The Hydrophobic Effect Studied by Using Interacting Colloidal Suspensions
Abstract
:1. Introduction
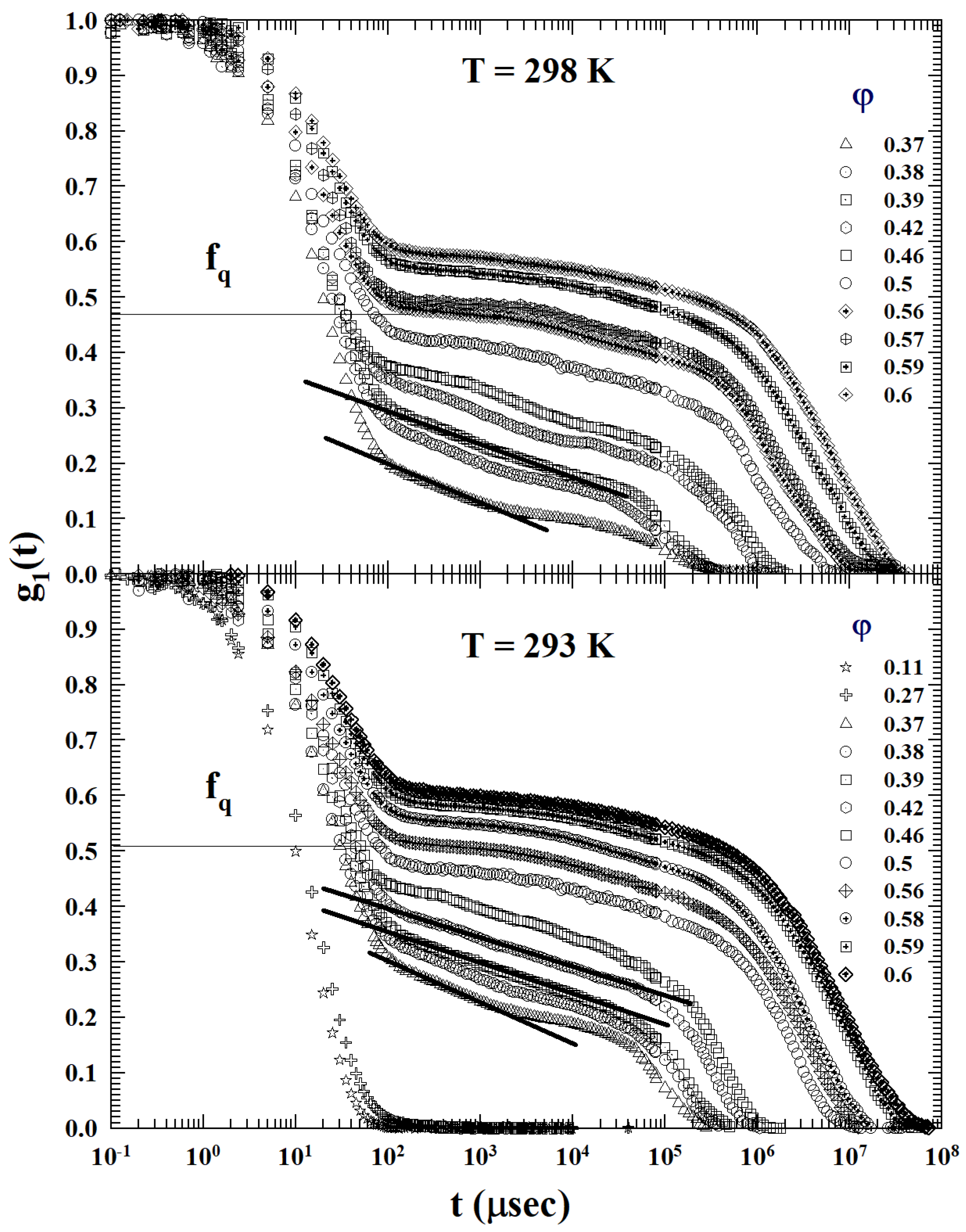
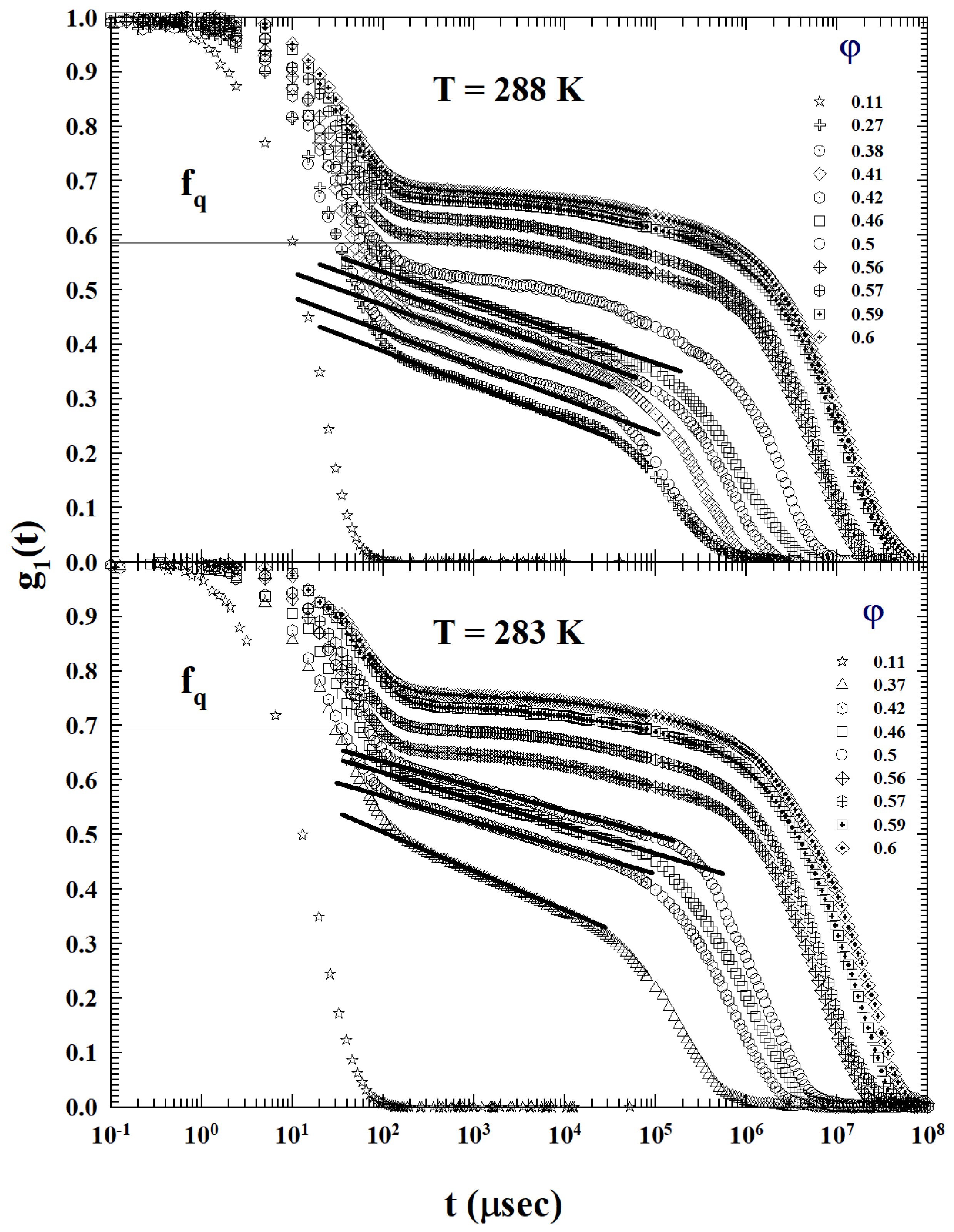
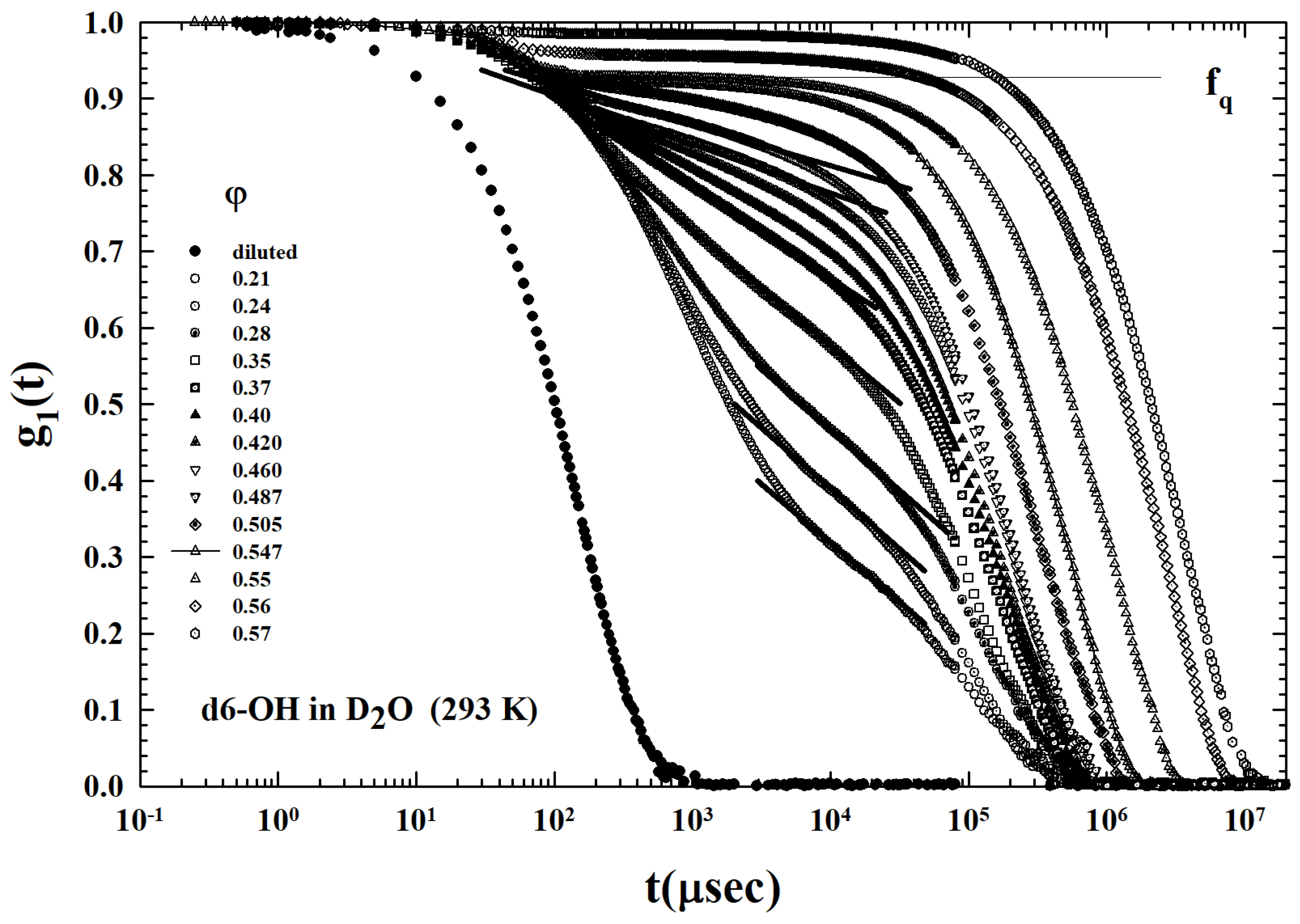
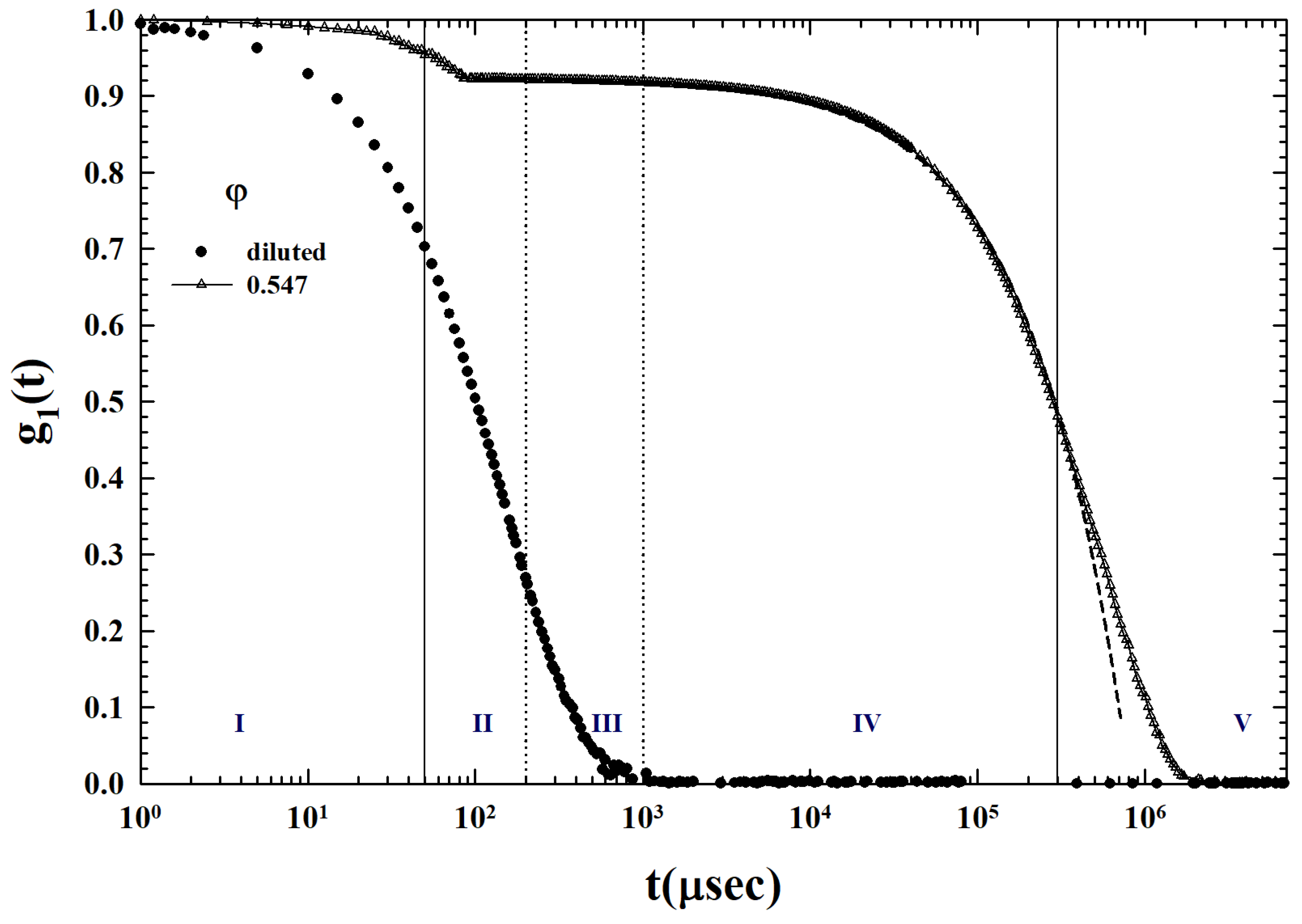
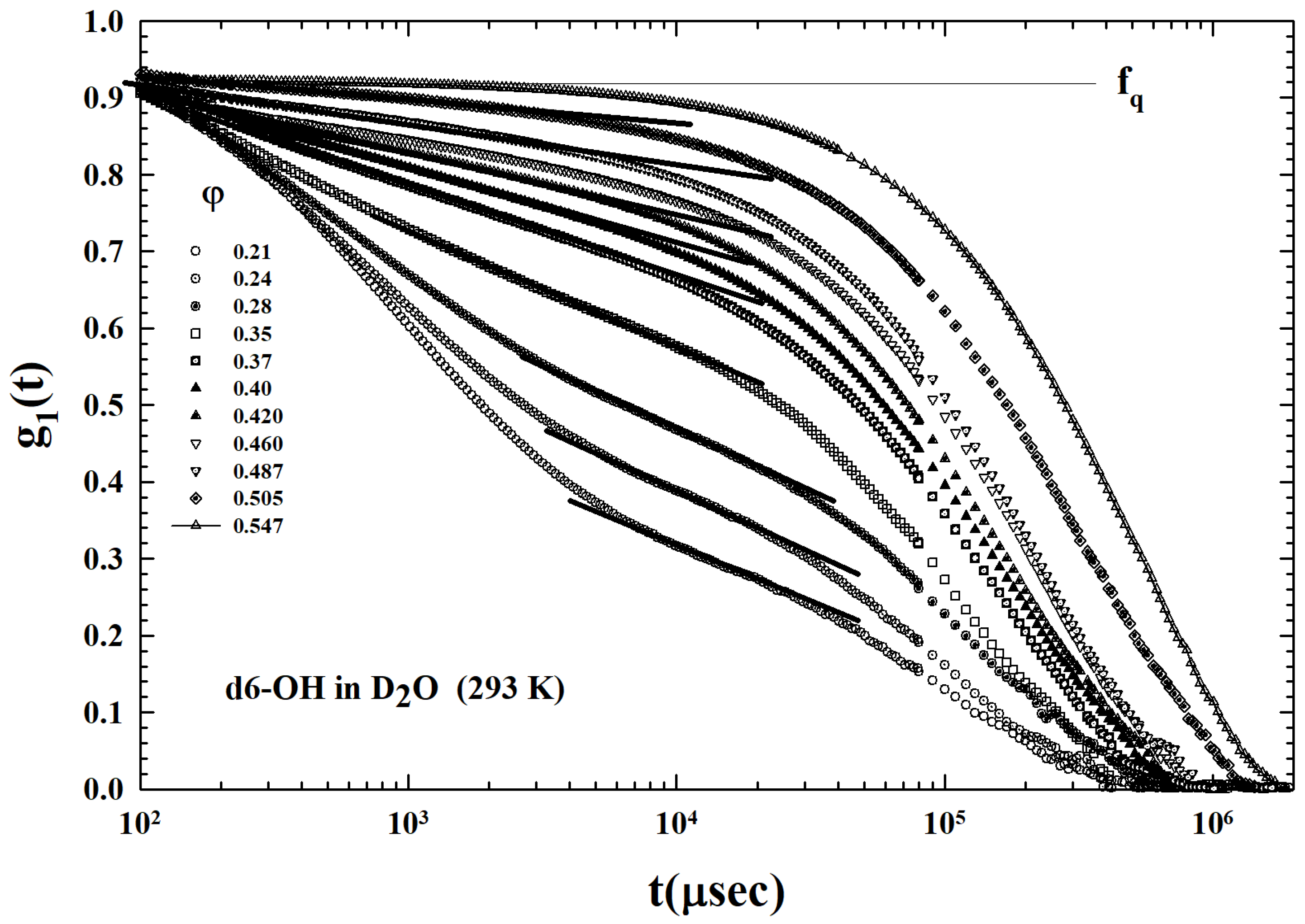
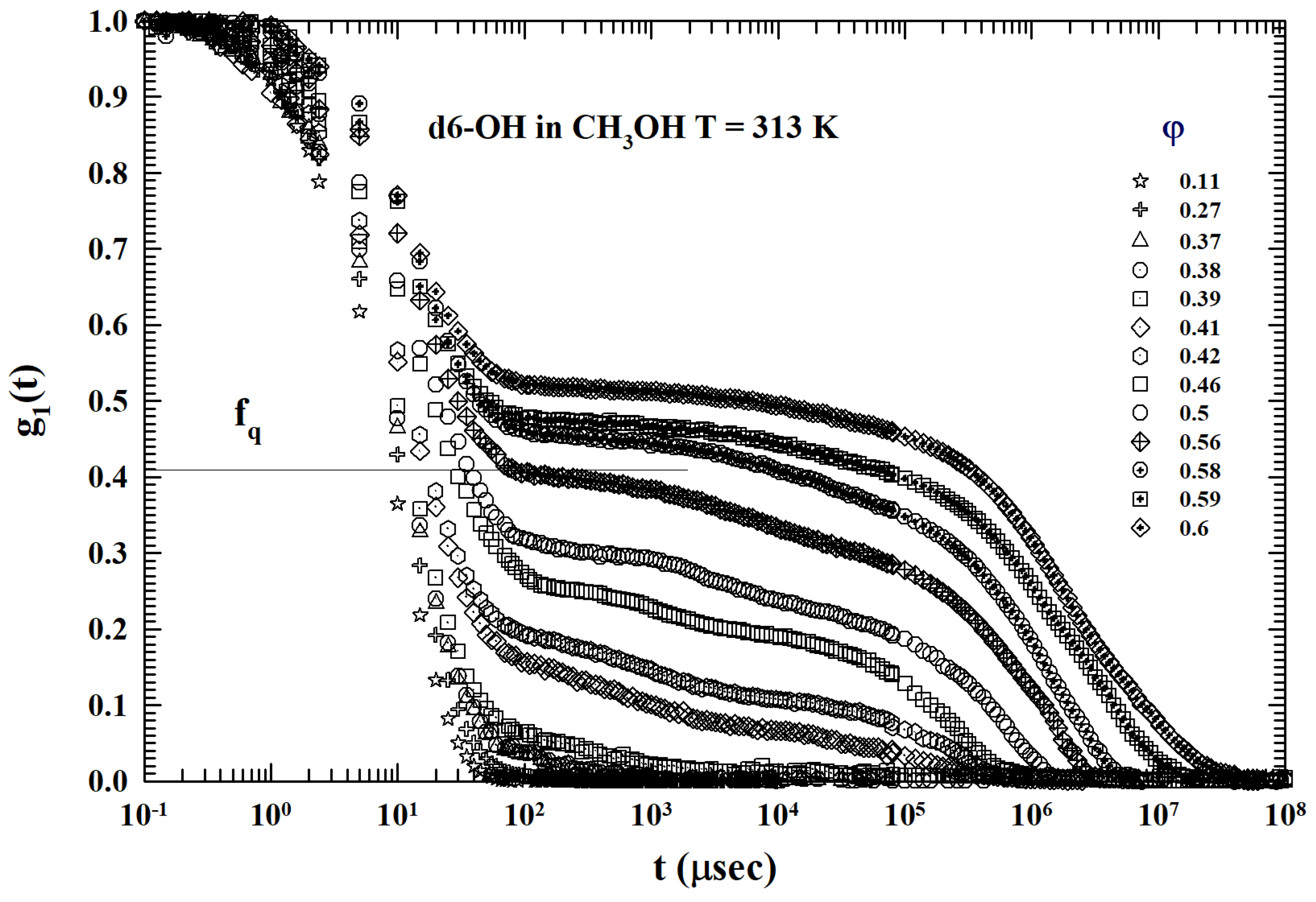
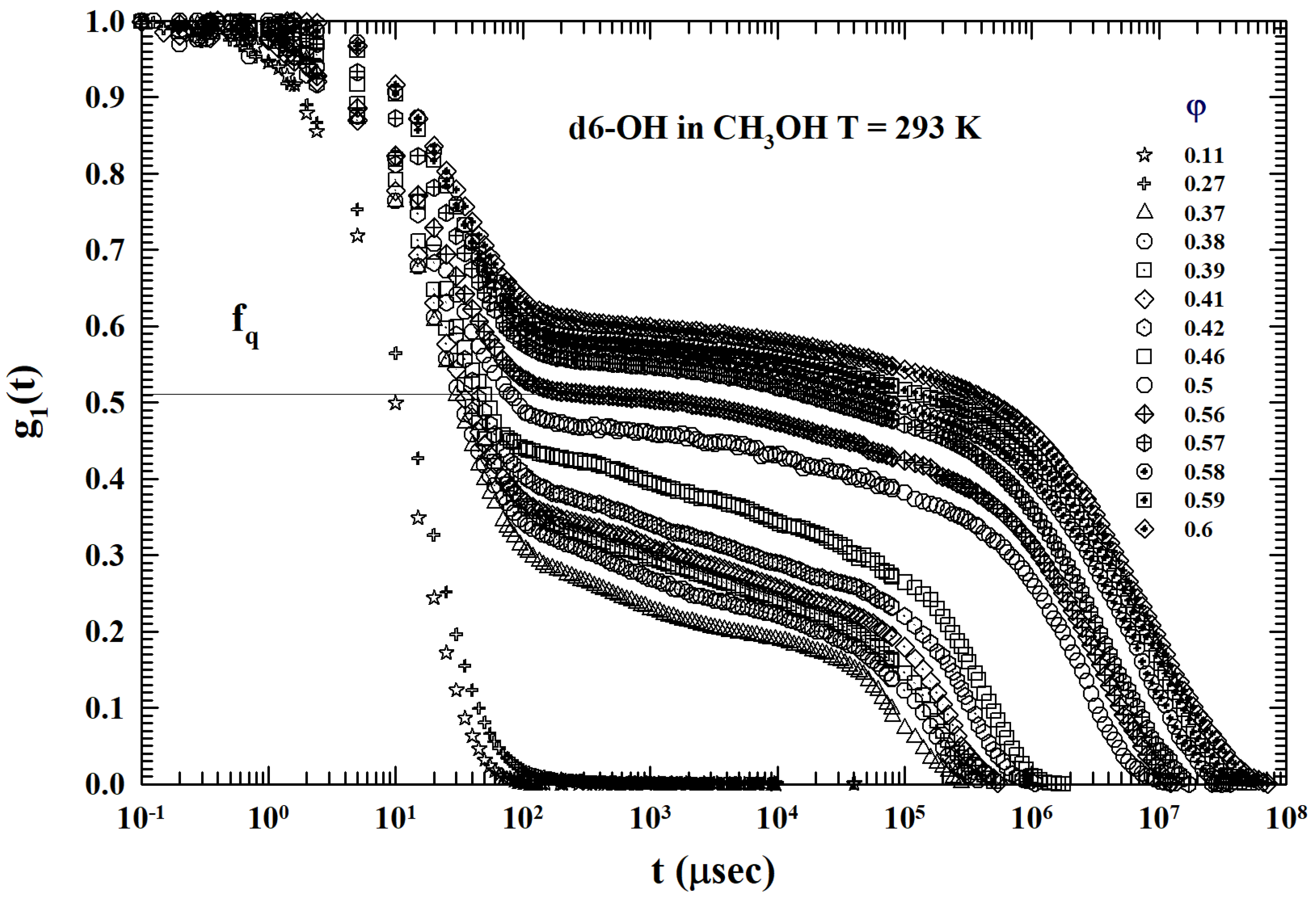
2. Results and Discussion
3. Materials and Methods
3.1. Samples and Experiment
3.2. Models and Results
3.3. Data Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stanley, H.E. Liquid Polymorphism; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Debenedetti, P.G. Metastable Liquids; Princeton University Press: Princeton, NJ, USA, 1997. [Google Scholar]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Baglioni, P.; Stanley, H.E.; Chen, S.H. A Possible Role of Water in the Protein Folding Process. J. Phys. Chem. B 2011, 115, 14280–14294. [Google Scholar] [CrossRef]
- Mallamace, F.; Corsaro, C.; Stanley, H.E. Possible relation of water structural relaxation to water anomalies. Proc. Natl. Acad. Sci. USA 2013, 110, 4899–4904. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.K.; Stanley, H.E. Thermal Conductivity Minimum: A New Water Anomaly. J. Phys. Chem. B 2011, 115, 14269–14273. [Google Scholar] [CrossRef] [Green Version]
- Mallamace, F.; Corsaro, C.; Stanley, H.E. A singular thermodynamically consistent temperature at the origin of the anomalous behavior of liquid water. Sci. Rep. 2012, 2, 993. [Google Scholar] [CrossRef] [Green Version]
- Mallamace, F. The liquid water polymorphism. Proc. Natl. Acad. Sci. USA 2009, 106, 15097–15098. [Google Scholar] [CrossRef] [Green Version]
