AmAtg2B-Mediated Lipophagy Regulates Lipolysis of Pupae in Apis mellifera
Abstract
:1. Introduction
2. Results
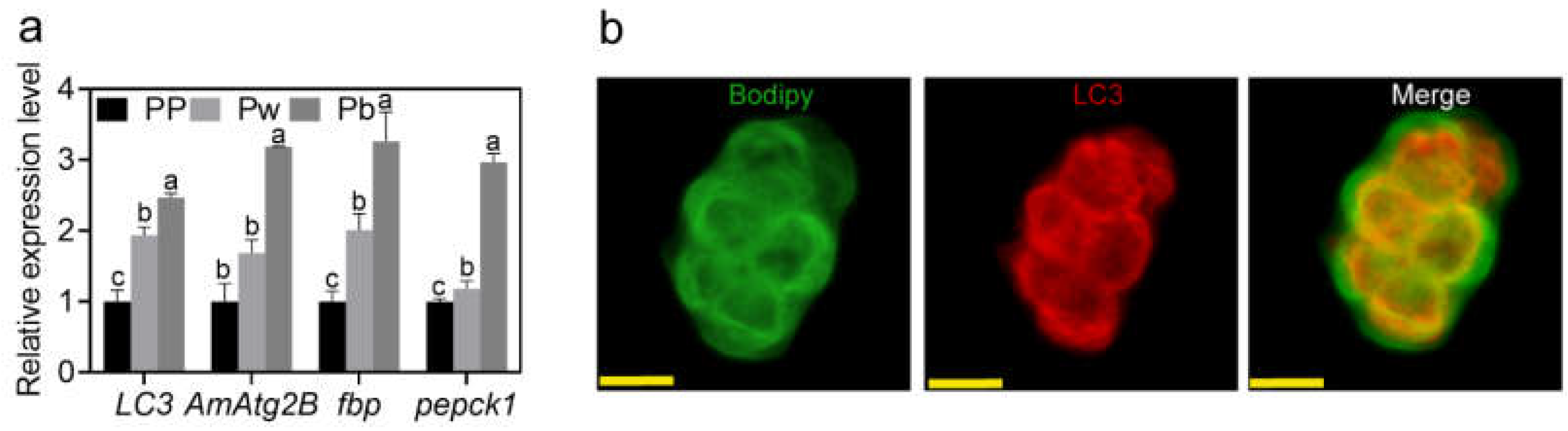
2.1. Lipophagy in the Fat Body of A. mellifera Pupae
2.2. Autophagy Is Functionally Involved in the Regulation of Pupal Lipid Metabolism in A. mellifera
2.3. Tissue Specificity of AmAtg2B and Structural and Functional Prediction of Its Protein
2.4. AmAtg2B Mediated Lipophagy Disorder Could Cause Abnormal Lipid Metabolism and Finally Lead to Abnormal Development of Pupae in A. mellifera
2.5. Molecular Mechanism of AmAtg2B Involved Lipophagy Affecting Pupal Lipid Lipolysis in A. mellifera
2.6. PC Rescued Abnormal Developmental Arrest of Pupae Induced by Anti-Atg2B
3. Discussion
4. Materials and Methods
4.1. Honeybee Samples and Specimens
4.2. RNA Preparation and Fluorescent Real-Time Quantitative PCR (qRT-PCR)
4.3. Western Blotting
4.4. Immunofluorescence Staining and BODIPY 493/503 Staining
4.5. Measurement of Enzymatic Activity Levels and TAG Content
4.6. Bioinformatics Analysis
4.7. LC-MS/MS Protein Identification and LC/MS Non-Targeted Lipidome Analysis
4.8. RNA INTERFERENCE
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.-Y.; Cooksey, B.A.; Baehrecke, E.H. Steroid Regulation of Midgut Cell Death during Drosophila Development. Dev. Biol. 2002, 250, 101–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, M.; Ohsumi, Y. Isolation and characterization of autophagy-defective mutants of Saccharomyces cerevisiae. FEBS Lett. 1993, 333, 169–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotani, T.; Kirisako, H.; Koizumi, M.; Ohsumi, Y.; Nakatogawa, H. The Atg2-Atg18 complex tethers pre-autophagosomal membranes to the endoplasmic reticulum for autophagosome formation. Proc. Natl. Acad. Sci. USA 2018, 115, 10363–10368. [Google Scholar] [CrossRef] [Green Version]
- Valverde, D.P.; Yu, S.; Boggavarapu, V.; Kumar, N.; Lees, J.A.; Walz, T.; Reinisch, K.M.; Melia, T.J. ATG2 transports lipids to promote autophagosome biogenesis. J. Cell Biol. 2019, 218, 1787–1798. [Google Scholar] [CrossRef] [Green Version]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Bartholomew, C.R.; Zhou, W.; Klionsky, D.J. Assaying autophagic activity in transgenic GFP-Lc3 and GFP-Gabarap zebrafish embryos. Autophagy 2009, 5, 520–526. [Google Scholar] [CrossRef] [Green Version]
- Yabu, T.; Imamura, S.; Mizusawa, N.; Touhata, K.; Yamashita, M. Induction of Autophagy by Amino Acid Starvation in Fish Cells. Mar. Biotechnol. 2012, 14, 491–501. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Han, S.-L.; Lu, D.-L.; Li, L.-Y.; Limbu, S.M.; Zhang, M.-L.; Du, Z.-Y.; Li, D.-L. Inhibited Lipophagy Suppresses Lipid Metabolism in Zebrafish Liver Cells. Front. Physiol. 2019, 10, 1077. [Google Scholar] [CrossRef]
