Morphological and Functional Remodeling of Vascular Endothelium in Cardiovascular Diseases
Abstract
1. Introduction
2. The Vascular Endothelium
3. Origin and Differentiation of the Vascular Endothelium
4. Role of the Endothelium in Vascular Physiology
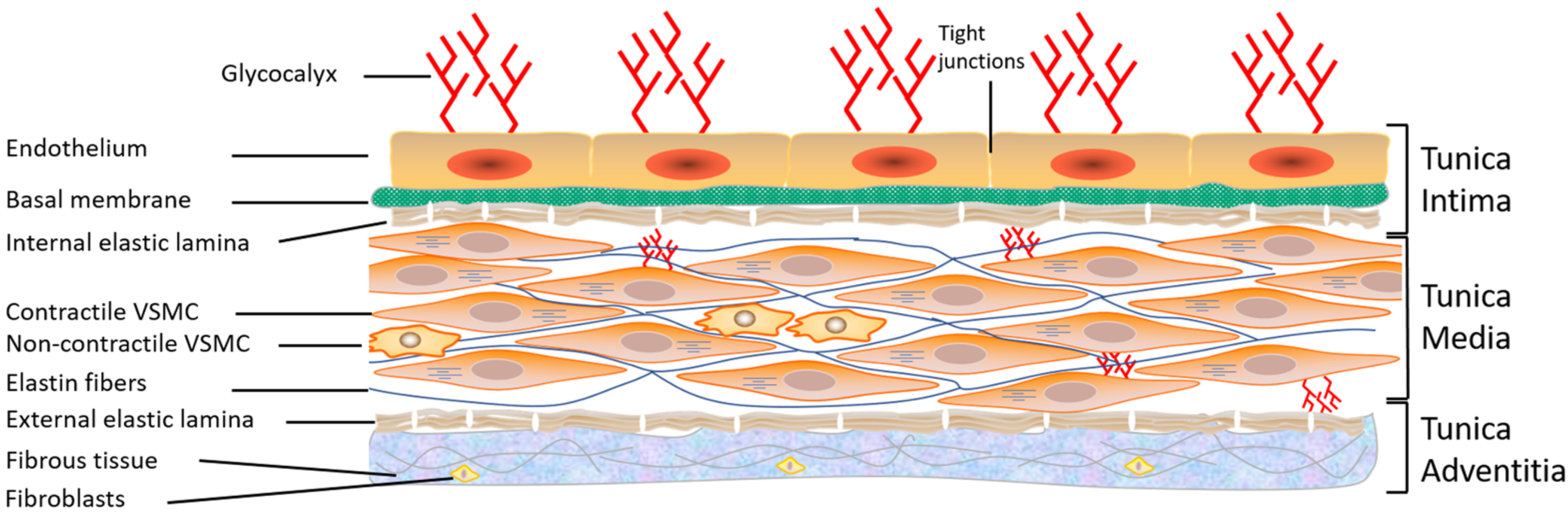
5. Structure of the Vascular Endothelium of Arteries and Veins
6. Role of the Endothelium in Vascular Activity
7. Inter-Endothelial Junctions
8. Ionic Transporters in Vascular Endothelium
9. Morphological and Functional Remodeling of Vascular Endothelium
10. Atherosclerosis
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mann, M.J.; Gibbons, G.H.; Tsao, P.S.; von der Leyen, H.E.; Cooke, J.P.; Buitrago, R.; Kernoff, R.; Dzau, V.J. Cell cycle inhibition preserves endothelial function in genetically engineered rabbit vein grafts. J. Clin. Investig. 1997, 99, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Abou Abdallah, N.; Simon, Y.; Jazzar, A.; Jacques, D. Vascular smooth muscle remodeling in health and disease. Can. J. Physiol. Pharmacol. 2021, 99, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Kotlyarov, S. Immune Function of Endothelial Cells: Evolutionary Aspects, Molecular Biology and Role in Atherogenesis. Int. J. Mol. Sci. 2022, 23, 9770. [Google Scholar] [CrossRef] [PubMed]
- Florey, L. The endothelial cell. Br. Med. J. 1966, 2, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.C., Jr. Coronary endothelial dysfunction in the hypertensive patient: From myocardial ischemia to heart failure. J. Hum. Hypertens. 1997, 11, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Samuesl, S.M.; Ravishankar, S.; Marei, I.; Arunachalam, G.; Ding, H. The endothelium: Influencing vascular smooth muscle in many ways. Can. J. Physiol. Pharmacol. 2012, 90, 713–738. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial cell dysfunction and the pathobiology of atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Chia, P.Y.; Teo, A.; Yeo, T.W. Overview of the assessment of endothelial function in humans. Front. Med. 2020, 7, 542567. [Google Scholar] [CrossRef]
- Santos-Gomes, J.; Le Ribeuz, H.; Brás-Silva, C.; Antigny, F.; Adão, R. Role of Ion Channel Remodeling in Endothelial Dysfunction Induced by Pulmonary Arterial Hypertension. Biomolecules 2022, 12, 484. [Google Scholar] [CrossRef]
- Ratajska, A.; Jankowska-Steifer, E.; Czarnowska, E.; Olkowski, R.; Gula, G.; Niderla-Bielińska, J.; Flaht-Zabost, A.; Jasińska, A. Vasculogenesis and Its Cellular Therapeutic Applications. Cells Tissues Organs 2017, 203, 141–152. [Google Scholar] [CrossRef]
- Ferguson, J.E., 3rd; Kelley, R.W.; Patterson, C. Mechanisms of endothelial differentiation in embryonic vasculogenesis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2246–2254. [Google Scholar] [CrossRef] [PubMed]
- Risau, W. Differentiation of endothelium. FASEB J. 1995, 9, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Atkins, G.B.; Jain, M.K. Role of Krüppel-like transcription factors in endothelial biology. Circ. Res. 2007, 100, 1686–1695. [Google Scholar] [CrossRef] [PubMed]
- Dyer, L.A.; Patterson, C. Development of the endothelium: An emphasis on heterogeneity. Semin. Thromb. Hemost. 2010, 36, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Ladak, S.S.; McQueen, L.W.; Layton, G.R.; Aujla, H.; Adebayo, A.; Zakkar, M. The Role of Endothelial Cells in the Onset, Development and Modulation of Vein Graft Disease. Cells 2022, 11, 3066. [Google Scholar] [CrossRef]
