Mitochondrial Transplantation in Mitochondrial Medicine: Current Challenges and Future Perspectives
Abstract
:1. Introduction
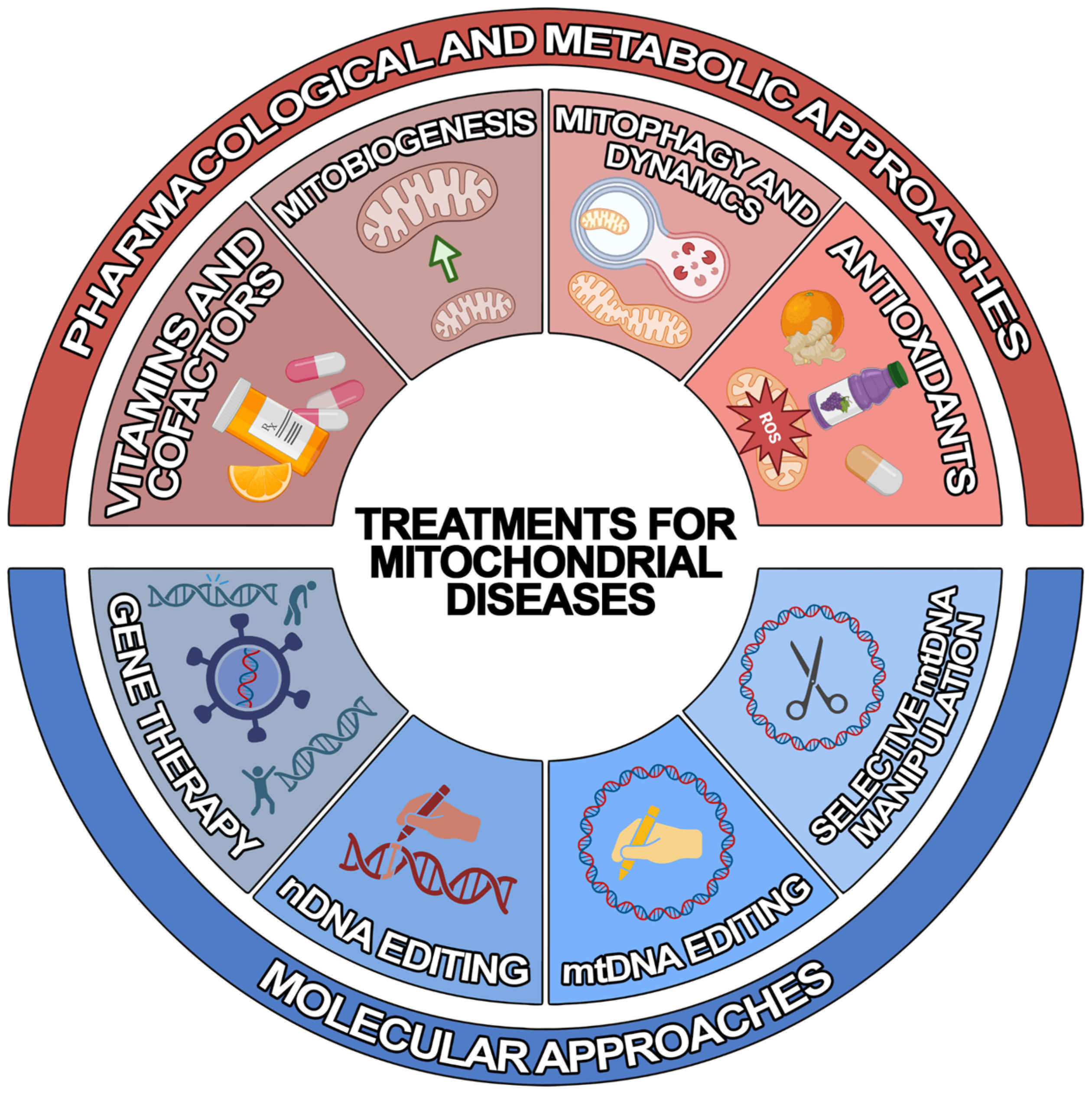
2. Treatments for Mitochondrial Diseases
2.1. Pharmacological and Metabolic Approaches
2.2. Molecular Approaches
3. Intercellular Transfer of Mitochondria
3.1. Mechanisms of Mitochondria Intercellular Transfer
3.1.1. Transfer via TNTs
3.1.2. Transfer via EVs
4. Mitochondrial Transplantation (MT)
4.1. In Vitro Methods for MT
4.2. In Vivo Methods for MT
5. Challenges and Future Prospective
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rossmann, M.P.; Dubois, S.M.; Agarwal, S.; Zon, L.I. Mitochondrial Function in Development and Disease. Dis. Model. Mech. 2021, 14, dmm048912. [Google Scholar] [CrossRef] [PubMed]
- Vinten-Johansen, J. Commentary: Mitochondria Are More than Just the Cells’ Powerhouse. J. Thorac. Cardiovasc. Surg. 2020, 160, e33–e34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacomello, M.; Pyakurel, A.; Glytsou, C.; Scorrano, L. The Cell Biology of Mitochondrial Membrane Dynamics. Nat. Rev. Mol. Cell Biol. 2020, 21, 204–224. [Google Scholar] [CrossRef]
- Whitley, B.N.; Engelhart, E.A.; Hoppins, S. Mitochondrial Dynamics and Their Potential as a Therapeutic Target. Mitochondrion 2019, 49, 269–283. [Google Scholar] [CrossRef]
- Roger, A.J.; Muñoz-Gómez, S.A.; Kamikawa, R. The Origin and Diversification of Mitochondria. Curr. Biol. 2017, 27, R1177–R1192. [Google Scholar] [CrossRef] [Green Version]
- Taanman, J.-W. The Mitochondrial Genome: Structure, Transcription, Translation and Replication. Biochim. Biophys. Acta (BBA) Bioenerg. 1999, 1410, 103–123. [Google Scholar] [CrossRef] [Green Version]
- Pagnamenta, A.T.; Wei, W.; Rahman, S.; Chinnery, P.F. Biparental Inheritance of Mitochondrial DNA Revisited. Nat. Rev. Genet. 2021, 22, 477–478. [Google Scholar] [CrossRef]
- Zeviani, M.; Viscomi, C. Mitochondrial Neurodegeneration. Cells 2022, 11, 637. [Google Scholar] [CrossRef]
- Pagliarini, D.J.; Calvo, S.E.; Chang, B.; Sheth, S.A.; Vafai, S.B.; Ong, S.-E.; Walford, G.A.; Sugiana, C.; Boneh, A.; Chen, W.K.; et al. A Mitochondrial Protein Compendium Elucidates Complex I Disease Biology. Cell 2008, 134, 112–123. [Google Scholar] [CrossRef] [Green Version]
- Bogenhagen, D.; Clayton, D.A. The Number of Mitochondrial Deoxyribonucleic Acid Genomes in Mouse L and Human HeLa Cells. Quantitative Isolation of Mitochondrial Deoxyribonucleic Acid. J. Biol. Chem. 1974, 249, 7991–7995. [Google Scholar] [CrossRef]
- Veltri, K.L.; Espiritu, M.; Singh, G. Distinct Genomic Copy Number in Mitochondria of Different Mammalian Organs. J. Cell. Physiol. 1990, 143, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Jevtic, V.; Kindle, P.; Avilov, S.V. SYBR Gold Dye Enables Preferential Labelling of Mitochondrial Nucleoids and Their Time-Lapse Imaging by Structured Illumination Microscopy. PLoS ONE 2018, 13, e0203956. [Google Scholar] [CrossRef] [PubMed]
- Bogenhagen, D.F.; Rousseau, D.; Burke, S. The Layered Structure of Human Mitochondrial DNA Nucleoids. J. Biol. Chem. 2008, 283, 3665–3675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazak, L.; Reyes, A.; Holt, I.J. Minimizing the Damage: Repair Pathways Keep Mitochondrial DNA Intact. Nat. Rev. Mol. Cell Biol. 2012, 13, 659–671. [Google Scholar] [CrossRef]
- Brown, W.M.; George, M.; Wilson, A.C. Rapid Evolution of Animal Mitochondrial DNA. Proc. Natl. Acad. Sci. USA 1979, 76, 1967–1971. [Google Scholar] [CrossRef] [Green Version]
- Pham, V.H.; Nguyen, V.L.; Jung, H.-E.; Cho, Y.-S.; Shin, J.-G. The Frequency of the Known Mitochondrial Variants Associated with Drug-Induced Toxicity in a Korean Population. BMC Med. Genom. 2022, 15, 3. [Google Scholar] [CrossRef]
- Paz, M.V.; Cotán, D.; Cordero, M.D.; Garrido Maraver, J.; Oropesa-Ávila, M.; de la Mata, M.; Pavón, A.D.; Alcócer Gómez, E.; de Lavera, I.; Sánchez Alcázar, J.A. The Role of Autophagy and Mitophagy in Mitochondrial Diseases. In Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 155–172. ISBN 978-0-12-802937-4. [Google Scholar]
- Ortiz, G.G.; Mireles-Ramírez, M.A.; González-Usigli, H.; Macías-Islas, M.A.; Bitzer-Quintero, O.K.; Torres-Sánchez, E.D.; Sánchez-López, A.L.; Ramírez-Jirano, J.; Ríos-Silva, M.; Torres-Mendoza, B. Mitochondrial Aging and Metabolism: The Importance of a Good Relationship in the Central Nervous System. In Mitochondrial DNA—New Insights; Seligmann, H., Ed.; InTech: London, UK, 2018; ISBN 978-1-78984-265-4. [Google Scholar]
- Wallace, D.C.; Singh, G.; Lott, M.T.; Hodge, J.A.; Schurr, T.G.; Lezza, A.M.; Elsas, L.J.; Nikoskelainen, E.K. Mitochondrial DNA Mutation Associated with Leber’s Hereditary Optic Neuropathy. Science 1988, 242, 1427–1430. [Google Scholar] [CrossRef]
- Holt, I.J.; Harding, A.E.; Morgan-Hughes, J.A. Deletions of Muscle Mitochondrial DNA in Patients with Mitochondrial Myopathies. Nature 1988, 331, 717–719. [Google Scholar] [CrossRef]
- Zeviani, M.; Tiranti, V.; Piantadosi, C. Mitochondrial Disorders. Medicine 1998, 77, 59–72. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondrial Genetic Medicine. Nat. Genet. 2018, 50, 1642–1649. [Google Scholar] [CrossRef]
- Zeviani, M.; Di Donato, S. Mitochondrial Disorders. Brain 2004, 127, 2153–2172. [Google Scholar] [CrossRef] [PubMed]
- Bottani, E.; Lamperti, C.; Prigione, A.; Tiranti, V.; Persico, N.; Brunetti, D. Therapeutic Approaches to Treat Mitochondrial Diseases: “One-Size-Fits-All” and “Precision Medicine” Strategies. Pharmaceutics 2020, 12, 1083. [Google Scholar] [CrossRef] [PubMed]
- Rahman, J.; Rahman, S. Mitochondrial Medicine in the Omics Era. Lancet 2018, 391, 2560–2574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial Diseases. Nat. Rev. Dis. Prim. 2016, 2, 16080. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Zarante, A.M.; Almannai, M.; Scaglia, F. Therapies for Mitochondrial Diseases and Current Clinical Trials. Mol. Genet. Metab. 2017, 122, 1–9. [Google Scholar] [CrossRef]
- Di Donfrancesco, A.; Massaro, G.; Di Meo, I.; Tiranti, V.; Bottani, E.; Brunetti, D. Gene Therapy for Mitochondrial Diseases: Current Status and Future Perspective. Pharmaceutics 2022, 14, 1287. [Google Scholar] [CrossRef]
- Slone, J.; Huang, T. The Special Considerations of Gene Therapy for Mitochondrial Diseases. NPJ Genom. Med. 2020, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Artika, I.M. Allotopic Expression of Mitochondrial Genes: Basic Strategy and Progress. Genes Dis. 2020, 7, 578–584. [Google Scholar] [CrossRef]