- Poole, P.H.; Sciortino, F.; Essmann, U.; Stanley, H.E. Phase-behavior of metastable water. Nature 1992, 360, 324–328. [Google Scholar] [CrossRef]
- Mallamace, D.; Corsaro, C.; Mallamace, F.; Stanley, H.E. Experimental tests for a liquid-liquid critical point in water. Sci. China Phys. Mech. Astron. 2020, 63, 127001. [Google Scholar] [CrossRef]
- Xu, L.; Kumar, P.; Buldyrev, S.V.; Chen, S.H.; Poole, P.H.; Sciortino, F.; Stanley, H.E. Relation between the Widom line and the dynamic crossover in systems with a liquid–liquid phase transition. Proc. Natl. Acad. Sci. USA 2005, 102, 16558–16562. [Google Scholar] [CrossRef] [Green Version]
- Mishima, O.; Stanley, H.E. The relationship between liquid, supercooled and glassy water. Nature 1998, 396, 329–335. [Google Scholar] [CrossRef]
- Chen, S.H.; Mallamace, F.; Mou, C.Y.; Broccio, M.; Corsaro, C.; Faraone, A.; Liu, L. The violation of the Stokes-Einstein relation in supercooled water. Proc. Natl. Acad. Sci. USA 2006, 103, 12974–12978. [Google Scholar] [CrossRef]
- Cerveny, S.; Mallamace, F.; Swenson, J.; Vogel, M.; Xu, L. Confined water as model of supercooled water. Chem. Rev. 2016, 116, 7608–7625. [Google Scholar] [CrossRef]
- Xu, L.M.; Mallamace, F.; Yan, Z.Y.; Starr, F.W.; Buldyrev, S.V.; Stanley, H.E. Appearance of a fractional Stokes-Einstein relation in water and a structural interpretation of its onset. Nat. Phys. 2009, 5, 565–569. [Google Scholar] [CrossRef]
- Mallamace, F.; Baglioni, P.; Corsaro, C.; Chen, S.H.; Mallamace, D.; Vasi, S.; Vasi, C.; Stanley, H.E. The influence of water on protein properties. J. Chem. Phys. 2014, 141, 165104. [Google Scholar] [CrossRef] [Green Version]