- Gratton, J.P.; Bernatchez, P.; Sessa, W.C. Caveolae and caveolins in the cardiovascular system. Circ. Res. 2004, 94, 1408–1417. [Google Scholar] [CrossRef]
- Sandoo, A.; Veldhuijzen van Zanten, J.J.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef]
- Van Hinsbergh, V.W. Endothelium—Role in Regulation of Coagulation and Inflammation; Springer: Amsterdam, The Netherlands, 2012; pp. 93–106. [Google Scholar]
- Mehrotra, D.; Wu, J.; Papangeli, I.; Chun, H.J. Endothelium as a gatekeeper of fatty acid transport. Trends Endocrinol. Metab. 2014, 25, 99–106. [Google Scholar] [CrossRef]
- Ghosh, A.; Gao, L.; Thakur, A.; Siu, P.M.; Lai, C.W. Role of free fatty acids in endothelial dysfunction. J. Biomed. Sci. 2017, 24, 50. [Google Scholar] [CrossRef]
- Northcott, J.M.; Czubryt, M.P.; Wigle, J.T. Vascular senescence and ageing: A role for the MEOX proteins in promoting endothelial dysfunction. Can. J. Physiol. Pharmacol. 2017, 95, 1067–1077. [Google Scholar] [CrossRef]
- Mallick, R.; Duttaroy, A.K. Modulation of endothelium function by fatty acids. Mol. Cell. Biochem. 2022, 477, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Stanek, A.; Fazeli, B.; Bartuś, S.; Sutkowska, E. The role of endothelium in physiological and pathological states: New data. BioMed. Res. Int. 2018, 2018, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Pi, X.; Xie, L.; Patterson, C. Emerging roles of vascular endothelium in metabolic homeostasis. Circ. Res. 2018, 123, 477–494. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; Jacques, D.; Bkaily, G. Angiotensin II receptors’ modulation of calcium homeostasis in human vascular endothelial cells. Can. J. Physiol. Pharmacol. 2017, 95, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Jacques, D.; Abdel-Karim Abdel-Malak, N.; Abou Abdallah, N.; Al-Khoury, J.; Bkaily, G. Difference in response to angiotensin II between left and right ventricular endocardial endothelial cells. Can. J. Physiol. Pharmacol. 2017, 95, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G. Biophysical and pharmacological properties of T-, L- and R-type Ca2+ channels. In Ionic Channels in Vascular Smooth Muscle; R.G. Landers Company: Georgetown, TX, USA, 1994. [Google Scholar]
- Gottlieb, A.I.; Langille, B.L.; Wong, M.K.; Kim, D.W. Structure and function of the endothelial cytoskeleton. Lab. Investig. 1991, 65, 123–137. [Google Scholar]
- Gaudette, S.; Hughes, D.; Boller, M. The endothelial glycocalyx: Structure and function in health and critical illness. J. Vet. Emerg. Crit. Care 2020, 30, 117–134. [Google Scholar] [CrossRef]
- Rajendran, P.; Rengarajan, T.; Thangavel, J.; Nishigaki, Y.; Sakthisekaran, D.; Sethi, G.; Nishigaki, I. The vascular endothelium and human diseases. Int. J. Biol. Sci. 2013, 9, 1057–1069. [Google Scholar] [CrossRef]
- Koçer, G.; Albino, I.M.C.; Verheijden, M.L.; Jonkheijm, P. Endothelial cell spreading on lipid bilayers with combined integrin and cadherin binding ligands. Bioorg. Med. Chem. 2022, 68, 116850. [Google Scholar] [CrossRef]
- Lampugnan, A.G.; Dejana, E. Interendothelial junctions: Structure, signaling and functional roles. Curr. Opin. Cell Biol. 1997, 9, 674–682. [Google Scholar] [CrossRef]
