Trimethylamine N-Oxide Response to a Mixed Macronutrient Tolerance Test in a Cohort of Healthy United States Adults
Abstract
:1. Introduction
2. Results
2.1. Participant and Mixed Macronutrient Tolerance Test Characteristics
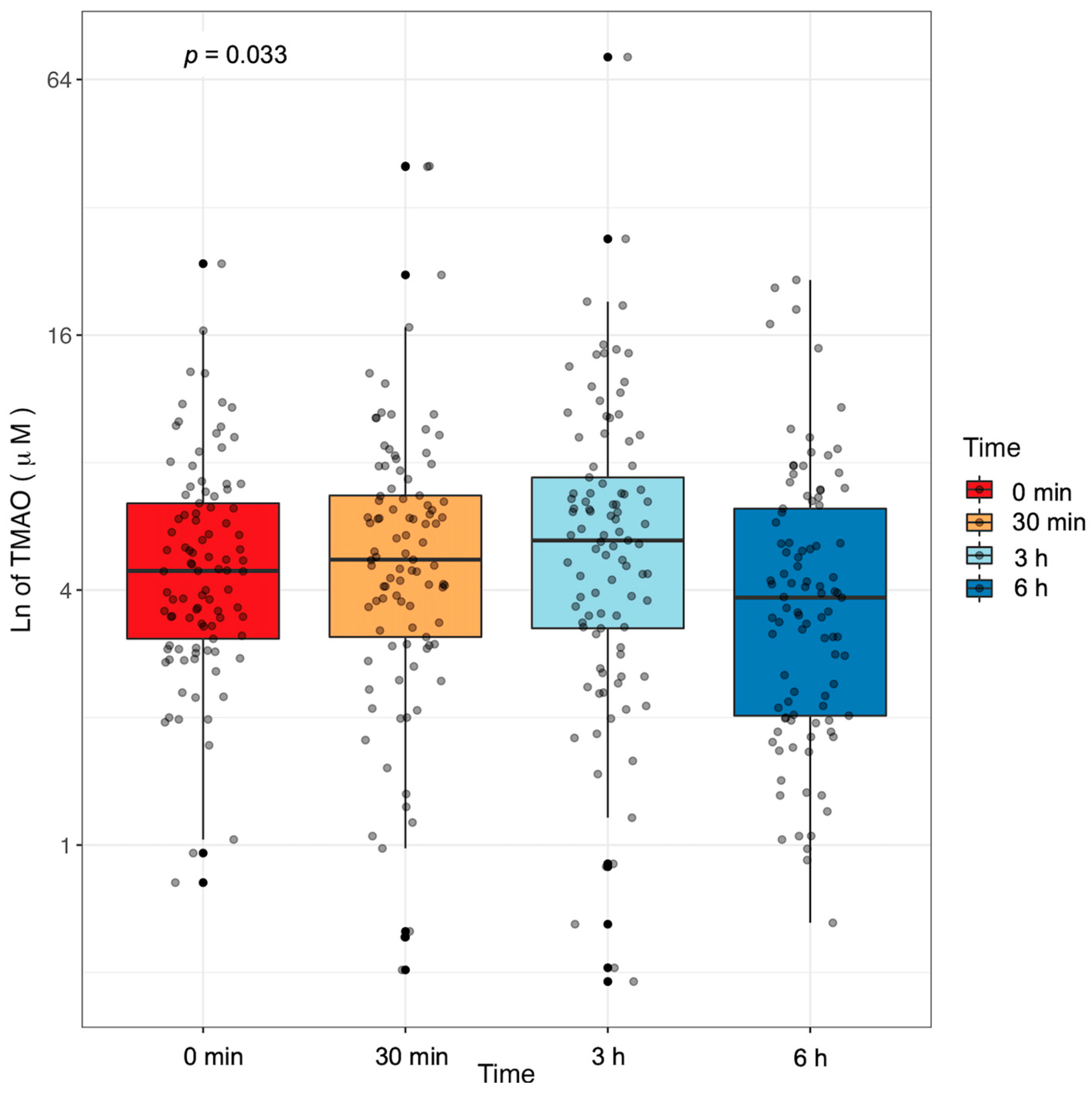
2.2. Plasma TMAO Concentration Changes in Response to an MMTT
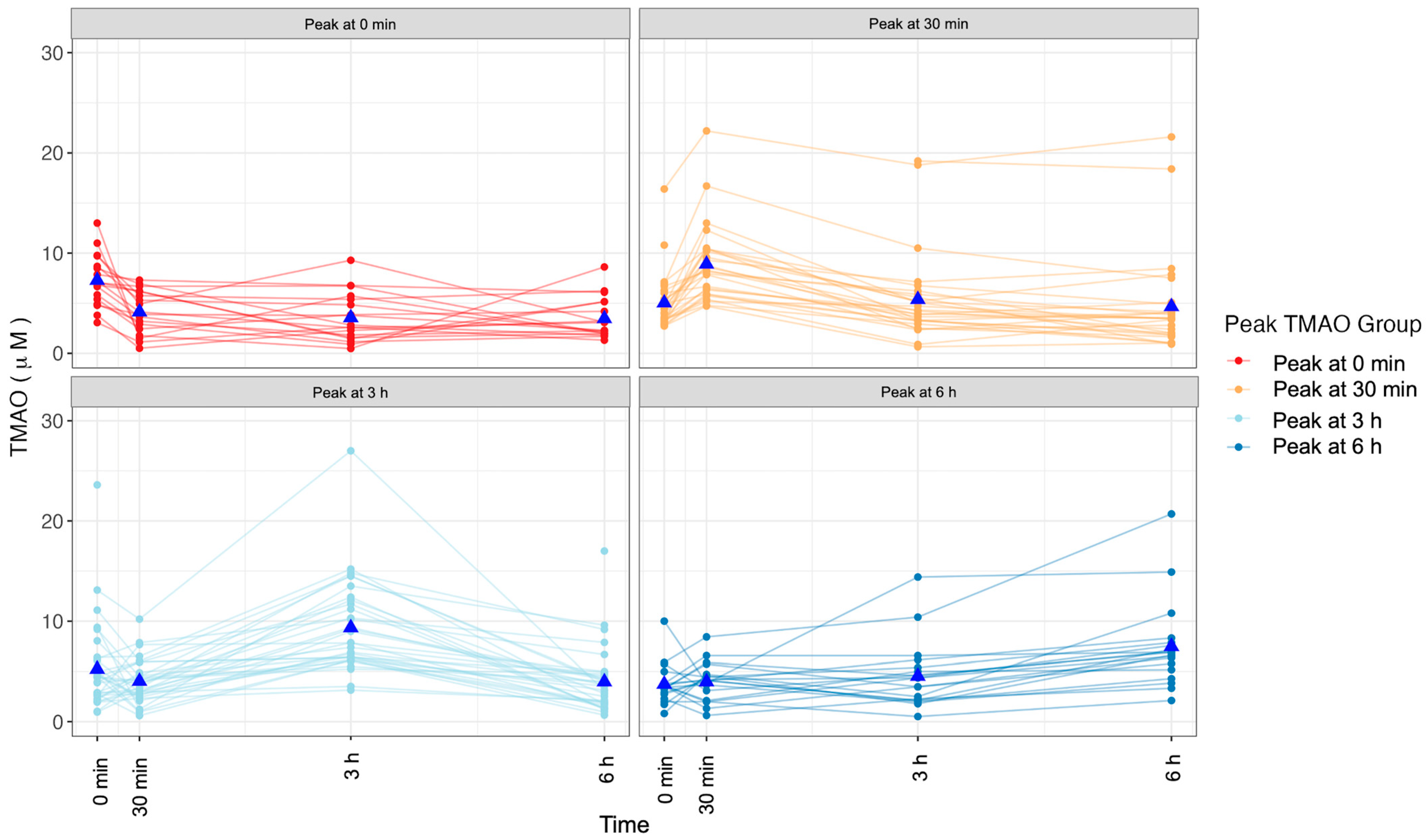
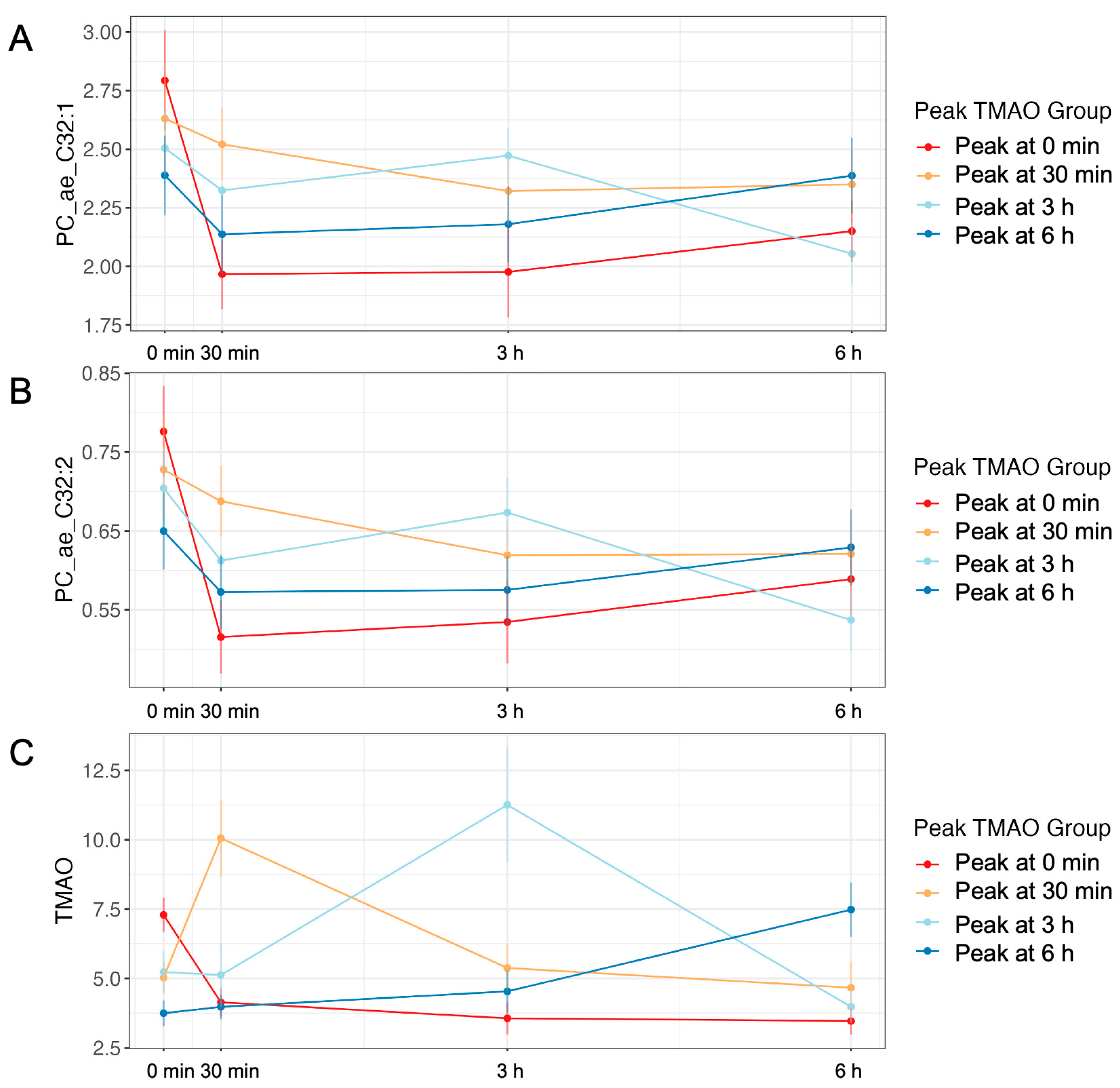
2.3. Changes in TMAO Concentration after an MMTT Display Variation
2.4. Relationships between Fecal Microbiome and Postprandial TMAO Response Are Weak
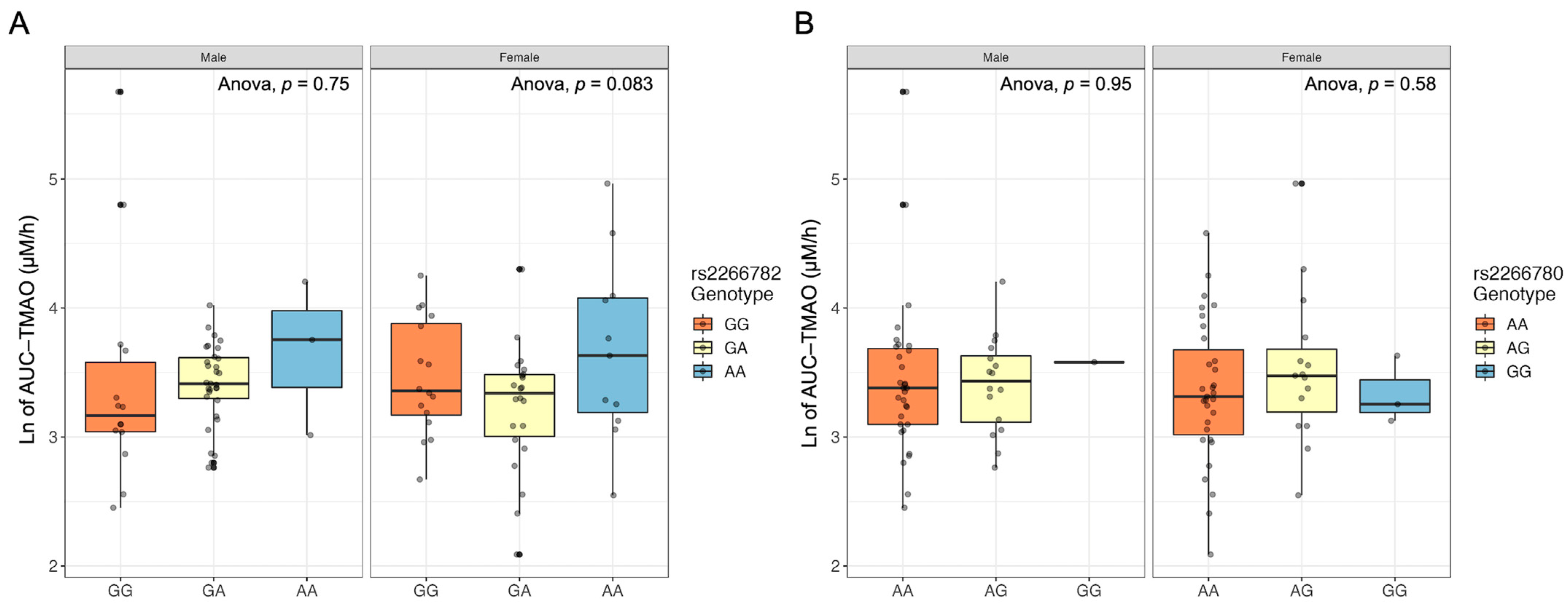
2.5. FMO3 Genotype Does Not Relate to Postprandial TMAO Response
2.6. The Plasma Metabolome Differs by TMAO-Response Group
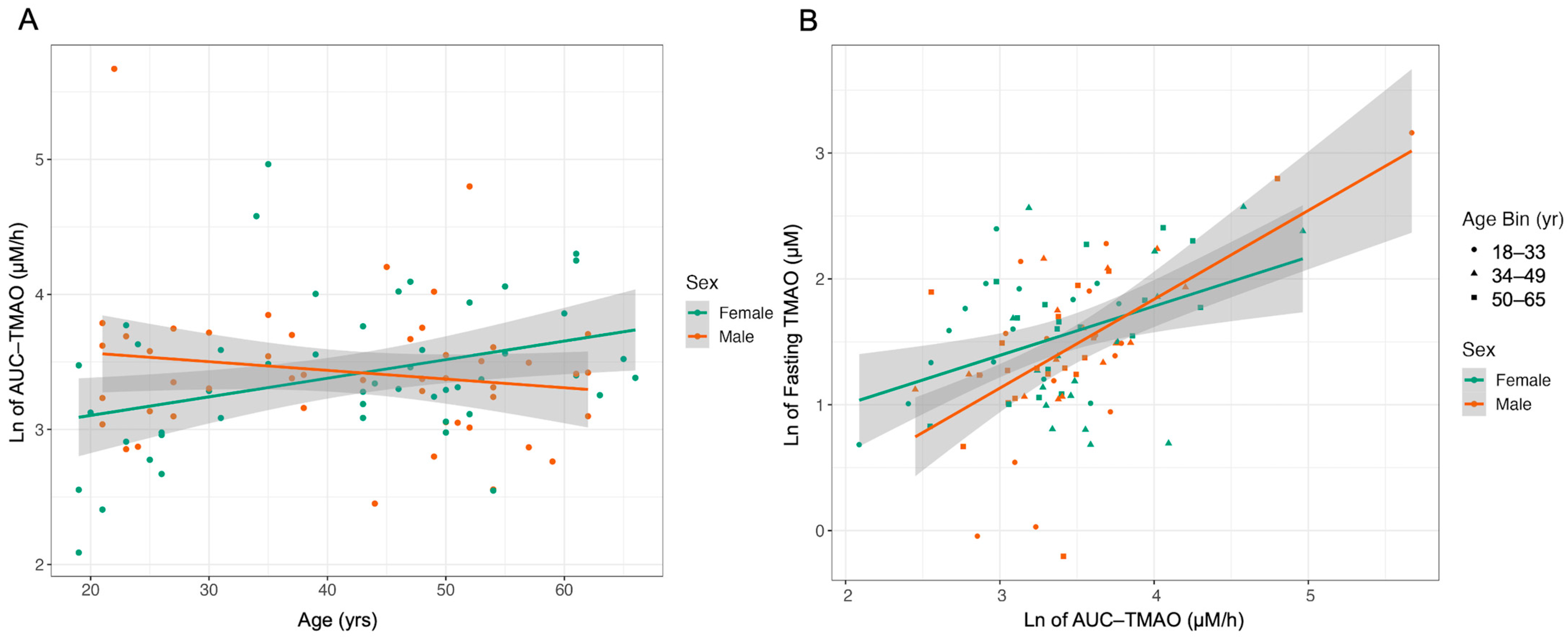
2.7. Postprandial Exposure to TMAO Relates to CVD Biomarkers
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Mixed Macronutrient Tolerance Test
4.3. Gut Microbiome
4.4. Plasma Metabolome
4.5. CVD Biomarkers
4.6. Total Exposure Quantification
4.7. Postprandial TMAO Response Patterns
4.8. FMO3 Genotype Assessment