- Reddy, P.; Ocampo, A.; Suzuki, K.; Luo, J.; Bacman, S.R.; Williams, S.L.; Sugawara, A.; Okamura, D.; Tsunekawa, Y.; Wu, J.; et al. Selective Elimination of Mitochondrial Mutations in the Germline by Genome Editing. Cell 2015, 161, 459–469. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Jiang, J.; Li, Z.; Liang, J.; Xiang, Y. Strategies for Mitochondrial Gene Editing. Comput. Struct. Biotechnol. J. 2021, 19, 3319–3329. [Google Scholar] [CrossRef]
- Falabella, M.; Minczuk, M.; Hanna, M.G.; Viscomi, C.; Pitceathly, R.D.S. Gene Therapy for Primary Mitochondrial Diseases: Experimental Advances and Clinical Challenges. Nat. Rev. Neurol. 2022, 18, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Mok, B.Y.; de Moraes, M.H.; Zeng, J.; Bosch, D.E.; Kotrys, A.V.; Raguram, A.; Hsu, F.; Radey, M.C.; Peterson, S.B.; Mootha, V.K.; et al. A Bacterial Cytidine Deaminase Toxin Enables CRISPR-Free Mitochondrial Base Editing. Nature 2020, 583, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.-I.; Lee, S.; Mok, Y.G.; Lim, K.; Lee, J.; Lee, J.M.; Chung, E.; Kim, J.-S. Targeted A-to-G Base Editing in Human Mitochondrial DNA with Programmable Deaminases. Cell 2022, 185, 1764–1776.e12. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Jiang, X.; Yang, Q.; Zhao, J.; Zhou, Q.; Zhou, Y. The Functions, Methods, and Mobility of Mitochondrial Transfer Between Cells. Front. Oncol. 2021, 11, 672781. [Google Scholar] [CrossRef]
- Rustom, A.; Saffrich, R.; Markovic, I.; Walther, P.; Gerdes, H.-H. Nanotubular Highways for Intercellular Organelle Transport. Science 2004, 303, 1007–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spees, J.L.; Olson, S.D.; Whitney, M.J.; Prockop, D.J. Mitochondrial Transfer between Cells Can Rescue Aerobic Respiration. Proc. Natl. Acad. Sci. USA 2006, 103, 1283–1288. [Google Scholar] [CrossRef] [Green Version]
- Torralba, D.; Baixauli, F.; Sánchez-Madrid, F. Mitochondria Know No Boundaries: Mechanisms and Functions of Intercellular Mitochondrial Transfer. Front. Cell Dev. Biol. 2016, 4, 107. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Park, J.-H.; Hayakawa, K. Therapeutic Use of Extracellular Mitochondria in CNS Injury and Disease. Exp. Neurol. 2020, 324, 113114. [Google Scholar] [CrossRef]
- Miliotis, S.; Nicolalde, B.; Ortega, M.; Yepez, J.; Caicedo, A. Forms of Extracellular Mitochondria and Their Impact in Health. Mitochondrion 2019, 48, 16–30. [Google Scholar] [CrossRef]
- Burdett, T.C.; Freeman, M.R. Astrocytes Eyeball Axonal Mitochondria. Science 2014, 345, 385–386. [Google Scholar] [CrossRef]
- Lyamzaev, K.G.; Nepryakhina, O.K.; Saprunova, V.B.; Bakeeva, L.E.; Pletjushkina, O.Y.; Chernyak, B.V.; Skulachev, V.P. Novel Mechanism of Elimination of Malfunctioning Mitochondria (Mitoptosis): Formation of Mitoptotic Bodies and Extrusion of Mitochondrial Material from the Cell. Biochim. Biophys. Acta (BBA) Bioenerg. 2008, 1777, 817–825. [Google Scholar] [CrossRef] [Green Version]
- Davis, C.O.; Kim, K.-Y.; Bushong, E.A.; Mills, E.A.; Boassa, D.; Shih, T.; Kinebuchi, M.; Phan, S.; Zhou, Y.; Bihlmeyer, N.A.; et al. Transcellular Degradation of Axonal Mitochondria. Proc. Natl. Acad. Sci. USA 2014, 111, 9633–9638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phinney, D.G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; St Croix, C.M.; Stolz, D.B.; Watkins, S.C.; Di, Y.P.; Leikauf, G.D.; et al. Mesenchymal Stem Cells Use Extracellular Vesicles to Outsource Mitophagy and Shuttle MicroRNAs. Nat. Commun. 2015, 6, 8472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Zhong, J.; Wang, L.; Chen, Y. Mitochondrial Transfer in Cardiovascular Disease: From Mechanisms to Therapeutic Implications. Front. Cardiovasc. Med. 2021, 8, 771298. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, S.; Peruzzotti-Jametti, L.; Frezza, C. Astrocyte Power Fuels Neurons during Stroke. Swiss Med. Wkly. 2016, 146, w14374. [Google Scholar] [CrossRef] [Green Version]
- Hayakawa, K.; Chan, S.J.; Mandeville, E.T.; Park, J.H.; Bruzzese, M.; Montaner, J.; Arai, K.; Rosell, A.; Lo, E.H. Protective Effects of Endothelial Progenitor Cell-Derived Extracellular Mitochondria in Brain Endothelium. Stem Cells 2018, 36, 1404–1410. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, K.A.; Yerkovich, S.T.; Hopkins, P.M.-A.; Chambers, D.C. Characterization of Intercellular Communication and Mitochondrial Donation by Mesenchymal Stromal Cells Derived from the Human Lung. Stem Cell Res. Ther. 2016, 7, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, A.-M.; Nakhle, J.; Griessinger, E.; Vignais, M.-L. Intercellular Mitochondria Trafficking Highlighting the Dual Role of Mesenchymal Stem Cells as Both Sensors and Rescuers of Tissue Injury. Cell Cycle 2018, 17, 712–721. [Google Scholar] [CrossRef]
- Liu, K.; Ji, K.; Guo, L.; Wu, W.; Lu, H.; Shan, P.; Yan, C. Mesenchymal Stem Cells Rescue Injured Endothelial Cells in an in vitro Ischemia-Reperfusion Model via Tunneling Nanotube like Structure-Mediated Mitochondrial Transfer. Microvasc. Res. 2014, 92, 10–18. [Google Scholar] [CrossRef]
- Kumar, V. A STING to Inflammation and Autoimmunity. J. Leukoc. Biol. 2019, 106, 171–185. [Google Scholar] [CrossRef]
- Rodríguez-Nuevo, A.; Díaz-Ramos, A.; Noguera, E.; Díaz-Sáez, F.; Duran, X.; Muñoz, J.P.; Romero, M.; Plana, N.; Sebastián, D.; Tezze, C.; et al. Mitochondrial DNA and TLR9 Drive Muscle Inflammation upon Opa1 Deficiency. EMBO J. 2018, 37, e96553. [Google Scholar] [CrossRef]
- Liu, K.; Zhou, Z.; Pan, M.; Zhang, L. Stem Cell-derived Mitochondria Transplantation: A Promising Therapy for Mitochondrial Encephalomyopathy. CNS Neurosci. Ther. 2021, 27, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, H.; Yao, Y.; Zhao, T.; Chen, Y.; Shen, Y.; Wang, L.; Zhu, Y. Stem Cell-Derived Mitochondria Transplantation: A Novel Strategy and the Challenges for the Treatment of Tissue Injury. Stem Cell Res. Ther. 2018, 9, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domhan, S.; Ma, L.; Tai, A.; Anaya, Z.; Beheshti, A.; Zeier, M.; Hlatky, L.; Abdollahi, A. Intercellular Communication by Exchange of Cytoplasmic Material via Tunneling Nano-Tube Like Structures in Primary Human Renal Epithelial Cells. PLoS ONE 2011, 6, e21283. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, Y. Membrane Nanotubes: Novel Communication between Distant Cells. Sci. China Life Sci. 2013, 56, 994–999. [Google Scholar] [CrossRef] [Green Version]