- Francke, W.W.; Cowin, P.; Grund, C.; Kuhn, C.; Kapprel, H.P. The endothelium junction. The plaque and its components. In Endothelial Cell Biology in Health and Disease; Siminescu, N., Simionescu, M., Eds.; Plenum Publishing Corp.: New York, NY, USA, 1988. [Google Scholar]
- Nguyen Duong, C.; Vestweber, D. Mechanisms ensuring endothelial junction integrity beyond Ve-cadherin. Front. Physiol. 2020, 11, 1–9. [Google Scholar]
- Seebach, J.; Klusmeier, N.; Schnittler, H. Autoregulatory “Multitasking” at Endothelial Cell Junctions by Junction-Associated Intermittent Lamellipodia Controls Barrier Properties. Front. Physiol. 2021, 11, 586921. [Google Scholar] [CrossRef] [PubMed]
- Charollais, A.; Gjinovci, A.; Huarte, J.; Bauquis, J.; Nadal, A.; Martin, F.; Andreu, E.; Sanchez-Andres, J.V.; Calabrese, A.; Bosco, D.; et al. Junctional communication of pancreatic beta cells contributes to the control of insulin secretion and glucose tolerance. J. Clin. Investig. 2000, 106, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Saez, J.C.; Branes, M.C.; Corvalan, L.A.; Eugenin, E.A.; Gonzalez, H.; Martinez, A.D.; Palisson, F. Gap junctions in cells of the immune system: Structure, regulation and possible functional roles. Braz. J. Med. Biol. Res. 2000, 33, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Morini, M.F.; Giampietro, C.; Corada, M.; Pisati, F.; Lavarone, E.; Cunha, S.I.; Conze, L.L.; O’Reilly, N.; Joshi, D.; Kjaer, S.; et al. VE-Cadherin-Mediated Epigenetic Regulation of Endothelial Gene Expression. Circ. Res. 2018, 122, 231–245. [Google Scholar] [CrossRef]
- Becker, C.G.; Murphy, G.E. Demonstration of contractile protein in endothelium and cells of the heart valves, endocardium, intima, arteriosclerotic plaques, and Aschoff bodies of rheumatic heart disease. Am. J. Pathol. 1969, 55, 1–37. [Google Scholar]
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef]
- Willingham, M.C.; Yamada, S.S.; Davies, P.J.; Rutherford, A.V.; Gallo, M.G.; Pastan, I. Intracellular localization of actin in cultured fibroblasts by electron microscopic immunocytochemistry. J. Histochem. Cytochem. 1981, 29, 17–37. [Google Scholar] [CrossRef]
- Majolée, J.; Kovačević, I.; Hordijk, P.L. Ubiquitin-based modifications in endothelial cell-cell contact and inflammation. J. Cell. Sci. 2019, 132, jcs227728. [Google Scholar] [CrossRef]
- Alonso, F.; Spuul, P.; Daubon, T.; Kramer, I.; Génot, E. Variations on the theme of podosomes: A matter of context. Biochim. Biophys. Acta Mol. Cell. Res. 2019, 1866, 545–553. [Google Scholar] [CrossRef]
- Bkaily, G.; Pothier, P.; D’Orléans-Juste, P.; Simaan, M.; Jacques, D.; Jaalouk, D.; Belzile, F.; Hassan, G.; Boutin, C.; Haddad, G.; et al. The use of confocal microscopy in the investigation of cell structure and function in the heart, vascular endothelium and smooth muscle cells. Mol. Cell. Biochem. 1997, 172, 171–194. [Google Scholar] [CrossRef] [PubMed]
- Cerutti, C.; Ridley, A.J. Endothelial cell-cell adhesion and signaling. Exp. Cell. Res. 2017, 358, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; D’Orleans-Juste, P.; Jacques, D. A new paradigm: Calcium independent and caveolae internalization dependent release of nitric oxide by the endothelial nitric oxide synthase. Circ. Res. 2006, 99, 793–794. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Nader, M.; Avedanian, L.; Choufani, S.; Jacques, D.; D’Orléans-Juste, P.; Al-Khoury, J. G-protein-coupled receptors, channels, and Na+–H+ exchanger in nuclear membranes of heart, hepatic, vascular endothelial, and smooth muscle cells. Can. J. Physiol. Pharmacol. 