Therapeutic Potential of Bioactive Components from Scutellaria baicalensis Georgi in Inflammatory Bowel Disease and Colorectal Cancer: A Review
Abstract
1. Introduction
2. IBD
2.1. CD and UC
2.2. Onset of CRC in Patients with IBD
2.3. IBD Treatment
2.4. Experimental Models of IBD
2.4.1. In Vitro and Ex Vivo IBD Models
2.4.2. In Vivo IBD Models
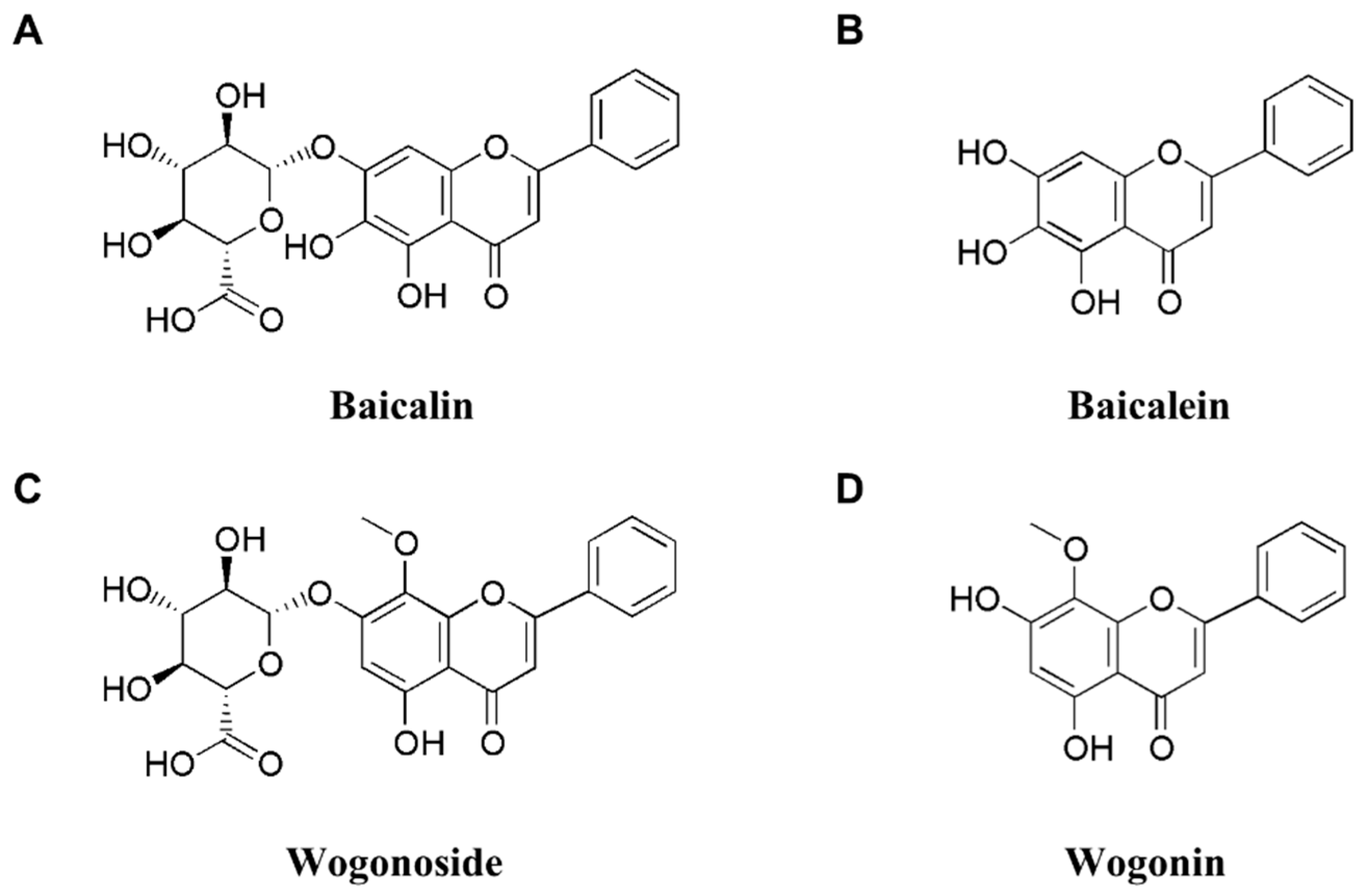
3. SBG
4. Therapeutic Benefits of SBG
4.1. Effect of SBG on IBD
4.1.1. Effect of SBG on COX-2
4.1.2. Effect of SBG on TLR4/NF-κB
4.2. Effect of SBG on CRC
4.2.1. Effect of SBG on Apoptosis in CRC
4.2.2. Effect of SBG on PI3K/AKT/mTOR in CRC
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, X.; Xie, L.; Long, J.; Liu, K.; Lu, J.; Liang, Y.; Cao, Y.; Dai, X.; Li, X. Therapeutic effect of baicalin on inflammatory bowel disease: A review. J. Ethnopharmacol. 2022, 283, 114749. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.; Soni, A.; Acharya, S. In vitro models and ex vivo systems used in inflammatory bowel disease. Vitr. Model. 2022, 1, 213–227. [Google Scholar] [CrossRef]
- Guan, Q. A Comprehensive review and update on the pathogenesis of inflammatory bowel disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L.; et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Hanauer, S.B. Inflammatory bowel disease: Epidemiology, pathogenesis, and therapeutic opportunities. Inflamm. Bowel. Dis. 2006, 12 (Suppl. 1), S3–S9. [Google Scholar] [CrossRef]
- The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: A systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 17–30. [CrossRef]
- Rajamäki, K.; Taira, A.; Katainen, R.; Välimäki, N.; Kuosmanen, A.; Plaketti, R.M.; Seppälä, T.T.; Ahtiainen, M.; Wirta, E.V.; Vartiainen, E.; et al. Genetic and epigenetic characteristics of inflammatory bowel disease-associated colorectal cancer. Gastroenterology 2021, 161, 592–607. [Google Scholar] [CrossRef]
- Nadeem, M.S.; Kumar, V.; Al-Abbasi, F.A.; Kamal, M.A.; Anwar, F. Risk of colorectal cancer in inflammatory bowel diseases. Semin. Cancer Biol. 2020, 64, 51–60. [Google Scholar] [CrossRef]
- Stidham, R.W.; Higgins, P.D. Colorectal cancer in inflammatory bowel disease. Clin. Colon Rectal Surg. 2018, 31, 168–178. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Jung, K.W.; Won, Y.J.; Kang, M.J.; Kong, H.J.; Im, J.S.; Seo, H.G. Prediction of cancer incidence and mortality in Korea, 2022. Cancer Res. Treat. 2022, 54, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.J.; Jang, J.Y.; Kwon, Y.H.; Lee, J.H.; Lee, S.; Park, Y.; Jung, Y.S.; Im, E.; Moon, H.R.; Chung, H.Y.; et al. MHY2245, a Sirtuin inhibitor, induces cell cycle arrest and apoptosis in HCT116 human colorectal cancer cells. Int. J. Mol. Sci. 2022, 23, 1590. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, Z.; Guo, J.; Li, W.; You, F. Effect of Chinese herbal medicine formula on progression-free survival among patients with metastatic colorectal cancer: Study protocol for a multi-center, double-blinded, randomized, placebo-controlled trial. PLoS One 2022, 17, e0275058. [Google Scholar] [CrossRef]
- Gao, Y.; Snyder, S.A.; Smith, J.N.; Chen, Y.C. Anticancer properties of baicalein: A review. Med. Chem. Res. 2016, 25, 1515–1523. [Google Scholar] [CrossRef]
- Xiang, L.; Gao, Y.; Chen, S.; Sun, J.; Wu, J.; Meng, X. Therapeutic potential of Scutellaria baicalensis Georgi in lung cancer therapy. Phytomedicine 2022, 95, 153727. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Fu, L.; Wang, P.; Yang, L.; Zhu, X.; Li, C.G. Drug-herb interactions between Scutellaria baicalensis and pharmaceutical drugs: Insights from experimental studies, mechanistic actions to clinical applications. Biomed. Pharmacother. 2021, 138, 111445. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.J.; Zhang, Z.Y.; Hu, J.; Guo, L.P.; Shao, A.J.; Huang, L.Q. Impacts of recent cultivation on genetic diversity pattern of a medicinal plant, Scutellaria baicalensis (Lamiaceae). BMC Genet 2010, 11, 29. [Google Scholar] [CrossRef]
- Wang, Z.L.; Wang, S.; Kuang, Y.; Hu, Z.M.; Qiao, X.; Ye, M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm. Biol. 2018, 56, 465–484. [Google Scholar] [CrossRef]
- Zhao, T.; Tang, H.; Xie, L.; Zheng, Y.; Ma, Z.; Sun, Q.; Li, X. Scutellaria baicalensis Georgi. (Lamiaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 2019, 71, 1353–1369. [Google Scholar] [CrossRef]
- Seyedian, S.S.; Nokhostin, F.; Malamir, M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life 2019, 12, 113–122. [Google Scholar] [CrossRef]
- Ungaro, R.; Mehandru, S.; Allen, P.B.; Peyrin-Biroulet, L.; Colombel, J.F. Ulcerative colitis. Lancet 2017, 389, 1756–1770. [Google Scholar] [CrossRef] [PubMed]
- Ocansey, D.K.W.; Zhang, L.; Wang, Y.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. Exosome-mediated effects and applications in inflammatory bowel disease. Biol. Rev. Camb. Philos. Soc. 2020, 95, 1287–1307. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Saikam, V.; Skrada, K.A.; Merlin, D.; Iyer, S.S. Inflammatory bowel disease biomarkers. Med. Res. Rev. 2022, 42, 1856–1887. [Google Scholar] [CrossRef] [PubMed]
- Høivik, M.L.; Moum, B.; Solberg, I.C.; Henriksen, M.; Cvancarova, M.; Bernklev, T. Work disability in inflammatory bowel disease patients 10 years after disease onset: Results from the IBSEN Study. Gut 2013, 62, 368–375. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Blanchard, J.F.; Rawsthorne, P.; Yu, N. The prevalence of extraintestinal diseases in inflammatory bowel disease: A population-based study. Am. J. Gastroenterol. 2001, 96, 1116–1122. [Google Scholar] [CrossRef]
- Kim, E.R.; Chang, D.K. Colorectal cancer in inflammatory bowel disease: The risk, pathogenesis, prevention and diagnosis. World J. Gastroenterol. 2014, 20, 9872–9881. [Google Scholar] [CrossRef]
- Porter, R.J.; Arends, M.J.; Churchhouse, A.M.D.; Din, S. Inflammatory bowel disease-associated colorectal cancer: Translational risks from mechanisms to medicines. J. Crohns Colitis 2021, 15, 2131–2141. [Google Scholar] [CrossRef]
- Lucafò, M.; Curci, D.; Franzin, M.; Decorti, G.; Stocco, G. Inflammatory bowel disease and risk of colorectal cancer: An overview from pathophysiology to pharmacological prevention. Front. Pharmacol. 2021, 12, 772101. [Google Scholar] [CrossRef]