- Ulgherait, M.; Midoun, A.M.; Park, S.J.; Gatto, J.A.; Tener, S.J.; Siewert, J.; Klickstein, N.; Canman, J.C.; Ja, W.W.; Shirasu-Hiza, M. Circadian autophagy drives iTRF-mediated longevity. Nature 2021, 598, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Stolz, A.; Ernst, A.; Dikic, I. Cargo recognition and trafficking in selective autophagy. Nature 2014, 16, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131–1135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Araujo, S.; Bomfim, L.; Araripe, L.O.; Bruno, R.; Ramos, I.; Gondim, K.C. Silencing of ATG6 and ATG8 promotes increased levels of triacylglycerol (TAG) in the fat body during prolonged starvation periods in the Chagas disease vector Rhodnius prolixus. Insect Biochem. Mol. Biol. 2020, 127, 103484. [Google Scholar] [CrossRef]
- Vaughan, M.; Berger, J.E.; Steinberg, D. Hormone-sensitive lipasde and monoglyceride lipase activities in adipose tissue. J. Biol. Chem. 1964, 239, 401–409. [Google Scholar] [CrossRef]
- Zimmermann, R.; Strauss, J.G.; Haemmerle, G.; Schoiswohl, G.; Birner-Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; et al. Fat Mobilization in Adipose Tissue Is Promoted by Adipose Triglyceride Lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef] [Green Version]
- Zechner, R.; Zimmermann, R.; Eichmann, T.O.; Kohlwein, S.D.; Haemmerle, G.; Lass, A.; Madeo, F. FAT SIGNALS—Lipases and Lipolysis in Lipid Metabolism and Signaling. Cell Metab. 2012, 15, 279–291. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, S.; Rodriguez-Navarro, J.A.; Arias, E.; Kiffin, R.; Sahu, S.; Schwartz, G.J.; Cuervo, A.M.; Singh, R. Autophagy in Hypothalamic AgRP Neurons Regulates Food Intake and Energy Balance. Cell Metab. 2011, 14, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Leonard, S.P.; Powell, J.E.; Perutka, J.; Geng, P.; Heckmann, L.C.; Horak, R.D.; Davies, B.W.; Ellington, A.D.; Barrick, J.E.; Moran, N.A. Engineered symbionts activate honey bee immunity and limit pathogens. Science 2020, 367, 573–576. [Google Scholar] [CrossRef]
- Ouimet, M.; Franklin, V.; Mak, E.; Liao, X.; Tabas, I.; Marcel, Y.L. Autophagy Regulates Cholesterol Efflux from Macrophage Foam Cells via Lysosomal Acid Lipase. Cell Metab. 2011, 13, 655–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khaldoun, S.A.; Emond-Boisjoly, M.-A.; Chateau, D.; Carrière, V.; Lacasa, M.; Rousset, M.; Demignot, S.; Morel, E. Autophagosomes contribute to intracellular lipid distribution in enterocytes. Mol. Biol. Cell 2014, 25, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Xiang, Y.; Wang, Y.; Baikati, K.; Cuervo, A.M.; Luu, Y.K.; Tang, Y.; Pessin, J.E.; Schwartz, G.J.; Czaja, M.J. Autophagy regulates adipose mass and differentiation in mice. J. Clin. Investig. 2009, 119, 3329–3339. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.N.; Bormann, J.; Le, G.T.T.; Stärkel, C.; Olsson, S.; Nosanchuk, J.D.; Giese, H.; Schäfer, W. Autophagy-related lipase FgATG15 of Fusarium graminearum is important for lipid turnover and plant infection. Fungal Genet. Biol. 2011, 48, 217–224. [Google Scholar] [CrossRef]