2006, 84, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Al-Khoury, J.; Jacques, D. Nuclear membranes GPCRs: Implication in cardiovascular health and diseases. Curr. Vasc. Pharmacol. 2014, 12, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, M.; Vachon, P.H.; D’Orléans-Juste, P.; Jacques, D.; Bkaily, G. Role of endothelin-1 and its receptors, ETA and ETB, in the survival of human vascular endothelial cells. Can. J. Physiol. Pharmacol. 2017, 95, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, P.O.; Lerman, L.O.; Lerman, A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 168–175. [Google Scholar] [CrossRef]
- Jules, F.; Avedanian, L.; Al-Khoury, J.; Keita, R.; Normand, A.; Bkaily, G.; Jacques, D. Nuclear Membranes ETB Receptors Mediate ET-1-induced Increase of Nuclear Calcium in Human Left Ventricular Endocardial Endothelial Cells. J. Cardiovasc. Pharmacol. 2015, 66, 50–57. [Google Scholar] [CrossRef]
- Nilius, B.; Droogmans, G. Ion channels and their functional role in vascular endothelium. Physiol. Rev. 2001, 81, 1415–1459. [Google Scholar] [CrossRef]
- Lüscher, T.F.; Barton, M. Biology of the endothelium. Clin. Cardiol. 1997, 20, 1415–1459. [Google Scholar] [CrossRef]
- Mombouli, J.V.; Vanhoutte, P.M. Endothelial dysfunction: From physiology to therapy. J. Mol. Cell. Cardiol. 1999, 31, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.A.; Risau, W.; Drexler, H.C. Regression of vessels in the tunica vasculosa lentis is initiated by coordinated endothelial apoptosis: A role for vascular endothelial growth factor as a survival factor for endothelium. Dev. Dyn. 1998, 213, 322–333. [Google Scholar] [CrossRef]
- Drexler, H. Endothelial dysfunction: Clinical implications. Prog. Cardiovasc. Dis. 1997, 39, 287–324. [Google Scholar] [CrossRef] [PubMed]
- Pacinella, G.; Ciaccio, A.M.; Tuttolomondo, A. Endothelial Dysfunction and Chronic Inflammation: The Cornerstones of Vascular Alterations in Age-Related Diseases. Int. J. Mol. Sci. 2022, 23, 15722. [Google Scholar] [CrossRef] [PubMed]
- Al-Khoury, J.; Jacques, D.; Bkaily, G. Hypotension in hereditary cardiomyopathy. Pflugers Arch. 2022, 474, 517–527. [Google Scholar] [CrossRef] [PubMed]
- de Wit, T.R.; van Mourik, J.A. Biosynthesis, processing and secretion of von Willebrand factor: Biological implications. Best Pract. Res. Clin. Haematol. 2001, 14, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, Z.M.; Landolfi, R. Platelet function and arterial thrombosis. Ital. Heart J. 2001, 2, 809–810. [Google Scholar]
- Agostini, S.; Lionetti, V. New insights into the non-hemostatic role of von Willebrand factor in endothelial protection. Can. J. Physiol. Pharmacol. 2017, 95, 1183–1189. [Google Scholar] [CrossRef]
- Wagner, D.D.; Marder, V.J. Biosynthesis of von Willebrand protein by human endothelial cells: Processing steps and their intracellular localization. J. Cell. Biol. 1984, 99, 2123–2130. [Google Scholar] [CrossRef]
- Sporn, L.A.; Rubin, P.; Marder, V.J.; Wagner, D.D. Irradiation induces release of von Willebrand protein from endothelial cells in culture. Blood 1984, 64, 567–570. [Google Scholar] [CrossRef]