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Psychogios, N.; Hau, D.D.; Peng, J.; Guo, A.C.; Mandal, R.; Bouatra, S.; Sinelnikov, I.; Krishnamurthy, R.; Eisner, R.; Gautam, B.; et al. The Human Serum Metabolome. PLoS ONE 2011, 6, e16957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Huda, M.N.; Bennett, B.J. Sequence Meets Function—Microbiota and Cardiovascular Disease. Cardiovasc. Res. 2022, 118, 399–412. [Google Scholar] [CrossRef]
- Stroeve, J.H.M.; van Wietmarschen, H.; Kremer, B.H.A.; van Ommen, B.; Wopereis, S. Phenotypic Flexibility as a Measure of Health: The Optimal Nutritional Stress Response Test. Genes Nutr. 2015, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Newman, J.W.; Krishnan, S.; Borkowski, K.; Adams, S.H.; Stephensen, C.B.; Keim, N.L. Assessing Insulin Sensitivity and Postprandial Triglyceridemic Response Phenotypes With a Mixed Macronutrient Tolerance Test. Front. Nutr. 2022, 9, 877696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. Gut Flora Metabolism of Phosphatidylcholine Promotes Cardiovascular Disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef] [Green Version]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal Microbiota Metabolism of L-Carnitine, a Nutrient in Red Meat, Promotes Atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taesuwan, S.; Cho, C.E.; Malysheva, O.V.; Bender, E.; King, J.H.; Yan, J.; Thalacker-Mercer, A.E.; Caudill, M.A. The Metabolic Fate of Isotopically Labeled Trimethylamine- N -Oxide (TMAO) in Humans. J. Nutr. Biochem. 2017, 45, 77–82. [Google Scholar] [CrossRef]
- Zhou, Z.; Jin, H.; Ju, H.; Sun, M.; Chen, H.; Li, L. Circulating Trimethylamine-N-Oxide and Risk of All-Cause and Cardiovascular Mortality in Patients With Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Front. Med. 2022, 9, 828343. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.C.; Org, E.; Buffa, J.A.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut Microbial Metabolite TMAO Enhances Platelet Hyperreactivity and Thrombosis Risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, R.; Ge, X.; Han, L.; Yu, P.; Gong, X.; Meng, Q.; Zhang, Y.; Fan, H.; Zheng, L.; Liu, Z.; et al. Gut Microbe–Generated Metabolite Trimethylamine N -oxide and the Risk of Diabetes: A Systematic Review and Dose-response Meta-analysis. Obes. Rev. 2019, 20, 883–894. [Google Scholar] [CrossRef]
