320. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Lu, M.M.; Zhang, L.; Whitsett, J.A.; Morrisey, E.E. GATA6 Regulates Differentiation of Distal Lung Epithelium. Development 2002, 129, 2233–2246. [Google Scholar] [CrossRef]
- Zhou, B.; Francis, T.A.; Yang, H.; Tseng, W.; Zhong, Q.; Frenkel, B.; Morrisey, E.E.; Ann, D.K.; Minoo, P.; Crandall, E.D.; et al. GATA-6 Mediates Transcriptional Activation of Aquaporin-5 through Interactions with Sp1. Am. J. Physiol. Cell Physiol. 2008, 295, C1141–C1150. [Google Scholar] [CrossRef] [Green Version]
- Flodby, P.; Li, C.; Liu, Y.; Wang, H.; Rieger, M.E.; Minoo, P.; Crandall, E.D.; Ann, D.K.; Borok, Z.; Zhou, B. Cell-Specific Expression of Aquaporin-5 (Aqp5) in Alveolar Epithelium Is Directed by GATA6/Sp1 via Histone Acetylation. Sci. Rep. 2017, 7, 3473. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, K.; Wang, X.; Bhawal, U.K. Dec1 Deficiency Restores the Age-Related Dysfunctions of Submandibular Glands. J. Physiol. Pharmacol. 2021, 72, 571–581. [Google Scholar] [CrossRef]
- Zuo, W.; Yang, H.; Li, N.; Ouyang, Y.; Xu, X.; Hong, J. Helicobacter Pylori Infection Activates Wnt/β-Catenin Pathway to Promote the Occurrence of Gastritis by Upregulating ASCL1 and AQP5. Cell Death Discov. 2022, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Arbeithuber, B.; Thuenauer, R.; Gravogl, Y.; Balogi, Z.; Römer, W.; Sonnleitner, A.; Tiemann-Boege, I. Aquaporin 5 Expression in Mouse Mammary Gland Cells Is Not Driven by Promoter Methylation. BioMed Res. Int. 2015, 2015, e460598. [Google Scholar] [CrossRef] [Green Version]
- Kiely, M.; Tse, L.A.; Koka, H.; Wang, D.; Lee, P.; Wang, F.; Wu, C.; Tsang, K.H.; Chan, W.-C.; Law, S.H.; et al. Age-Related DNA Methylation in Paired Normal and Tumour Breast Tissue in Chinese Breast Cancer Patients. Epigenetics 2021, 16, 677–691. [Google Scholar] [CrossRef]
- Chen, G.; Song, H.; Yang, Z.; Du, T.; Zheng, Y.; Lu, Z.; Zhang, K.; Wei, D. AQP5 Is a Novel Prognostic Biomarker in Pancreatic Adenocarcinoma. Front. Oncol. 2022, 12, 890193. [Google Scholar] [CrossRef]
- Thangavel, J.; Malik, A.B.; Elias, H.K.; Rajasingh, S.; Simpson, A.D.; Sundivakkam, P.K.; Vogel, S.M.; Xuan, Y.-T.; Dawn, B.; Rajasingh, J. Combinatorial Therapy with Acetylation and Methylation Modifiers Attenuates Lung Vascular Hyperpermeability in Endotoxemia-Induced Mouse Inflammatory Lung Injury. Am. J. Pathol. 2014, 184, 2237–2249. [Google Scholar] [CrossRef] [Green Version]
- Rump, K.; Unterberg, M.; Dahlke, A.; Nowak, H.; Koos, B.; Bergmann, L.; Siffert, W.; Schäfer, S.T.; Peters, J.; Adamzik, M.; et al. DNA Methylation of a NF-ΚB Binding Site in the Aquaporin 5 Promoter Impacts on Mortality in Sepsis. Sci. Rep. 2019, 9, 18511. [Google Scholar] [CrossRef] [Green Version]
- Motegi, K.; Azuma, M.; Tamatani, T.; Ashida, Y.; Sato, M. Expression of Aquaporin-5 in and Fluid Secretion from Immortalized Human Salivary Gland Ductal Cells by Treatment with 5-Aza-2’-Deoxycytidine: A Possibility for Improvement of Xerostomia in Patients with Sjögren’s Syndrome. Lab. Investig. 2005, 85, 342–353. [Google Scholar] [CrossRef] [Green Version]
- Nomura, J.; Horie, I.; Seto, M.; Nagai, K.; Hisatsune, A.; Miyata, T.; Isohama, Y. All-Trans Retinoic Acid Increases Expression of Aquaporin-5 and Plasma Membrane Water Permeability via Transactivation of Sp1 in Mouse Lung Epithelial Cells. Biochem. Biophys. Res. Commun. 2006, 351, 1048–1053. [Google Scholar] [CrossRef]
- Yamamura, Y.; Aota, K.; Yamanoi, T.; Kani, K.; Takano, H.; Momota, Y.; Motegi, K.; Azuma, M. DNA Demethylating Agent Decitabine Increases AQP5 Expression and Restores Salivary Function. J. Dent. Res. 2012, 91, 612–617. [Google Scholar] [CrossRef]