- Utgaard, J.O.; Jahnsen, F.L.; Bakka, A.; Brandtzaeg, P.; Haraldsen, G. Rapid secretion of prestored interleukin 8 from Weibel-Palade bodies of microvascular endothelial cells. J. Exp. Med. 1998, 188, 1751–1756. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.D.; Urban-Pickering, M.; Marder, V.J. Von Willebrand protein binds to extracellular matrices independently of collagen. Proc. Natl. Acad. Sci. USA 1984, 81, 471–475. [Google Scholar] [CrossRef] [PubMed]
- DeMali, K.A.; Adams, J.C. Cell-cell and cell-matrix interactions. Mol. Biol. Cell. 2012, 23, 965. [Google Scholar] [CrossRef]
- Dejana, E. Perspectives series: Cell adhesion in vascular biology. Endothelial adherens junctions: Implications in the control of vascular permeability and angiogenesis. J. Clin. Investig. 1996, 98, 1949–1953. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.P.; Wang, J.; Zhou, S.X.; Huang, D.C.; Qi, G.H.; Chen, G.T. Ginger polysaccharides enhance intestinal immunity by modulating gut microbiota in cyclophosphamide-induced immunosuppressed mice. Int. J. Biol. Macromol. 2022, 223, 1308–1319. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, E.E.; Lynch, R.D. Tight junctions. Their structure, composition, and function. Circ. Res. 1984, 55, 723–733. [Google Scholar] [CrossRef]
- Simionescu, N.; Simionescu, M. Cellular interactions of lipoproteins with the vascular endothelium: Endocytosis and transcytosis. Targeted Diagn. Ther. 1991, 5, 45–95. [Google Scholar]
- Claassen, J.A.H.R.; Thijssen, D.H.J.; Panerai, R.B.; Faraci, F.M. Regulation of cerebral blood flow in humans: Physiology and clinical implications of autoregulation. Physiol. Rev. 2021, 101, 1487–1559. [Google Scholar] [CrossRef]
- Pries, A.R.; Kuebler, W.M. Normal endothelium. Handb. Exp. Pharmacol. 2006, 176, 1–40. [Google Scholar]
- Kojimahara, M.; Yamazaki, K.; Ooneda, G. Ultrastructural study of hemangiomas. 1. Capillary hemangioma of the skin. Acta Pathol. Jpn. 1981, 31, 105–115. [Google Scholar]
- Zhang, X.; Gong, P.; Zhao, Y.; Wan, T.; Yuan, K.; Xiong, Y.; Wu, M.; Zha, M.; Li, Y.; Jiang, T.; et al. Endothelial caveolin-1 regulates cerebral thrombo-inflammation in acute ischemia/reperfusion injury. BioMedicine 2022, 84, 104275. [Google Scholar] [CrossRef] [PubMed]
- Ramasubramanian, B.; Kim, J.; Ke, Y.; Li, Y.; Zhang, C.O.; Promnares, K.; Tanaka, K.A.; Birukov, K.G.; Karki, P.; Birukova, A.A. Mechanisms of pulmonary endothelial permeability and inflammation caused by extracellular histone subunits H3 and H4. FASEB J. 2022, 36, e22470. [Google Scholar] [CrossRef] [PubMed]
- Marcinczyk, N.; Misztal, T.; Gromotowicz-Poplawska, A.; Zebrowska, A.; Rusak, T.; Radziwon, P.; Chabielska, E. Utility of Platelet Endothelial Cell Adhesion Molecule 1 in the Platelet Activity Assessment in Mouse and Human Blood. Int. J. Mol. Sci. 2021, 22, 9611. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.K.; Sapkota, A.; Milner, R. The impact of genetic manipulation of laminin and integrins at the blood-brain barrier. Fluids Barriers CNS 2022, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.F. Endothelial Ion Channels and Cell-Cell Communication in the Microcirculation. Front. Physiol. 2022, 13, 805149. [Google Scholar] [CrossRef] [PubMed]
- Hautefort, A.; Pfenniger, A.; Kwak, B.R. Endothelial connexins in vascular function. Vasc. Biol. 2019, 1, H117–H124. [Google Scholar] [CrossRef]
- Pogoda, K.; Kameritsch, P.; Mannell, H.; Pohl, U. Connexins in the control of vasomotor function. Acta Physiol. 2019, 225, e13108. [Google Scholar] [CrossRef]