- Kameyama, H.; Nagahashi, M.; Shimada, Y.; Tajima, Y.; Ichikawa, H.; Nakano, M.; Sakata, J.; Kobayashi, T.; Narayanan, S.; Takabe, K.; et al. Genomic characterization of colitis-associated colorectal cancer. World J. Surg. Oncol. 2018, 16, 121. [Google Scholar] [CrossRef]
- Potack, J.; Itzkowitz, S.H. Colorectal cancer in inflammatory bowel disease. Gut Liver 2008, 2, 61–73. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, S.; Li, J. Treatment of inflammatory bowel disease: A comprehensive review. Front. Med. 2021, 8, 765474. [Google Scholar] [CrossRef] [PubMed]
- Na, S.Y.; Moon, W. Perspectives on current and novel treatments for inflammatory bowel disease. Gut Liver 2019, 13, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Rangel, E.B. Tacrolimus in pancreas transplant: A focus on toxicity, diabetogenic effect and drug-drug interactions. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1585–1605. [Google Scholar] [CrossRef] [PubMed]
- Reinisch, W.; Sandborn, W.J.; Hommes, D.W.; D’Haens, G.; Hanauer, S.; Schreiber, S.; Panaccione, R.; Fedorak, R.N.; Tighe, M.B.; Huang, B.; et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: Results of a randomised controlled trial. Gut 2011, 60, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Sandborn, W.J.; van Assche, G.; Reinisch, W.; Colombel, J.F.; D’Haens, G.; Wolf, D.C.; Kron, M.; Tighe, M.B.; Lazar, A.; Thakkar, R.B. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012, 142, e42. [Google Scholar] [CrossRef] [PubMed]
- Rutgeerts, P.; Sandborn, W.J.; Feagan, B.G.; Reinisch, W.; Olson, A.; Johanns, J.; Travers, S.; Rachmilewitz, D.; Hanauer, S.B.; Lichtenstein, G.R.; et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2005, 353, 2462–2476. [Google Scholar] [CrossRef]
- Targan, S.R.; Hanauer, S.B.; van Deventer, S.J.; Mayer, L.; Present, D.H.; Braakman, T.; DeWoody, K.L.; Schaible, T.F.; Rutgeerts, P.J. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s disease cA2 study group. N. Engl. J. Med. 1997, 337, 1029–1035. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Sandborn, W.J.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.; Panaccione, R.; Wolf, D.; Pollack, P. Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: The CLASSIC-I trial. Gastroenterology 2006, 130, 323–333; quiz 591. [Google Scholar] [CrossRef]
- Schreiber, S.; Khaliq-Kareemi, M.; Lawrance, I.C.; Thomsen, O.; Hanauer, S.B.; McColm, J.; Bloomfield, R.; Sandborn, W.J. Maintenance therapy with certolizumab pegol for Crohn’s disease. N. Engl. J. Med. 2007, 357, 239–250. [Google Scholar] [CrossRef]
- Sandborn, W.J.; Hanauer, S.B.; Rutgeerts, P.; Fedorak, R.N.; Lukas, M.; MacIntosh, D.G.; Panaccione, R.; Wolf, D.; Kent, J.D.; Bittle, B.; et al. Adalimumab for maintenance treatment of Crohn’s disease: Results of the CLASSIC II trial. Gut 2007, 56, 1232–1239. [Google Scholar] [CrossRef]
- Hanauer, S.B.; Feagan, B.G.; Lichtenstein, G.R.; Mayer, L.F.; Schreiber, S.; Colombel, J.F.; Rachmilewitz, D.; Wolf, D.C.; Olson, A.; Bao, W.; et al. Maintenance infliximab for Crohn’s disease: The ACCENT I randomised trial. Lancet 2002, 359, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Allez, M.; Karmiris, K.; Louis, E.; Van Assche, G.; Ben-Horin, S.; Klein, A.; Van der Woude, J.; Baert, F.; Eliakim, R.; Katsanos, K.; et al. Report of the ECCO pathogenesis workshop on anti-TNF therapy failures in inflammatory bowel diseases: Definitions, frequency and pharmacological aspects. J. Crohns Colitis 2010, 4, 355–366. [Google Scholar] [CrossRef]
- D’Haens, G.R.; Panaccione, R.; Higgins, P.D.; Vermeire, S.; Gassull, M.; Chowers, Y.; Hanauer, S.B.; Herfarth, H.; Hommes, D.W.; Kamm, M.; et al. The London position statement of the world congress of gastroenterology on biological therapy for IBD with the European Crohn’s and colitis organization: When to start, when to stop, which drug to choose, and how to predict response? Am. J. Gastroenterol. 2011, 106, 199–212; quiz 213. [Google Scholar] [CrossRef]
- Ben-Horin, S.; Chowers, Y. Review article: Loss of response to anti-TNF treatments in Crohn’s disease. Aliment. Pharmacol. Ther. 2011, 33, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Toruner, M.; Loftus, E.V., Jr.; Harmsen, W.S.; Zinsmeister, A.R.; Orenstein, R.; Sandborn, W.J.; Colombel, J.F.; Egan, L.J. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology 2008, 134, 929–936. [Google Scholar] [CrossRef]
- Andersen, N.N.; Jess, T. Risk of infections associated with biological treatment in inflammatory bowel disease. World J. Gastroenterol. 2014, 20, 16014–16019. [Google Scholar] [CrossRef]
- Siegel, C.A.; Marden, S.M.; Persing, S.M.; Larson, R.J.; Sands, B.E. Risk of lymphoma associated with combination anti-tumor necrosis factor and immunomodulator therapy for the treatment of Crohn’s disease: A meta-analysis. Clin. Gastroenterol. Hepatol. 2009, 7, 874–881. [Google Scholar] [CrossRef]
- Lea, T. Caco-2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 103–111. [Google Scholar]
- Lozoya-Agullo, I.; Araújo, F.; González-Álvarez, I.; Merino-Sanjuán, M.; González-Álvarez, M.; Bermejo, M.; Sarmento, B. Usefulness of Caco-2/HT29-MTX and Caco-2/HT29-MTX/Raji B coculture models to predict intestinal and colonic permeability compared to Caco-2 monoculture. Mol. Pharm. 2017, 14, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Borgonetti, V.; Cocetta, V.; Biagi, M.; Carnevali, I.; Governa, P.; Montopoli, M. Anti-inflammatory activity of a fixed combination of probiotics and herbal extract in an in-vitro model of intestinal inflammation by stimulating Caco-2 cells with LPS-conditioned THP-1 cells medium. Minerva Pediatr. 2022, 74, 511–518. [Google Scholar] [CrossRef]
- Andrews, C.; McLean, M.H.; Durum, S.K. Cytokine tuning of intestinal epithelial function. Front. Immunol. 2018, 9, 1270. [Google Scholar] [CrossRef] [PubMed]
- Weber, L.; Kuck, K.; Jürgenliemk, G.; Heilmann, J.; Lipowicz, B.; Vissiennon, C. Anti-inflammatory and barrier-stabilising effects of myrrh, coffee charcoal and chamomile flower extract in a co-culture cell model of the intestinal mucosa. Biomolecules 2020, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Lanevskij, K.; Didziapetris, R. Physicochemical QSAR analysis of passive permeability across Caco-2 monolayers. J. Pharm. Sci. 2019, 108, 78–86. [Google Scholar] [CrossRef]