- Resnik, N.; Prezelj, T.; De Luca, G.M.R.; Manders, E.; Polishchuk, R.; Veranič, P.; Kreft, M.E. Helical Organization of Microtubules Occurs in a Minority of Tunneling Membrane Nanotubes in Normal and Cancer Urothelial Cells. Sci. Rep. 2018, 8, 17133. [Google Scholar] [CrossRef]
- Dupont, M.; Souriant, S.; Lugo-Villarino, G.; Maridonneau-Parini, I.; Vérollet, C. Tunneling Nanotubes: Intimate Communication between Myeloid Cells. Front. Immunol. 2018, 9, 43. [Google Scholar] [CrossRef] [Green Version]
- Driscoll, J.; Gondaliya, P.; Patel, T. Tunneling Nanotube-Mediated Communication: A Mechanism of Intercellular Nucleic Acid Transfer. Int. J. Mol. Sci. 2022, 23, 5487. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.-H.; Xiao, H.; Wu, J.-M.; He, K.-M.; Lv, Z.-Z.; Li, Z.-J.; Xu, M.; Zhang, Y.-Y. Mitochondria Are Transported along Microtubules in Membrane Nanotubes to Rescue Distressed Cardiomyocytes from Apoptosis. Cell Death Dis. 2018, 9, 81. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.; Mukherjee, S.; Pattnaik, B.; Kumar, M.; Singh, S.; Kumar, M.; Rehman, R.; Tiwari, B.K.; Jha, K.A.; Barhanpurkar, A.P.; et al. Miro1 Regulates Intercellular Mitochondrial Transport & Enhances Mesenchymal Stem Cell Rescue Efficacy. EMBO J. 2014, 33, 994–1010. [Google Scholar] [CrossRef] [PubMed]
- Tang, B. MIRO GTPases in Mitochondrial Transport, Homeostasis and Pathology. Cells 2015, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Drab, M.; Stopar, D.; Kralj-Iglič, V.; Iglič, A. Inception Mechanisms of Tunneling Nanotubes. Cells 2019, 8, 626. [Google Scholar] [CrossRef] [Green Version]
- Vignais, M.-L.; Caicedo, A.; Brondello, J.-M.; Jorgensen, C. Cell Connections by Tunneling Nanotubes: Effects of Mitochondrial Trafficking on Target Cell Metabolism, Homeostasis, and Response to Therapy. Stem Cells Int. 2017, 2017, 6917941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaronson, S. The Synthesis of Extracellular Macromolecules and Membranes by a Population of the Phytoflagellate Ochromonas Danica 1: Extracellular Secretion by Ochromonas. Limnol. Oceanogr. 1971, 16, 1–9. [Google Scholar] [CrossRef]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B Lymphocytes Secrete Antigen-Presenting Vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Kroemer, G.; Zitvogel, L. Extracellular Vesicles: Masters of Intercellular Communication and Potential Clinical Interventions. J. Clin. Investig. 2016, 126, 1139–1143. [Google Scholar] [CrossRef] [Green Version]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and Challenges of Extracellular Vesicle-Based Drug Delivery System: Considering Cell Source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef] [Green Version]
- Mir, B.; Goettsch, C. Extracellular Vesicles as Delivery Vehicles of Specific Cellular Cargo. Cells 2020, 9, 1601. [Google Scholar] [CrossRef]
- Peruzzotti-Jametti, L.; Bernstock, J.D.; Willis, C.M.; Manferrari, G.; Rogall, R.; Fernandez-Vizarra, E.; Williamson, J.C.; Braga, A.; van den Bosch, A.; Leonardi, T.; et al. Neural Stem Cells Traffic Functional Mitochondria via Extracellular Vesicles. PLoS Biol. 2021, 19, e3001166. [Google Scholar] [CrossRef]
- Ratajczak, M.Z.; Ratajczak, J. Horizontal Transfer of RNA and Proteins between Cells by Extracellular Microvesicles: 14 Years Later. Clin. Transl. Med. 2016, 5, 7. [Google Scholar] [CrossRef]
- Mao, J.; Li, C.; Wu, F.; She, Z.; Luo, S.; Chen, X.; Wen, C.; Tian, J. MSC-EVs Transferring Mitochondria and Related Components: A New Hope for the Treatment of Kidney Disease. Front. Immunol. 2022, 13, 978571. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.N.; Das, S.R.; Emin, M.T.; Wei, M.; Sun, L.; Westphalen, K.; Rowlands, D.J.; Quadri, S.K.; Bhattacharya, S.; Bhattacharya, J. Mitochondrial Transfer from Bone-Marrow–Derived Stromal Cells to Pulmonary Alveoli Protects against Acute Lung Injury. Nat. Med. 2012, 18, 759–765. [Google Scholar] [CrossRef] [Green Version]
- Hough, K.P.; Trevor, J.L.; Strenkowski, J.G.; Wang, Y.; Chacko, B.K.; Tousif, S.; Chanda, D.; Steele, C.; Antony, V.B.; Dokland, T.; et al. Exosomal Transfer of Mitochondria from Airway Myeloid-Derived Regulatory Cells to T Cells. Redox Biol. 2018, 18, 54–64. [Google Scholar] [CrossRef]
- Hayakawa, K.; Esposito, E.; Wang, X.; Terasaki, Y.; Liu, Y.; Xing, C.; Ji, X.; Lo, E.H. Transfer of Mitochondria from Astrocytes to Neurons after Stroke. Nature 2016, 535, 551–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todkar, K.; Chikhi, L.; Desjardins, V.; El-Mortada, F.; Pépin, G.; Germain, M. Selective Packaging of Mitochondrial Proteins into Extracellular Vesicles Prevents the Release of Mitochondrial DAMPs. Nat. Commun. 2021, 12, 1971. [Google Scholar] [CrossRef]
- Fuentes, P.; Sesé, M.; Guijarro, P.J.; Emperador, M.; Sánchez-Redondo, S.; Peinado, H.; Hümmer, S.; Ramón y Cajal, S. ITGB3-Mediated Uptake of Small Extracellular Vesicles Facilitates Intercellular Communication in Breast Cancer Cells. Nat. Commun. 2020, 11, 4261. [Google Scholar] [CrossRef]
- Van Niel, G.; D’Angelo, G.; Raposo, G. Shedding Light on the Cell Biology of Extracellular Vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tan, J.; Miao, Y.; Zhang, Q. The Effect of Extracellular Vesicles on the Regulation of Mitochondria under Hypoxia. Cell Death Dis. 2021, 12, 358. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Liang, M.-Z.; Chen, L. Current Progress of Mitochondrial Transplantation That Promotes Neuronal Regeneration. Transl. Neurodegener. 2019, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Tria, F.D.K.; Brueckner, J.; Skejo, J.; Xavier, J.C.; Kapust, N.; Knopp, M.; Wimmer, J.L.E.; Nagies, F.S.P.; Zimorski, V.; Gould, S.B.; et al. Gene Duplications Trace Mitochondria to the Onset of Eukaryote Complexity. Genome Biol. Evol. 2021, 13, evab055. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, Y.; Qi, Z.; Cao, L.; Ding, S. Mitochondrial Transfer/Transplantation: An Emerging Therapeutic Approach for Multiple Diseases. Cell Biosci. 2022, 12, 66. [Google Scholar] [CrossRef]
- Park, A.; Oh, M.; Lee, S.J.; Oh, K.-J.; Lee, E.-W.; Lee, S.C.; Bae, K.-H.; Han, B.S.; Kim, W.K. Mitochondrial Transplantation as a Novel Therapeutic Strategy for Mitochondrial Diseases. Int. J. Mol. Sci. 2021, 22, 4793. [Google Scholar] [CrossRef]
- Lightowlers, R.N.; Chrzanowska-Lightowlers, Z.M.; Russell, O.M. Mitochondrial Transplantation—A Possible Therapeutic for Mitochondrial Dysfunction? EMBO Rep. 2020, 21, e50964. [Google Scholar] [CrossRef]
