Effect of Clinical and Laboratory Parameters on HDL Particle Composition
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Impact of Serum Lipid Parameters on the HDL Lipidome
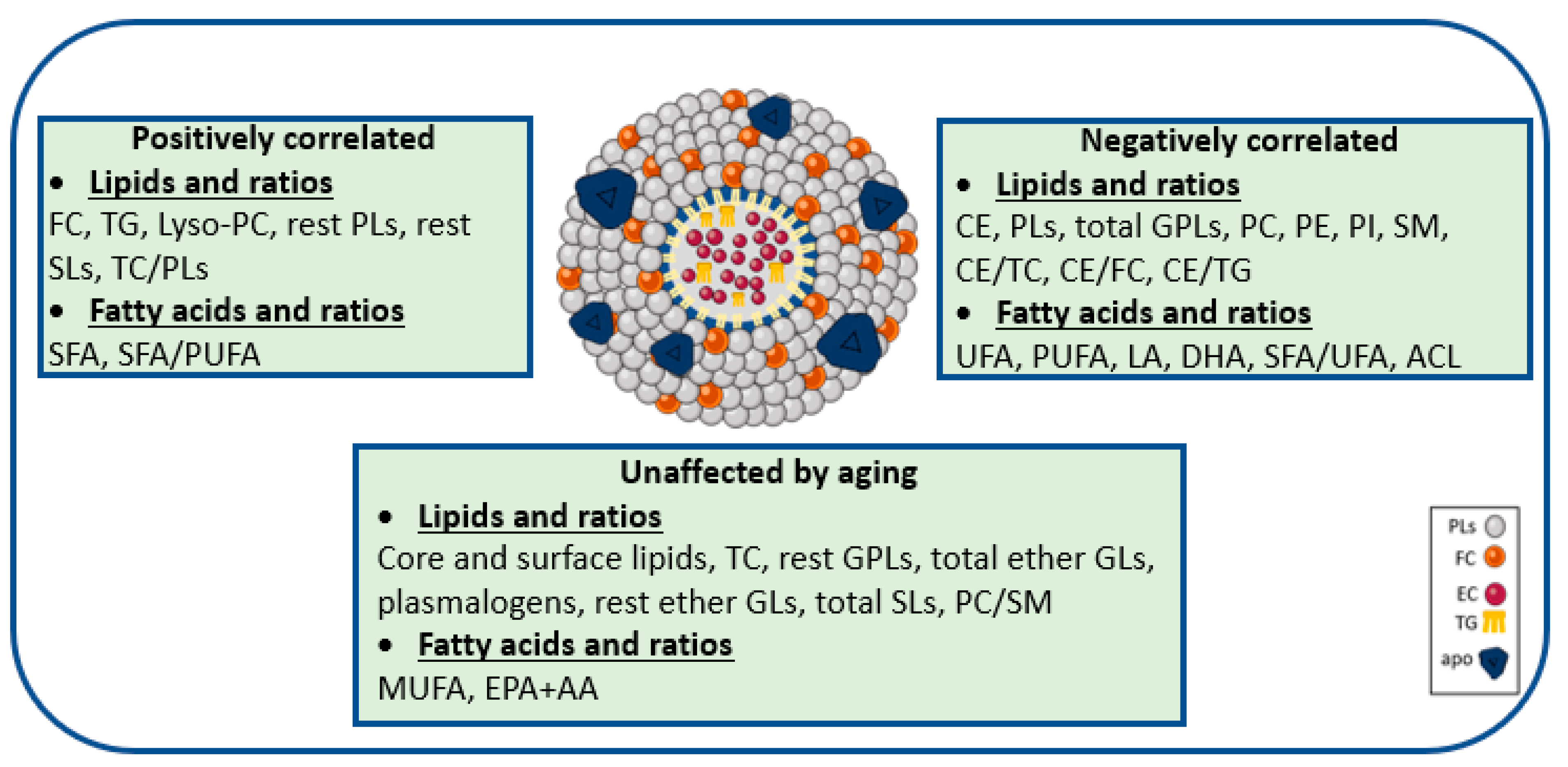
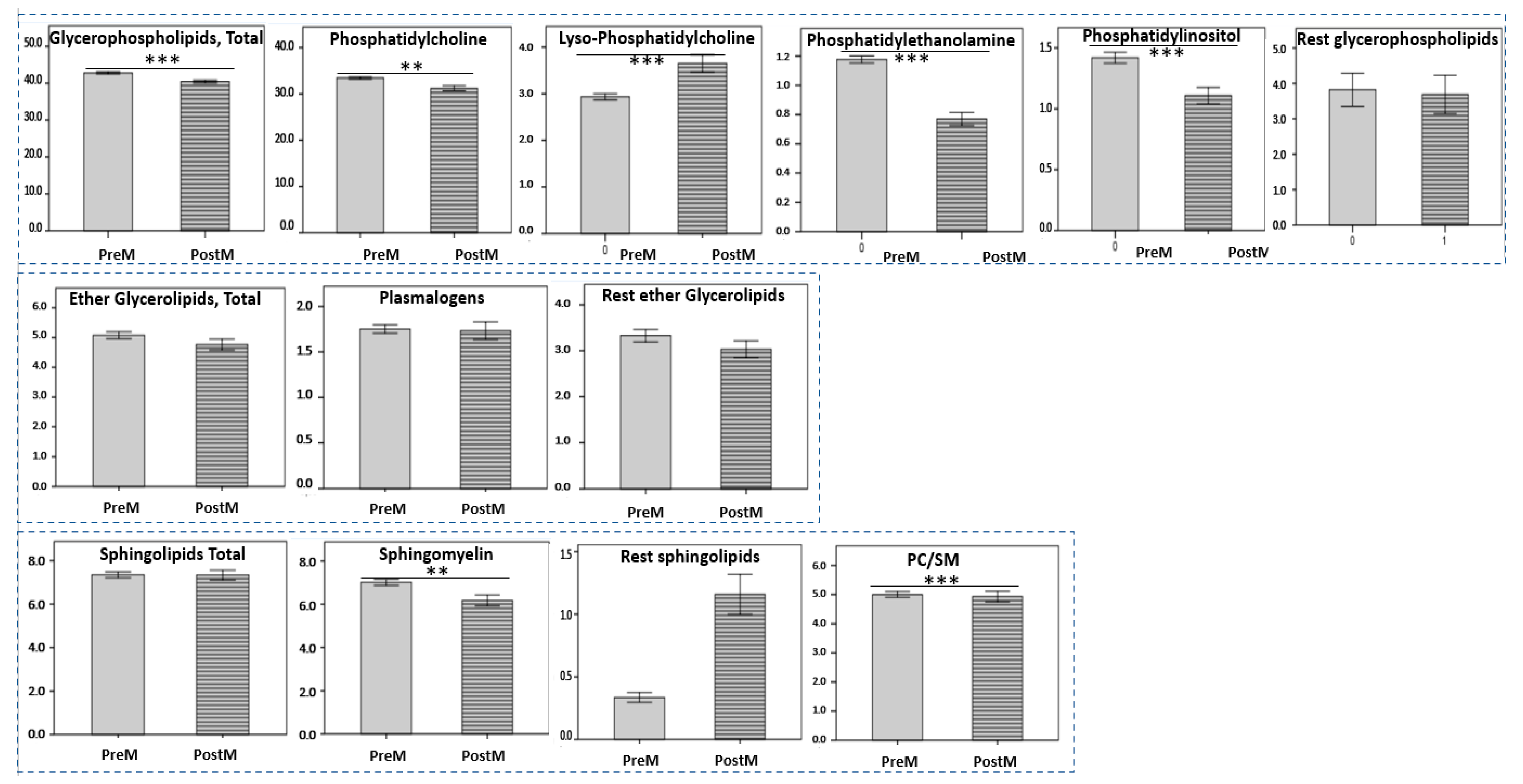
3.2. Aging-Specific Changes in HDL Lipidome
3.3. Gender-Specific Changes in HDL Lipidome
3.4. Menopause Status-Specific Changes in HDL Lipidome
4. Materials and Methods
4.1. HDL Lipidome Analysis by NMR Spectroscopy
4.2. Statistical Analysis of Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schoch, L.; Badimon, L.; Vilahur, G. Unraveling the Complexity of HDL Remodeling: On the Hunt to Restore HDL Quality. Biomedicines 2021, 9, 805. [Google Scholar] [CrossRef] [PubMed]
- von Eckardstein, A.; Nordestgaard, B.G.; Remaley, A.T.; Catapano, A.L. High-density lipoprotein revisited: Biological functions and clinical relevance. Eur. Heart J. 2022, ehac605. [Google Scholar] [CrossRef] [PubMed]
- Kostara, C.E.; Karakitsou, K.S.; Florentin, M.; Bairaktari, E.T.; Tsimihodimos, V. Progressive, Qualitative, and Quantitative Alterations in HDL Lipidome from Healthy Subjects to Patients with Prediabetes and Type 2 Diabetes. Metabolites 2022, 12, 683. [Google Scholar] [CrossRef]
- Kostara, C.E.; Ferrannini, E.; Bairaktari, E.T.; Papathanasiou, A.; Elisaf, M.; Tsimihodimos, V. Early Signs of Atherogenic Features in the HDL Lipidomes of Normolipidemic Patients Newly Diagnosed with Type 2 Diabetes. Int. J. Mol. Sci. 2020, 21, 8835. [Google Scholar] [CrossRef]
- de la Llera Moya, M.; McGillicuddy, F.C.; Hinkle, C.C.; Byrne, M.; Joshi, M.R.; Nguyen, V.; Tabita-Martinez, J.; Wolfe, M.L.; Badellino, K.; Pruscino, L.; et al. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis 2012, 222, 390–394. [Google Scholar] [CrossRef] [Green Version]
- Mocciaro, G.; D’Amore, S.; Jenkins, B.; Kay, R.; Murgia, A.; Herrera-Marcos, L.V.; Neun, S.; Sowton, A.P.; Hall, Z.; Palma-Duran, S.A.; et al. Lipidomic Approaches to Study HDL Metabolism in Patients with Central Obesity Diagnosed with Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 6786. [Google Scholar] [CrossRef]
- Bonizzi, A.; Piuri, G.; Corsi, F.; Cazzola, R.; Mazzucchelli, S. HDL Dysfunctionality: Clinical Relevance of Quality Rather Than Quantity. Biomedicines 2021, 9, 729. [Google Scholar] [CrossRef]