- Tyml, K. Role of connexins in microvascular dysfunction during inflammation. Can. J. Physiol. Pharmacol. 2011, 89, 1–12. [Google Scholar] [CrossRef]
- Johnstone, S.; Isakson, B.; Locke, D. Biological and biophysical properties of vascular connexin channels. Int. Rev. Cell. Mol. Biol. 2009, 278, 69–118. [Google Scholar]
- Brisset, A.C.; Isakson, B.E.; Kwak, B.R. Connexins in vascular physiology and pathology. Antioxid. Redox Signal. 2009, 11, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Bean, B.P. Two kind of Ca2+ ion canine atrial cells. Differences in kinetics selectivity and pharmacology. J. Gen. Physiol. 1985, 86, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G.; Sculptoreanu, A.; Wang, S.; Nader, M.; Hazzouri, K.M.; Jacques, D.; Regoli, D.; D’Orleans-Juste, P.; Avedanian, L. Angiotensin II-induced increase of T-type Ca2+ current and decrease of L-type Ca2+ current in heart cells. Peptides 2005, 26, 1410–1417. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Klepper, M. Voltage-dependent and agonist-activated ionic currents in vascular endothelial cells: A review. Blood Vessel. 1990, 27, 169–183. [Google Scholar] [CrossRef]
- Bkaily, G.; Jaalouk, D.; Jacques, D.; Economos, D.; Hassan, G.; Simaan, M.; Regoli, D.; Pothier, P. Bradykinin activates R-, T-, and L-type Ca2+ channels and induces a sustained increase of nuclear Ca2+ in aortic vascular smooth muscle cells. Can. J. Physiol. Pharmacol. 1997, 75, 652–660. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.F.; Hodgkin, A.L.; Ridgway, E.B. Depolarization and calcium entry in squid giant axons. J. Physiol. 1971, 218, 709–755. [Google Scholar] [CrossRef] [PubMed]
- DiPolo, R. Calcium influx in internally dialyzed squid giant axons. J. Gen. Physiol. 1979, 73, 91–113. [Google Scholar] [CrossRef]
- Bkaily, G.; Economos, D.; Potvin, L.; Ardilouze, J.L.; Marriott, C.; Corcos, J.; Bonneau, D.; Fong, C.N. Blockade of insulin sensitive steady-state R-type Ca2+ channel by PN 200-110 in heart and vascular smooth muscle. Mol. Cell. Biochem. 1992, 117, 93–106. [Google Scholar] [CrossRef]
- Bkaily, G.; D’Orléans-Juste, P.; Naik, R.; Pérodin, J.; Stankova, J.; Abdulnour, E.; Rola-Pleszczynski, M. PAF activation of a voltage-gated R-type Ca2+ channel in human and canine aortic endothelial cells. Br. J. Pharmacol. 1993, 110, 519–520. [Google Scholar] [CrossRef]
- Moccia, F.; Berra-Romani, R.; Tanzi, F. Update on vascular endothelial Ca2+ signalling: A tale of ion channels, pumps and transporters. World J. Biol. Chem. 2012, 3, 127–158. [Google Scholar] [CrossRef]
- Becchetti, A.; Munaron, L.; Arcangeli, A. The role of ion channels and transporters in cell proliferation and cancer. Front. Physiol. 2013, 4, 312. [Google Scholar] [CrossRef]
- Kwan, H.Y.; Huang, Y.; Yao, X. TRP channels in endothelial function and dysfunction. BBA Mol. Basis Dis. 2007, 1772, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Moccia, F.; Tanzi, F.; Munaron, L. Endothelial remodelling and intracellular calcium machinery. Curr. Mol. Med. 2014, 14, 457–480. [Google Scholar] [CrossRef] [PubMed]
- Bkaily, G. Regulation of Ca2+ channels in VSM by monocyte-released factors. In Ionic Channels in Vascular Smooth Muscle; R.G. Landers Company: Georgetown, TX, USA, 1994. [Google Scholar]