- Kurusu, T.; Koyano, T.; Hanamata, S.; Kubo, T.; Noguchi, Y.; Yagi, C.; Nagata, N.; Yamamoto, T.; Ohnishi, T.; Okazaki, Y.; et al. OsATG7 is required for autophagy-dependent lipid metabolism in rice postmeiotic anther development. Autophagy 2014, 10, 878–888. [Google Scholar] [CrossRef]
- Lapierre, L.R.; Gelino, S.; Meléndez, A.; Hansen, M. Autophagy and Lipid Metabolism Coordinately Modulate Life Span in Germline-less C. elegans. Curr. Biol. 2011, 21, 1507–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Rourke, E.J.; Ruvkun, G. MXL-3 and HLH-30 transcriptionally link lipolysis and autophagy to nutrient availability. Nat. Cell Biol. 2013, 15, 668–676. [Google Scholar] [CrossRef] [Green Version]
- van Zutphen, T.; Todde, V.; de Boer, R.; Kreim, M.; Hofbauer, H.F.; Wolinski, H.; Veenhuis, M.; van der Klei, I.J.; Kohlwein, S.D. Lipid droplet autophagy in the yeast Saccharomyces cerevisiae. Mol. Biol. Cell 2014, 25, 290–301. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.-W.; Miao, Y.-H.; Chang, Y.-S. A sterol-enriched vacuolar microdomain mediates stationary phase lipophagy in budding yeast. J. Cell Biol. 2014, 206, 357–366. [Google Scholar] [CrossRef] [Green Version]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Wang, C.-W. Lipid droplets, lipophagy, and beyond. Biochim. Et Biophys. Acta 2016, 1861, 793–805. [Google Scholar] [CrossRef] [PubMed]
- Von, G. Ber den formwechsel einiger zellorganelle bei der bildung der reservestoffe im fettkoerper von drosophila-larven [on variation in some cell organelles during formation of reserve substances in fatty bodies of drosophila larvae. Z. Fur Zellforsch. Und Mikrosk. Anat. 1963, 61, 56–95. [Google Scholar]
- Mulakkal, N.C.; Nagy, P.; Takats, S.; Tusco, R.; Juhász, G.; Nezis, I.P. Autophagy in Drosophila: From Historical Studies to Current Knowledge. BioMed. Res. Int. 2014, 2014, 273473. [Google Scholar] [CrossRef] [Green Version]
- Wigglesworth, V.B. Cytological Changes in the Fat Body of Rhodnius During Starvation, Feeding And Oxygen Want. J. Cell Sci. 1967, 2, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Rusten, T.E.; Lindmo, K.; Juhász, G.; Sass, M.; Seglen, P.O.; Brech, A.; Stenmark, H. Programmed Autophagy in the Drosophila Fat Body Is Induced by Ecdysone through Regulation of the PI3K Pathway. Dev. Cell 2004, 7, 179–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McPhee, C.K.; Baehrecke, E.H. Autophagy in Drosophila melanogaster. Biochim. Biophys. Acta 2009, 1793, 1452–1460. [Google Scholar] [CrossRef] [Green Version]
- Jung, W.; Liu, C.; Yu, Y.; Chang, Y.; Lien, W.; Chao, H.; Huang, S.; Kuo, C.; Ho, H.; Chan, C. Lipophagy prevents activity-dependent neurodegeneration due to dihydroceramide accumulation in vivo. EMBO Rep. 2017, 18, 1150–1165. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Neufeld, T.P.; O’Connor, M.B. A Tissue- and Temporal-Specific Autophagic Switch Controls Drosophila Pre-metamorphic Nutritional Checkpoints. Curr. Biol. 2019, 29, 2840–2851.e4. [Google Scholar] [CrossRef]