- Jarc, T.; Novak, M.; Hevir, N.; Rižner, T.L.; Kreft, M.E.; Kristan, K. Demonstrating suitability of the Caco-2 cell model for BCS-based biowaiver according to the recent FDA and ICH harmonised guidelines. J. Pharm. Pharmacol. 2019, 71, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Zhu, H.; Hu, L.; Yu, B.; Zhan, X.; Yuan, Y.; Zhou, P. Characterization of the intestinal absorption of morroniside from Cornus officinalis Sieb. et Zucc via a Caco-2 cell monolayer model. PLoS One 2020, 15, e0227844. [Google Scholar] [CrossRef]
- Zhou, G.; Kong, W.S.; Li, Z.C.; Xie, R.F.; Yu, T.Y.; Zhou, X. Effects of qing chang suppository powder and its ingredients on IL-17 signal pathway in HT-29 cells and DSS-induced Mice. Phytomedicine 2021, 87, 153573. [Google Scholar] [CrossRef] [PubMed]
- Zweibaum, A.; Laburthe, M.; Grasset, E.; Louvard, D. Use of cultured cell lines in studies of intestinal cell differentiation and function. Compr. Physiol. 2010, 223–255. [Google Scholar] [CrossRef]
- Adamczak, M.I.; Hagesaether, E.; Smistad, G.; Hiorth, M. An in vitro study of mucoadhesion and biocompatibility of polymer coated liposomes on HT29-MTX mucus-producing cells. Int. J. Pharm. 2016, 498, 225–233. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, L.; Winter, D.C.; Aherne, C.M. The role of organoids as a novel platform for modeling of inflammatory bowel disease. Front. Pediatr. 2021, 9, 624045. [Google Scholar] [CrossRef]
- Nozaki, K.; Mochizuki, W.; Matsumoto, Y.; Matsumoto, T.; Fukuda, M.; Mizutani, T.; Watanabe, M.; Nakamura, T. Co-culture with intestinal epithelial organoids allows efficient expansion and motility analysis of intraepithelial lymphocytes. J. Gastroenterol. 2016, 51, 206–213. [Google Scholar] [CrossRef]
- Noel, G.; Baetz, N.W.; Staab, J.F.; Donowitz, M.; Kovbasnjuk, O.; Pasetti, M.F.; Zachos, N.C. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci. Rep. 2017, 7, 45270. [Google Scholar] [CrossRef]
- Poletti, M.; Arnauts, K.; Ferrante, M.; Korcsmaros, T. Organoid-based models to study the role of host-microbiota interactions in IBD. J. Crohns Colitis 2021, 15, 1222–1235. [Google Scholar] [CrossRef]
- Dutta, D.; Clevers, H. Organoid culture systems to study host-pathogen interactions. Curr. Opin. Immunol. 2017, 48, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kayisoglu, O.; Weiss, F.; Niklas, C.; Pierotti, I.; Pompaiah, M.; Wallaschek, N.; Germer, C.T.; Wiegering, A.; Bartfeld, S. Location-specific cell identity rather than exposure to GI microbiota defines many innate immune signalling cascades in the gut epithelium. Gut 2021, 70, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Ojo, B.A.; VanDussen, K.L.; Rosen, M.J. The promise of patient-derived colon organoids to model Ulcerative Colitis. Inflamm. Bowel. Dis. 2022, 28, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Hong, S.N.; Kim, E.R.; Chang, D.K.; Kim, Y.H. Epithelial regeneration ability of Crohn’s disease assessed using patient-derived intestinal organoids. Int. J. Mol. Sci. 2021, 22, 6013. [Google Scholar] [CrossRef]
- Lesuffleur, T.; Barbat, A.; Dussaulx, E.; Zweibaum, A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990, 50, 6334–6343. [Google Scholar]
- Pan, F.; Han, L.; Zhang, Y.; Yu, Y.; Liu, J. Optimization of Caco-2 and HT29 co-culture in vitro cell models for permeability studies. Int.J. Food Sci. Nutr. 2015, 66, 680–685. [Google Scholar] [CrossRef]
- Kleiveland, C.R. Co-cultivation of Caco-2 and HT-29MTX. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; Chapter 13; p. 135. [Google Scholar]
- Ponce de León-Rodríguez, M.D.C.; Guyot, J.P.; Laurent-Babot, C. Intestinal in vitro cell culture models and their potential to study the effect of food components on intestinal inflammation. Crit. Rev. Food Sci. Nutr. 2019, 59, 3648–3666. [Google Scholar] [CrossRef]
- Sawasvirojwong, S.; Kittayaruksakul, S. Constitutive androstane receptor inhibits Ca2+-dependent Cl− secretion in intestinal epithelial cells. J. Physiol. 2019, 32, 47–52. [Google Scholar]
- Baxter, E.W.; Graham, A.E.; Re, N.A.; Carr, I.M.; Robinson, J.I.; Mackie, S.L.; Morgan, A.W. Standardized protocols for differentiation of THP-1 cells to macrophages with distinct M(IFNγ+LPS), M(IL-4) and M(IL-10) phenotypes. J. Immunol. Methods 2020, 478, 112721. [Google Scholar] [CrossRef]
- Zhen, D.; Xuan, T.Q.; Hu, B.; Bai, X.; Fu, D.N.; Wang, Y.; Wu, Y.; Yang, J.; Ma, Q. Pteryxin attenuates LPS-induced inflammatory responses and inhibits NLRP3 inflammasome activation in RAW264.7 cells. J. Ethnopharmacol. 2022, 284, 114753. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.; Smart, K.; Somerville, M.S.; Lauder, S.N.; Appanna, G.; Horwood, J.; Sunder Raj, L.; Srivastava, B.; Durai, D.; Scurr, M.J.; et al. The Ussing chamber system for measuring intestinal permeability in health and disease. BMC Gastroenterol. 2019, 19, 98. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Cao, W.; Xia, M.; Pan, S.; Xu, X. Transport and interactions of co-incubated bi-functional flavonoids through inhibiting the function of P-Glycoprotein (P-gp) using KB/multidrug-resistant (MDR) cells and rat everted gut sacs. J. Agric. Food Chem. 2022, 70, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Ghiboub, M.; Donkers, J.M.; van de Steeg, E.; van Tol, E.A.F.; Hakvoort, T.B.M.; de Jonge, W.J. The progress of intestinal epithelial models from cell lines to gut-on-chip. Int. J. Mol. Sci. 2021, 22, 13472. [Google Scholar] [CrossRef] [PubMed]
- Beaurivage, C.; Kanapeckaite, A.; Loomans, C.; Erdmann, K.S.; Stallen, J.; Janssen, R.A.J. Development of a human primary gut-on-a-chip to model inflammatory processes. Sci. Rep. 2020, 10, 21475. [Google Scholar] [CrossRef] [PubMed]
- Katsandegwaza, B.; Horsnell, W.; Smith, K. Inflammatory bowel disease: A review of pre-clinical murine models of human Disease. Int. J. Mol. Sci. 2022, 23, 9344. [Google Scholar] [CrossRef]
- Wirtz, S.; Popp, V.; Kindermann, M.; Gerlach, K.; Weigmann, B.; Fichtner-Feigl, S.; Neurath, M.F. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat. Protoc. 2017, 12, 1295–1309. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2015, 104, 15.25.1–15.25.14. [Google Scholar] [CrossRef]
- Singh, K.N.M.; Koli, P.G. Animal models for preclinical drug research on ulcerative colitis: A review. J. Sci. Soc. 2018, 45, 80–83. [Google Scholar] [CrossRef]
- Kiesler, P.; Fuss, I.J.; Strober, W. Experimental models of inflammatory bowel diseases. Cell. Mol. Gastroenterol. Hepatol. 2015, 1, 154–170. [Google Scholar] [CrossRef]