- Bennett, B.J.; de Aguiar Vallim, T.Q.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-Oxide, a Metabolite Associated with Atherosclerosis, Exhibits Complex Genetic and Dietary Regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, C.E.; Taesuwan, S.; Malysheva, O.V.; Bender, E.; Tulchinsky, N.F.; Yan, J.; Sutter, J.L.; Caudill, M.A. Trimethylamine-N-Oxide (TMAO) Response to Animal Source Foods Varies among Healthy Young Men and Is Influenced by Their Gut Microbiota Composition: A Randomized Controlled Trial. Mol. Nutr. Food Res. 2017, 61, 1600324. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.E.; Aardema, N.D.J.; Bunnell, M.L.; Larson, D.P.; Aguilar, S.S.; Bergeson, J.R.; Malysheva, O.V.; Caudill, M.A.; Lefevre, M. Effect of Choline Forms and Gut Microbiota Composition on Trimethylamine-N-Oxide Response in Healthy Men. Nutrients 2020, 12, 2220. [Google Scholar] [CrossRef]
- Krishnan, S.; O’Connor, L.E.; Wang, Y.; Gertz, E.R.; Campbell, W.W.; Bennett, B.J. Adopting a Mediterranean-Style Eating Pattern with Low, but Not Moderate, Unprocessed, Lean Red Meat Intake Reduces Fasting Serum Trimethylamine N-Oxide (TMAO) in Adults Who Are Overweight or Obese. Br. J. Nutr. 2021, 128, 1738–1746. [Google Scholar] [CrossRef] [PubMed]