- Demarco, R.S.; Uyemura, B.S.; Jones, D.L. EGFR Signaling Stimulates Autophagy to Regulate Stem Cell Maintenance and Lipid Homeostasis in the Drosophila Testis. Cell Rep. 2020, 30, 1101–1116.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguila, J.R.; Suszko, J.; Gibbs, A.G.; Hoshizaki, D.K. The role of larval fat cells in adult Drosophila melanogaster. J. Exp. Biol. 2007, 210, 956–963. [Google Scholar] [CrossRef] [Green Version]
- Zheng, H.; Yang, X.; Xi, Y. Fat body remodeling and homeostasis control in Drosophila. Life Sci. 2016, 167, 22–31. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.P.; Cruz-Landim, C. Morphology and function of insect fat body cells: A review. Biociências 2003, 11, 195–205. [Google Scholar] [CrossRef]
- Olofsson, S.-O.; Boström, P.; Andersson, L.; Rutberg, M.; Perman, J.; Borén, J. Lipid droplets as dynamic organelles connecting storage and efflux of lipids. Biochim. Biophys. Acta 2009, 1791, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, S.G.; Bakula, D.; Frickey, T.; Cezanne, A.; Brigger, D.; Tschan, M.P.; Robenek, H.; Proikas-Cezanne, T. Lipid droplet and early autophagosomal membrane targeting of Atg2A and Atg14L in human tumor cells. J. Lipid Res. 2014, 55, 1267–1278. [Google Scholar] [CrossRef] [Green Version]
- Shibata, M.; Yoshimura, K.; Furuya, N.; Koike, M.; Ueno, T.; Komatsu, M.; Arai, H.; Tanaka, K.; Kominami, E.; Uchiyama, Y. The MAP1-LC3 conjugation system is involved in lipid droplet formation. Biochem. Biophys. Res. Commun. 2009, 382, 419–423. [Google Scholar] [CrossRef]
- Velikkakath, A.K.G.; Nishimura, T.; Oita, E.; Ishihara, N.; Mizushima, N. Mammalian Atg2 proteins are essential for autophagosome formation and important for regulation of size and distribution of lipid droplets. Mol. Biol. Cell 2012, 23, 896–909. [Google Scholar] [CrossRef]
- Yamamoto, Y.-H.; Noda, T. Autophagosome formation in relation to the endoplasmic reticulum. J. Biomed. Sci. 2020, 27, 97. [Google Scholar] [CrossRef]
- Harding, T.M.; Morano, K.; Scott, S.V.; Klionsky, D.J. Isolation and characterization of yeast mutants in the cytoplasm to vacuole protein targeting pathway. J. Cell Biol. 1995, 131, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Scott, R.C.; Schuldiner, O.; Neufeld, T.P. Role and Regulation of Starvation-Induced Autophagy in the Drosophila Fat Body. Dev. Cell 2004, 7, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Bartok, O.; Teesalu, M.; Ashwall-Fluss, R.; Pandey, V.; Hanan, M.; Rovenko, B.M.; Poukkula, M.; Havula, E.; Moussaieff, A.; Vodala, S.; et al. The transcription factor Cabut coordinates energy metabolism and the circadian clock in response to sugar sensing. EMBO J. 2015, 34, 1538–1553. [Google Scholar] [CrossRef] [Green Version]
- Yoshinari, Y.; Kosakamoto, H.; Kamiyama, T.; Hoshino, R.; Matsuoka, R.; Kondo, S.; Tanimoto, H.; Nakamura, A.; Obata, F.; Niwa, R. The sugar-responsive enteroendocrine neuropeptide F regulates lipid metabolism through glucagon-like and insulin-like hormones in Drosophila melanogaster. Nat. Commun. 2021, 12, 4818. [Google Scholar] [CrossRef] [PubMed]