- Safran, S.A. Statistical Thermodynamics of Surfaces, Interfaces and Membranes; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 2005, 437, 640–647. [Google Scholar] [CrossRef]
- Widom, D.; Ben-Amotz, D. Note on the energy density in the solvent induced by a solute. Proc. Natl. Acad. Sci. USA 2006, 103, 18887–18890. [Google Scholar] [CrossRef] [Green Version]
- Jeffrey, G.A.; Saenger, W. Hydrogen Bonding in Biological Structures; Springer: Berlin, Germany, 1991. [Google Scholar]
- van Erp, T.S.; Meijer, E.J. Ab initio molecular dynamics study of aqueous solvation of ethanol and ethylene. J. Chem. Phys. 2003, 118, 8831. [Google Scholar] [CrossRef] [Green Version]
- Young, T. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 65–87. [Google Scholar]
- Ashbaugh, H.S.; Pratt, L.R. Scaled particle theory and the length scales of hydrophobicity. Rev. Mod. Phys. 2006, 78, 159–178. [Google Scholar] [CrossRef]
- Ball, P. Water as an Active Constituent in Cell Biology. Chem. Rev. 2008, 108, 74–108. [Google Scholar] [CrossRef]
- Garde, S.; Patel, A.J. Unraveling the hydrophobic effect, one molecule at a time. Proc. Natl. Acad. Sci. USA 2011, 108, 16491–16492. [Google Scholar] [CrossRef] [Green Version]
- Hummer, G. Under water’s influence. Nat. Chem. 2010, 2, 906–907. [Google Scholar] [CrossRef] [PubMed]
- Stillinger, F.H. Supercooled liquids, glass transitions, and the Kauzmann paradox. J. Chem. Phys. 1988, 88, 7818. [Google Scholar] [CrossRef] [Green Version]
- Rajamani, S.; Truskett, T.M.; Garde, S. Hydrophobic hydration from small to large lengthscales: Understanding and manipulating the crossover. Proc. Natl. Acad. Sci. USA 2005, 102, 9475–9480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Gennes, P.G. Scaling Concepts in Polymer Physics; Cornell University Press: Ithaca, NY, USA, 1979. [Google Scholar]