- Kontush, A.; Lhomme, M.; Chapman, M.J. Unraveling the complexities of the HDL lipidome. J. Lipid Res. 2013, 54, 2950–2963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohatgi, A.; Westerterp, M.; von Eckardstein, A.; Remaley, A.; Rye, K.A. HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research. Circulation 2021, 143, 2293–2309. [Google Scholar] [CrossRef]
- Salazar, J.; Olivar, L.C.; Ramos, E.; Chavez-Castillo, M.; Rojas, J.; Bermudez, V. Dysfunctional High-Density Lipoprotein: An Innovative Target for Proteomics and Lipidomics. Cholesterol 2015, 2015, 296417. [Google Scholar] [CrossRef]
- Lydic, T.A.; Goo, Y.H. Lipidomics unveils the complexity of the lipidome in metabolic diseases. Clin. Transl. Med. 2018, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mika, A.; Kaczynski, Z.; Stepnowski, P.; Kaczor, M.; Proczko-Stepaniak, M.; Kaska, L.; Sledzinski, T. Potential Application of (1)H NMR for Routine Serum Lipidome Analysis -Evaluation of Effects of Bariatric Surgery. Sci. Rep. 2017, 7, 15530. [Google Scholar] [CrossRef] [Green Version]
- Berezhnoy, G.; Laske, C.; Trautwein, C. Quantitative NMR-Based Lipoprotein Analysis Identifies Elevated HDL-4 and Triglycerides in the Serum of Alzheimer’s Disease Patients. Int. J. Mol. Sci. 2022, 23, 12472. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Vosegaard, T.; Guo, Z. Applications of nuclear magnetic resonance in lipid analyses: An emerging powerful tool for lipidomics studies. Prog. Lipid Res. 2017, 68, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.; Jankowski, V.; Bender, G.; Zewinger, S.; Rye, K.A.; van der Vorst, E.P.C. Interaction between high-density lipoproteins and inflammation: Function matters more than concentration! Adv. Drug Deliv. Rev. 2020, 159, 94–119. [Google Scholar] [CrossRef]
- Kostara, C.E.; Tsimihodimos, V.; Elisaf, M.S.; Bairaktari, E.T. NMR-Based Lipid Profiling of High Density Lipoprotein Particles in Healthy Subjects with Low, Normal, and Elevated HDL-Cholesterol. J. Proteome Res. 2017, 16, 1605–1616. [Google Scholar] [CrossRef]
- Holzer, M.; Trieb, M.; Konya, V.; Wadsack, C.; Heinemann, A.; Marsche, G. Aging affects high-density lipoprotein composition and function. Biochim. Biophys. Acta 2013, 1831, 1442–1448. [Google Scholar] [CrossRef] [Green Version]
- Rye, K.A.; Barter, P.J. Regulation of high-density lipoprotein metabolism. Circ. Res. 2014, 114, 143–156. [Google Scholar] [CrossRef] [Green Version]
- Calabresi, L.; Franceschini, G. Lecithin:cholesterol acyltransferase, high-density lipoproteins, and atheroprotection in humans. Trends Cardiovasc. Med. 2010, 20, 50–53. [Google Scholar] [CrossRef]