- Grönke, S.; Mildner, A.; Fellert, S.; Tennagels, N.; Petry, S.; Müller, G.; Jäckle, H.; Kühnlein, R.P. Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab. 2005, 1, 323–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.; Jung, J.; Yunusbaev, U.; Ilyasov, R.A.; Kwon, H.W. Characterization and its implication of a novel taste receptor detecting nutrients in the honey bee, Apis mellifera. Sci. Rep. 2019, 9, 17004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfisterer, S.G.; Bakula, D.; Proikas-Cezanne, T.; Robenek, H.; Proikas-Cezanne, T. WIPI β-propellers at the crossroads of autophagosome and lipid droplet dynamics. Biochem. Soc. Trans. 2014, 42, 1414–1417. [Google Scholar] [CrossRef]
- Muñoz-Braceras, S.; Calvo, R.; Escalante, R. TipC and the chorea-acanthocytosis protein VPS13A regulate autophagy in Dictyostelium and human HeLa cells. Autophagy 2015, 11, 918–927. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Leonzino, M.; Hancock-Cerutti, W.; Horenkamp, F.A.; Li, P.; Lees, J.A.; Wheeler, H.; Reinisch, K.M.; De Camilli, P. VPS13A and VPS13C are lipid transport proteins differentially localized at ER contact sites. J. Cell Biol. 2018, 217, 3625–3639. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Evans, T.D.; Jeong, S.-J.; Razani, B. Classical and alternative roles for autophagy in lipid metabolism. Curr. Opin. Infect. Dis. 2018, 29, 203–211. [Google Scholar] [CrossRef]
- Singh, R.; Cuervo, A.M. Lipophagy: Connecting Autophagy and Lipid Metabolism. Int. J. Cell Biol. 2012, 2012, 282041. [Google Scholar] [CrossRef] [Green Version]
- Brutscher, L.M.; Flenniken, M.L. RNAi and Antiviral Defense in the Honey Bee. J. Immunol. Res. 2015, 2015, 941897. [Google Scholar] [CrossRef] [Green Version]
- Kniazeva, M.; Shen, H.; Euler, T.; Wang, C.; Han, M. Regulation of maternal phospholipid composition and IP3-dependent embryonic membrane dynamics by a specific fatty acid metabolic event in C. elegans. Genes Dev. 2012, 26, 554–566. [Google Scholar] [CrossRef] [Green Version]
- Watts, J.L.; Ristow, M. Lipid and Carbohydrate Metabolism in Caenorhabditis elegans. Genetics 2017, 207, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hu, Y.; Wang, Y.; Fu, L.; Xu, X.; Li, C.; Xu, J.; Li, C.; Zhang, L.; Yang, R.; et al. mmBCFA C17iso ensures endoplasmic reticulum integrity for lipid droplet growth. J. Cell Biol. 2021, 220, e202102122. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, A.S.; Coleman, R.A.; Kraemer, F.B.; McManaman, J.L.; Obin, M.S.; Puri, V.; Yan, Q.-W.; Miyoshi, H.; Mashek, D.G. The role of lipid droplets in metabolic disease in rodents and humans. J. Clin. Investig. 2011, 121, 2102–2110. [Google Scholar] [CrossRef] [Green Version]