- Lee, B.H.; Yegnasubramanian, S.; Lin, X.; Nelson, W.G. Procainamide Is a Specific Inhibitor of DNA Methyltransferase 1. J. Biol. Chem. 2005, 280, 40749–40756. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Wang, J.; Sun, J.; Shen, L.; Liu, M.; Zhao, E. Procaine Stimulates Aquaporin-5 Expression in Human Salivary Gland Ductal Cells via the Suppression of DNA Methyltransferase-1. Mol. Med. Rep. 2018, 17, 7996–8002. [Google Scholar] [CrossRef]
- Bennett, L.B.; Schnabel, J.L.; Kelchen, J.M.; Taylor, K.H.; Guo, J.; Arthur, G.L.; Papageorgio, C.N.; Shi, H.; Caldwell, C.W. DNA Hypermethylation Accompanied by Transcriptional Repression in Follicular Lymphoma. Genes Chromosomes Cancer 2009, 48, 828–841. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, T.; Eto, K.; Kadoya, Y. Downregulation of Ten-Eleven Translocation-2 Triggers Epithelial Differentiation during Organogenesis. Differentiation 2022, 125, 45–53. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.Y.; Chepelev, I.; Zhu, Q.; Wei, G.; Zhao, K.; Wang, R.-F. Stage-Dependent and Locus-Specific Role of Histone Demethylase Jumonji D3 (JMJD3) in the Embryonic Stages of Lung Development. PLoS Genet. 2014, 10, e1004524. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Chen, L.; Zhang, X.; Zhou, Q.; Li, J.-M.; Berger, S.; Borok, Z.; Zhou, B.; Xiao, Z.; Yin, H.; et al. Aqp5 Is a New Transcriptional Target of Dot1a and a Regulator of Aqp2. PLoS ONE 2013, 8, e53342. [Google Scholar] [CrossRef]
- Nielsen, J.; Kwon, T.-H.; Praetorius, J.; Frøkiaer, J.; Knepper, M.A.; Nielsen, S. Aldosterone Increases Urine Production and Decreases Apical AQP2 Expression in Rats with Diabetes Insipidus. Am. J. Physiol. Renal. Physiol. 2006, 290, F438–F449. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Chen, M.; Zhang, Y.; Peng, P.; Li, J.; Xin, X. MiR-96 and MiR-330 Overexpressed and Targeted AQP5 in Lipopolysaccharide-Induced Rat Lung Damage of Disseminated Intravascular Coagulation. Blood Coagul. Fibrinolysis 2014, 25, 731–737. [Google Scholar] [CrossRef]
- Jin, Y.; Yu, G.; Peng, P.; Zhang, Y.; Xin, X. Down-Regulated Expression of AQP5 on Lung in Rat DIC Model Induced by LPS and Its Effect on the Development of Pulmonary Edema. Pulm. Pharmacol. Ther. 2013, 26, 661–665. [Google Scholar] [CrossRef]
- Li, Z.; Ma, L.; Di, L.; Lin, X. MicroRNA-1271-5p Alleviates the Malignant Development of Hepatitis B Virus-mediated Liver Cancer via Binding to AQP5. Mol. Med. Rep. 2021, 23, 386. [Google Scholar] [CrossRef]
- Zhou, C.; Kong, W.; Ju, T.; Xie, Q.; Zhai, L. MiR-185-3p Mimic Promotes the Chemosensitivity of CRC Cells via AQP5. Cancer Biol. Ther. 2020, 21, 790–798. [Google Scholar] [CrossRef]
- Park, E.-J.; Jung, H.J.; Choi, H.-J.; Jang, H.-J.; Park, H.-J.; Nejsum, L.N.; Kwon, T.-H. Exosomes Co-Expressing AQP5-Targeting MiRNAs and IL-4 Receptor-Binding Peptide Inhibit the Migration of Human Breast Cancer Cells. FASEB J. 2020, 34, 3379–3398. [Google Scholar] [CrossRef]
- Yao, Q.; Ke, H.-J.; Yang, Q.; Liao, G.-Y.; Liu, P. Study on the Mechanism of MicroRNA551b-5p in Severe Acute Pancreatitis Capillary Leakage Syndrome. Dis. Markers 2022, 2022, 6373757. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Iida, H.; Ishida, H. The Muscarinic Acetylcholine Receptor-Stimulated Increase in Aquaporin-5 Levels in the Apical Plasma Membrane in Rat Parotid Acinar Cells Is Coupled with Activation of Nitric Oxide/CGMP Signal Transduction. Mol. Pharmacol. 2002, 61, 1423–1434. [Google Scholar] [CrossRef] [Green Version]