- Juhl, A.D.; Wustner, D. Pathways and Mechanisms of Cellular Cholesterol Efflux-Insight From Imaging. Front. Cell Dev. Biol. 2022, 10, 834408. [Google Scholar] [CrossRef]
- Schwendeman, A.; Sviridov, D.O.; Yuan, W.; Guo, Y.; Morin, E.E.; Yuan, Y.; Stonik, J.; Freeman, L.; Ossoli, A.; Thacker, S.; et al. The effect of phospholipid composition of reconstituted HDL on its cholesterol efflux and anti-inflammatory properties. J. Lipid Res. 2015, 56, 1727–1737. [Google Scholar] [CrossRef] [Green Version]
- Baker, P.W.; Rye, K.A.; Gamble, J.R.; Vadas, M.A.; Barter, P.J. Phospholipid composition of reconstituted high density lipoproteins influences their ability to inhibit endothelial cell adhesion molecule expression. J. Lipid Res. 2000, 41, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.A.; Stolzing, A. The role of lipid metabolism in aging, lifespan regulation, and age-related disease. Aging Cell 2019, 18, e13048. [Google Scholar] [CrossRef] [Green Version]
- Chung, K.W. Advances in Understanding of the Role of Lipid Metabolism in Aging. Cells 2021, 10, 880. [Google Scholar] [CrossRef]
- Walter, M. Interrelationships among HDL metabolism, aging, and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Sparks, D.L.; Davidson, W.S.; Lund-Katz, S.; Phillips, M.C. Effects of the neutral lipid content of high density lipoprotein on apolipoprotein A-I structure and particle stability. J. Biol. Chem. 1995, 270, 26910–26917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamarche, B.; Rashid, S.; Lewis, G.F. HDL metabolism in hypertriglyceridemic states: An overview. Clin. Chim. Acta 1999, 286, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Curtiss, L.K.; Bonnet, D.J.; Rye, K.A. The conformation of apolipoprotein A-I in high-density lipoproteins is influenced by core lipid composition and particle size: A surface plasmon resonance study. Biochemistry 2000, 39, 5712–5721. [Google Scholar] [CrossRef] [PubMed]
- Berrougui, H.; Isabelle, M.; Cloutier, M.; Grenier, G.; Khalil, A. Age-related impairment of HDL-mediated cholesterol efflux. J. Lipid Res. 2007, 48, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, T.; Kobayashi, T.; Kamata, K. Role of lysophosphatidylcholine (LPC) in atherosclerosis. Curr. Med. Chem. 2007, 14, 3209–3220. [Google Scholar] [CrossRef]