- Mizoguchi, E.; Low, D.; Ezaki, Y.; Okada, T. Recent updates on the basic mechanisms and pathogenesis of inflammatory bowel diseases in experimental animal models. Intest. Res. 2020, 18, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.J.; Xu, B.; Huang, S.W.; Luo, X.; Deng, X.L.; Luo, S.; Liu, C.; Wang, Q.; Chen, J.Y.; Zhou, L. Baicalin prevents LPS-induced activation of TLR4/NF-κB p65 pathway and inflammation in mice via inhibiting the expression of CD14. Acta Pharmacol. Sin. 2021, 42, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Surh, Y.J.; Do, S.G.; Shin, E.; Shim, K.S.; Lee, C.K.; Na, H.K. Baicalein inhibits dextran sulfate sodium-induced mouse colitis. J. Cancer Prev. 2019, 24, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Zhang, K.; Zhang, K.; Guo, Z.; Liu, Q.; Wang, L.; Wang, X.; Qiu, Z.; Wang, G.; Zhang, J.; et al. Antiviral activity and underlying mechanisms of baicalin against avian infectious bronchitis virus in vitro. Avian Pathol. 2022, 51, 574–589. [Google Scholar] [CrossRef]
- Duan, C.; Matsumura, S.; Kariya, N.; Nishimura, M.; Shimono, T. In vitro antibacterial activities of Scutellaria baicalensis Georgi against cariogenic bacterial. Pediatr. Dent. J. 2007, 17, 58–64. [Google Scholar] [CrossRef]
- Gao, Z.; Huang, K.; Xu, H. Protective effects of flavonoids in the roots of Scutellaria baicalensis Georgi against hydrogen peroxide-induced oxidative stress in HS-SY5Y cells. Pharmacol. Res. 2001, 43, 173–178. [Google Scholar] [CrossRef]
- Dou, J.; Wang, Z.; Ma, L.; Peng, B.; Mao, K.; Li, C.; Su, M.; Zhou, C.; Peng, G. Baicalein and baicalin inhibit colon cancer using two distinct fashions of apoptosis and senescence. Oncotarget 2018, 9, 20089–20102. [Google Scholar] [CrossRef]
- Han, C.; Xing, G.; Zhang, M.; Zhong, M.; Han, Z.; He, C.; Liu, X. Wogonoside inhibits cell growth and induces mitochondrial-mediated autophagy-related apoptosis in human colon cancer cells through the PI3K/AKT/mTOR/p70S6K signaling pathway. Oncol. Lett. 2018, 15, 4463–4470. [Google Scholar] [CrossRef]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents wogonin, baicalein and baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- Liao, H.; Ye, J.; Gao, L.; Liu, Y. The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother. 2021, 133, 110917. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, Z.; Zhang, H.; Zhen, Z.; Calway, T.; Wang, Y.; Yuan, C.S.; Wang, C.Z. Pretreatment of baicalin and wogonoside with glycoside hydrolase: A promising approach to enhance anticancer potential. Oncol. Rep. 2013, 30, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, R.; Gupta, A.; Pandey, A.K. Role of baicalin as a potential therapeutic agent in hepatobiliary and gastrointestinal disorders: A review. World J. Gastroenterol. 2022, 28, 3047–3062. [Google Scholar] [CrossRef] [PubMed]
- Noh, K.; Kang, Y.; Nepal, M.R.; Jeong, K.S.; Oh, D.G.; Kang, M.J.; Lee, S.; Kang, W.; Jeong, H.G.; Jeong, T.C. Role of intestinal microbiota in baicalin-induced drug interaction and its pharmacokinetics. Molecules 2016, 21, 337. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Z.; Wan, J.Y.; Zhang, C.F.; Lu, F.; Chen, L.; Yuan, C.S. Deglycosylation of wogonoside enhances its anticancer potential. J. Cancer Res. Ther. 2018, 14, S594–S599. [Google Scholar] [CrossRef]
- Heo, S.H.; Song, J.; Kim, B.J.; Kim, H.; Chang, D.I. Rationale and design to assess the efficacy and safety of HT047 in patients with acute ischemic stroke: A multicenter, randomized, double-blind, placebo-controlled, parallel-group, phase II trial. Medicine 2019, 98, e17655. [Google Scholar] [CrossRef]
- Zhang, C.L.; Zhang, S.; He, W.X.; Lu, J.L.; Xu, Y.J.; Yang, J.Y.; Liu, D. Baicalin may alleviate inflammatory infiltration in dextran sodium sulfate-induced chronic ulcerative colitis via inhibiting IL-33 expression. Life Sci. 2017, 186, 125–132. [Google Scholar] [CrossRef]
- Feng, J.; Guo, C.; Zhu, Y.; Pang, L.; Yang, Z.; Zou, Y.; Zheng, X. Baicalin down regulates the expression of TLR4 and NFkB-p65 in colon tissue in mice with colitis induced by dextran sulfate sodium. Int. J. Clin. Exp. Med. 2014, 7, 4063–4072. [Google Scholar]
- Dou, W.; Mukherjee, S.; Li, H.; Venkatesh, M.; Wang, H.; Kortagere, S.; Peleg, A.; Chilimuri, S.S.; Wang, Z.T.; Feng, Y.; et al. Alleviation of gut inflammation by Cdx2/Pxr pathway in a mouse model of chemical colitis. PLoS One 2012, 7, e36075. [Google Scholar] [CrossRef]
- Zhu, L.; Xu, L.Z.; Zhao, S.; Shen, Z.F.; Shen, H.; Zhan, L.B. Protective effect of baicalin on the regulation of Treg/Th17 balance, gut microbiota and short-chain fatty acids in rats with ulcerative colitis. Appl. Microbiol. Biotechnol. 2020, 104, 5449–5460. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, H.; Gu, P.Q.; Liu, Y.J.; Zhang, L.; Cheng, J.F. Baicalin alleviates TNBS-induced colitis by inhibiting PI3K/AKT pathway activation. Exp. Ther. Med. 2020, 20, 581–590. [Google Scholar] [CrossRef]
- Shen, J.; Cheng, J.; Zhu, S.; Zhao, J.; Ye, Q.; Xu, Y.; Dong, H.; Zheng, X. Regulating effect of baicalin on IKK/IKB/NF-kB signaling pathway and apoptosis-related proteins in rats with ulcerative colitis. Int. Immunopharmacol. 2019, 73, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Feng, L.; Zhang, Z.H.; Jia, X.B. The anti-inflammation effect of baicalin on experimental colitis through inhibiting TLR4/NF-κB pathway activation. Int. Immunopharmacol. 2014, 23, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Cao, X.; Zhang, R.; Li, Y.X.; Xu, Z.L.; Zhang, D.G.; Wang, L.S.; Wang, J.Y. Protective effect of baicalin against experimental colitis via suppression of oxidant stress and apoptosis. Pharmacogn. Mag. 2016, 12, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Dai, S.X.; Chi, H.G.; Li, T.; He, Z.W.; Wang, J.; Ye, C.G.; Huang, G.L.; Zhao, B.; Li, W.Y.; et al. Baicalin attenuates TNBS-induced colitis in rats by modulating the Th17/Treg paradigm. Arch. Pharm. Res. 2015, 38, 1873–1887. [Google Scholar] [CrossRef]
- Liang, S.; Deng, X.; Lei, L.; Zheng, Y.; Ai, J.; Chen, L.; Xiong, H.; Mei, Z.; Cheng, Y.C.; Ren, Y. The comparative study of the therapeutic effects and mechanism of baicalin, baicalein, and their combination on ulcerative colitis rat. Front. Pharmacol. 2019, 10, 1466. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, V.; Ferlazzo, N.; Currò, M.; Isola, G.; Matarese, M.; Bertuccio, M.P.; Caccamo, D.; Matarese, G.; Ientile, R. Baicalin-induced autophagy preserved LPS-stimulated intestinal cells from inflammation and alterations of paracellular permeability. Int. J. Mol. Sci. 2021, 22, 2315. [Google Scholar] [CrossRef]