- Clark, M.A.; Shay, J.W. Mitochondrial Transformation of Mammalian Cells. Nature 1982, 295, 605–607. [Google Scholar] [CrossRef]
- Caicedo, A.; Aponte, P.M.; Cabrera, F.; Hidalgo, C.; Khoury, M. Artificial Mitochondria Transfer: Current Challenges, Advances, and Future Applications. Stem Cells Int. 2017, 2017, 7610414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, M.P.; Attardi, G. Injection of Mitochondria into Human Cells Leads to a Rapid Replacement of the Endogenous Mitochondrial DNA. Cell 1988, 52, 811–819. [Google Scholar] [CrossRef]
- Pinkert, C.A.; Irwin, M.H.; Johnson, L.W.; Moffatt, R.J. Mitochondria Transfer into Mouse Ova by Microinjection. Transgenic Res. 1997, 6, 379–383. [Google Scholar] [CrossRef]
- Reznichenko, A.; Huyser, C.; Pepper, M. Mitochondrial Transfer: Implications for Assisted Reproductive Technologies. Appl. Transl. Genom. 2016, 11, 40–47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali Pour, P.; Kenney, M.C.; Kheradvar, A. Bioenergetics Consequences of Mitochondrial Transplantation in Cardiomyocytes. J. Am. Heart Assoc. 2020, 9, e014501. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Kami, D.; Maeda, R.; Shikuma, A.; Gojo, S. Generation of Somatic Mitochondrial DNA-Replaced Cells for Mitochondrial Dysfunction Treatment. Sci. Rep. 2021, 11, 10897. [Google Scholar] [CrossRef] [PubMed]
- Reilly, W.M.; Obara, C.J. Advances in Confocal Microscopy and Selected Applications. In Confocal Microscopy; Brzostowski, J., Sohn, H., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2021; Volume 2304, pp. 1–35. ISBN 978-1-07-161401-3. [Google Scholar]
- King, M.P.; Attardi, G. Human Cells Lacking MtDNA: Repopulation with Exogenous Mitochondria by Complementation. Science 1989, 246, 500–503. [Google Scholar] [CrossRef] [PubMed]
- Gomzikova, M.O.; James, V.; Rizvanov, A.A. Mitochondria Donation by Mesenchymal Stem Cells: Current Understanding and Mitochondria Transplantation Strategies. Front. Cell Dev. Biol. 2021, 9, 653322. [Google Scholar] [CrossRef]
- Chang, J.-C.; Hoel, F.; Liu, K.-H.; Wei, Y.-H.; Cheng, F.-C.; Kuo, S.-J.; Tronstad, K.J.; Liu, C.-S. Peptide-Mediated Delivery of Donor Mitochondria Improves Mitochondrial Function and Cell Viability in Human Cybrid Cells with the MELAS A3243G Mutation. Sci. Rep. 2017, 7, 10710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H.; Kami, D.; Maeda, R.; Murata, Y.; Jo, J.; Kitani, T.; Tabata, Y.; Matoba, S.; Gojo, S. TAT-dextran–Mediated Mitochondrial Transfer Enhances Recovery from Models of Reperfusion Injury in Cultured Cardiomyocytes. J. Cell. Mol. Med. 2020, 24, 5007–5020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caicedo, A.; Fritz, V.; Brondello, J.-M.; Ayala, M.; Dennemont, I.; Abdellaoui, N.; de Fraipont, F.; Moisan, A.; Prouteau, C.A.; Boukhaddaoui, H.; et al. MitoCeption as a New Tool to Assess the Effects of Mesenchymal Stem/Stromal Cell Mitochondria on Cancer Cell Metabolism and Function. Sci. Rep. 2015, 5, 9073. [Google Scholar] [CrossRef] [Green Version]
- Cabrera, F.; Ortega, M.; Velarde, F.; Parra, E.; Gallardo, S.; Barba, D.; Soto, L.; Peña, G.; Pedroza, L.A.; Jorgensen, C.; et al. Primary Allogeneic Mitochondrial Mix (PAMM) Transfer/Transplant by MitoCeption to Address Damage in PBMCs Caused by Ultraviolet Radiation. BMC Biotechnol. 2019, 19, 42. [Google Scholar] [CrossRef]
- Kim, M.J.; Hwang, J.W.; Yun, C.-K.; Lee, Y.; Choi, Y.-S. Delivery of Exogenous Mitochondria via Centrifugation Enhances Cellular Metabolic Function. Sci. Rep. 2018, 8, 3330. [Google Scholar] [CrossRef] [Green Version]
- Ulger, O.; Kubat, G.B. Therapeutic Applications of Mitochondrial Transplantation. Biochimie 2022, 195, 1–15. [Google Scholar] [CrossRef]
- Wu, T.-H.; Sagullo, E.; Case, D.; Zheng, X.; Li, Y.; Hong, J.S.; TeSlaa, T.; Patananan, A.N.; McCaffery, J.M.; Niazi, K.; et al. Mitochondrial Transfer by Photothermal Nanoblade Restores Metabolite Profile in Mammalian Cells. Cell Metab. 2016, 23, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.-C.; Wu, T.-H.; Clemens, D.L.; Lee, B.-Y.; Wen, X.; Horwitz, M.A.; Teitell, M.A.; Chiou, P.-Y. Massively Parallel Delivery of Large Cargo into Mammalian Cells with Light Pulses. Nat. Methods 2015, 12, 439–444. [Google Scholar] [CrossRef]
- Sercel, A.J.; Patananan, A.N.; Man, T.; Wu, T.-H.; Yu, A.K.; Guyot, G.W.; Rabizadeh, S.; Niazi, K.R.; Chiou, P.-Y.; Teitell, M.A. Stable Transplantation of Human Mitochondrial DNA by High-Throughput, Pressurized Isolated Mitochondrial Delivery. eLife 2021, 10, e63102. [Google Scholar] [CrossRef] [PubMed]
- Kubat, G.B.; Ulger, O.; Akin, S. Requirements for Successful Mitochondrial Transplantation. J. Biochem. Mol. Toxicol. 2021, 35, e22898. [Google Scholar] [CrossRef] [PubMed]
- Gäbelein, C.G.; Feng, Q.; Sarajlic, E.; Zambelli, T.; Guillaume-Gentil, O.; Kornmann, B.; Vorholt, J.A. Mitochondria Transplantation between Living Cells. PLoS Biol. 2022, 20, e3001576. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, G.; Santoso, M.R.; Tada, Y.; Li, A.M.; Vaskova, E.; Jung, J.-H.; O’Brien, C.; Egan, E.; Ye, J.; Yang, P.C. Mitochondria-Rich Extracellular Vesicles From Autologous Stem Cell–Derived Cardiomyocytes Restore Energetics of Ischemic Myocardium. J. Am. Coll. Cardiol. 2021, 77, 1073–1088. [Google Scholar] [CrossRef]
- McCully, J.D.; Cowan, D.B.; Pacak, C.A.; Toumpoulis, I.K.; Dayalan, H.; Levitsky, S. Injection of Isolated Mitochondria during Early Reperfusion for Cardioprotection. Am. J. Physiol.-Heart Circ. Physiol. 2009, 296, H94–H105. [Google Scholar] [CrossRef] [Green Version]
- Masuzawa, A.; Black, K.M.; Pacak, C.A.; Ericsson, M.; Barnett, R.J.; Drumm, C.; Seth, P.; Bloch, D.B.; Levitsky, S.; Cowan, D.B.; et al. Transplantation of Autologously Derived Mitochondria Protects the Heart from Ischemia-Reperfusion Injury. Am. J. Physiol.-Heart Circ. Physiol. 2013, 304, H966–H982. [Google Scholar] [CrossRef]
- Cowan, D.B.; Yao, R.; Akurathi, V.; Snay, E.R.; Thedsanamoorthy, J.K.; Zurakowski, D.; Ericsson, M.; Friehs, I.; Wu, Y.; Levitsky, S.; et al. Intracoronary Delivery of Mitochondria to the Ischemic Heart for Cardioprotection. PLoS ONE 2016, 11, e0160889. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Ito, M.; Arai, M.; Hibino, M.; Tsujioka, T.; Harashima, H. Challenges in Promoting Mitochondrial Transplantation Therapy. Int. J. Mol. Sci. 2020, 21, 6365. [Google Scholar] [CrossRef]
- Emani, S.M.; Piekarski, B.L.; Harrild, D.; del Nido, P.J.; McCully, J.D. Autologous Mitochondrial Transplantation for Dysfunction after Ischemia-Reperfusion Injury. J. Thorac. Cardiovasc. Surg. 2017, 154, 286–289. [Google Scholar] [CrossRef] [Green Version]