- Kitchen, P.; Öberg, F.; Sjöhamn, J.; Hedfalk, K.; Bill, R.M.; Conner, A.C.; Conner, M.T.; Törnroth-Horsefield, S. Plasma Membrane Abundance of Human Aquaporin 5 Is Dynamically Regulated by Multiple Pathways. PLoS ONE 2015, 10, e0143027. [Google Scholar] [CrossRef]
- Wang, Z.; Schey, K.L. Proteomic Analysis of S-Palmitoylated Proteins in Ocular Lens Reveals Palmitoylation of AQP5 and MP20. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5648–5658. [Google Scholar] [CrossRef] [Green Version]
- Blaskovic, S.; Blanc, M.; van der Goot, F.G. What Does S-Palmitoylation Do to Membrane Proteins? FEBS J 2013, 280, 2766–2774. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Schey, K.L. Proteomic Analysis of Lipid Raft-Like Detergent-Resistant Membranes of Lens Fiber Cells. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8349–8360. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, Y.; Yuan, Z.; Inoue, N.; Skowronski, M.T.; Nakae, Y.; Shono, M.; Cho, G.; Yasui, M.; Agre, P.; Nielsen, S. Identification of AQP5 in Lipid Rafts and Its Translocation to Apical Membranes by Activation of M3 MAChRs in Interlobular Ducts of Rat Parotid Gland. Am. J. Physiol. Cell Physiol. 2005, 289, C1303–C1311. [Google Scholar] [CrossRef]
- Garfias, Y.; Navas, A.; Pérez-Cano, H.J.; Quevedo, J.; Villalvazo, L.; Zenteno, J.C. Comparative Expression Analysis of Aquaporin-5 (AQP5) in Keratoconic and Healthy Corneas. Mol. Vis. 2008, 14, 756–761. [Google Scholar]
- Barandika, O.; Ezquerra-Inchausti, M.; Anasagasti, A.; Vallejo-Illarramendi, A.; Llarena, I.; Bascaran, L.; Alberdi, T.; De Benedetti, G.; Mendicute, J.; Ruiz-Ederra, J. Increased Aquaporin 1 and 5 Membrane Expression in the Lens Epithelium of Cataract Patients. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 2015–2021. [Google Scholar] [CrossRef]
- Satoh, K.; Narita, T.; Matsuki-Fukushima, M.; Okabayashi, K.; Ito, T.; Senpuku, H.; Sugiya, H. E2f1-Deficient NOD/SCID Mice Have Dry Mouth Due to a Change of Acinar/Duct Structure and the down-Regulation of AQP5 in the Salivary Gland. Pflug. Arch. 2013, 465, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Villandre, J.; White, V.; Lear, T.B.; Chen, Y.; Tuncer, F.; Vaiz, E.; Tuncer, B.; Lockwood, K.; Camarco, D.; Liu, Y.; et al. A Repurposed Drug Screen for Compounds Regulating Aquaporin 5 Stability in Lung Epithelial Cells. Front. Pharmacol. 2022, 13, 828643. [Google Scholar] [CrossRef] [PubMed]
- Fenton, R.A.; Murali, S.K.; Moeller, H.B. Advances in Aquaporin-2 Trafficking Mechanisms and Their Implications for Treatment of Water Balance Disorders. Am. J. Physiol. Cell Physiol. 2020, 319, C1–C10. [Google Scholar] [CrossRef] [PubMed]
- Centrone, M.; Ranieri, M.; Di Mise, A.; D’Agostino, M.; Venneri, M.; Ferrulli, A.; Valenti, G.; Tamma, G. AQP2 Trafficking in Health and Diseases: An Updated Overview. Int. J. Biochem. Cell Biol. 2022, 149, 106261. [Google Scholar] [CrossRef]
- Gresz, V.; Kwon, T.-H.; Gong, H.; Agre, P.; Steward, M.C.; King, L.S.; Nielsen, S. Immunolocalization of AQP-5 in Rat Parotid and Submandibular Salivary Glands after Stimulation or Inhibition of Secretion in Vivo. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G151–G161. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, Y.; Skowronski, M.T.; Inoue, N.; Ishida, H. Alpha(1)-Adrenoceptor-Induced Trafficking of Aquaporin-5 to the Apical Plasma Membrane of Rat Parotid Cells. Biochem. Biophys. Res. Commun. 1999, 265, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Cho, G.; Bragiel, A.M.; Wang, D.; Pieczonka, T.D.; Skowronski, M.T.; Shono, M.; Nielsen, S.; Ishikawa, Y. Activation of Muscarinic Receptors in Rat Parotid Acinar Cells Induces AQP5 Trafficking to Nuclei and Apical Plasma Membrane. Biochim. Biophys. Acta 2015, 1850, 784–793. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Eguchi, T.; Skowronski, M.T.; Ishida, H. Acetylcholine Acts on M3 Muscarinic Receptors and Induces the Translocation of Aquaporin5 Water Channel via Cytosolic Ca2+ Elevation in Rat Parotid Glands. Biochem. Biophys. Res. Commun. 1998, 245, 835–840. [Google Scholar] [CrossRef]