- Tao, Y.; Zhan, S.; Wang, Y.; Zhou, G.; Liang, H.; Chen, X.; Shen, H. Baicalin, the major component of traditional Chinese medicine Scutellaria baicalensis induces colon cancer cell apoptosis through inhibition of oncomiRNAs. Sci. Rep. 2018, 8, 14477. [Google Scholar] [CrossRef]
- Yang, B.; Bai, H.; Sa, Y.; Zhu, P.; Liu, P. Inhibiting EMT, stemness and cell cycle involved in baicalin-induced growth inhibition and apoptosis in colorectal cancer cells. J. Cancer 2020, 11, 2303–2317. [Google Scholar] [CrossRef]
- Ma, W.; Liu, X.; Du, W. Baicalin induces apoptosis in SW480 cells through downregulation of the SP1 transcription factor. Anticancer Drugs 2019, 30, 153–158. [Google Scholar] [CrossRef]
- Chen, W.C.; Kuo, T.H.; Tzeng, Y.S.; Tsai, Y.C. Baicalin induces apoptosis in SW620 human colorectal carcinoma cells in vitro and suppresses tumor growth in vivo. Molecules 2012, 17, 3844–3857. [Google Scholar] [CrossRef]
- Li, H.; Dong, W.; Hou, J.; He, D. Effects of baicalin regulating PI3K/AKT/GSK-3β pathway on biological behavior of human colon cancer cells. Chin. Arch. TCM. Med. 2021, 39, 241–245. [Google Scholar] [CrossRef]
- Yan, G.; Chen, L.; Wang, H.; Wu, S.; Li, S.; Wang, X. Baicalin inhibits LPS-induced inflammation in RAW264.7 cells through miR-181b/HMGB1/TRL4/NF-κB pathway. Am. J. Transl. Res. 2021, 13, 10127–10141. [Google Scholar] [PubMed]
- Zhu, W.; Jin, Z.; Yu, J.; Liang, J.; Yang, Q.; Li, F.; Shi, X.; Zhu, X.; Zhang, X. Baicalin ameliorates experimental inflammatory bowel disease through polarization of macrophages to an M2 phenotype. Int. Immunopharmacol. 2016, 35, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Jin, G.B.; Cho, S.; Cyong, J.C. Evaluation of the anti-inflammatory effect of baicalein on dextran sulfate sodium-induced colitis in mice. Planta Med. 2002, 68, 268–271. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Chen, Y.; Huang, S.; Wang, X.; Luo, S.; Su, Y.; Zhou, L.; Luo, X. Baicalein restores the balance of Th17/Treg cells via aryl hydrocarbon receptor to attenuate colitis. Mediators Inflamm. 2020, 2020, 5918587. [Google Scholar] [CrossRef]
- Luo, X.; Yu, Z.; Deng, C.; Zhang, J.; Ren, G.; Sun, A.; Mani, S.; Wang, Z.; Dou, W. Baicalein ameliorates TNBS-induced colitis by suppressing TLR4/MyD88 signaling cascade and NLRP3 inflammasome activation in mice. Sci. Rep. 2017, 7, 16374. [Google Scholar] [CrossRef]
- Wang, C.Z.; Zhang, C.F.; Chen, L.; Anderson, S.; Lu, F.; Yuan, C.S. Colon cancer chemopreventive effects of baicalein, an active enteric microbiome metabolite from baicalin. Int. J. Oncol. 2015, 47, 1749–1758. [Google Scholar] [CrossRef]
- Kim, D.H.; Hossain, M.A.; Kang, Y.J.; Jang, J.Y.; Lee, Y.J.; Im, E.; Yoon, J.H.; Kim, H.S.; Chung, H.Y.; Kim, N.D. Baicalein, an active component of Scutellaria baicalensis Georgi, induces apoptosis in human colon cancer cells and prevents AOM/DSS-induced colon cancer in mice. Int. J. Oncol. 2013, 43, 1652–1658. [Google Scholar] [CrossRef]
- Havermann, S.; Chovolou, Y.; Humpf, H.U.; Wätjen, W. Modulation of the Nrf2 signalling pathway in Hct116 colon carcinoma cells by baicalein and its methylated derivative negletein. Pharm. Biol. 2016, 54, 1491–1502. [Google Scholar] [CrossRef]
- Chen, Z.; Hou, R.; Gao, S.; Song, D.; Feng, Y. Baicalein inhibits proliferation activity of human colorectal cancer cells HCT116 through downregulation of Ezrin. Cell Physiol. Biochem. 2018, 49, 2035–2046. [Google Scholar] [CrossRef]
- Su, M.Q.; Zhou, Y.R.; Rao, X.; Yang, H.; Zhuang, X.H.; Ke, X.J.; Peng, G.Y.; Zhou, C.L.; Shen, B.Y.; Dou, J. Baicalein induces the apoptosis of HCT116 human colon cancer cells via the upregulation of DEPP/Gadd45a and activation of MAPKs. Int. J. Oncol. 2018, 53, 750–760. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Kim, H.J.; Kim, H.R.; Lee, S.H.; Cho, S.D.; Choi, C.S.; Nam, J.S.; Jung, J.Y. Antitumor actions of baicalein and wogonin in HT-29 human colorectal cancer cells. Mol. Med. Rep. 2012, 6, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Rui, X.; Yan, X.I.; Zhang, K. Baicalein inhibits the migration and invasion of colorectal cancer cells via suppression of the AKT signaling pathway. Oncol. Lett. 2016, 11, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Chai, Y.; Xu, J.; Yan, B. The anti-metastatic effect of baicalein on colorectal cancer. Oncol. Rep. 2017, 37, 2317–2323. [Google Scholar] [CrossRef]
- Qi, Z.; Yin, F.; Lu, L.; Shen, L.; Qi, S.; Lan, L.; Luo, L.; Yin, Z. Baicalein reduces lipopolysaccharide-induced inflammation via suppressing JAK/STATs activation and ROS production. Inflamm. Res. 2013, 62, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Fan, G.W.; Zhang, Y.; Jiang, X.; Zhu, Y.; Wang, B.; Su, L.; Cao, W.; Zhang, H.; Gao, X. Anti-inflammatory activity of baicalein in LPS-stimulated RAW264.7 macrophages via estrogen receptor and NF-κB-dependent pathways. Inflammation 2013, 36, 1584–1591. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, H.J.; Lee, J.Y.; Kim, D.H.; Kang, M.S.; Park, W. Anti-inflammatory effect of baicalein on polyinosinic⁻polycytidylic acid-induced RAW 264.7 mouse macrophages. Viruses 2018, 10, 224. [Google Scholar] [CrossRef]
- Huang, S.; Fu, Y.; Xu, B.; Liu, C.; Wang, Q.; Luo, S.; Nong, F.; Wang, X.; Huang, S.; Chen, J.; et al. Wogonoside alleviates colitis by improving intestinal epithelial barrier function via the MLCK/pMLC2 pathway. Phytomedicine 2020, 68, 153179. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Yao, J.; Zhao, L.; Wu, Z.; Wang, Y.; Pan, D.; Miao, H.; Guo, Q.; Lu, N. Wogonoside protects against dextran sulfate sodium-induced experimental colitis in mice by inhibiting NF-κB and NLRP3 inflammasome activation. Biochem. Pharmacol. 2015, 94, 142–154. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, Y.; Wang, X.; Zhao, L.; Li, W.; Ding, Y.; Kong, L.; Guo, Q.; Lu, N. Wogonoside prevents colitis-associated colorectal carcinogenesis and colon cancer progression in inflammation-related microenvironment via inhibiting NF-κB activation through PI3K/Akt pathway. Oncotarget 2016, 7, 34300–34315. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Tang, Y.Z.; Liu, Y.H. Wogonoside displays anti-inflammatory effects through modulating inflammatory mediator expression using RAW264.7 cells. J. Ethnopharmacol. 2013, 148, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Dou, F.; Song, H.; Liu, T. Anti-ulcerative effects of wogonin on ulcerative colitis induced by dextran sulfate sodium via Nrf2/TLR4/NF-κB signaling pathway in BALB/c mice. Environ. Toxicol. 2022, 37, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Wang, H.; Pang, J.; Ji, L.; Han, J.; Wang, Y.; Qi, X.; Liu, Z.; Lu, L. Prevention of wogonin on colorectal cancer tumorigenesis by regulating p53 nuclear translocation. Front. Pharmacol. 2018, 9, 1356. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhao, L.; Zhao, Q.; Zhao, Y.; Sun, Y.; Zhang, Y.; Miao, H.; You, Q.D.; Hu, R.; Guo, Q.L. NF-κB and Nrf2 signaling pathways contribute to wogonin-mediated inhibition of inflammation-associated colorectal carcinogenesis. Cell Death Dis. 2014, 5, e1283. [Google Scholar] [CrossRef]