- Kaza, A.K.; Wamala, I.; Friehs, I.; Kuebler, J.D.; Rathod, R.H.; Berra, I.; Ericsson, M.; Yao, R.; Thedsanamoorthy, J.K.; Zurakowski, D.; et al. Myocardial Rescue with Autologous Mitochondrial Transplantation in a Porcine Model of Ischemia/Reperfusion. J. Thorac. Cardiovasc. Surg. 2017, 153, 934–943. [Google Scholar] [CrossRef]
- Moskowitzova, K.; Shin, B.; Liu, K.; Ramirez-Barbieri, G.; Guariento, A.; Blitzer, D.; Thedsanamoorthy, J.K.; Yao, R.; Snay, E.R.; Inkster, J.A.H.; et al. Mitochondrial Transplantation Prolongs Cold Ischemia Time in Murine Heart Transplantation. J. Heart Lung Transplant. 2019, 38, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Saeed, M.Y.; Esch, J.J.; Guariento, A.; Blitzer, D.; Moskowitzova, K.; Ramirez-Barbieri, G.; Orfany, A.; Thedsanamoorthy, J.K.; Cowan, D.B.; et al. A Novel Biological Strategy for Myocardial Protection by Intracoronary Delivery of Mitochondria: Safety and Efficacy. JACC Basic Transl. Sci. 2019, 4, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Blitzer, D.; Guariento, A.; Doulamis, I.P.; Shin, B.; Moskowitzova, K.; Barbieri, G.R.; Orfany, A.; del Nido, P.J.; McCully, J.D. Delayed Transplantation of Autologous Mitochondria for Cardioprotection in a Porcine Model. Ann. Thorac. Surg. 2020, 109, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Guariento, A.; Blitzer, D.; Doulamis, I.; Shin, B.; Moskowitzova, K.; Orfany, A.; Ramirez-Barbieri, G.; Staffa, S.J.; Zurakowski, D.; del Nido, P.J.; et al. Preischemic Autologous Mitochondrial Transplantation by Intracoronary Injection for Myocardial Protection. J. Thorac. Cardiovasc. Surg. 2020, 160, e15–e29. [Google Scholar] [CrossRef] [PubMed]
- Weixler, V.; Lapusca, R.; Grangl, G.; Guariento, A.; Saeed, M.Y.; Cowan, D.B.; del Nido, P.J.; McCully, J.D.; Friehs, I. Autogenous Mitochondria Transplantation for Treatment of Right Heart Failure. J. Thorac. Cardiovasc. Surg. 2021, 162, e111–e121. [Google Scholar] [CrossRef] [PubMed]
- Doulamis, I.P.; Guariento, A.; Duignan, T.; Orfany, A.; Kido, T.; Zurakowski, D.; del Nido, P.J.; McCully, J.D. Mitochondrial Transplantation for Myocardial Protection in Diabetic Hearts. Eur. J. Cardio-Thorac. Surg. 2020, 57, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gao, R.; Li, W.; Zhao, Y.; Yang, H.; Chen, H.; Jiang, H.; Dong, Z.; Hu, J.; Liu, J.; et al. Alda-1 Treatment Promotes the Therapeutic Effect of Mitochondrial Transplantation for Myocardial Ischemia-Reperfusion Injury. Bioact. Mater. 2021, 6, 2058–2069. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, H.; Gao, R.; Qu, Y.; Huang, Y.; Zhang, N.; Hu, S.; Fan, F.; Zou, Y.; Hu, K.; et al. Intravenous Transplantation of an Ischemic-Specific Peptide-TPP-Mitochondrial Compound Alleviates Myocardial Ischemic Reperfusion Injury. ACS Nano 2023. [Google Scholar] [CrossRef]
- Lin, H.-C.; Liu, S.-Y.; Lai, H.-S.; Lai, I.-R. Isolated Mitochondria Infusion Mitigates Ischemia-Reperfusion Injury of the Liver in Rats. Shock 2013, 39, 304–310. [Google Scholar] [CrossRef]
- Fu, A.; Shi, X.; Zhang, H.; Fu, B. Mitotherapy for Fatty Liver by Intravenous Administration of Exogenous Mitochondria in Male Mice. Front. Pharmacol. 2017, 8, 241. [Google Scholar] [CrossRef]
- Shi, X.; Bai, H.; Zhao, M.; Li, X.; Sun, X.; Jiang, H.; Fu, A. Treatment of Acetaminophen-Induced Liver Injury with Exogenous Mitochondria in Mice. Transl. Res. 2018, 196, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Khouri-Farah, N.; Wu, C.H.; Wu, G.Y. Targeted Delivery of Mitochondria to the Liver in Rats. J. Gastroenterol. Hepatol. 2020, 35, 2241–2247. [Google Scholar] [CrossRef]
- Ko, S.; Chen, Y.; Sung, P.; Chiang, J.Y.; Chu, Y.; Huang, C.; Huang, C.; Yip, H. Hepatic 31 P-magnetic Resonance Spectroscopy Identified the Impact of Melatonin-pretreated Mitochondria in Acute Liver Ischaemia-reperfusion Injury. J. Cell. Mol. Med. 2020, 24, 10088–10099. [Google Scholar] [CrossRef] [PubMed]
- Ulger, O.; Kubat, G.B.; Cicek, Z.; Celik, E.; Atalay, O.; Suvay, S.; Ozler, M. The Effects of Mitochondrial Transplantation in Acetaminophen-Induced Liver Toxicity in Rats. Life Sci. 2021, 279, 119669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Hou, Y.; Zhou, W.; Keerthiga, R.; Fu, A. Mitochondrial Transplantation Therapy Inhibit Carbon Tetrachloride-induced Liver Injury through Scavenging Free Radicals and Protecting Hepatocytes. Bioeng. Transl. Med. 2021, 6, 69–83. [Google Scholar] [CrossRef]
- Su, Y.; Zhu, L.; Yu, X.; Cai, L.; Lu, Y.; Zhang, J.; Li, T.; Li, J.; Xia, J.; Xu, F.; et al. Mitochondrial Transplantation Attenuates Airway Hyperresponsiveness by Inhibition of Cholinergic Hyperactivity. Theranostics 2016, 6, 1244–1260, Erratum in Theranostics 2019, 9, 1385–1386. [Google Scholar] [CrossRef] [Green Version]
- Fu, A.; Hou, Y.; Yu, Z.; Zhao, Z.; Liu, Z. Healthy Mitochondria Inhibit the Metastatic Melanoma in Lungs. Int. J. Biol. Sci. 2019, 15, 2707–2718. [Google Scholar] [CrossRef]
- Moskowitzova, K.; Orfany, A.; Liu, K.; Ramirez-Barbieri, G.; Thedsanamoorthy, J.K.; Yao, R.; Guariento, A.; Doulamis, I.P.; Blitzer, D.; Shin, B.; et al. Mitochondrial Transplantation Enhances Murine Lung Viability and Recovery after Ischemia-Reperfusion Injury. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2020, 318, L78–L88. [Google Scholar] [CrossRef]
- de Carvalho, L.R.P.; Abreu, S.C.; de Castro, L.L.; Andrade da Silva, L.H.; Silva, P.M.; Vieira, J.B.; Santos, R.T.; Cabral, M.R.; Khoury, M.; Weiss, D.J.; et al. Mitochondria-Rich Fraction Isolated From Mesenchymal Stromal Cells Reduces Lung and Distal Organ Injury in Experimental Sepsis. Crit. Care Med. 2021, 49, e880–e890. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Roan, J.-N.; Fang, S.-Y.; Chiu, M.-H.; Cheng, T.-T.; Huang, C.-C.; Lin, M.-W.; Lam, C.-F. Transplantation of Viable Mitochondria Improves Right Ventricular Performance and Pulmonary Artery Remodeling in Rats with Pulmonary Arterial Hypertension. J. Thorac. Cardiovasc. Surg. 2022, 163, e361–e373. [Google Scholar] [CrossRef]