- Boutagy, N.E.; Neilson, A.P.; Osterberg, K.L.; Smithson, A.T.; Englund, T.R.; Davy, B.M.; Hulver, M.W.; Davy, K.P. Short-Term High-Fat Diet Increases Postprandial Trimethylamine-N-Oxide in Humans. Nutr. Res. 2015, 35, 858–864. [Google Scholar] [CrossRef]
- Baldiviez, L.M.; Keim, N.L.; Laugero, K.D.; Hwang, D.H.; Huang, L.; Woodhouse, L.R.; Burnett, D.J.; Zerofsky, M.S.; Bonnel, E.L.; Allen, L.H.; et al. Design and Implementation of a Cross-Sectional Nutritional Phenotyping Study in Healthy US Adults. BMC Nutr. 2017, 3, 79. [Google Scholar] [CrossRef] [Green Version]
- U.S. Census Bureau QuickFacts: California. Available online: https://www.census.gov/quickfacts/CA (accessed on 23 November 2022).
- Cashman, J.R.; Akerman, B.R.; Forrest, S.M.; Treacy, E.P. Population-specific polymorphisms of the human fmo3 gene: Significance for detoxication. Drug Metab. Dispos. 2000, 28, 169–173. [Google Scholar]
- DbSNP Short Genetic Variants Rs2266782. Available online: https://www.ncbi.nlm.nih.gov/snp/rs2266782 (accessed on 25 October 2022).
