Dihomo-γ-Linolenic Acid (20:3n-6)—Metabolism, Derivatives, and Potential Significance in Chronic Inflammation
Abstract
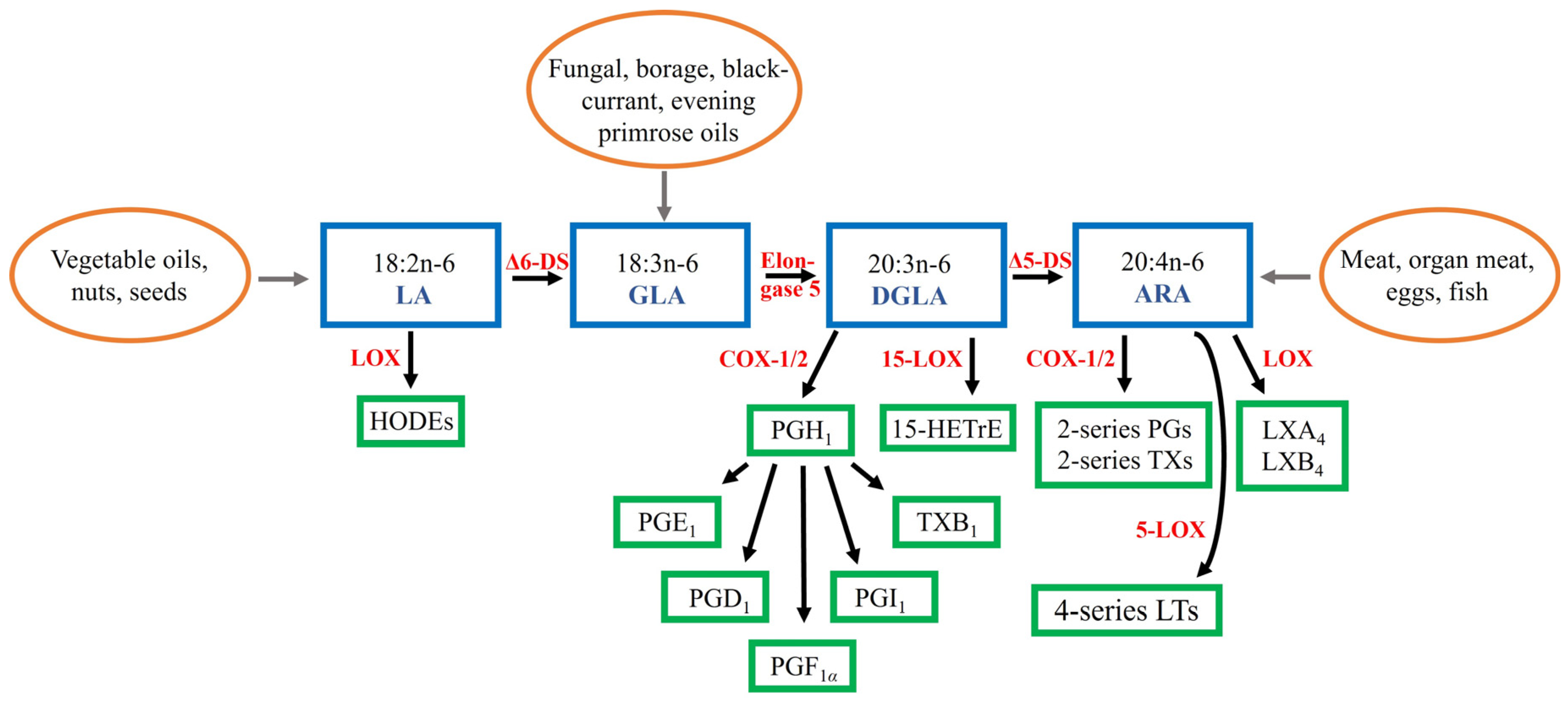
:1. Introduction
2. Literature Search
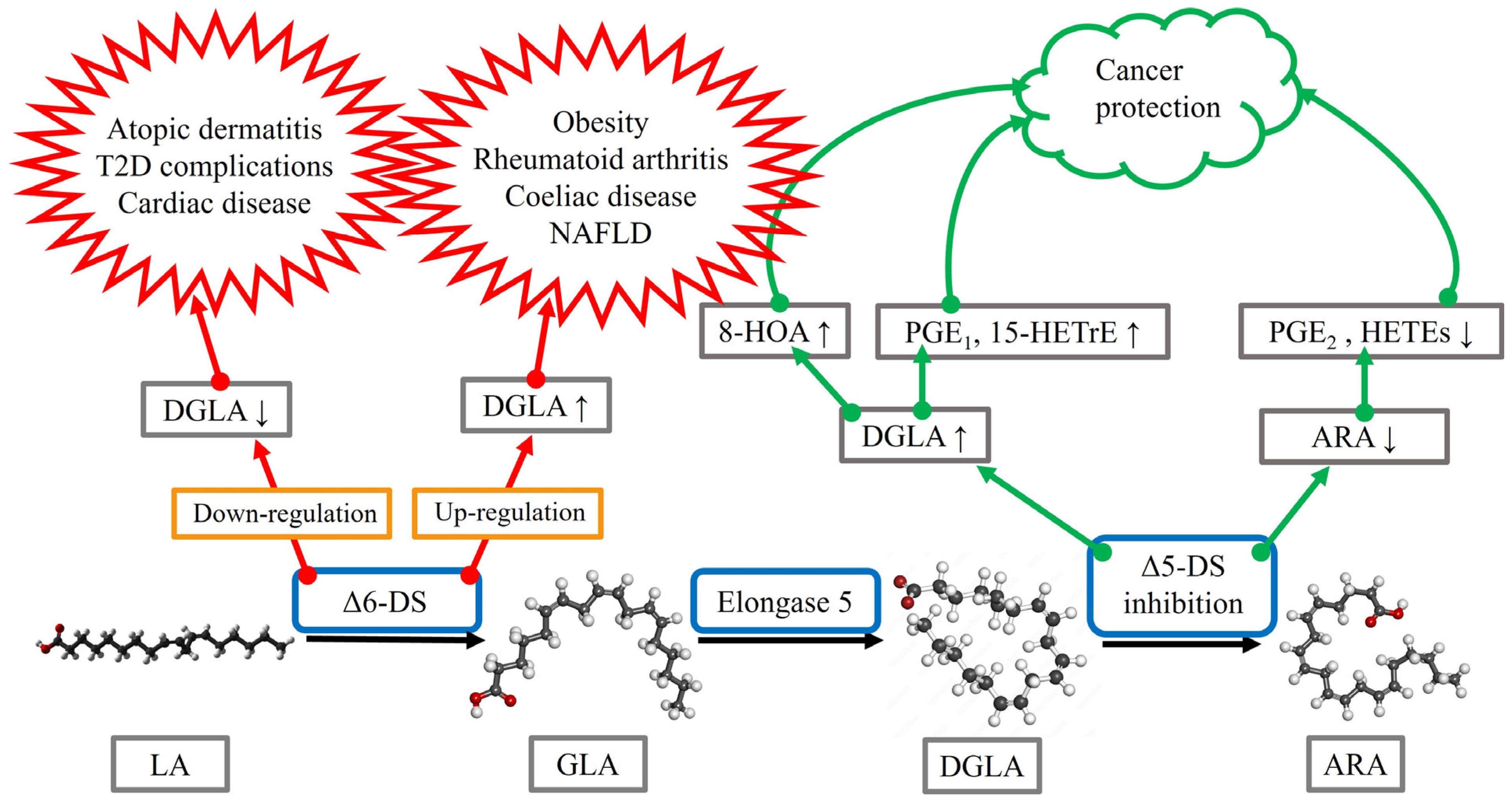
3. DGLA in Inflammatory Conditions
| Condition/Disease | Finding | Reference |
|---|---|---|
| Obesity | Increased DGLA levels in overweight/obese subjects | [11] |
| High maternal DGLA levels predict childhood body fat | [21] | |
| Type 2 diabetes | Direct relationship between DGLA and HOMA-IR | [13] |
| (T2D) | Increased DGLA levels in overweight/obese subjects with T2D | [12] |
| High DGLA levels associated with an increased risk of T2D | [15] | |
| Inverse relationship with complications of T2D | [16,17,19] | |
| Cardiovascular | DGLA supplement reduces atherosclerosis development | [22] |
| diseases | Low DGLA levels worsen disease prognosis | [23,24] |
| Hepatic diseases | High DGLA levels associated with disease development | [25,26,27] |
| High maternal DGLA levels predict childhood lipidosis | [28] | |
| Gastrointestinal | Increased DGLA levels in Crohn’s disease | [29] |
| diseases | Increased DGLA levels in coeliac disease | [30] |
| Arthritis | Increased DGLA levels in rheumatoid arthritis | [31,32,33] |
| DGLA exposure reduces synovial cell proliferation | [34] | |
| GLA supplement reduces rheumatoid arthritis symptoms | [8] | |
| Bronchial asthma | Increased DGLA levels in disease cases | [35] |
| Inverse relationship with lung function parameters | [36] | |
| GLA supplement reduces clinical symptoms | [37] | |
| Atopic dermatitis | Reduced GLA and DGLA levels in disease cases | [38] |
| GLA and DGLA supplements improve clinical signs | [38,39] | |
| Cancers | Anti-proliferative effects of GLA and DGLA | [40] |
4. Potential Applications of DGLA to the Prevention and Treatment of Inflammatory Conditions
4.1. Potential Dietary Sources of DGLA
4.2. Therapeutic Potential of DGLA Supplementation or Suppression of DGLA Metabolism
4.3. Potential Risks of Dietary DGLA Administration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARA | arachidonic acid |
| BALF | bronchoalveolar lavage fluid |
| COX | cyclooxygenase |
| DGLA | dihomo-γ-linolenic acid |
| DHA | docosahexaenoic acid |
| ELOVL | fatty acid elongase |
| EPA | eicosapentaenoic acid |
| EV | extracellular vesicle |
| FA | fatty acid |
| FADS | fatty acid desaturase |
| GLA | γ-linolenic acid |