- Flory, P. Principles of Polymer Chemistry; Cornell University Press: Ithaca, NY, USA, 1953. [Google Scholar]
- Zhang, X.; Zhu, Y.; Granick, S. Hydrophobicity at a Janus interface. Science 2002, 295, 663–666. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Margulis, C.J.; Berne, B.J. Hydrophobic hydration and molecular association in methanol–water mixtures studied by microwave dielectric analysis. Proc. Natl. Acad. Sci. USA 2003, 100, 11953. [Google Scholar] [CrossRef] [Green Version]
- Rasaiah, J.C.; Garde, S.; Hummer, G. Water in Nonpolar Confinement: From Nanotubes to Proteins and Beyond. Annu. Rev. Phys. Chem. 2008, 59, 713–740. [Google Scholar] [CrossRef] [Green Version]
- Ben-Amoz, D. Hydrophobic ambivalence: Teetering on the edge of randomness. J. Phys. Chem. Lett. 2015, 6, 1696–1791. [Google Scholar] [CrossRef]
- Stillinger, F.H. Structure in aqueous solutions of nonpolar solutes from the standpoint of scaled particle theory. J. Sol. Chem. 1973, 2, 141–158. [Google Scholar] [CrossRef]
- Silvera Batista, C.A.; Larson, R.G.; Kotov, N.A. Nonadditivity of nanoparticle interactions. Science 2015, 350, 1242477. [Google Scholar] [CrossRef] [Green Version]
- Davis, J.G.; Gierszal, K.P.; Wang, P.; Ben-Amotzm, D. Water structural transformation at molecular hydrophobic interfaces. Nature 2012, 491, 582. [Google Scholar] [CrossRef]
- Du, Q.; Freysz, E.; Shen, Y.R. Surface Vibrational Spectroscopic Studies of Hydrogen Bonding and Hydrophobicity. Science 1994, 264, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Ide, M.; Maeda, Y.; Kitano, H. Effect of hydrophobicity of amino acids on the structure of water. J. Phys. Chem. B 1997, 101, 7022. [Google Scholar] [CrossRef]