- Davidson, W.S.; Gillotte, K.L.; Lund-Katz, S.; Johnson, W.J.; Rothblat, G.H.; Phillips, M.C. The effect of high density lipoprotein phospholipid acyl chain composition on the efflux of cellular free cholesterol. J. Biol. Chem. 1995, 270, 5882–5890. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Rutkowsky, J.M.; Snodgrass, R.G.; Ono-Moore, K.D.; Schneider, D.A.; Newman, J.W.; Adams, S.H.; Hwang, D.H. Saturated fatty acids activate TLR-mediated proinflammatory signaling pathways. J. Lipid Res. 2012, 53, 2002–2013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, C.; Ramirez, R.; Delgado-Lista, J.; Yubero-Serrano, E.M.; Perez-Martinez, P.; Carracedo, J.; Garcia-Rios, A.; Rodriguez, F.; Gutierrez-Mariscal, F.M.; Gomez, P.; et al. Mediterranean diet reduces endothelial damage and improves the regenerative capacity of endothelium. Am. J. Clin. Nutr. 2011, 93, 267–274. [Google Scholar] [CrossRef] [Green Version]
- Woudstra, T.D.; Drozdowski, L.A.; Wild, G.E.; Clandinin, M.T.; Agellon, L.B.; Thomson, A.B. The age-related decline in intestinal lipid uptake is associated with a reduced abundance of fatty acid-binding protein. Lipids 2004, 39, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Field, P.A.; Gibbons, G.F. Decreased hepatic expression of the low-density lipoprotein (LDL) receptor and LDL receptor-related protein in aging rats is associated with delayed clearance of chylomicrons from the circulation. Metab. Clin. Exp. 2000, 49, 492–498. [Google Scholar] [CrossRef]
- Freedman, D.S.; Otvos, J.D.; Jeyarajah, E.J.; Shalaurova, I.; Cupples, L.A.; Parise, H.; D’Agostino, R.B.; Wilson, P.W.; Schaefer, E.J. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: The Framingham Study. Clin. Chem. 2004, 50, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Pascot, A.; Lemieux, I.; Bergeron, J.; Tremblay, A.; Nadeau, A.; Prud’homme, D.; Couillard, C.; Lamarche, B.; Despres, J.P. HDL particle size: A marker of the gender difference in the metabolic risk profile. Atherosclerosis 2002, 160, 399–406. [Google Scholar] [CrossRef]
- Badeau, R.M.; Metso, J.; Kovanen, P.T.; Lee-Rueckert, M.; Tikkanen, M.J.; Jauhiainen, M. The impact of gender and serum estradiol levels on HDL-mediated reverse cholesterol transport. Eur. J. Clin. Investig. 2013, 43, 317–323. [Google Scholar] [CrossRef]
- Anagnostis, P.; Stevenson, J.C.; Crook, D.; Johnston, D.G.; Godsland, I.F. Effects of menopause, gender and age on lipids and high-density lipoprotein cholesterol subfractions. Maturitas 2015, 81, 62–68. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Chen, X.; Nasr, A.N.; Billheimer, J.; Brooks, M.M.; McConnell, D.; Orchard, T.J.; Crawford, S.L.; Matthews, K.A.; Rader, D.J. HDL (High-Density Lipoprotein) Subclasses, Lipid Content, and Function Trajectories Across the Menopause Transition: SWAN-HDL Study. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 951–961. [Google Scholar] [CrossRef]