- Bragiel, A.M.; Wang, D.; Pieczonka, T.D.; Shono, M.; Ishikawa, Y. Mechanisms Underlying Activation of A₁-Adrenergic Receptor-Induced Trafficking of AQP5 in Rat Parotid Acinar Cells under Isotonic or Hypotonic Conditions. Int. J. Mol. Sci. 2016, 17, 1022. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.-H.; Park, S.-H.; Moon, Y.W.; Hwang, S.; Kim, D.; Jo, S.-H.; Oh, S.B.; Kim, J.S.; Jahng, J.W.; Lee, J.-H.; et al. Histamine H1 Receptor Induces Cytosolic Calcium Increase and Aquaporin Translocation in Human Salivary Gland Cells. J. Pharmacol. Exp. Ther. 2009, 330, 403–412. [Google Scholar] [CrossRef]
- Koffman, J.S.; Arnspang, E.C.; Marlar, S.; Nejsum, L.N. Opposing Effects of CAMP and T259 Phosphorylation on Plasma Membrane Diffusion of the Water Channel Aquaporin-5 in Madin-Darby Canine Kidney Cells. PLoS ONE 2015, 10, e0133324. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yao, C.; Hasegawa, T.; Akamatsu, T.; Yoshimura, H.; Hosoi, K. Effects of Isoproterenol on Aquaporin 5 Levels in the Parotid Gland of Mice in Vivo. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E100–E108. [Google Scholar] [CrossRef] [PubMed]
- Collaco, A.M.; Jakab, R.L.; Hoekstra, N.E.; Mitchell, K.A.; Brooks, A.; Ameen, N.A. Regulated Traffic of Anion Transporters in Mammalian Brunner’s Glands: A Role for Water and Fluid Transport. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G258–G275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvin, M.N.; Kurabuchi, S.; Murdiastuti, K.; Yao, C.; Kosugi-Tanaka, C.; Akamatsu, T.; Kanamori, N.; Hosoi, K. Subcellular Redistribution of AQP5 by Vasoactive Intestinal Polypeptide in the Brunner’s Gland of the Rat Duodenum. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G1283–G1291. [Google Scholar] [CrossRef]
- Sidhaye, V.; Hoffert, J.D.; King, L.S. CAMP Has Distinct Acute and Chronic Effects on Aquaporin-5 in Lung Epithelial Cells. J. Biol. Chem. 2005, 280, 3590–3596. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.S.; Varadaraj, M.; Yerramilli, V.S.; Menon, A.G.; Varadaraj, K. Spatial Expression of Aquaporin 5 in Mammalian Cornea and Lens, and Regulation of Its Localization by Phosphokinase A. Mol. Vis. 2012, 18, 957–967. [Google Scholar]
- Kosugi-Tanaka, C.; Li, X.; Yao, C.; Akamatsu, T.; Kanamori, N.; Hosoi, K. Protein Kinase A-Regulated Membrane Trafficking of a Green Fluorescent Protein-Aquaporin 5 Chimera in MDCK Cells. Biochim. Biophys. Acta 2006, 1763, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.; Chae, Y.K.; Jang, S.J.; Kim, M.S.; Baek, J.H.; Park, J.C.; Trink, B.; Ratovitski, E.; Lee, T.; Park, B.; et al. Membrane Trafficking of AQP5 and CAMP Dependent Phosphorylation in Bronchial Epithelium. Biochem. Biophys. Res. Commun. 2008, 366, 321–327. [Google Scholar] [CrossRef]
- Woo, J.; Lee, J.; Chae, Y.K.; Kim, M.S.; Baek, J.H.; Park, J.C.; Park, M.J.; Smith, I.M.; Trink, B.; Ratovitski, E.; et al. Overexpression of AQP5, a Putative Oncogene, Promotes Cell Growth and Transformation. Cancer Lett. 2008, 264, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Karabasil, M.R.; Hasegawa, T.; Azlina, A.; Purwanti, N.; Purevjav, J.; Yao, C.; Akamatsu, T.; Hosoi, K. Trafficking of GFP-AQP5 Chimeric Proteins Conferred with Unphosphorylated Amino Acids at Their PKA-Target Motif ((152)SRRTS) in MDCK-II Cells. J. Med. Investig. 