- Ge, Z.; Cahill, D.G.; Braun, P.V. Thermal Conductance of Hydrophilic and Hydrophobic Interfaces. Phys. Rev. Lett. 2006, 96, 186101. [Google Scholar] [CrossRef]
- Gallagher, K.R.; Sharp, K.A. A new angle on heat capacity changes in hydrophobic solvation. J. Am. Chem. Soc. 2003, 125, 9853–9860. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Chiba, A.; Nozaki, R. Hydrophobic hydration and molecular association in methanol–water mixtures studied by microwave dielectric analysis. J. Chem. Phys. 2000, 112, 2924–2932. [Google Scholar] [CrossRef]
- Russo, D.; Murarka, R.K.; Copley, J.R.D.; Head-Gordon, T. Molecular view of water dynamics near model peptides. J. Phys. Chem. B 2005, 109, 12966. [Google Scholar] [CrossRef] [Green Version]
- Meyer, E.E.; Lin, Q.; Hassenkam, T.; Oroudjev, E.; Israelachvili, J.N. Origin of the long-range attraction between surfactant-coated surfaces. Proc. Natl. Acad. Sci. USA 2005, 102, 6839–6842. [Google Scholar] [CrossRef] [Green Version]
- Mallamace, F.; Corsaro, C.; Mallamace, D.; Vasi, S.; Vasi, C.; Baglioni, P.; Buldyrev, S.V.; Chen, S.H.; Stanley, H.E. Energy landscape in protein folding and unfolding. Proc. Natl. Acad. Sci. USA 2016, 113, 3159–3163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hertz, H.G.; Zeidler, M.D. Relaxationszeitmessungen zur Frage der Hydratation unpolarer Gruppen in wäßriger Lösung. Ber. Bunsen Ges. Phys. Chem. 1964, 68, 821. [Google Scholar] [CrossRef]