| HETE | hydroxyeicosatetraenoic acid |
| 15-HETrE | 15-(S)-hydroxy-8,11,13-eicosatrienoic acid |
| 8-HOA | 8-hydroxyoctanoic acid |
| HOMA-IR | homeostatic model assessment of insulin resistance |
| LA | linoleic acid |
| LOX | lipoxygenase |
| LT | leukotriene |
| LXA4 | lipoxin A4 |
| NAFLD | non-alcoholic fatty liver disease |
| OA | osteoarthritis |
| PG | prostaglandin |
| PL | phospholipid |
| PUFA | polyunsaturated fatty acid |
| RA | rheumatoid arthritis |
| SF | synovial fluid |
| SPM | specialized pro-resolving mediator |
| T2D | type 2 diabetes |
| VSMC | vascular smooth muscle cell |
References
- Fan, Y.-Y.; Chapkin, R.S. Importance of dietary γ-linolenic acid in human health and nutrition. J. Nutr. 1998, 128, 1411–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sergeant, S.; Rahbar, E.; Chilton, F.H. Gamma-linolenic acid, dihommo-gamma linolenic, eicosanoids and inflammatory processes. Eur. J. Pharmacol. 2016, 785, 77–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, U.N. “Cell membrane theory of senescence” and the role of bioactive lipids in aging, and aging associated diseases and their therapeutic implications. Biomolecules 2021, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Innes, J.K.; Calder, P.C. Omega-6 fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strassburg, K.; Huijbrechts, A.M.L.; Kortekaas, K.A.; Lindeman, J.H.; Pedersen, T.L.; Dane, A.; Berger, R.; Brenkman, A.; Hankemeier, T.; van Duynhoven, J.; et al. Quantitative profiling of oxylipins through comprehensive LC-MS/MS analysis: Application in cardiac surgery. Anal. Bioanal. Chem. 2012, 404, 1413–1426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mustonen, A.-M.; Nieminen, P. Fatty acids and oxylipins in osteoarthritis and rheumatoid arthritis—A complex field with significant potential for future treatments. Curr. Rheumatol. Rep. 2021, 23, 41. [Google Scholar] [CrossRef] [PubMed]
- Wendell, S.G.; Baffi, C.; Holguin, F. Fatty acids, inflammation, and asthma. J. Allergy Clin. Immunol. 2014, 133, 1255–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macfarlane, G.J.; El-Metwally, A.; De Silva, V.; Ernst, E.; Dowds, G.L.; Moots, R.J.; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of rheumatoid arthritis: A systematic review. Rheumatology 2011, 50, 1672–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bamford, J.T.M.; Ray, S.; Musekiwa, A.; van Gool, C.; Humphreys, R.; Ernst, E. Oral evening primrose oil and borage oil for eczema. Cochrane Database Syst. Rev. 2013, 4, CD004416. [Google Scholar] [CrossRef]