2009, 56, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, T.; Azlina, A.; Javkhlan, P.; Yao, C.; Akamatsu, T.; Hosoi, K. Novel Phosphorylation of Aquaporin-5 at Its Threonine 259 through CAMP Signaling in Salivary Gland Cells. Am. J. Physiol. Cell Physiol. 2011, 301, C667–C678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellner, R.B.; Cotrim, A.P.; Hong, S.; Swaim, W.D.; Baum, B.J. Localization of AQP5/AQP8 Chimeras in MDCK-II Cells: Exchange of the N- and C-Termini. Biochem. Biophys. Res. Commun. 2005, 330, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Wellner, R.B.; Hong, S.; Cotrim, A.P.; Swaim, W.D.; Baum, B.J. Modifying the NH2 and COOH Termini of Aquaporin-5: Effects on Localization in Polarized Epithelial Cells. Tissue Eng. 2005, 11, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Muroi, S.-I.; Isohama, Y. C-Terminal Domain of Aquaporin-5 Is Required to Pass Its Protein Quality Control and Ensure Its Trafficking to Plasma Membrane. Int. J. Mol. Sci. 2021, 22, 13461. [Google Scholar] [CrossRef]
- Roche, J.V.; Törnroth-Horsefield, S. Aquaporin Protein-Protein Interactions. Int. J. Mol. Sci. 2017, 18, 2255. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.; Kang, J.Y.; Kim, M.J.; Shin, D.M.; Hong, J.H. Carbonic Anhydrase 12 Mutation Modulates Membrane Stability and Volume Regulation of Aquaporin 5. J. Enzym. Inhib. Med. Chem. 2019, 34, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Bandyopadhyay, B.C.; Bandyopadhyay, B.; Nakamoto, T.; Singh, B.; Liedtke, W.; Melvin, J.E.; Ambudkar, I. A Role for AQP5 in Activation of TRPV4 by Hypotonicity: Concerted Involvement of AQP5 and TRPV4 in Regulation of Cell Volume Recovery. J. Biol. Chem. 2006, 281, 15485–15495. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, D.; Yu, L.; Wang, M. Expression Patterns of Conjunctival Mucin 5AC and Aquaporin 5 in Response to Acute Dry Eye Stress. PLoS ONE 2017, 12, e0187188. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, Y.; Tsuzaka, K.; Takeuchi, T.; Sasaki, Y.; Tsubota, K. Altered Distribution of Aquaporin 5 and Its C-Terminal Binding Protein in the Lacrimal Glands of a Mouse Model for Sjögren’s Syndrome. Curr. Eye Res. 2008, 33, 621–629. [Google Scholar] [CrossRef]
- Chivasso, C.; Nesverova, V.; Järvå, M.; Blanchard, A.; Rose, K.L.; Öberg, F.K.; Wang, Z.; Martin, M.; Lhotellerie, F.; Zindy, E.; et al. Unraveling Human AQP5-PIP Molecular Interaction and Effect on AQP5 Salivary Glands Localization in SS Patients. Cells 2021, 10, 2108. [Google Scholar] [CrossRef]
- Chivasso, C.; Hagströmer, C.J.; Rose, K.L.; Lhotellerie, F.; Leblanc, L.; Wang, Z.; Moscato, S.; Chevalier, C.; Zindy, E.; Martin, M.; et al. Ezrin Is a Novel Protein Partner of Aquaporin-5 in Human Salivary Glands and Shows Altered Expression and Cellular Localization in Sjögren’s Syndrome. Int. J. Mol. Sci. 2021, 22, 9213. [Google Scholar] [CrossRef] [PubMed]
- Muroi, S.-I.; Isohama, Y. Ezrin Regulates Ca2+ Ionophore-Induced Plasma Membrane Translocation of Aquaporin-5. Int. J. Mol. Sci. 2021, 22, 13505. [Google Scholar] [CrossRef] [PubMed]
- Tada, J.; Sawa, T.; Yamanaka, N.; Shono, M.; Akamatsu, T.; Tsumura, K.; Parvin, M.N.; Kanamori, N.; Hosoi, K. Involvement of Vesicle-Cytoskeleton Interaction in AQP5 Trafficking in AQP5-Gene-Transfected HSG Cells. Biochem. Biophys. Res. Commun. 1999, 266, 443–447. [Google Scholar] [CrossRef]
- Gletten, R.B.; Cantrell, L.S.; Bhattacharya, S.; Schey, K.L. Lens Aquaporin-5 Inserts Into Bovine Fiber Cell Plasma Membranes Via Unconventional Protein Secretion. Investig. Ophthalmol. Vis. Sci. 2022, 63, 5. [Google Scholar] [CrossRef] [PubMed]