- Stevenson, J.C.; Crook, D.; Godsland, I.F. Influence of age and menopause on serum lipids and lipoproteins in healthy women. Atherosclerosis 1993, 98, 83–90. [Google Scholar] [CrossRef] [PubMed]
- El Khoudary, S.R.; Aggarwal, B.; Beckie, T.M.; Hodis, H.N.; Johnson, A.E.; Langer, R.D.; Limacher, M.C.; Manson, J.E.; Stefanick, M.L.; Allison, M.A.; et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e506–e532. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, M.E.; Karas, R.H. Molecular and cellular basis of cardiovascular gender differences. Science 2005, 308, 1583–1587. [Google Scholar] [CrossRef] [Green Version]
- Mendelsohn, M.E.; Karas, R.H. The protective effects of estrogen on the cardiovascular system. N. Engl. J. Med. 1999, 340, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Lamon-Fava, S.; Ordovas, J.M.; Schaefer, E.J. Estrogen increases apolipoprotein (apo) A-I secretion in hep G2 cells by modulating transcription of the apo A-I gene promoter. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 2960–2965. [Google Scholar] [CrossRef] [Green Version]
- Dubey, R.K.; Oparil, S.; Imthurn, B.; Jackson, E.K. Sex hormones and hypertension. Cardiovasc. Res. 2002, 53, 688–708. [Google Scholar] [CrossRef] [Green Version]
- Palmisano, B.T.; Zhu, L.; Eckel, R.H.; Stafford, J.M. Sex differences in lipid and lipoprotein metabolism. Mol. Metab. 2018, 15, 45–55. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]

| Mean ± SD | Range | |
|---|---|---|
| n | 90 | |
| Age, years | 49.5 ± 12.7 | 30–77 |
| Gender, M/F | 44/46 | |
| TC (mg/dL) | 171 ± 20 | 117–200 |
| TG (mg/dL) | 96 ± 30 | 40–150 |
| HDL-C (mg/dL) | 49 ± 9 | 25–71 |
| LDL-C (mg/dL) | 101 ± 23 | 54–160 |
| non-HDL-C (mg/dL) | 122 ± 21 | 63–171 |
| apoAI (mg/dL) | 136 ± 25 | 80–188 |
| apo B (mg/dL) | 81 ± 17 | 39–116 |
| HDL-C/apoAI | 0.4 ± 0.05 | 0.3–0.5 |
| LDL-C/apoB | 1.2 ± 0.2 | 1.0–1.8 |
| non-HDL-C/apoB | 1.5 ± 0.2 | 1.2–2.2 |
| Major Lipid Classes | Mean ± SD | Range |
|---|---|---|
| Cholesterol, total (TC) | 41.9± 1.6 | 38.1–45.8 |
| free (FC) | 7.9 ± 1.0 | 6.5–11.4 |
| esterified (CE) | 34.0 ± 1.1 | 31.0–36.1 |
| Triglycerides (TG) | 4.1± 0.9 | 2.3–6.5 |
| Phospholipids (PLs), total | 54.0± 1.8 | 49.3–59.5 |
| Core Lipids, total | 38.0 ± 1.2 | 33.8–41.3 |
| Surface Lipids, total | 62.0 ± 1.2 | 58.7–66.2 |