- Munaron, L. Intracellular calcium, endothelial cells and angiogenesis. Recent Pat. Anticancer Drug Discov. 2006, 1, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Shichiri, M.; Kato, H.; Marumo, F.; Hirata, Y. Endothelin-1 as an autocrine/paracrine apoptosis survival factor for endothelial cells. Hypertension 1997, 30, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.M.; Levin, E.R.; Pedram, A.; Frank, H.J. Insulin stimulates production and secretion of endothelin from bovine endothelial cells. Diabetes 1993, 42, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Elkayam, U.; Khan, S.; Mehboob, A.; Ahsan, N. mpaired endothelium-mediated vasodilation in heart failure: Clinical evidence and the potential for therapy. J. Card. Fail. 2002, 8, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, M.W.; Goodfellow, J.; Jones, C.J.; Luddington, L.A.; Lewis, M.J.; Henderson, A.H. Endothelial control of arterial distensibility is impaired in chronic heart failure. Circulation 1995, 92, 3212–3219. [Google Scholar] [CrossRef]
- Bkaily, G.; Simon, Y.; Jazzar, A.; Najibeddine, H.; Normand, A.; Jacques, D. High Na+ Salt Diet and Remodeling of Vascular Smooth Muscle and Endothelial Cells. Biomedicines 2021, 9, 883. [Google Scholar] [CrossRef]
- Bkaily, G.; Simon, Y.; Menkovic, I.; Bkaily, C.; Jacques, D. High salt-induced hypertrophy of human vascular smooth muscle cells associated with a decrease in glycocalyx. J. Mol. Cell. Cardiol. 2018, 125, 1–5. [Google Scholar] [CrossRef]
- Jacques, D.; Bkaily, G. Endocardial endothelial cell hypertrophy takes place during the development of hereditary cardiomyopathy. Mol. Cell. Biochem. 2019, 453, 157–161. [Google Scholar] [CrossRef]
- Hassan, G.S.; Jacques, D.; D’Orléans-Juste, P.; Magder, S.; Bkaily, G. Physical contact between human vascular endothelial and smooth muscle cells modulates cytosolic and nuclear calcium homeostasis. Can. J. Physiol. Pharmacol. 2018, 96, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Spagnoli, L.G.; Pietra, G.G.; Villaschi, S.; Johns, L.W. Morphometric analysis of gap junctions in regenerating arterial endothelium. Lab. Invest. 1982, 46, 139–148. [Google Scholar] [PubMed]
- Spagnoli, L.G.; Villaschi, S.; Neri, L.; Palmieri, G. Gap junctions in myo-endothelial bridges of rabbit carotid arteries. Experientia 1982, 38, 124–125. [Google Scholar] [CrossRef] [PubMed]
- Glassberg, M.K.; Bern, M.M.; Coughlin, S.R.; Haudenschild, C.C.; Hoyer, L.W.; Antoniades, H.N.; Zetter, B.R. Cultured endothelial cells derived from the human iliac arteries. Vitr. Plant 1982, 18, 859–866. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bkaily, G.; Jacques, D. Morphological and Functional Remodeling of Vascular Endothelium in Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 1998. https://doi.org/10.3390/ijms24031998
Bkaily G, Jacques D. Morphological and Functional Remodeling of Vascular Endothelium in Cardiovascular Diseases. International Journal of Molecular Sciences. 2023; 24(3):1998. https://doi.org/10.3390/ijms24031998
Chicago/Turabian StyleBkaily, Ghassan, and Danielle Jacques. 2023. "Morphological and Functional Remodeling of Vascular Endothelium in Cardiovascular Diseases" International Journal of Molecular Sciences 24, no. 3: 1998. https://doi.org/10.3390/ijms24031998
APA StyleBkaily, G., & Jacques, D. (2023). Morphological and Functional Remodeling of Vascular Endothelium in Cardiovascular Diseases. International Journal of Molecular Sciences, 24(3), 1998. https://doi.org/10.3390/ijms24031998