- Perreault, M.; Roke, K.; Badawi, A.; Nielsen, D.E.; Abdelmagid, S.A.; El-Sohemy, A.; Ma, D.W.L.; Mutch, D.M. Plasma levels of 14:0, 16:0, 16:1n-7, and 20:3n-6 are positively associated, but 18:0 and 18:2n-6 are inversely associated with markers of inflammation in young healthy adults. Lipids 2014, 49, 255–263. [Google Scholar] [CrossRef]
- Fekete, K.; Györei, E.; Lohner, S.; Verduci, E.; Agostoni, C.; Decsi, T. Long-chain polyunsaturated fatty acid status in obesity: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 488–497. [Google Scholar] [CrossRef]
- Ni, Y.; Zhao, L.; Yu, H.; Ma, X.; Bao, Y.; Rajani, C.; Loo, L.W.M.; Shvetsov, Y.B.; Yu, H.; Chen, T.; et al. Circulating unsaturated fatty acids delineate the metabolic status of obese individuals. EBioMedicine 2015, 2, 1513–1522. [Google Scholar] [CrossRef] [Green Version]
- Tsurutani, Y.; Inoue, K.; Sugisawa, C.; Saito, J.; Omura, M.; Nishikawa, T. Increased serum dihomo-γ-linolenic acid levels are associated with obesity, body fat accumulation, and insulin resistance in Japanese patients with type 2 diabetes. Intern. Med. 2018, 57, 2929–2935. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Stein, T.P.; Steer, R.A.; Scholl, T.O. Individual free fatty acids have unique associations with inflammatory biomarkers, insulin resistance and insulin secretion in healthy and gestational diabetic pregnant women. BMJ Open Diab. Res. Care 2019, 7, e000632. [Google Scholar] [CrossRef] [Green Version]
- Alhazmi, A.; Stojanovski, E.; Garg, M.L.; McEvoy, M. Fasting whole blood fatty acid profile and risk of type 2 diabetes in adults: A nested case control study. PLoS ONE 2014, 9, e97001. [Google Scholar] [CrossRef] [Green Version]
- Das, U.N. Essential fatty acid metabolism in patients with essential hypertension, diabetes mellitus and coronary heart disease. Prostaglandins Leukot. Essent. Fatty Acids 1995, 52, 387–391. [Google Scholar] [CrossRef]
- Okamura, T.; Nakajima, H.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; Nakanishi, N.; Hamaguchi, M.; Asano, M.; Yamazaki, M.; et al. Low circulating dihomo-gamma-linolenic acid is associated with diabetic retinopathy: A cross sectional study of KAMOGAWA-DM cohort study. Endocr. J. 2021, 68, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Pitel, S.; Raccah, D.; Gerbi, A.; Pieroni, G.; Vague, P.; Coste, T.C. At low doses, a γ-linolenic acid-lipoic acid conjugate is more effective than docosahexaenoic acid-enriched phospholipids in preventing neuropathy in diabetic rats. J. Nutr. 2007, 137, 368–372. [Google Scholar] [CrossRef] [Green Version]