- He, L.; Lu, N.; Dai, Q.; Zhao, Y.; Zhao, L.; Wang, H.; Li, Z.; You, Q.; Guo, Q. Wogonin induced G1 cell cycle arrest by regulating Wnt/β-catenin signaling pathway and inactivating CDK8 in human colorectal cancer carcinoma cells. Toxicology 2013, 312, 36–47. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, L.; Wu, Y.; Dai, Q.; Zhou, Y.; Li, Z.; Yang, L.; Guo, Q.; Lu, N. Selective anti-tumor activity of wogonin targeting the Warburg effect through stablizing p53. Pharmacol. Res. 2018, 135, 49–59. [Google Scholar] [CrossRef]
- Tan, H.; Li, X.; Yang, W.H.; Kang, Y. A flavone, wogonin from Scutellaria baicalensis inhibits the proliferation of human colorectal cancer cells by inducing of autophagy, apoptosis and G2/M cell cycle arrest via modulating the PI3K/AKT and STAT3 signalling pathways. J. BUON 2019, 24, 1143–1149. [Google Scholar]
- Chi, Y.S.; Cheon, B.S.; Kim, H.P. Effect of wogonin, a plant flavone from Scutellaria radix, on the suppression of cyclooxygenase-2 and the induction of inducible nitric oxide synthase in lipopolysaccharide-treated RAW 264.7 cells. Biochem. Pharmacol. 2001, 61, 1195–1203. [Google Scholar] [CrossRef]
- Wakabayashi, I.; Yasui, K. Wogonin inhibits inducible prostaglandin E(2) production in macrophages. Eur. J. Pharmacol. 2000, 406, 477–481. [Google Scholar] [CrossRef]
- Wu, Q.; Xie, S.; Zhu, Y.; Chen, J.; Tian, J.; Xiong, S.; Wu, C.; Ye, Y.; Peng, Y. Wogonin strengthens the therapeutic effects of mesenchymal stem cells in DSS-induced colitis via promoting IL-10 Production. Oxid. Med. Cell Longev. 2021, 2021, 5527935. [Google Scholar] [CrossRef]
- Wang, W.; Xia, T.; Yu, X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-κB pathway in vitro. Inflamm. Res. 2015, 64, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Huynh, D.L.; Ngau, T.H.; Nguyen, N.H.; Tran, G.B.; Nguyen, C.T. Potential therapeutic and pharmacological effects of wogonin: An updated review. Mol. Biol. Rep. 2020, 47, 9779–9789. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, A.; Reimund, J.M.; Chasserot, S.; Muller, C.D.; Andriantsitohaina, R. Cyclooxygenase-2 expression and role of vasoconstrictor prostanoids in small mesenteric arteries from patients with Crohn’s disease. Circulation 2003, 107, 1407–1410. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Zhang, Z.J.; Cao, C.B.; Wang, N.; Liu, F.F.; Peng, J.Q.; Ren, X.J.; Qian, J. The TLR4/NF-κB signaling pathway mediates the growth of colon cancer. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 3834–3843. [Google Scholar] [PubMed]
- Capece, D.; Verzella, D.; Flati, I.; Arboretto, P.; Cornice, J.; Franzoso, G. NF-κB: Blending metabolism, immunity, and inflammation. Trends Immunol. 2022, 43, 757–775. [Google Scholar] [CrossRef]
- Kim, D.H.; Sung, B.; Chung, H.Y.; Kim, N.D. Modulation of colitis-associated colon tumorigenesis by baicalein and betaine. J. Cancer Prev. 2014, 19, 153–160. [Google Scholar] [CrossRef]
- Toiyama, Y.; Araki, T.; Yoshiyama, S.; Hiro, J.; Miki, C.; Kusunoki, M. The expression patterns of Toll-like receptors in the ileal pouch mucosa of postoperative ulcerative colitis patients. Surg. Today 2006, 36, 287–290. [Google Scholar] [CrossRef]
- Lu, Y.; Li, X.; Liu, S.; Zhang, Y.; Zhang, D. Toll-like receptors and inflammatory bowel disease. Front. Immunol. 2018, 9, 72. [Google Scholar] [CrossRef]
- Dejban, P.; Nikravangolsefid, N.; Chamanara, M.; Dehpour, A.; Rashidian, A. The role of medicinal products in the treatment of inflammatory bowel diseases (IBD) through inhibition of TLR4/NF-kappaB pathway. Phytother. Res. 2021, 35, 835–845. [Google Scholar] [CrossRef]
- Zhang, W.; Shen, X.; Xie, L.; Chu, M.; Ma, Y. MicroRNA-181b regulates endotoxin tolerance by targeting IL-6 in macrophage RAW264.7 cells. J. Inflamm. 2015, 12, 18. [Google Scholar] [CrossRef]
- Michel, H.E.; Menze, E.T. Tetramethylpyrazine guards against cisplatin-induced nephrotoxicity in rats through inhibiting HMGB1/TLR4/NF-κB and activating Nrf2 and PPAR-γ signaling pathways. Eur. J. Pharmacol. 2019, 857, 172422. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A target for anticancer therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Lopez, J.; Tait, S.W. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Bertheloot, D.; Latz, E.; Franklin, B.S. Necroptosis, pyroptosis and apoptosis: An intricate game of cell death. Cell Mol. Immunol. 2021, 18, 1106–1121. [Google Scholar] [CrossRef]
- Dogan, E.; Kara, H.G.; Kosova, B.; Cetintas, V.B. Metastasis; Sergi, C.M., Ed.; Exon Publications: Brisbane, Australia, 2022; Chapter 12; p. 163. [Google Scholar]
- Kaloni, D.; Diepstraten, S.T.; Strasser, A.; Kelly, G.L. BCL-2 protein family: Attractive targets for cancer therapy. Apoptosis 2022. [Google Scholar] [CrossRef]
- Salcher, S.; Hagenbuchner, J.; Geiger, K.; Seiter, M.A.; Rainer, J.; Kofler, R.; Hermann, M.; Kiechl-Kohlendorfer, U.; Ausserlechner, M.J.; Obexer, P. C10ORF10/DEPP, a transcriptional target of FOXO3, regulates ROS-sensitivity in human neuroblastoma. Mol. Cancer 2014, 13, 224. [Google Scholar] [CrossRef]
- Sanaei, M.J.; Baghery Saghchy Khorasani, A.; Pourbagheri-Sigaroodi, A.; Shahrokh, S.; Zali, M.R.; Bashash, D. The PI3K/Akt/mTOR axis in colorectal cancer: Oncogenic alterations, non-coding RNAs, therapeutic opportunities, and the emerging role of nanoparticles. J. Cell Physiol. 2022, 237, 1720–1752. [Google Scholar] [CrossRef]
- Narayanankutty, A. PI3K/ Akt/ mTOR pathway as a therapeutic target for colorectal cancer: A review of preclinical and clinical evidence. Curr. Drug Targets 2019, 20, 1217–1226. [Google Scholar] [CrossRef]