- Gee, H.Y.; Kim, J.; Lee, M.G. Unconventional Secretion of Transmembrane Proteins. Semin. Cell Dev. Biol. 2018, 83, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Chiaradia, E.; Urbanelli, L.; Emiliani, C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int. J. Mol. Sci. 2020, 21, 2576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrova, R.S.; Bavana, N.; Zhao, R.; Schey, K.L.; Donaldson, P.J. Changes to Zonular Tension Alters the Subcellular Distribution of AQP5 in Regions of Influx and Efflux of Water in the Rat Lens. Investig. Ophthalmol. Vis. Sci. 2020, 61, 36. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, X.; Mao, Q.; Zhang, Y.; Cong, X.; Zhang, X.; Zhang, Z.; Wu, L.; Xiang, R.; Yu, G. Aquaporin 5 Is Degraded by Autophagy in Diabetic Submandibular Gland. Sci. China Life Sci. 2018, 61, 1049–1059. [Google Scholar] [CrossRef]
- Azlina, A.; Javkhlan, P.; Hiroshima, Y.; Hasegawa, T.; Yao, C.; Akamatsu, T.; Hosoi, K. Roles of Lysosomal Proteolytic Systems in AQP5 Degradation in the Submandibular Gland of Rats Following Chorda Tympani Parasympathetic Denervation. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 299, G1106–G1117. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Azlina, A.; Karabasil, M.R.; Purwanti, N.; Hasegawa, T.; Yao, C.; Akamatsu, T.; Hosoi, K. Degradation of Submandibular Gland AQP5 by Parasympathetic Denervation of Chorda Tympani and Its Recovery by Cevimeline, an M3 Muscarinic Receptor Agonist. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 295, G112–G123. [Google Scholar] [CrossRef] [Green Version]
- Kudou, M.; Shiozaki, A.; Kosuga, T.; Shimizu, H.; Ichikawa, D.; Konishi, H.; Morimura, R.; Komatsu, S.; Ikoma, H.; Fujiwara, H.; et al. Heat Shock Exerts Anticancer Effects on Liver Cancer via Autophagic Degradation of Aquaporin 5. Int. J. Oncol. 2017, 50, 1857–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ono Minagi, H.; Sarper, S.E.; Kurosaka, H.; Kuremoto, K.-I.; Taniuchi, I.; Sakai, T.; Yamashiro, T. Runx1 Mediates the Development of the Granular Convoluted Tubules in the Submandibular Glands. PLoS ONE 2017, 12, e0184395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Huo, D.; Zhu, H.; Xu, Q.; Gao, C.; Chen, W.; Zhang, Y. Deciphering the Structure, Function, Expression and Regulation of Aquaporin-5 in Cancer Evolution. Oncol. Lett. 2021, 21, 309. [Google Scholar] [CrossRef] [PubMed]
- Dajani, S.; Saripalli, A.; Sharma-Walia, N. Water Transport Proteins-Aquaporins (AQPs) in Cancer Biology. Oncotarget 2018, 9, 36392–36405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, H.H.; Login, F.H.; Park, J.-Y.; Kwon, T.-H.; Nejsum, L.N. Immunohistochemical Evalulation of Activated Ras and Rac1 as Potential Downstream Effectors of Aquaporin-5 in Breast Cancer in Vivo. Biochem. Biophys. Res. Commun. 2017, 493, 1210–1216. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.J.; Park, J.-Y.; Jeon, H.-S.; Kwon, T.-H. Aquaporin-5: A Marker Protein for Proliferation and Migration of Human Breast Cancer Cells. PLoS ONE 2011, 6, e28492. [Google Scholar] [CrossRef] [Green Version]
- Chae, Y.K.; Woo, J.; Kim, M.-J.; Kang, S.K.; Kim, M.S.; Lee, J.; Lee, S.K.; Gong, G.; Kim, Y.H.; Soria, J.C.; et al. Expression of Aquaporin 5 (AQP5) Promotes Tumor Invasion in Human Non Small Cell Lung Cancer. PLoS ONE 2008, 3, e2162. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Z.; Song, Y.; Zhang, P.; Hu, J.; Bai, C. Expression of Aquaporin 5 Increases Proliferation and Metastasis Potential of Lung Cancer. J. Pathol. 2010, 221, 210–220. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Chong, T.; Chen, H.; Li, H.; Li, G.; Zhai, X.; Li, Y. Over-Expression of a Poor Prognostic Marker in Prostate Cancer: AQP5 Promotes Cells Growth and Local Invasion. World J. Surg. Oncol. 2014, 12, 284. [Google Scholar] [CrossRef] [Green Version]