- Ben Khedher, M.R.; Bouhajja, H.; Haj Ahmed, S.; Abid, M.; Jamoussi, K.; Hammami, M. Role of disturbed fatty acids metabolism in the pathophysiology of diabetic erectile dysfunction. Lipids Health Dis. 2017, 16, 241. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-J.; Huang, L.-L.; Su, H.; Chen, Y.-X.; Huang, J.; He, C.; Li, P.; Yang, D.-Z.; Wan, J.-B. Characterizing plasma phospholipid fatty acid profiles of polycystic ovary syndrome patients with and without insulin resistance using GC–MS and chemometrics approach. J. Pharm. Biomed. Anal. 2014, 95, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Vidakovic, A.J.; Gishti, O.; Voortman, T.; Felix, J.F.; Williams, M.A.; Hofman, A.; Demmelmair, H.; Koletzko, B.; Tiemeier, H.; Jaddoe, V.W.V.; et al. Maternal plasma PUFA concentrations during pregnancy and childhood adiposity: The Generation R Study. Am. J. Clin. Nutr. 2016, 103, 1017–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takai, S.; Jin, D.; Kawashima, H.; Kimura, M.; Shiraishi-Tateishi, A.; Tanaka, T.; Kakutani, S.; Tanaka, K.; Kiso, Y.; Miyazaki, M. Anti-atherosclerotic effects of dihomo-γ-linolenic acid in ApoE-deficient mice. J. Atheroscler. Thromb. 2009, 16, 480–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouchi, S.; Miyazaki, T.; Shimada, K.; Sugita, Y.; Shimizu, M.; Murata, A.; Kato, T.; Aikawa, T.; Suda, S.; Shiozawa, T.; et al. Decreased circulating dihomo-gamma-linolenic acid levels are associated with total mortality in patients with acute cardiovascular disease and acute decompensated heart failure. Lipids Health Dis. 2017, 16, 150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsen, D.W.T.; Myhre, P.L.; Kalstad, A.; Schmidt, E.B.; Arnesen, H.; Seljeflot, I. Serum levels of dihomo-gamma (γ)-linolenic acid (DGLA) are inversely associated with linoleic acid and total death in elderly patients with a recent myocardial infarction. Nutrients 2021, 13, 3475. [Google Scholar] [CrossRef] [PubMed]
- Puri, P.; Wiest, M.M.; Cheung, O.; Mirshahi, F.; Sargeant, C.; Min, H.-K.; Contos, M.J.; Sterling, R.K.; Fuchs, M.; Zhou, H.; et al. The plasma lipidomic signature of nonalcoholic steatohepatitis. Hepatology 2009, 50, 1827–1838. [Google Scholar] [CrossRef] [Green Version]