- Huang, D.M.; Chandler, D. Temperature and length scale dependence of hydrophobic effects and their possible implications for protein folding. Proc. Natl. Acad. Sci. USA 2000, 97, 8324–8327. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.J.; Xi, X.K.; Kleinhammes, A.; Wu, Y. Temperature-induced hydrophobic-hydrophilic transition observed by water adsorption. Science 2008, 322, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Mallamace, D.; Chen, S.H.; Corsaro, C.; Fazio, E.; Mallamace, F.; Stanley, H.E. Hydrophilic and hydrophobic competition in water methanol soutions. Sci. China Phys. Mech. Astr. 2019, 62, 107003. [Google Scholar] [CrossRef]
- Berne, B.J.; Pecora, R. Dynamic Light Scattering: With Applications to Chemistry, Biology, and Physics; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Stanley, H.E. Introduction to Phase Transition and Critical Phenomena; Oxford University Press: Oxford, UK, 1971. [Google Scholar]
- Mori, H. A Continued-Fraction Representation of the Time-Correlation Functions. Prog. Theor. Phys. 1965, 34, 399–416. [Google Scholar] [CrossRef] [Green Version]
- Götze, W. Complex Dynamics of Glass-Forming Liquids a Mode-Coupling Theory; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Mallamace, F.; Mensitieri, G.; Salzano de Luna, M.; Lanzafame, P.; Papanikolaou, G.; Mallamace, D. The Interplay between the Theories of Mode Coupling and of Percolation Transition in Attractive Colloidal Systems. Int. J. Mol. Sci. 2022, 23, 5316. [Google Scholar] [CrossRef] [PubMed]
- Bergenholtz, J.; Fuchs, M. Nonergodicity transitions in colloidal suspensions with attractive interactions. Phys. Rev. E 1999, 59, 5706. [Google Scholar] [CrossRef] [Green Version]