- Jang, J.Y.; Im, E.; Kim, N.D. Mechanism of resveratrol-induced programmed cell death and new drug discovery against cancer: A Review. Int. J. Mol. Sci. 2022, 23, 13689. [Google Scholar] [CrossRef]
| Disease | Animal/Cell Models | Dose and Times | Up-Regulation | Down-Regulation | Refs. |
|---|---|---|---|---|---|
| UC | DSS-induced UC mice | 100 mg/kg, 5 days | CD14, IL-6 | [84] | |
| 150 mg/kg/d, 30 days | IκB-α | MPO, NO, IL-1β, IL-6, TNF-α, IL-33, NF-κB p65, p-NF-κB p65, p-IκB-α | [98] | ||
| 100 mg/kg, twice daily for 7 days | IL-10 | TLR2, TLR4, TLR9, NF-κB p65, IL-6, IL-13, TNF-α, | [99] | ||
| 20 mg/kg, 13 days | CDX2, MDR1 (colon), Cyp3a11 (colon) | TNF-α, IL-6 | [100] | ||
| TNBS-induced UC rat | 100 mg/kg, every 2 days for 14 days | ZO-1, occludin, SOD, GSH, IL-10, Foxp3, acetic acid (fecal), propionic acid (fecal), butyric acid (fecal) | MUC2, ROS, MDA, Th17/Treg, IL-17, RORγt | [101] | |
| 100 mg/kg/d, 14 days | IL-10, ZO-1, β-catenin | p-PI3K/PI3K, p-AKT/AKT, TNF-α, IL-6, IL-1β | [102] | ||
| 90 mg/kg, 4 weeks | SOD, CAT, GSH-Px | MDA, IL-1β, MPO, PGE2, TNF-α, caspase-3 and -9, Bcl-2/Bax, Cyt c, NF-κB p65, p-IKKβ/IKKβ, p-IKBα/IKBα | [103] | ||
| 4 mL baicalin liquid (5 mg/mL), 15 days | MPO activity, ICAM-1, MCP-1, COX-2, TNF-α, IL-1β, IL-6 | [104] | |||
| 120 mg/kg, 15 days | CAT, GSH-Px, SOD | MDA, apoptosis rate, ROS | [105] | ||
| 10 mL/kg, 7 days | Foxp3 | MPO, TNF-α, IL-1β, IL-12, IFN-γ, IL-17, IL-6, IL-10, TGF-β, RORγt | [106] | ||
| TNBS-induced UC rat (intestinal mucosal cell) | 100 mg/kg/d, 14 days | Bcl-2 | caspase-3 and -9, FasL, Bax | [102] | |
| UC rats of various complex factors | 100 mg/kg, 7 days | SOD, ATP | AST, ALT, GGT, TG, MDA, NO, cAMP/cGMP, IL-6, IL-1β, IL-17, NF-κB p65, p38 | [107] | |
| CRC | HT29 cells (LPS-treated) | 50 μM, 2 or 24 h | CD14, MyD88, NF-κB p65 | [84] | |
| 100 ng/mL, 30 min | IL-10, Bcl-2, ZO-1, β-catenin | p-PI3K/PI3K, p-AKT/AKT, TNF-α, IL-6, IL-1β, caspase-3 and -9, FasL, Bax | [102] | ||
| 10 μg/mL, 30 min | LC3, ATG5, BECN1, claudin 1 | TNF-α, IL-1β, NF-κB p65 | [108] | ||
| HT29 cells | 150 μM, 48 h | cleaved of caspase-3, apoptosis, HIC1, PDCD4, PTEN, E2F2, E-cadherin | miR-10a, miR-23a, miR-30c, miR-31, miR-151a, miR-205, Bcl-2, c-Myc | [109] | |
| RKO and HCT116 cells | 100 μg/mL, 48 h | cleaved of caspase-3, -8, -9, and PARP-1, E-cadherin, Cytokeratin 18, Claudin 1, Smad7 | XIAP, NF-κB, survivin, Bcl-2, p-AKT(Ser473), cyclin D1, cyclin E1, cyclin B1, N-Cadherin, vimentin, snail, slug, twist, TGF-β1, p-Smad2/3, Smad2/3/4, CD133, CD44, SOX2, OCT4, Nanog | [110] | |
| SW480 cells | 400 μg/mL, 24 or 48 h | apoptosis, cleaved of caspase-3 and PARP-1 | Sp1 | [111] | |
| SW620 cells | 200 µM, 24 h | sub-G1, caspase-3, -8, and -9 activity, ROS | [112] | ||
| 50 μmol/L, 48 h | p-PI3K, p-AKT, p-GSK-3β | [113] | |||
| HCT116, SW480, and HT29 cells | 20 µM, 72 h | apoptotic cells, p-ERK, p-p38 | [89] | ||
| HCT116 xenograft | 200 mg/kg, 32 days | E-cadherin, cleaved of caspase-3 and PARP-1 | Ki67, vimentin, N-cadherin, CD44, CD133, cyclin D1, cyclin B1 | [110] | |
| HT29 xenograft | 100 mg/kg, 3 weeks | cleaved of caspase-3, miR-204 | Ki67, c-Myc, miR-10a, miR-23a, miR-30c, miR-31, miR-151a | [109] | |
| Miscellaneous | RAW 264.7cells (LPS-treated) | 50 μM, 2 or 24 h | NO, IL-6, MHC II, MyD88, NF-κB p65, CD14 | [84] | |
| 1.0 μmol/L, 24 h | HMGB1, TLR4, p-IκBα, p-p65, p65 (nuclear), IL-1β, IL-6, COX, iNOS | [114] | |||
| p-p65/p-65, p-IKBα/IKBα | [103] | ||||
| 1.0 × 10−5 M | CAT, GSH-Px, SOD, Bcl-2 | MDA, apoptosis rate, TGF-β1, caspase-3 and -9, cleaved caspase-3 and -9, Bax, Fas, FasL | [105] | ||
| 5 × 10−4 M | IκB | TLR4, NF-κB, p-NF-κB, p-IκB, ICAM-1, MCP-1, COX-2, TNF-α, IL-1β, IL-6 | [104] | ||
| LPS-treated mice | 100 mg/kg (i.p.), 3 days | CD14, IL-6 | [84] | ||
| Mouse peritoneal macrophages (LPS-treated) | 50 μM, 24 h | IL-10, Arg-1, IRF4 | TNF-α, IL-23, IRF5, M1/M2 macrophage ratio | [115] |
| Disease | Animal/Cell Models | Dose and Times | Up-Regulation | Down-Regulation | Refs. |
|---|---|---|---|---|---|
| UC | DSS-induced UC mice | 25 mg/kg, 7 days | IκBα | COX-2, iNOS, cyclin D1, p-IκBα, p-p65, p65, IKKβ, NF-κB, p-STAT3, STAT3 | [85] |
| 20 mg/kg, 10 days | IL-4 | IFN-γ | [116] | ||
| 40 mg/kg, 10 days | CYP1A1 | IL-6, IL-10, IL-17, IL-22, TNF-α, TNF-β | [117] | ||
| 20 mg/kg, 13 days | CDX2, MDR1 (colon), Cyp3a11 (colon) | TNF-α, IL-6 | [100] | ||
| UC rats of various complex factors | 100 mg/kg, 7 days | SOD, ATP | AST, ALT, GGT, TG, MDA, NO, cAMP/cGMP, IL-6, IL-1β, IL-17, NF-κB p65, p38 | [107] | |
| CD | TNBS-induced CD mice | 20 mg/kg/d, 10 days | iNOS, ICAM-1, MCP-1, COX-2, TNF-α, IL-1β, TLR4, MyD88, p-p65, p-IκBα, p-p38 | [118] | |
| CRC | HCT116 cells | 60 µM, 48 h | apoptosis, caspase-3 and - 9 | [119] | |
| 100 µM, 24 h | cleavage of PARP, PPARγ | pro-caspase-3, -8, and -9, p-IκBα, iNOS, p50 (nuclear), p65 (nuclear), MMP-2 and -9 | [120] | ||
| 40 µM, 4 h | p-Nrf2/Nrf2 | [121] | |||
| 40 µmol/L, 24 or 48 h | p53, p21 | Ezrin, cyclin D1, CDK4 | [122] | ||
| HCT116, A549, and Panc-1 cells | 40 µM, 48 h | apoptotic rate, cleaved of caspase-3 and -9, DEPP, GADD45A, p-JNK, p-ERK, p-p38 | [123] | ||
| HCT116, SW480, and HT29 cells | 20 µM, 72 h | apoptotic cells, hTERT, p-ERK, p-p38 | [89] | ||
| HT29 cells | 100 µM, 48 h | cleavage of PARP and caspase-3, Bax, p53 | Bcl-2, p-AKT, p-caspase- 9, p-GSK-3β, survivin, cyclin D1, cyclin B1 | [124] | |
| HT29 xenograft | 10 mg/kg three times/week, 43 days | apoptotic cells, p53, p21 | [124] | ||
| LS174T cells | 25 µM, 48 h | PXR, CDX2 | [100] | ||
| DLD-1 cells | 30 µM, 24 h | MMP-2 and -9, p-AKT | [125] | ||
| HT29 and DLD1 cells | 30 µM, 24 h | MMP-2 and -9, p-ERK | [126] | ||
| DLD1 xenograft | 20 mg/kg/day, 21 days | MMP-2 and -9, p-ERK | [126] | ||
| HCT116 xenograft | 50 mg/kg, 3 weekly cycle | Ki67 | [122] | ||
| Miscellaneous | RAW 264.7cells (LPS-treated) | 50 μM, 2 h or 48 h | TLR4, MyD88, IRAK-1, COX-2, NO, iNOS, p-JNK, p-ERK1/2, p-p38, MD-2/TLR4 | [118] | |
| 80 μM, 2 h | p-ERK, p-p38, p-JNK, p- JAK1, p-JAK2 | iNOS, TNF-α, IL-6, IL-1β, NO, PGE2, p-STAT1(Tyr701), p-STAT3(Tyr705), p-STAT3(Ser727) | [127] | ||
| 10 μM, 2 h | TNF-α, COX-2, iNOS, NO, IL-1β, PGE2, p-IκBα | [128] | |||