- DbSNP Short Genetiic Variants Rs2266780. Available online: https://www.ncbi.nlm.nih.gov/snp/rs2266780 (accessed on 25 October 2022).
- Voshol, P.J.; Minich, D.M.; Havinga, R.; Elferink, R.P.J.O.; Verkade, H.J.; Groen, A.K.; Kuipers, F. Postprandial Chylomicron Formation and Fat Absorption in Multidrug Resistance Gene 2 P-Glycoprotein–Deficient Mice. Gastroenterology 2000, 118, 173–182. [Google Scholar] [CrossRef]
- James, K.L.; Gertz, E.R.; Cervantes, E.; Bonnel, E.L.; Stephensen, C.B.; Kable, M.E.; Bennett, B.J. Diet, Fecal Microbiome, and Trimethylamine N-Oxide in a Cohort of Metabolically Healthy United States Adults. Nutrients 2022, 14, 1376. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal Microbial Metabolism of Phosphatidylcholine and Cardiovascular Risk. N. Engl. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.; You, T.; Li, J.; Pan, T.; Xiang, L.; Han, Y.; Zhu, L. Circulating Trimethylamine N-Oxide and the Risk of Cardiovascular Diseases: A Systematic Review and Meta-Analysis of 11 Prospective Cohort Studies. J. Cell. Mol. Med. 2018, 22, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.A.; Benton, T.Z.; Bennett, B.J.; Jacobs, D.R.; Lloyd-Jones, D.M.; Gross, M.D.; Carr, J.J.; Gordon-Larsen, P.; Zeisel, S.H. Microbiota-Dependent Metabolite Trimethylamine N-Oxide and Coronary Artery Calcium in the Coronary Artery Risk Development in Young Adults Study (CARDIA). J. Am. Heart Assoc. 2016, 5, e003970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andraos, S.; Jones, B.; Lange, K.; Clifford, S.A.; Thorstensen, E.B.; Kerr, J.A.; Wake, M.; Saffery, R.; Burgner, D.P.; O’Sullivan, J.M. Trimethylamine N-Oxide (TMAO) Is Not Associated with Cardiometabolic Phenotypes and Inflammatory Markers in Children and Adults. Curr. Dev. Nutr. 2021, 5, nzaa179. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Li, X.S.; Wu, Y.; Wang, Z.; Khaw, K.-T.; Wareham, N.J.; Nieuwdorp, M.; Boekholdt, S.M.; Hazen, S.L. Plasma Trimethylamine N-Oxide (TMAO) Levels Predict Future Risk of Coronary Artery Disease in Apparently Healthy Individuals in the EPIC-Norfolk Prospective Population Study. Am. Heart J. 2021, 236, 80–86. [Google Scholar] [CrossRef]
- Ferrell, M.; Bazeley, P.; Wang, Z.; Levison, B.S.; Li, X.S.; Jia, X.; Krauss, R.M.; Knight, R.; Lusis, A.J.; Garcia-Garcia, J.C.; et al. Fecal Microbiome Composition Does Not Predict Diet-Induced TMAO Production in Healthy Adults. J. Am. Heart Assoc. 2021, 10, e021934. [Google Scholar] [CrossRef]
- Schadt, E.E.; Molony, C.; Chudin, E.; Hao, K.; Yang, X.; Lum, P.Y.; Kasarskis, A.; Zhang, B.; Wang, S.; Suver, C.; et al. Mapping the Genetic Architecture of Gene Expression in Human Liver. PLoS Biol. 2008, 6, e107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schugar, R.C.; Gliniak, C.M.; Osborn, L.J.; Massey, W.; Sangwan, N.; Horak, A.; Banerjee, R.; Orabi, D.; Helsley, R.N.; Brown, A.L.; et al. Gut Microbe-Targeted Choline Trimethylamine Lyase Inhibition Improves Obesity via Rewiring of Host Circadian Rhythms. eLife 2022, 11, e63998. [Google Scholar] [CrossRef]
- Astafev, A.A.; Patel, S.A.; Kondratov, R.V. Calorie Restriction Effects on Circadian Rhythms in Gene Expression Are Sex Dependent. Sci. Rep. 2017, 7, 9716. [Google Scholar] [CrossRef] [Green Version]
- GTExPortal. Available online: https://www.gtexportal.org/home/gene/FMO (accessed on 31 October 2022).