- Huang, P.-J.; Kuo, C.-C.; Lee, H.-C.; Shen, C.-I.; Cheng, F.-C.; Wu, S.-F.; Chang, J.-C.; Pan, H.-C.; Lin, S.-Z.; Liu, C.-S.; et al. Transferring Xenogenic Mitochondria Provides Neural Protection against Ischemic Stress in Ischemic Rat Brains. Cell Transpl. 2016, 25, 913–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Ma, Z.; Yan, C.; Pu, K.; Wu, M.; Bai, J.; Li, Y.; Wang, Q. Muscle-Derived Autologous Mitochondrial Transplantation: A Novel Strategy for Treating Cerebral Ischemic Injury. Behav. Brain Res. 2019, 356, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Zeng, J.; Zheng, Y.; Li, T.; Ren, J.; Chen, K.; Zhang, Q.; Xie, R.; Xu, F.; Zhu, J. Mitochondrial Transplantation Attenuates Cerebral Ischemia-Reperfusion Injury: Possible Involvement of Mitochondrial Component Separation. Oxidative Med. Cell. Longev. 2021, 2021, 1006636. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Lo, E.H.; Hayakawa, K. Placental Mitochondria Therapy for Cerebral Ischemia-Reperfusion Injury in Mice. Stroke 2020, 51, 3142–3146. [Google Scholar] [CrossRef] [PubMed]
- Orrego, M.A.; Levy, S.; Kelly, C.; Arroyo, G.; Toribio, L.; García, H.H.; Walker, M. Procedimiento Para La Infusión de Mitocondrias Autólogas Por La Arteria Carótida En El Cerebro Porcino. Rev. Peru. Med. Exp. Salud Pública 2021, 38, 345–351. [Google Scholar] [CrossRef]
- Pourmohammadi-Bejarpasi, Z.; Roushandeh, A.M.; Saberi, A.; Rostami, M.K.; Toosi, S.M.R.; Jahanian-Najafabadi, A.; Tomita, K.; Kuwahara, Y.; Sato, T.; Roudkenar, M.H. Mesenchymal Stem Cells-Derived Mitochondria Transplantation Mitigates I/R-Induced Injury, Abolishes I/R-Induced Apoptosis, and Restores Motor Function in Acute Ischemia Stroke Rat Model. Brain Res. Bull. 2020, 165, 70–80. [Google Scholar] [CrossRef]
- Hosseini, L.; Karimipour, M.; Seyedaghamiri, F.; Abolhasanpour, N.; Sadigh-Eteghad, S.; Mahmoudi, J.; Farhoudi, M. Intranasal Administration of Mitochondria Alleviated Cognitive Impairments and Mitochondrial Dysfunction in the Photothrombotic Model of MPFC Stroke in Mice. J. Stroke Cerebrovasc. Dis. 2022, 31, 106801. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, Y.; Li, Q.; Sun, D.; Dong, X.; Li, X.; Xin, W.; Zhang, J. Effects of Brain-Derived Mitochondria on the Function of Neuron and Vascular Endothelial Cell After Traumatic Brain Injury. World Neurosurg. 2020, 138, e1–e9. [Google Scholar] [CrossRef]
- Chang, J.-C.; Wu, S.-L.; Liu, K.-H.; Chen, Y.-H.; Chuang, C.-S.; Cheng, F.-C.; Su, H.-L.; Wei, Y.-H.; Kuo, S.-J.; Liu, C.-S. Allogeneic/Xenogeneic Transplantation of Peptide-Labeled Mitochondria in Parkinson’s Disease: Restoration of Mitochondria Functions and Attenuation of 6-Hydroxydopamine–Induced Neurotoxicity. Transl. Res. 2016, 170, 40–56.e3. [Google Scholar] [CrossRef]
- Shi, X.; Zhao, M.; Fu, C.; Fu, A. Intravenous Administration of Mitochondria for Treating Experimental Parkinson’s Disease. Mitochondrion 2017, 34, 91–100. [Google Scholar] [CrossRef]
- Chang, J.-C.; Chao, Y.-C.; Chang, H.-S.; Wu, Y.-L.; Chang, H.-J.; Lin, Y.-S.; Cheng, W.-L.; Lin, T.-T.; Liu, C.-S. Intranasal Delivery of Mitochondria for Treatment of Parkinson’s Disease Model Rats Lesioned with 6-Hydroxydopamine. Sci. Rep. 2021, 11, 10597. [Google Scholar] [CrossRef] [PubMed]
- Robicsek, O.; Ene, H.M.; Karry, R.; Ytzhaki, O.; Asor, E.; McPhie, D.; Cohen, B.M.; Ben-Yehuda, R.; Weiner, I.; Ben-Shachar, D. Isolated Mitochondria Transfer Improves Neuronal Differentiation of Schizophrenia-Derived Induced Pluripotent Stem Cells and Rescues Deficits in a Rat Model of the Disorder. Schizophr. Bull. 2018, 44, 432–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitzan, K.; Benhamron, S.; Valitsky, M.; Kesner, E.E.; Lichtenstein, M.; Ben-Zvi, A.; Ella, E.; Segalstein, Y.; Saada, A.; Lorberboum-Galski, H.; et al. Mitochondrial Transfer Ameliorates Cognitive Deficits, Neuronal Loss, and Gliosis in Alzheimer’s Disease Mice. J. Alzheimer’s Dis. 2019, 72, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wei, D.; Li, Z.; Guo, H.; Wu, Y.; Feng, J. Hippocampal Mitochondrial Transplantation Alleviates Age-Associated Cognitive Decline via Enhancing Wnt Signaling and Neurogenesis. Comput. Intell. Neurosci. 2022, 2022, 9325302. [Google Scholar] [CrossRef]
- Bobkova, N.V.; Zhdanova, D.Y.; Belosludtseva, N.V.; Penkov, N.V.; Mironova, G.D. Intranasal Administration of Mitochondria Improves Spatial Memory in Olfactory Bulbectomized Mice. Exp. Biol. Med. 2022, 247, 416–425. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, T.; Tang, W.; Ma, Z.; Pu, K.; Xu, F.; Chang, H.; Zhao, G.; Gao, W.; Li, Y.; et al. Transplantation of Platelet-Derived Mitochondria Alleviates Cognitive Impairment and Mitochondrial Dysfunction in Db/Db Mice. Clin. Sci. 2020, 134, 2161–2175. [Google Scholar] [CrossRef]
- Wang, Y.; Ni, J.; Gao, C.; Xie, L.; Zhai, L.; Cui, G.; Yin, X. Mitochondrial Transplantation Attenuates Lipopolysaccharide- Induced Depression-like Behaviors. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 93, 240–249. [Google Scholar] [CrossRef]
- Javani, G.; Babri, S.; Farajdokht, F.; Ghaffari-Nasab, A.; Mohaddes, G. Mitochondrial Transplantation Improves Anxiety- and Depression-like Behaviors in Aged Stress-Exposed Rats. Mech. Ageing Dev. 2022, 202, 111632. [Google Scholar] [CrossRef]
- Lin, M.-W.; Fang, S.-Y.; Hsu, J.-Y.C.; Huang, C.-Y.; Lee, P.-H.; Huang, C.-C.; Chen, H.-F.; Lam, C.-F.; Lee, J.-S. Mitochondrial Transplantation Attenuates Neural Damage and Improves Locomotor Function After Traumatic Spinal Cord Injury in Rats. Front. Neurosci. 2022, 16, 800883. [Google Scholar] [CrossRef]
- Gollihue, J.L.; Patel, S.P.; Eldahan, K.C.; Cox, D.H.; Donahue, R.R.; Taylor, B.K.; Sullivan, P.G.; Rabchevsky, A.G. Effects of Mitochondrial Transplantation on Bioenergetics, Cellular Incorporation, and Functional Recovery after Spinal Cord Injury. J. Neurotrauma 2018, 35, 1800–1818. [Google Scholar] [CrossRef]
- Fang, S.-Y.; Roan, J.-N.; Lee, J.-S.; Chiu, M.-H.; Lin, M.-W.; Liu, C.-C.; Lam, C.-F. Transplantation of Viable Mitochondria Attenuates Neurologic Injury after Spinal Cord Ischemia. J. Thorac. Cardiovasc. Surg. 2021, 161, e337–e347. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yu, Z.; Hou, Y.; Zhang, L.; Fu, A. Improvement of Cognitive and Motor Performance with Mitotherapy in Aged Mice. Int. J. Biol. Sci. 2020, 16, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Nascimento-dos-Santos, G.; de-Souza-Ferreira, E.; Lani, R.; Faria, C.C.; Araújo, V.G.; Teixeira-Pinheiro, L.C.; Vasconcelos, T.; Gonçalo, T.; Santiago, M.F.; Linden, R.; et al. Neuroprotection from Optic Nerve Injury and Modulation of Oxidative Metabolism by Transplantation of Active Mitochondria to the Retina. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165686. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Ma, Z.; Ma, H.; Li, Q.; Zhai, Q.; Jiang, T.; Zhang, Z.; Wang, Q. Mitochondrial Transplantation Attenuates Brain Dysfunction in Sepsis by Driving Microglial M2 Polarization. Mol. Neurobiol. 2020, 57, 3875–3890. [Google Scholar] [CrossRef] [PubMed]