- Pust, A.; Kylies, D.; Hube-Magg, C.; Kluth, M.; Minner, S.; Koop, C.; Grob, T.; Graefen, M.; Salomon, G.; Tsourlakis, M.C.; et al. Aquaporin 5 Expression Is Frequent in Prostate Cancer and Shows a Dichotomous Correlation with Tumor Phenotype and PSA Recurrence. Hum. Pathol. 2016, 48, 102–110. [Google Scholar] [CrossRef]
- Song, T.; Yang, H.; Ho, J.C.M.; Tang, S.C.W.; Sze, S.C.W.; Lao, L.; Wang, Y.; Zhang, K.Y. Expression of Aquaporin 5 in Primary Carcinoma and Lymph Node Metastatic Carcinoma of Non-Small Cell Lung Cancer. Oncol. Lett. 2015, 9, 2799–2804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.K.; Chae, Y.K.; Woo, J.; Kim, M.S.; Park, J.C.; Lee, J.; Soria, J.C.; Jang, S.J.; Sidransky, D.; Moon, C. Role of Human Aquaporin 5 in Colorectal Carcinogenesis. Am. J. Pathol. 2008, 173, 518–525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Zhao, C.; Chen, D.; Zhou, Z. Overexpression of AQP5 in Cervical Cancer: Correlation with Clinicopathological Features and Prognosis. Med. Oncol. 2012, 29, 1998–2004. [Google Scholar] [CrossRef] [PubMed]
- Jensen, H.H.; Holst, M.R.; Login, F.H.; Morgen, J.J.; Nejsum, L.N. Ectopic Expression of Aquaporin-5 in Noncancerous Epithelial MDCK Cells Changes Cellular Morphology and Actin Fiber Formation without Inducing Epithelial-to-Mesenchymal Transition. Am. J. Physiol.-Cell Physiol. 2018, 314, C654–C661. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.; Lee, J.; Kim, M.S.; Jang, S.J.; Sidransky, D.; Moon, C. The Effect of Aquaporin 5 Overexpression on the Ras Signaling Pathway. Biochem. Biophys. Res. Commun. 2008, 367, 291–298. [Google Scholar] [CrossRef]
- Direito, I.; Madeira, A.; Brito, M.A.; Soveral, G. Aquaporin-5: From Structure to Function and Dysfunction in Cancer. Cell. Mol. Life Sci. 2016, 73, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Hein, A.L.; Post, C.M.; Sheinin, Y.M.; Lakshmanan, I.; Natarajan, A.; Enke, C.A.; Batra, S.K.; Ouellette, M.M.; Yan, Y. RAC1 GTPase Promotes the Survival of Breast Cancer Cells in Response to Hyper-Fractionated Radiation Treatment. Oncogene 2016, 35, 6319–6329. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Zhang, J.-N.; Chen, W.-L.; Wang, G.-S.; Mao, Q.; Li, S.-Q.; Xiong, W.-H.; Lin, Y.-Y.; Ge, J.-W.; Li, X.-X.; et al. Effects of AQP5 Gene Silencing on Proliferation, Migration and Apoptosis of Human Glioma Cells through Regulating EGFR/ERK/ P38 MAPK Signaling Pathway. Oncotarget 2017, 8, 38444–38455. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, Y.; Hisatsune, A.; Katsuki, H.; Horie, I.; Isohama, Y. Aquaporin 5 Increases Keratinocyte-Derived Chemokine Expression and NF-ΚB Activity through ERK Activation. Biochem. Biophys. Res. Commun. 2014, 448, 355–360. [Google Scholar] [CrossRef]
- Wang, W.; Li, Q.; Yang, T.; Li, D.; Ding, F.; Sun, H.; Bai, G. RNA Interference-Mediated Silencing of Aquaporin (AQP)-5 Hinders Angiogenesis of Colorectal Tumor by Suppressing the Production of Vascular Endothelial Growth Factor. Neoplasma 2018, 65, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Li, Q.; Yang, T.; Li, D.; Ding, F.; Sun, H.; Bai, G. Anti-Cancer Effect of Aquaporin 5 Silencing in Colorectal Cancer Cells in Association with Inhibition of Wnt/β-Catenin Pathway. Cytotechnology 2018, 70, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ma, T.; Zhang, C.; Zhang, H.; Bai, L.; Kong, L.; Luo, J. Down-Regulation of Aquaporin 5-Mediated Epithelial-Mesenchymal Transition and Anti-Metastatic Effect by Natural Product Cairicoside E in Colorectal Cancer. Mol. Carcinog. 