- Dantas Machado, A.C.; Brown, S.D.; Lingaraju, A.; Sivaganesh, V.; Martino, C.; Chaix, A.; Zhao, P.; Pinto, A.F.M.; Chang, M.W.; Richter, R.A.; et al. Diet and Feeding Pattern Modulate Diurnal Dynamics of the Ileal Microbiome and Transcriptome. Cell Rep. 2022, 40, 111008. [Google Scholar] [CrossRef]
- Falconi, C.A.; Junho, C.V.d.C.; Fogaça-Ruiz, F.; Vernier, I.C.S.; da Cunha, R.S.; Stinghen, A.E.M.; Carneiro-Ramos, M.S. Uremic Toxins: An Alarming Danger Concerning the Cardiovascular System. Front. Physiol. 2021, 12, 686249. [Google Scholar] [CrossRef]
- Vollmers, C.; Gill, S.; DiTacchio, L.; Pulivarthy, S.R.; Le, H.D.; Panda, S. Time of Feeding and the Intrinsic Circadian Clock Drive Rhythms in Hepatic Gene Expression. Proc. Natl. Acad. Sci. USA 2009, 106, 21453–21458. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Kang, J.; Kim, S.H.; Chung, H.S.; Kim, Y.J.; Yu, J.M.; Cho, S.T.; Oh, C.-M.; Kim, T. Beneficial Effects of Time-Restricted Eating on Metabolic Diseases: A Systemic Review and Meta-Analysis. Nutrients 2020, 12, 1267. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide Promotes Vascular Inflammation Through Signaling of Mitogen-Activated Protein Kinase and Nuclear Factor-κB. J. Am. Heart Assoc. 2016, 5, e002767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, D.M.; Wang, Z.; Lee, R.; Meng, Y.; Che, N.; Charugundla, S.; Qi, H.; Wu, J.; Pan, C.; Brown, J.M.; et al. Flavin Containing Monooxygenase 3 Exerts Broad Effects on Glucose and Lipid Metabolism and Atherosclerosis. J. Lipid Res. 2015, 56, 22–37. [Google Scholar] [CrossRef] [Green Version]
- Cashman, J.; Camp, K.; Fakharzadeh, S.; Fennessey, P.; Hines, R.; Mamer, O.; Mitchell, S.; Preti, G.; Schlenk, D.; Smith, R. Biochemical and Clinical Aspects of the Human Flavin-Containing Monooxygenase Form 3 (FMO3) Related to Trimethylaminuria. CDM 2003, 4, 151–170. [Google Scholar] [CrossRef]
- Kable, M.E.; Chin, E.L.; Storms, D.; Lemay, D.G.; Stephensen, C.B. Tree-Based Analysis of Dietary Diversity Captures Associations between Fiber Intake and Gut Microbiota Composition in a Healthy U.S. Adult Cohort. J. Nutr. 2021, 152, 779–788. [Google Scholar] [CrossRef]
- Wang, Y.E.; Kirschke, C.P.; Woodhouse, L.R.; Bonnel, E.L.; Stephensen, C.B.; Bennett, B.J.; Newman, J.W.; Keim, N.L.; Huang, L. SNPs in Apolipoproteins Contribute to Sex-Dependent Differences in Blood Lipids before and after a High-Fat Dietary Challenge in Healthy U.S. Adults. BMC Nutr. 2022, 8, 95. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet 2011, 17, 3. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]

| Male | Female | p-Value | |
|---|---|---|---|
| n | 49 | 48 | |
| Age (yr) | 41.86 (14.01) | 41.71 (13.56) | 0.958 |
| BMI (kg/m2) | 28.66 (5.52) | 28.19 (5.41) | 0.673 |
| Ethnicity (%) | 0.919 | ||
| Caucasian | 32 (65.3) | 33 (70.2) | |
| Hispanic | 9 (18.4) | 5 (10.6) | |
| African American | 1 (2.0) | 2 (4.3) | |
| Asian | 2 (4.1) | 2 (4.3) | |
| Multiple | 4 (8.2) | 4 (8.5) | |
| Other | 1 (2.0) | 1 (2.1) | |
| Cystatin C (µM) | 0.84 (0.14) | 0.90 (0.14) | 0.036 |
| TMAO (µM) | 5.41 (2.96) | 5.12 (3.87) | 0.679 |
| Glucose (mg/dL) | 92.93 (6.85) | 95.80 (11.96) | 0.152 |
| Insulin (pM) | 69.20 (42.79) | 76.62 (100.28) | 0.638 |
| Triglycerides (mg/dL) | 94.42 (38.55) | 111.77 (60.86) | 0.098 |
| HDL-C (mg/dL) | 58.58 (18.79) | 44.66 (11.97) | <0.001 |
| LDL-C (mg/dL) | 107.65 (22.09) | 106.86 (30.80) | 0.885 |