| RAW 264.7 cells (polyinosinic–polycytidylic-acid-treated) | 100 µM, 24 h | NO, calcium release, IL-1α, IL-6, G-CSF, GM-CSF, VEGF, MCP-1, IP-10, LIX, RANTES, STAT1, LIX, STAT3, CHOP, Fas | [129] | ||
| THP-1 cells (LPS-treated) | 50 μM, 2 h | iNOS, COX-2, IL-1α, IL-1β, NLRP3, ASC, caspase-1 | [118] | ||
| EL-4 cells | 50 μM, 24 h | CYP1A1, AHR (nuclear) | AHR (cytosol) | [117] | |
| colonic lamina propria lymphocyte | 40 mg/kg, 10 days | CD4+CD25+Foxp3+ T cells | CD4+IL-17+ T cells | [117] | |
| mesenteric lymph of mice | 40 mg/kg, 10 days | CD4+CD25+Foxp3+ T cells | CD4+IL-17+ T cells | [117] |
| Disease | Animal/Cell Models | Dose and Times | Up-Regulation | Down-Regulation | Refs. |
|---|---|---|---|---|---|
| UC | DSS-induced UC mice | 50 mg/kg, 10 days | occludin, ZO-1, Claudin1, MLC2 | IL-13, IFN-γ, MLCK, p-MLC2 | [130] |
| 50 mg/kg, 10 days | NF-κB p65 (cytosol) | MPO activity, iNOS activity, CD11b+F4/80+ monocyte/macrophage, CD11b+Gr-1+ neutrophils, IL-1β, TNF-α, and IL-6 (serum, colonic), IL-18, IFN-γ, and MIP-1α (colonic), NF-κB p65 (nucleus), p-IkBα (cytosol), p-p65, NF-kB DNA binding activity, cleaved caspase-1 and IL-1β, NLRP3, ASC, caspase-1 activity | [131] | ||
| THP-1 cells (LPS-treated) | 50 µM, 6 h | NF-κB p65 (cytosol) | IL-1β, IL-6, TNF-α, NF-κB p65 (nucleus), p-IkBα (cytosol), p-p65, NF-kB DNA binding activity, NLRP3, pro-caspase-1, cleaved caspase-1 and IL-1β, NLRP3, ASC, caspase-1 activity | [131] | |
| Caco-2 cells (TNF-α exposure) | 50 µM, 72 h | occludin, ZO-1, Claudin1, TER, MLC2, F-actin | FD4, MLCK, p-MLC2 | [130] | |
| CRC | AOM/DSS mouse | 100 mg/kg, 105 days | apoptotic cells, NF-κB (cytoplasm) | neutrophil (Gr-1+ positive cells), macrophage (F4/80+ positive cells), Ki-67, BrdU, PCNA, IL-1β, IL-6, TNF-α, NF-κB, NF-κB (nucleus), p-p65, PI3K, p-AKT/AKT, p-IKKα/IKKα, p-IkBα/ IkBα, cyclin D1, survivin | [132] |
| HCT116 and HT29 cells (conditioned media from LPS-treated THP-1 cells) | 150 µM, 24 h | NF-κB (cytoplasm) | PCNA, p-IKKα/IKKα, p-IκBα/IκBα, cyclin D1, survivin, NF-κB (nucleus), NF-κB fluorescence, NF-κB luciferase activity, PI3K, p-AKT/AKT | [132] | |
| LOVO cells | 62.5 µM, 48 h | caspase-3 and -9, bax, LC3, p62 | Bcl-2, PI3K, p-AKT, p-mTOR, p-p70S6K | [90] | |
| Miscellaneous | RAW 264.7 cells (LPS-treated) | 50 μM, 24 h | NO, PGE2, TNF-α, IL-6, iNOS, COX-2 | [133] |
| Disease | Animal/Cell Models | Dose and Times | Up-Regulation | Down-Regulation | Refs. |
|---|---|---|---|---|---|
| UC | DSS-induced UC mice | 30 mg/kg, 7 days | GST, GSH, SOD, IL-10 (tissue), Bax, caspase-3 and -9, Nrf2, HO-1 | MPO, NO, TBARS, TNF-α (serum, tissue), IL-6 (serum, tissue), PGE2 (tissue), Bcl-2, COX-2, iNOS, TLR4, p65 | [134] |
| CRC | AOM/DSS mouse | 100 mg/kg, 25 weeks | cleavage of caspase-3 and -9, IRE1α, PDI | [135] | |
| 60 mg/kg, 105 days | IOD of Nrf2-positive cells (surrounding tissues), NF-kB p65 (cytoplasm), Nrf2 (nuclear) | IOD of BrdU-, PCNA-, and NF-kB-positive cells (surrounding tissues, tumor tissues), IOD of IL-6- and 1β-positive cells, IOD of Nrf2-positive cells (tumor tissues), NF-kB p65 (nuclear) | [136] | ||
| HCT116 cells (cocultured with LPS-treated THP-1 cells) | 100 µM, 12 h | IL-6, IL1β, PCNA, NF-kB (nuclear), p-IκB, p-IKK(α/β) | [136] | ||
| HCT116 cells (LPS-treated) | 50 µM, 8 h | Nrf2 (nuclear), NQO-1, HO-1 | NF-kB (nuclear), p-IκB, p-IKKα/β, p-p38, p-ERK, p-JNK, p-AKT, PI3K, Nrf2 (cytoplasm) | [136] | |
| HCT116 cells | 40 µM, 24 h | p21, p27, E2F1, p-Rb, GSK-3β, AXIN, p-β-catenin (Ser33/37/Thr41), apoptotic rate | cyclin A, cyclin E, cyclin D1, CDK2, CDK4, CDK8, Rb, Wnt3a, Dvl2, LRP6, p-GSK-3β, β-catenin, β-catenin (cytosolic), β-catenin (nuclear), TCF1, TCF3, TCF4, LEF1, c-Myc, survivin | [137] | |
| 10 µM, 72 h | cleavage of caspase-3 and -9, apoptotic cells, IRE1α, calnexin, PDI, CHOP, DDIT3, XBP1, p53(Ser376), p53(Ser376) (cytoplasm), p53(Ser315) | colony formation rate, LC3-II, ATG12, p53(Ser376) (nuclear), p-p53(Ser15) (nuclear/ cytoplasm) | [135] | ||
| 40 µM, 24 h | p53, TIGAR, p-p53(Ser15), p-p53(Ser20), Ac-p53(Lys382) | glucose uptake, lactate generation, ATP production, PGM, GLUT1, HK2, PDHK1, LDHA, MDM2, | [138] | ||
| HCT116 cells xenograft | 60 mg/kg | E2F1 | β-catenin, cyclin D1, c–Myc, TCF3 | [137] | |
| HT29 cells | 100 µM, 48 h | cleavage of PARP and caspase-3, Bax, p53, | Bcl-2, p-AKT, p-caspase-9, p-GSK-3β, survivin, cyclin D1, cyclin B1 | [124] | |
| HT29 xenograft | 10 mg/kg three times/week, 43 days | apoptotic cells, p53, p21 | [124] | ||
| SW48 cells | 16 μM | LC3-II, cleavage of caspase-3, -8, -9, and PARP, PI3K | p-PI3K, p-AKT, p-STAT3(Tyr705), p-STAT3(Ser727) | [139] | |
| SW-480 cells | 60 μM, 48 h | apoptotic cells, caspases-3, -8, and -9 activity | [96] | ||
| THP-1 cells (LPS-treated) | 100 µM | Nrf2 (nuclear), Keap1, HO-1 | Nrf2 (cytosolic) | [136] | |
| Miscellaneous | RAW 264.7 cells (LPS-treated) | 100 µM, 24 h | NO, PGE2, iNOS, COX-2 | [140] | |
| 50 µM, 24 h | PGE2, COX-2, COX-2 activity | [141] | |||
| DSS + MSCs | 10 mg/kg, 8 days | IL-10 | TNF-α | [142] | |
| MSCs + LPS | 50 μM, 24 h | ROS, HIF-1α, IL-10, p-GSK-3β, p-GSK-3β/GSK-3β, p-AKT, p-AKT/AKT | [142] | ||
| Caco-2 cells (LPS-treated) | 50 μM | TEER, ZO-1, claudin-1, IκB | FD4, FD10, FD20, IL-1β, IL-6, IL-8, iNOS, COX-2, NF-κB p65, p-IκB, TLR4, MyD88, p-TAK1 | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.Y.; Im, E.; Kim, N.D. Therapeutic Potential of Bioactive Components from Scutellaria baicalensis Georgi in Inflammatory Bowel Disease and Colorectal Cancer: A Review. Int. J. Mol. Sci. 2023, 24, 1954. https://doi.org/10.3390/ijms24031954
Jang JY, Im E, Kim ND. Therapeutic Potential of Bioactive Components from Scutellaria baicalensis Georgi in Inflammatory Bowel Disease and Colorectal Cancer: A Review. International Journal of Molecular Sciences. 2023; 24(3):1954. https://doi.org/10.3390/ijms24031954
Chicago/Turabian StyleJang, Jung Yoon, Eunok Im, and Nam Deuk Kim. 2023. "Therapeutic Potential of Bioactive Components from Scutellaria baicalensis Georgi in Inflammatory Bowel Disease and Colorectal Cancer: A Review" International Journal of Molecular Sciences 24, no. 3: 1954. https://doi.org/10.3390/ijms24031954
APA StyleJang, J. Y., Im, E., & Kim, N. D. (2023). Therapeutic Potential of Bioactive Components from Scutellaria baicalensis Georgi in Inflammatory Bowel Disease and Colorectal Cancer: A Review. International Journal of Molecular Sciences, 24(3), 1954. https://doi.org/10.3390/ijms24031954