2017, 56, 2692–2705. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Cui, X.; Li, W.; Lin, W.; Li, Y. AQP5: A Novel Biomarker That Predicts Poor Clinical Outcome in Colorectal Cancer. Oncol. Rep. 2014, 32, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yang, T.; Li, D.; Ding, F.; Bai, G.; Wang, W.; Sun, H. Knockdown of Aquaporin-5 Sensitizes Colorectal Cancer Cells to 5-Fluorouracil via Inhibition of the Wnt-β-Catenin Signaling Pathway. Biochem. Cell Biol. 2018, 96, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFβ Pathway for Cancer Therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Di, G.; Wang, Y.; Chong, D.; Cao, X.; Chen, P. Aquaporin 5 Facilitates Corneal Epithelial Wound Healing and Nerve Regeneration by Reactivating Akt Signaling Pathway. Am. J. Pathol. 2021, 191, 1974–1985. [Google Scholar] [CrossRef]
- He, Z.; Dong, W.; Hu, J.; Ren, X. AQP5 Promotes Hepatocellular Carcinoma Metastasis via NF-ΚB-Regulated Epithelial-Mesenchymal Transition. Biochem. Biophys. Res. Commun. 2017, 490, 343–348. [Google Scholar] [CrossRef]
- Jensen, H.H.; Login, F.H.; Koffman, J.S.; Kwon, T.-H.; Nejsum, L.N. The Role of Aquaporin-5 in Cancer Cell Migration: A Potential Active Participant. Int. J. Biochem. Cell Biol. 2016, 79, 271–276. [Google Scholar] [CrossRef]
- Preciado-Patt, L.; Levartowsky, D.; Prass, M.; Hershkoviz, R.; Lider, O.; Fridkin, M. Inhibition of Cell Adhesion to Glycoproteins of the Extracellular Matrix by Peptides Corresponding to Serum Amyloid A. Toward Understanding the Physiological Role of an Enigmatic Protein. Eur. J. Biochem. 1994, 223, 35–42. [Google Scholar] [CrossRef]
- Ortiz, M.A.; Mikhailova, T.; Li, X.; Porter, B.A.; Bah, A.; Kotula, L. Src Family Kinases, Adaptor Proteins and the Actin Cytoskeleton in Epithelial-to-Mesenchymal Transition. Cell Commun. Signal. 2021, 19, 67. [Google Scholar] [CrossRef]
- Ingley, E. Functions of the Lyn Tyrosine Kinase in Health and Disease. Cell Commun. Signal. 2012, 10, 21. [Google Scholar] [CrossRef] [Green Version]
- Giubellino, A.; Burke, T.R.; Bottaro, D.P. Grb2 Signaling in Cell Motility and Cancer. Expert Opin. Ther. Targets 2008, 12, 1021–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, D.M. SRC Family Kinases in Cell Volume Regulation. Am. J. Physiol.-Cell Physiol. 2005, 288, C483–C493. [Google Scholar] [CrossRef] [PubMed]
- Sidhaye, V.K.; Chau, E.; Srivastava, V.; Sirimalle, S.; Balabhadrapatruni, C.; Aggarwal, N.R.; D’Alessio, F.R.; Robinson, D.N.; King, L.S. A Novel Role for Aquaporin-5 in Enhancing Microtubule Organization and Stability. PLoS ONE 2012, 7, e38717. [Google Scholar] [CrossRef]
- Kawedia, J.D.; Nieman, M.L.; Boivin, G.P.; Melvin, J.E.; Kikuchi, K.-I.; Hand, A.R.; Lorenz, J.N.; Menon, A.G. Interaction between Transcellular and Paracellular Water Transport Pathways through Aquaporin 5 and the Tight Junction Complex. Proc. Natl. Acad. Sci. USA 2007, 104, 3621–3626. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Agostino, C.; Parisis, D.; Chivasso, C.; Hajiabbas, M.; Soyfoo, M.S.; Delporte, C. Aquaporin-5 Dynamic Regulation. Int. J. Mol. Sci. 2023, 24, 1889. https://doi.org/10.3390/ijms24031889
D’Agostino C, Parisis D, Chivasso C, Hajiabbas M, Soyfoo MS, Delporte C. Aquaporin-5 Dynamic Regulation. International Journal of Molecular Sciences. 2023; 24(3):1889. https://doi.org/10.3390/ijms24031889
Chicago/Turabian StyleD’Agostino, Claudia, Dorian Parisis, Clara Chivasso, Maryam Hajiabbas, Muhammad Shahnawaz Soyfoo, and Christine Delporte. 2023. "Aquaporin-5 Dynamic Regulation" International Journal of Molecular Sciences 24, no. 3: 1889. https://doi.org/10.3390/ijms24031889