- Gukovskaya, A.S.; Pandol, S.J.; Gukovsky, I. New insights into the pathways initiating and driving pancreatitis. Curr. Opin. Gastroenterol. 2016, 32, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Goldman, S.; Baerga, R.; Zhao, Y.; Komatsu, M.; Jin, S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 19860–19865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juhász, G.; Csikós, G.; Sinka, R.; Erdélyi, M.; Sass, M. The Drosophila homolog of Aut1 is essential for autophagy and development. FEBS Lett. 2003, 543, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Thummel, C.S. Steroid-triggered death by autophagy. BioEssays News Rev. Mol. Cell Dev. Biol. 2001, 23, 677–682. [Google Scholar] [CrossRef]
- Mailler, E.; Guardia, C.M.; Bai, X.; Jarnik, M.; Williamson, C.D.; Li, Y.; Maio, N.; Golden, A.; Bonifacino, J.S. The autophagy protein ATG9A enables lipid mobilization from lipid droplets. Nat. Commun. 2021, 12, 6750. [Google Scholar] [CrossRef]
- Bekbulat, F.; Schmitt, D.; Feldmann, A.; Huesmann, H.; Eimer, S.; Juretschke, T.; Beli, P.; Behl, C.; Kern, A. RAB18 Loss Interferes With Lipid Droplet Catabolism and Provokes Autophagy Network Adaptations. J. Mol. Biol. 2020, 432, 1216–1234. [Google Scholar] [CrossRef]
- Li, D.; Zhao, Y.G.; Li, D.; Zhao, H.; Huang, J.; Miao, G.; Feng, D.; Liu, P.; Li, D.; Zhang, H. The ER-Localized Protein DFCP1 Modulates ER-Lipid Droplet Contact Formation. Cell Rep. 2019, 27, 343–358.e5. [Google Scholar] [CrossRef] [Green Version]
- Dupont, N.; Chauhan, S.; Arko-Mensah, J.; Castillo, E.F.; Masedunskas, A.; Weigert, R.; Robenek, H.; Proikas-Cezanne, T.; Deretic, V. Neutral Lipid Stores and Lipase PNPLA5 Contribute to Autophagosome Biogenesis. Curr. Biol. 2014, 24, 609–620. [Google Scholar] [CrossRef]
- Shpilka, T.; Welter, E.; Borovsky, N.; Amar, N.; Mari, M.; Reggiori, F.; Elazar, Z. Lipid droplets and their component triglycerides and steryl esters regulate autophagosome biogenesis. EMBO J. 2015, 34, 2117–2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morigny, P.; Boucher, J.; Arner, P.; Langin, D. Lipid and glucose metabolism in white adipocytes: Pathways, dysfunction and therapeutics. Nat. Rev. Endocrinol. 2021, 17, 276–295. [Google Scholar] [CrossRef] [PubMed]
- Solinas, G.; Borén, J.; Dulloo, A.G. De novo lipogenesis in metabolic homeostasis: More friend than foe? Mol. Metab. 2015, 4, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Green, C.R.; Wallace, M.; Divakaruni, A.S.; A Phillips, S.; Murphy, A.N.; Ciaraldi, T.P.; Metallo, C.M. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol. 2016, 12, 15–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, M.; Green, C.R.; Roberts, L.S.; Lee, Y.M.; McCarville, J.L.; Sanchez-Gurmaches, J.; Meurs, N.; Gengatharan, J.M.; Hover, J.D.; Phillips, S.A.; et al. Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat. Chem. Biol. 2018, 14, 1021–1031. [Google Scholar] [CrossRef]
- Zhao, S.; Torres, A.; Henry, R.A.; Trefely, S.; Wallace, M.; Lee, J.V.; Carrer, A.; Sengupta, A.; Campbell, S.L.; Kuo, Y.-M.; et al. ATP-Citrate Lyase Controls a Glucose-to-Acetate Metabolic Switch. Cell Rep. 2016, 17, 1037–1052. [Google Scholar] [CrossRef] [Green Version]