| Ratio | ||
| CE/TC | 0.8 ± 0.0 | 0.7–0.8 |
| CE/FC | 4.3 ± 0.5 | 3.0–5.2 |
| CE/TG | 8.7 ± 1.8 | 4.8–13.5 |
| TC/PLs | 0.8 ± 0.1 | 0.6–0.9 |
| Phospholipids | Mean± SD | Range |
| Total Glycerophospholipids (GPLs) | 41.8± 1.9 | 36.8–46.2 |
| Phosphatidylcholine (PC) | 33.1 ± 2.3 | 22.4–37.6 |
| Lysophosphatidylcholine (LysoPC) | 3.2 ± 0.7 | 1.8–5.2 |
| Phosphatidylethanolamine (PE) | 1.0 ± 0.2 | 0.4–1.5 |
| Phosphatidylinositol (PI) | 1.4 ± 0.4 | 0.4–3.8 |
| Rest GPLs a | 3.1 ± 2.2 | 0.1–8.8 |
| Total Ether Glycerolipids (Ether GLs) | 4.9± 0.7 | 3.1–6.7 |
| Plasmalogens | 1.8 ± 0.3 | 1.2–3.1 |
| Rest ether GLs b | 3.1 ± 0.8 | 1.1–5.3 |
| Total Sphingolipids (SLs) | 7.3± 0.9 | 5.2–9.7 |
| Sphingomyelin (SM) | 6.5 ± 1.1 | 2.9–8.5 |
| Rest SLs c | 0.8 ± 0.4 | 0.1–4.8 |
| Ratio | ||
| PC/SM | 4.9 ± 0.6 | 3.6–7.3 |
| Fatty Acid Pattern | Mean ± SD | Range |
|---|---|---|
| Saturated fatty acids (SFA) | 36.8 ± 6.6 | 20.0–51.6 |
| Unsaturated fatty acids (UFA) | 63.2 ± 6.6 | 48.4–80.0 |
| Monounsaturated fatty acids (MUFA) | 8.6 ± 4.9 | 0.1–22.1 |
| Polyunsaturated fatty acids (PUFA) | 54.6 ± 5.8 | 36.1–73.5 |
| Linoleic acid (LA) | 19.4 ± 2.8 | 13.4–27.3 |
| Eicosapentaenoic + arachidonic acid (EPA + AA) | 10.7 ± 1.6 | 6.1–16.0 |
| Docosahexaenoic acid (DHA) | 3.7 ± 0.7 | 2.3–6.0 |
| Ratio | ||
| SFA/UFA | 0.6 ± 0.2 | 0.3–1.1 |
| SFA/PUFA | 0.7 ± 0.2 | 0.3–1.4 |
| Average chain length (ACL) | 16.2 ± 1.6 | 11.9–20.5 |
| HDL Lipidome | Serum Lipid and Apolipoprotein Profile | |||||||
|---|---|---|---|---|---|---|---|---|
| TC | TG | HDL-C | ApoAI | HDL-C/apoAI | LDL-C | non-HDL-C | Apo B | |
| TC | 0.07 * | −0.08 | 0.02 | 0.05 | −0.16 | −0.02 | −0.06 | −0.09 |
| - | - | - | - | - | - | - | - | |
| FC | 0.11 | 0.13 | 0.02 | 0.15 | −0.31 | −0.16 | −0.11 | −0.17 |
| - | - | - | - | 0.006 | - | - | - | |
| CE | 0.02 | −0.23 | 0.05 | −0.07 | 0.05 | 0.12 | 0.02 | 0.02 |
| - | 0.03 | - | - | - | - | - | - | |
| TG | −0.08 | 0.54 | 0.08 | 0.34 | −0.43 | −0.31 | −0.11 | −0.24 |
| - | 0.001 | - | 0.003 | 0.001 | 0.003 | - | 0.04 | |
| Total PLs | 0.09 | −0.19 | −0.02 | −0.19 | 0.33 | 0.16 | 0.10 | 0.19 |
| - | - | - | - | 0.004 | - | - | - | |
| Total GPLs | 0.07 | −0.09 | −0.15 | −0.18 | 0.19 | 0.16 | 0.13 | 0.19 |
| - | - | - | - | - | - | - | - | |
| PC | −0.11 | 0.01 | −0.03 | −0.01 | 0.10 | −0.08 | −0.09 | −0.10 |
| - | - | - | - | - | - | - | - | |
| LysoPC | −0.16 | 0.05 | 0.01 | 0.18 | −0.28 | −0.18 | −0.17 | −0.36 |
| - | - | - | - | 0.02 | - | - | 0.001 | |