- Mallamace, F.; Corsaro, L.C.; Vasi, C.; Vasino, S.; Mallamace, D.; Chen, S.H. The dynamical fragile-to-strong crossover in attractive colloidal systems. J. Non Cryst. Sol. 2015, 407, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeldt, S.; Dingenouts, N.; Pötschke, D.; Ballauff, M.; Berresheim, A.J.; Müllen, K.; Lindner, P. Analysis of the Spatial Dimensions of Fully Aromatic Dendrimers. Angew. Chem. Int. Ed. 2004, 43, 109–112. [Google Scholar] [CrossRef]
- van Mengen, W.; Mortensen, T.C.; Williams, S.R.; Müller, J. Measurement of the self-intermediate scattering function of suspensions of hard spherical particles near the glass transition. Phys. Rev. E 1988, 58, 6073–6085. [Google Scholar] [CrossRef]
- Pusey, P.N.; van Mengen, W. Observation of a glass transition in suspensions of spherical colloidal particles. Phys. Rev. Lett. 1987, 59, 2083. [Google Scholar] [CrossRef]
- Dawson, K.A.; Foffi, G.; Fuchs, M.; Götze, W.; Sciortino, F.; Sperl, M.; Tartaglia, P.; Voigtmann, T.; Zaccarelli, E. Higher-order glass-transition singularities in colloidal systems with attractive interactions. Phys. Rev. E 2000, 63, 011401. [Google Scholar] [CrossRef] [Green Version]
- Zaccarelli, E.; Foffi, G.; Dawson, K.A.; Sciortino, F.; Tartaglia, P. Mechanical properties of a model of attractive colloidal solutions. Phys. Rev. E 2001, 63, 031501. [Google Scholar] [CrossRef] [PubMed]
- Fabbian, L.; Götze, W.; Sciortino, F.; Tartaglia, P.; Thiery, F. Ideal glass-glass transitions and logarithmic decay of correlations in a simple system. Phys. Rev. E 1999, 59, R1347. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.H.; Chen, W.R.; Mallamace, F. The glass-to-glass transition and its end point in a copolymer micellar system. Science 2003, 300, 619–622. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.H. Connections of activated hopping processes with the breakdown of the Stokes-Einstein relation and with aspects of dynamical heterogeneities. Phys. Rev. E 2008, 78, 041501. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.H.; Chen, S.H.; Mallamace, F. A possible scenario for the fragile-to-strong dynamic crossover predicted by the extended mode-coupling theory for glass transition. J. Phys. Cond. Matter 2009, 21, 504101. [Google Scholar] [CrossRef] [PubMed]
- Mallamace, F.; Branca, C.; Corsaro, B.C.; Leone, N.; Spoorem, J.; Chen, S.H.; Stanley, E.H. Transport properties of glass-forming liquids suggest that dynamic crossover temperature is as importantas the glass transition temperature. Proc. Natl. Acad. Sci. USA 2010, 107, 22457–22462. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallamace, F.; Mensitieri, G.; Salzano de Luna, M.; Mallamace, D. The Hydrophobic Effect Studied by Using Interacting Colloidal Suspensions. Int. J. Mol. Sci. 2023, 24, 2003. https://doi.org/10.3390/ijms24032003
Mallamace F, Mensitieri G, Salzano de Luna M, Mallamace D. The Hydrophobic Effect Studied by Using Interacting Colloidal Suspensions. International Journal of Molecular Sciences. 2023; 24(3):2003. https://doi.org/10.3390/ijms24032003
Chicago/Turabian StyleMallamace, Francesco, Giuseppe Mensitieri, Martina Salzano de Luna, and Domenico Mallamace. 2023. "The Hydrophobic Effect Studied by Using Interacting Colloidal Suspensions" International Journal of Molecular Sciences 24, no. 3: 2003. https://doi.org/10.3390/ijms24032003
APA StyleMallamace, F., Mensitieri, G., Salzano de Luna, M., & Mallamace, D. (2023). The Hydrophobic Effect Studied by Using Interacting Colloidal Suspensions. International Journal of Molecular Sciences, 24(3), 2003. https://doi.org/10.3390/ijms24032003