- Vance, J.E.; Tasseva, G. Formation and function of phosphatidylserine and phosphatidylethanolamine in mammalian cells. Biochim. Biophys. Acta 2013, 1831, 543–554. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.-T.; Xu, B.-H. Diversity in life history of queen and worker honey bees, Apis mellifera L. J. Asia-Pacific Entomol. 2015, 18, 145–149. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Zhang, W.; Liu, Z.; Xu, B.; Wang, H. Methionine as a methyl donor regulates caste differentiation in the European honey bee (Apis mellifera). Insect Sci. 2021, 28, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, W.G.; Fernandes, K.M.; Santana, W.C.; Martins, G.F.; Zanuncio, J.C.; Serrão, J.E. Post-embryonic changes in the hindgut of honeybee Apis mellifera workers: Morphology, cuticle deposition, apoptosis, and cell proliferation. Dev. Biol. 2017, 431, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods (San Diego Calif.) 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Guidugli, K.R.; Nascimento, A.M.; Amdam, G.V.; Barchuk, A.R.; Omholt, S.; Simões, Z.L.P.; Hartfelder, K. Vitellogenin regulates hormonal dynamics in the worker caste of a eusocial insect. FEBS Lett. 2005, 579, 4961–4965. [Google Scholar] [CrossRef] [Green Version]
- Wass, S.; Karmiloff-Smith, A. The missing developmental dimension in the network perspective. Behav. Brain Sci. 2010, 33, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Porollo, A.; Meller, J. Prediction-based fingerprints of protein-protein interactions. Proteins Struct. Funct. Bioinform. 2007, 66, 630–645. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Pan, J.; Di, Y.-Q.; Liu, W.; Hou, L.; Wang, J.-X.; Zhao, X.-F. Protein kinase C delta phosphorylates ecdysone receptor B1 to promote gene expression and apoptosis under 20-hydroxyecdysone regulation. Proc. Natl. Acad. Sci. USA 2017, 114, E7121–E7130. [Google Scholar] [CrossRef] [Green Version]
- Zamore, P.D.; Tuschl, T.; A Sharp, P.; Bartel, D.P. RNAi: Double-Stranded RNA Directs the ATP-Dependent Cleavage of mRNA at 21 to 23 Nucleotide Intervals. Cell 2000, 101, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Elias-Neto, M.; Soares, M.P.; Simões, Z.L.; Hartfelder, K.; Bitondi, M.M. Developmental characterization, function and regulation of a Laccase2 encoding gene in the honey bee, Apis mellifera (Hymenoptera, Apinae). Insect Biochem. Mol. Biol. 2010, 40, 241–251. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.-F.; Wang, H.-F.; Wang, Y.; Liu, Z.-G.; Xu, B.-H. AmAtg2B-Mediated Lipophagy Regulates Lipolysis of Pupae in Apis mellifera. Int. J. Mol. Sci. 2023, 24, 2096. https://doi.org/10.3390/ijms24032096
Chen W-F, Wang H-F, Wang Y, Liu Z-G, Xu B-H. AmAtg2B-Mediated Lipophagy Regulates Lipolysis of Pupae in Apis mellifera. International Journal of Molecular Sciences. 2023; 24(3):2096. https://doi.org/10.3390/ijms24032096
Chicago/Turabian StyleChen, Wen-Feng, Hong-Fang Wang, Ying Wang, Zhen-Guo Liu, and Bao-Hua Xu. 2023. "AmAtg2B-Mediated Lipophagy Regulates Lipolysis of Pupae in Apis mellifera" International Journal of Molecular Sciences 24, no. 3: 2096. https://doi.org/10.3390/ijms24032096