| PE | −0.15 | −0.26 | −0.22 | −0.31 | 0.11 | 0.04 | −0.05 | −0.03 |
| - | 0.02 | 0.04 | 0.006 | - | - | - | - | |
| PI | 0.04 | −0.04 | −0.19 | −0.25 | 0.10 | 0.12 | 0.12 | 0.21 |
| - | - | - | 0.03 | - | - | - | - | |
| Rest GPLs | 0.23 | −0.07 | −0.03 | −0.11 | 0.11 | 0.25 | 0.25 | 0.32 |
| 0.03 | - | - | - | - | 0.02 | 0.02 | 0.004 | |
| Ether GLs | −0.02 | −0.03 | −0.01 | −0.10 | 0.05 | −0.03 | −0.02 | 0.11 |
| - | - | - | - | - | - | - | - | |
| Plasmalogens | −0.21 | 0.26 | −0.13 | 0.10 | −0.23 | −0.26 | −0.15 | −0.23 |
| 0.04 | 0.01 | - | - | 0.04 | 0.01 | - | - | |
| Rest ether GLs | 0.06 | −0.13 | 0.05 | −0.13 | 0.14 | 0.07 | 0.04 | 0.19 |
| - | - | - | - | - | - | - | - | |
| Total SLs | 0.07 | −0.17 | 0.28 | 0.05 | 0.26 | 0.01 | −0.06 | −0.11 |
| - | - | 0.008 | - | 0.02 | - | - | - | |
| SM | 0.05 | −0.39 | −0.03 | −0.23 | 0.26 | 0.14 | −0.03 | 0.01 |
| - | 0.001 | - | - | 0.02 | - | - | - | |
| Rest SLs | 0.12 | 0.31 | 0.31 | 0.33 | −0.08 | −0.16 | −0.02 | −0.11 |
| - | 0.003 | 0.003 | 0.004 | - | - | - | - | |
| Core Lipids | −0.05 | 0.17 | 0.01 | 0.17 | −0.24 | −0.11 | −0.06 | −0.14 |
| - | - | - | - | 0.03 | - | - | - | |
| Surface Lipids | 0.05 | −0.17 | −0.01 | 0.17 | 0.24 | 0.11 | 0.06 | 0.14 |
| - | - | - | - | 0.03 | - | - | - | |
| CE/TC | 0.10 | −0.20 | −0.04 | −0.17 | 0.32 | 0.19 | 0.12 | 0.18 |
| - | 0.04 | - | - | 0.005 | - | - | - | |
| CE/FC | 0.08 | −0.23 | −0.04 | −0.19 | 0.33 | 0.18 | 0.10 | 0.18 |
| - | 0.03 | - | - | 0.004 | - | - | - | |
| CE/TG | 0.08 | −0.62 | −0.07 | −0.33 | 0.41 | 0.33 | 0.11 | 0.21 |
| - | 0.001 | - | 0.004 | 0.001 | 0.001 | - | - | |
| TC/PLs | −0.09 | 0.05 | 0.001 | 0.12 | −0.26 | −0.09 | −0.08 | −0.14 |
| - | - | - | - | 0.03 | - | - | - | |
| PC/SM | 0.22 | −0.02 | 0.16 | 0.28 | −0.06 | 0.15 | 0.14 | −0.01 |
| 0.04 | - | - | 0.02 | - | - | - | - | |
| Major Lipid Classes | Total | 30–39 Years | 40–49 Years | 50–59 Years | ≥60 Years | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | p | Males | Females | Males | Females | Males | Females | Males | Females | |
| n | 44 | 46 | 14 | 13 | 11 | 12 | 8 | 7 | 11 | 14 | |
| Cholesterol, Total | 41.71 ± 1.32 | 42.08 ± 1.81 | NS | 41.71 ± 0.72 | 41.14 ± 0.83 | 41.73 ± 1.28 | 42.01 ± 1.44 | 42.60 ± 1.75 | 43.06 ± 1.87 | 41.03 ± 1.39 | 42.52 ± 2.40 |
| Free | 7.85 ± 0.73 | 8.03 ± 1.24 | NS | 7.37 ± 0.33 | 6.76 ± 0.15 *** | 7.66 ± 0.55 | 7.74 ± 0.48 | 8.73 ± 1.01 | 8.97 ± 0.72 | 8.00 ± 0.36 | 8.98 ± 1.31 * |