- Adlimoghaddam, A.; Benson, T.; Albensi, B.C. Mitochondrial Transfusion Improves Mitochondrial Function Through Up-Regulation of Mitochondrial Complex II Protein Subunit SDHB in the Hippocampus of Aged Mice. Mol. Neurobiol. 2022, 59, 6009–6017. [Google Scholar] [CrossRef]
- Norat, P.; Soldozy, S.; Sokolowski, J.D.; Gorick, C.M.; Kumar, J.S.; Chae, Y.; Yağmurlu, K.; Prada, F.; Walker, M.; Levitt, M.R.; et al. Mitochondrial Dysfunction in Neurological Disorders: Exploring Mitochondrial Transplantation. NPJ Regen. Med. 2020, 5, 22. [Google Scholar] [CrossRef]
- Jacoby, E.; Bar-Yosef, O.; Gruber, N.; Lahav, E.; Varda-Bloom, N.; Bolkier, Y.; Bar, D.; Blumkin, M.B.-Y.; Barak, S.; Eisenstein, E.; et al. Mitochondrial Augmentation of Hematopoietic Stem Cells in Children with Single Large-Scale Mitochondrial DNA Deletion Syndromes. Sci. Transl. Med. 2022, 14, eabo3724. [Google Scholar] [CrossRef]
- Rehman, M.U.; Sehar, N.; Dar, N.J.; Khan, A.; Arafah, A.; Rashid, S.; Rashid, S.M.; Ganaie, M.A. Mitochondrial Dysfunctions, Oxidative Stress and Neuroinflammation as Therapeutic Targets for Neurodegenerative Diseases: An Update on Current Advances and Impediments. Neurosci. Biobehav. Rev. 2022, 144, 104961. [Google Scholar] [CrossRef]
- Nascimento-dos-Santos, G.; de-Souza-Ferreira, E.; Linden, R.; Galina, A.; Petrs-Silva, H. Mitotherapy: Unraveling a Promising Treatment for Disorders of the Central Nervous System and Other Systemic Conditions. Cells 2021, 10, 1827. [Google Scholar] [CrossRef]
- Picone, P.; Nuzzo, D. Promising Treatment for Multiple Sclerosis: Mitochondrial Transplantation. Int. J. Mol. Sci. 2022, 23, 2245. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Nuevo, A.; Zorzano, A. The Sensing of Mitochondrial DAMPs by Non-Immune Cells. Cell Stress 2019, 3, 195–207. [Google Scholar] [CrossRef]
- Rickenbach, C.; Gericke, C. Specificity of Adaptive Immune Responses in Central Nervous System Health, Aging and Diseases. Front. Neurosci. 2022, 15, 806260. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Xu, H.; Bishawi, M.; Feng, F.; Samy, K.; Truskey, G.; Barbas, A.S.; Kirk, A.D.; Brennan, T.V. Circulating Mitochondria in Organ Donors Promote Allograft Rejection. Am. J. Transplant. 2019, 19, 1917–1929. [Google Scholar] [CrossRef]
- Pollara, J.; Edwards, R.W.; Lin, L.; Bendersky, V.A.; Brennan, T.V. Circulating Mitochondria in Deceased Organ Donors Are Associated with Immune Activation and Early Allograft Dysfunction. JCI Insight 2018, 3, e121622. [Google Scholar] [CrossRef]
- Zhang, T.; Miao, C. Mitochondrial Transplantation as a Promising Therapy for Mitochondrial Diseases. Acta Pharm. Sin. B 2022, S2211383522004257. [Google Scholar] [CrossRef]
- Dela Cruz, C.S.; Kang, M.-J. Mitochondrial Dysfunction and Damage Associated Molecular Patterns (DAMPs) in Chronic Inflammatory Diseases. Mitochondrion 2018, 41, 37–44. [Google Scholar] [CrossRef]
- Fernández-Vizarra, E.; Ferrín, G.; Pérez-Martos, A.; Fernández-Silva, P.; Zeviani, M.; Enríquez, J.A. Isolation of Mitochondria for Biogenetical Studies: An Update. Mitochondrion 2010, 10, 253–262. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, H. Common Methods in Mitochondrial Research (Review). Int. J. Mol. Med. 2022, 50, 126. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, W.B.; Harwood, C.L.; Prajapati, P.; Springer, J.E.; Saatman, K.E.; Sullivan, P.G. Fractionated Mitochondrial Magnetic Separation for Isolation of Synaptic Mitochondria from Brain Tissue. Sci. Rep. 2019, 9, 9656. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.-C.; Bergamini, C.; Fato, R.; Pon, L.A.; Pallotti, F. Isolation of Mitochondria from Cells and Tissues. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 155, pp. 3–31. ISBN 978-0-12-820228-9. [Google Scholar]
- McCully, J.D.; Cowan, D.B.; Emani, S.M.; del Nido, P.J. Mitochondrial Transplantation: From Animal Models to Clinical Use in Humans. Mitochondrion 2017, 34, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Nukala, V.N.; Singh, I.N.; Davis, L.M.; Sullivan, P.G. Cryopreservation of Brain Mitochondria: A Novel Methodology for Functional Studies. J. Neurosci. Methods 2006, 152, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, R.; Andreyev, A.; Murphy, A.N.; Perkins, G.A.; Ellisman, M.H.; Newmeyer, D.D. Mitochondria Frozen with Trehalose Retain a Number of Biological Functions and Preserve Outer Membrane Integrity. Cell Death Differ. 2007, 14, 616–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burrello, J.; Monticone, S.; Gai, C.; Gomez, Y.; Kholia, S.; Camussi, G. Stem Cell-Derived Extracellular Vesicles and Immune-Modulation. Front. Cell Dev. Biol. 2016, 4, 83. [Google Scholar] [CrossRef] [Green Version]
- Sugiura, A.; McLelland, G.; Fon, E.A.; McBride, H.M. A New Pathway for Mitochondrial Quality Control: Mitochondrial-derived Vesicles. EMBO J. 2014, 33, 2142–2156. [Google Scholar] [CrossRef] [Green Version]
- Picca, A.; Guerra, F.; Calvani, R.; Coelho-Junior, H.J.; Bossola, M.; Landi, F.; Bernabei, R.; Bucci, C.; Marzetti, E. Generation and Release of Mitochondrial-Derived Vesicles in Health, Aging and Disease. J. Clin. Med. 2020, 9, 1440. [Google Scholar] [CrossRef]
- Deus, C.M.; Tavares, H.; Beatriz, M.; Mota, S.; Lopes, C. Mitochondrial Damage-Associated Molecular Patterns Content in Extracellular Vesicles Promotes Early Inflammation in Neurodegenerative Disorders. Cells 2022, 11, 2364. [Google Scholar] [CrossRef]
- Dawson, E.R.; Patananan, A.N.; Sercel, A.J.; Teitell, M.A. Stable Retention of Chloramphenicol-Resistant MtDNA to Rescue Metabolically Impaired Cells. Sci. Rep. 2020, 10, 14328. [Google Scholar] [CrossRef]
- Cowan, D.B.; Yao, R.; Thedsanamoorthy, J.K.; Zurakowski, D.; del Nido, P.J.; McCully, J.D. Transit and Integration of Extracellular Mitochondria in Human Heart Cells. Sci. Rep. 2017, 7, 17450. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.-C.; Liu, K.-H.; Li, Y.-C.; Kou, S.-J.; Wei, Y.-H.; Chuang, C.-S.; Hsieh, M.; Liu, C.-S. Functional Recovery of Human Cells Harbouring the Mitochondrial DNA Mutation MERRF A8344G via Peptide-Mediated Mitochondrial Delivery. Neurosignals 2013, 21, 160–173. [Google Scholar] [CrossRef]
- Ni, H.-M.; Williams, J.A.; Ding, W.-X. Mitochondrial Dynamics and Mitochondrial Quality Control. Redox Biol. 2015, 4, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, M.P.; Gleichmann, M.; Cheng, A. Mitochondria in Neuroplasticity and Neurological Disorders. Neuron 2008, 60, 748–766. [Google Scholar] [CrossRef] [Green Version]