| Total Cholesterol (mg/dL) | 175.11 (30.93) | 164.51 (29.90) | 0.091 |
| Ingredient | Amount (g) | Weight % |
|---|---|---|
| Pasteurized liquid egg white | 2105.9 | 70.20 |
| Palm oil shortening | 456.5 | 15.22 |
| Granulated white sugar | 421.4 | 14.05 |
| Cellulose gum | 8.3 | 0.28 |
| Xanthan gum | 4.7 | 0.16 |
| Pure vanilla extract | 1.0 | 0.03 |
| Almond extract | 1.0 | 0.03 |
| Imitation butter flavor | 1.0 | 0.03 |
| Peak 0 m | Peak 30 m | Peak 3 h | Peak 6 h | p-Value | |
|---|---|---|---|---|---|
| n (Female) | 18 (10) | 27 (16) | 33 (15) | 19 (8) | 0.597 |
| Age (y) | 36.67 (14.74) | 42.67 (14.50) | 42.36 (12.14) | 44.37 (14.04) | 0.345 |
| BMI (kg/m2) | 28.87 (4.80) | 27.36 (5.45) | 28.40 (5.98) | 29.58 (5.14) | 0.576 |
| 0 min | 7.29 (2.55) A | 5.03 (2.92) A,B | 5.23 (4.35) A,B | 3.74 (1.97) B | 0.015 |
| 30 min | 4.14 (2.14) A | 10.05 (7.13) B | 5.12 (6.61) A | 3.97 (1.90) A,C | <0.001 |
| 3 h | 3.56 (2.43) A | 5.37 (4.41) A,C | 11.26 (11.88) B | 4.53 (3.27) A | 0.001 |
| 6 h | 3.46 (2.02) A | 4.66 (4.88) A,B | 3.97 (3.23) A | 7.48 (4.24) B | 0.006 |
| AUCTMAO (µM/h) | 23.01 (9.01) A | 38.11 (29.46) B | 45.90 (46.71) B | 30.57 (16.58) A,B | <0.001 |
| rs2266782, G > A (n = 97) | |||||
|---|---|---|---|---|---|
| rs2266780, A > G (n = 97) | GG | GA | AA | Total | |
| AA | 30 | 26 | 6 | 62 | |
| AG | 0 | 26 | 5 | 31 | |
| GG | 0 | 1 | 3 | 4 | |
| Total | 30 | 53 | 14 | 97 | |
| rs2266782, G > A (µM/h) | |||||
| rs2266780, A > G (µM/h) | GG (WT) | GA | AA | Average | |
| AA | 42.01 (51.66) | 28.17 (11.01) | 48.54 (27.60) | 39.57 | |
| AG | NA | 31.98 (11.63) | 60.24 (51.92) | 46.11 | |
| GG | NA | 35.86 (NA) | 28.80 (7.90) | 32.33 | |
| Average | 42.01 | 32.0 | 45.86 | ||
| r | P-Adj | |
|---|---|---|
| PC_ae_C32.2 | 0.291 | 0.004 |
| TLCA | 0.265 | 0.009 |
| PC_ae_C32.1 | 0.250 | 0.013 |
| lysoPC_a_C14.0 | −0.240 | 0.018 |
| p-Cresol SO4 | 0.237 | 0.019 |
| PC_aa_C40.1 | −0.235 | 0.021 |
| Indoxyl SO4 | 0.226 | 0.026 |
| lysoPC_a_C16.0 | −0.190 | 0.062 |
| PC_ae_C40.2 | 0.176 | 0.085 |
| PC_ae_C30.2 | 0.160 | 0.116 |
| PC_aa_C40.2 | −0.158 | 0.122 |
| PC_ae_C40.5 | 0.145 | 0.155 |
| lysoPC_a_C24.0 | −0.141 | 0.167 |
| lysoPC_a_C28.1 | 0.135 | 0.187 |
| PC_ae_C30.0 | 0.134 | 0.191 |
| TDCA | 0.128 | 0.211 |
| lysoPC_a_C18.0 | −0.124 | 0.226 |
| GUDCA | −0.123 | 0.230 |
| TCDCA | 0.122 | 0.236 |
| PC_aa_C28.1 | 0.121 | 0.236 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
James, K.L.; Gertz, E.R.; Kirschke, C.P.; Allayee, H.; Huang, L.; Kable, M.E.; Newman, J.W.; Stephensen, C.B.; Bennett, B.J. Trimethylamine N-Oxide Response to a Mixed Macronutrient Tolerance Test in a Cohort of Healthy United States Adults. Int. J. Mol. Sci. 2023, 24, 2074. https://doi.org/10.3390/ijms24032074
James KL, Gertz ER, Kirschke CP, Allayee H, Huang L, Kable ME, Newman JW, Stephensen CB, Bennett BJ. Trimethylamine N-Oxide Response to a Mixed Macronutrient Tolerance Test in a Cohort of Healthy United States Adults. International Journal of Molecular Sciences. 2023; 24(3):2074. https://doi.org/10.3390/ijms24032074
Chicago/Turabian StyleJames, Kristen L., Erik R. Gertz, Catherine P. Kirschke, Hooman Allayee, Liping Huang, Mary E. Kable, John W. Newman, Charles B. Stephensen, and Brian J. Bennett. 2023. "Trimethylamine N-Oxide Response to a Mixed Macronutrient Tolerance Test in a Cohort of Healthy United States Adults" International Journal of Molecular Sciences 24, no. 3: 2074. https://doi.org/10.3390/ijms24032074