| Esterified | 33.86 ± 1.01 | 34.05 ± 1.23 | NS | 34.34 ± 0.54 | 34.38 ± 0.74 | 34.07 ± 1.04 | 34.27 ± 1.18 | 33.87 ± 0.93 | 34.09 ± 1.46 | 33.03 ± 1.10 | 33.54 ± 1.47 |
| Triglycerides (TG) | 4.06 ± 0.74 | 4.10 ± 0.97 | NS | 3.75 ± 0.57 | 3.43 ± 0.37 | 3.75 ± 0.60 | 3.45 ± 0.42 | 4.19 ± 0.73 | 4.43 ± 0.89 | 4.69 ± 0.70 | 5.12 ± 0.76 * |
| Phospholipids (PLs), Total | 54.23 ± 1.32 | 53.82 ± 2.19 | NS | 54.54 ± 0.69 | 55.43 ± 0.79 ** | 54.52 ± 1.55 | 54.54 ± 1.77 | 53.21 ± 1.38 | 52.51 ± 2.17 | 54.28 ± 1.43 | 52.36 ± 2.21 * |
| Core Lipids, Total | 37.92 ± 0.99 | 38.15 ± 1.35 | NS | 38.08 ± 0.65 | 37.81 ± 0.73 | 37.81 ± 1.32 | 37.72 ± 1.50 | 38.05 ± 0.77 | 38.51 ± 1.81 | 37.72 ± 1.20 | 38.66 ± 1.34 * |
| Surface Lipids, Total | 62.08 ± 0.99 | 61.85 ± 1.35 | NS | 61.92 ± 0.64 | 62.19 ± 0.73 | 62.19 ± 1.32 | 62.28 ± 1.50 | 61.95 ± 0.77 | 61.49 ± 1.81 | 62.28 ± 1.20 | 61.34 ± 1.34 * |
| Ratios | |||||||||||
| CE/TC | 0.81 ± 0.01 | 0.81 ± 0.02 | NS | 0.82 ± 0.00 | 0.84 ± 0.00 *** | 0.82 ± 0.01 | 0.82 ± 0.01 | 0.80 ± 0.02 | 0.79 ± 0.01 | 0.81 ± 0.00 | 0.79 ± 0.02 * |
| CE/FC | 4.35 ± 0.37 | 4.33 ± 0.62 | NS | 4.66 ± 0.18 | 5.08 ± 0.11 *** | 4.47 ± 0.32 | 4.44 ± 0.25 | 3.91 ± 0.36 | 3.81 ± 0.28 | 4.13 ± 0.12 | 3.79 ± 0.46 * |
| CE/TG | 8.63 ± 1.68 | 8.74 ± 1.96 | NS | 9.37 ± 1.52 | 10.13 ± 1.21 | 9.32 ± 1.54 | 10.08 ± 1.25 | 8.35 ± 1.66 | 7.95 ± 1.51 | 7.19 ± 1.11 | 6.70 ± 1.08 |
| TC/PLs | 0.77 ± 0.04 | 0.78 ± 0.07 | NS | 0.76 ± 0.02 | 0.74 ± 0.02 * | 0.77 ± 0.04 | 0.77 ± 0.05 | 0.80 ± 0.05 | 0.82 ± 0.07 | 0.76 ± 0.04 | 0.82 ± 0.08 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostara, C.E.; Bairaktari, E.T.; Tsimihodimos, V. Effect of Clinical and Laboratory Parameters on HDL Particle Composition. Int. J. Mol. Sci. 2023, 24, 1995. https://doi.org/10.3390/ijms24031995
Kostara CE, Bairaktari ET, Tsimihodimos V. Effect of Clinical and Laboratory Parameters on HDL Particle Composition. International Journal of Molecular Sciences. 2023; 24(3):1995. https://doi.org/10.3390/ijms24031995
Chicago/Turabian StyleKostara, Christina E., Eleni T. Bairaktari, and Vasilis Tsimihodimos. 2023. "Effect of Clinical and Laboratory Parameters on HDL Particle Composition" International Journal of Molecular Sciences 24, no. 3: 1995. https://doi.org/10.3390/ijms24031995
APA StyleKostara, C. E., Bairaktari, E. T., & Tsimihodimos, V. (2023). Effect of Clinical and Laboratory Parameters on HDL Particle Composition. International Journal of Molecular Sciences, 24(3), 1995. https://doi.org/10.3390/ijms24031995