- Losón, O.C.; Song, Z.; Chen, H.; Chan, D.C. Fis1, Mff, MiD49, and MiD51 Mediate Drp1 Recruitment in Mitochondrial Fission. Mol. Biol. Cell 2013, 24, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Chan, D.C. Mitochondria: Dynamic Organelles in Disease, Aging, and Development. Cell 2006, 125, 1241–1252. [Google Scholar] [CrossRef] [Green Version]
- Burgstaller, J.P.; Johnston, I.G.; Poulton, J. Mitochondrial DNA Disease and Developmental Implications for Reproductive Strategies. Mol. Hum. Reprod. 2015, 21, 11–22. [Google Scholar] [CrossRef] [Green Version]
- Chinnery, P.F.; Craven, L.; Mitalipov, S.; Stewart, J.B.; Herbert, M.; Turnbull, D.M. The Challenges of Mitochondrial Replacement. PLoS Genet. 2014, 10, e1004315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soledad, R.B.; Charles, S.; Samarjit, D. The Secret Messages between Mitochondria and Nucleus in Muscle Cell Biology. Arch. Biochem. Biophys. 2019, 666, 52–62. [Google Scholar] [CrossRef]
- St. John, J.C. Genomic Balance: Two Genomes Establishing Synchrony to Modulate Cellular Fate and Function. Cells 2019, 8, 1306. [Google Scholar] [CrossRef] [Green Version]
- Picard, M.; Shirihai, O.S. Mitochondrial Signal Transduction. Cell Metab. 2022, 34, 1620–1653. [Google Scholar] [CrossRef]
- Nagao, Y.; Totsuka, Y.; Atomi, Y.; Kaneda, H.; Lindahl, K.F.; Imai, H.; Yonekawa, H. Decreased Physical Performance of Congenic Mice with Mismatch between the Nuclear and the Mitochondrial Genome. Genes Genet. Syst. 1998, 73, 21–27. [Google Scholar] [CrossRef]
- Acton, B.M.; Lai, I.; Shang, X.; Jurisicova, A.; Casper, R.F. Neutral Mitochondrial Heteroplasmy Alters Physiological Function in Mice1. Biol. Reprod. 2007, 77, 569–576. [Google Scholar] [CrossRef] [PubMed]

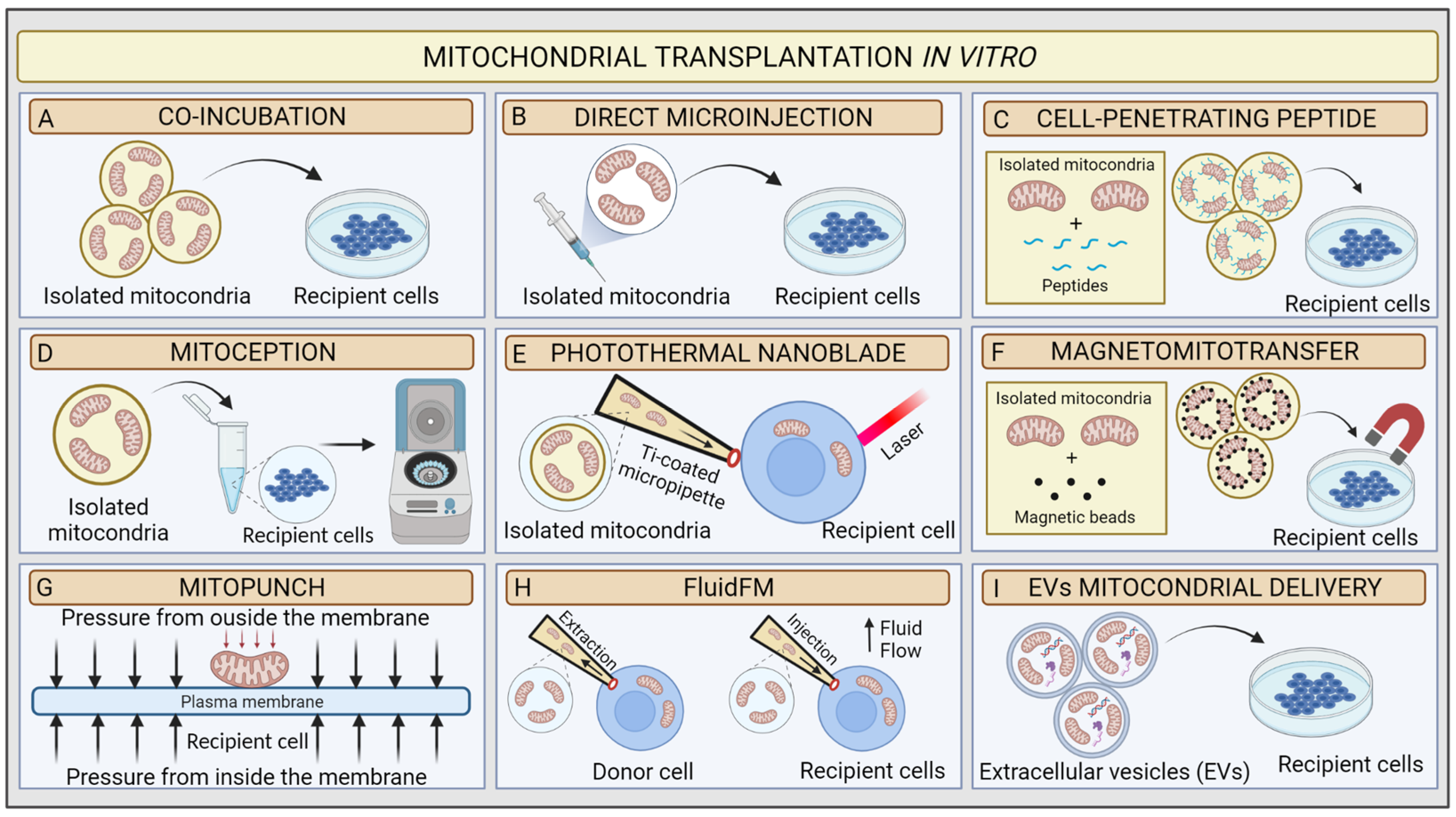
| In Vitro MT Methods | Pros | Cons | Remarks |
|---|---|---|---|
| Co-incubation | Reduced manipulation Broad number of transplanted recipient cells Easy to realise | Low accuracy Dependence on physiological state and uptake capacity of recipient cells Mitochondria dose-dependent High risk of mitochondria damage | mtDNA retention up to 12 passages Moderate transfer Efficiency |
| Microinjection | Successful regardless the physiological state and uptake capacity of the target cells | Potentially harmful for the target Limited number of cells that can be transplanted per experiment High risk of mitochondria damage | mtDNA is retained from 6–10 weeks after treatment |
| Cell-penetrating peptide | Low manipulation Increased uptake capacity rate of target cells | Unknown effect of Pep-1 on mitochondrial function High risk of mitochondria damage | mtDNA is retained 11 days after Treatment |
| MitoCeption | Time saving Successful regardless the physiological state and uptake capacity of the target cells | High manipulation Potentially harmful for the target Mitochondria dose-dependent High risk of mitochondria damage | mtDNA retention not known Moderate transfer Efficiency |
| Photothermal nanoblade | Rapid massively delivery Very accurate | High manipulation Specific equipment High risk of mitochondria damaging Limited number of transplanted cells | Stable retained 2% transfer efficiency |
| Magnetomitotransfer | Rapid massively delivery Very accurate | Specific supplies High risk of mitochondria damage | mtDNA retention not known High transfer Efficiency |
| Mitopunch | Rapid massively delivery | Not suitable for all cell types (only those attaching PET filter) Dependent on nDNA-mtDNA mismatch High risk of mitochondria damage | Stable retention Moderate transfer Efficiency |
| FluidFM | Mitochondria and cellular integrity preservation Minimally invasive | Specific equipment High cost | High transfer efficiency mtDNA retention is not known |
| EVs mitochondrial delivery | Low manipulation Mitochondrial and cellular integrity preservation Easy to realise | Mitochondria-rich-EV isolation | mtDNA retention not known |
| Targeted Organs | Species | Disease | Route of Administration | Studies Reference |
|---|---|---|---|---|
| Heart | Rabbits, pigs, rats, mice, piglets, humans | Heart regional/global ischaemia; heterotopic heart transplantation; right heart failure. | Local direct injection; intracoronary injection. | [107,108,109,110,112,113,114,115,116,117,118,119,120,121] |
| Liver | Rats, mice | Partial liver ischaemia; fatty liver; acetaminophen/carbon-tetrachloride-induced liver injury. | Intrasplenic injection; intravenously injection. | [122,123,124,125,126,127,128] |
| Lung | Rats, mice | Airway hyperresponsiveness; melanoma lung metastasis; acute lung ischaemia–reperfusion; pulmonary hypertension, experimental sepsis. | Intratracheally injection; intravenously injection; intracoronary injection; pulmonary artery injection. | [129,130,131,132,133] |
| Brain | Rats, mice, pigs | Stroke; Parkinson’s; schizophrenia; Alzheimer’s; age-associated cognitive decline, depression; spinal cord injury; optic nerve crush. | Intracerebral injection; systemic injection; intrathecal injection; intranasal injection; intracerebroventricular injection. | [71,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159] |
| Blood | Human | Single large-scale mtDNA deletion syndromes. | Intravenous reinfusion of CD34+ ex vivo transplanted cells. | [160] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Amato, M.; Morra, F.; Di Meo, I.; Tiranti, V. Mitochondrial Transplantation in Mitochondrial Medicine: Current Challenges and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 1969. https://doi.org/10.3390/ijms24031969
D’Amato M, Morra F, Di Meo I, Tiranti V. Mitochondrial Transplantation in Mitochondrial Medicine: Current Challenges and Future Perspectives. International Journal of Molecular Sciences. 2023; 24(3):1969. https://doi.org/10.3390/ijms24031969
Chicago/Turabian StyleD’Amato, Marco, Francesca Morra, Ivano Di Meo, and Valeria Tiranti. 2023. "Mitochondrial Transplantation in Mitochondrial Medicine: Current Challenges and Future Perspectives" International Journal of Molecular Sciences 24, no. 3: 1969. https://doi.org/10.3390/ijms24031969
APA StyleD’Amato, M., Morra, F., Di Meo, I., & Tiranti, V. (2023). Mitochondrial Transplantation in Mitochondrial Medicine: Current Challenges and Future Perspectives. International Journal of Molecular Sciences, 24(3), 1969. https://doi.org/10.3390/ijms24031969