- Mäkelä, T.N.K.; Tuomainen, T.-P.; Hantunen, S.; Virtanen, J.K. Associations of serum n-3 and n-6 polyunsaturated fatty acids with prevalence and incidence of nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2022, 116, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Kawamoto, T.; Tamura, R. Predictive value of serum dihomo-γ-linolenic acid level and estimated Δ-5 desaturase activity in patients with hepatic steatosis. Obes. Res. Clin. Pract. 2017, 11, 34–43. [Google Scholar] [CrossRef]
- Wahab, R.J.; Jaddoe, V.W.V.; Mezzoiuso, A.G.; Gaillard, R. Maternal polyunsaturated fatty acid concentrations during pregnancy and childhood liver fat accumulation. Clin. Nutr. 2022, 41, 847–854. [Google Scholar] [CrossRef]
- Scoville, E.A.; Allaman, M.M.; Adams, D.W.; Motley, A.K.; Peyton, S.C.; Ferguson, S.L.; Horst, S.N.; Williams, C.S.; Beaulieu, D.B.; Schwartz, D.A.; et al. Serum polyunsaturated fatty acids correlate with serum cytokines and clinical disease activity in Crohn’s disease. Sci. Rep. 2019, 9, 2882. [Google Scholar] [CrossRef] [Green Version]
- Riezzo, G.; Ferreri, C.; Orlando, A.; Martulli, M.; D’Attoma, B.; Russo, F. Lipidomic analysis of fatty acids in erythrocytes of coeliac patients before and after a gluten-free diet intervention: A comparison with healthy subjects. Br. J. Nutr. 2014, 112, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Bruderlein, H.; Daniel, R.; Boismenu, D.; Julien, N.; Couture, F. Fatty acid profiles of serum phospholipids in patients suffering rheumatoid arthritis. Prog. Lipid Res. 1981, 20, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Esteve, M.; Olivé, A.; Klaassen, J.; Cabré, E.; Tena, X.; Fernández-Bañares, F.; Pastor, C.; Gassull, M.A. Abnormal fatty acid pattern in rheumatoid arthritis. A rationale for treatment with marine and botanical lipids. J. Rheumatol. 2000, 27, 298–303. [Google Scholar] [PubMed]
- Mustonen, A.-M.; Tollis, S.; Käkelä, R.; Lehenkari, P.; Sihvo, S.P.; Nieminen, P. Increased n-6 polyunsaturated fatty acids indicate pro- and anti-inflammatory lipid modifications in synovial membranes with rheumatoid arthritis. Unpublished work, 2023.
- Baker, D.G.; Krakauer, K.A.; Tate, G.; Laposata, M.; Zurier, R.B. Suppression of human synovial cell proliferation by dihomo-γ-linolenic acid. Arthritis Rheum. 1989, 32, 1273–1281. [Google Scholar] [CrossRef]
- Woods, R.K.; Raven, J.M.; Walters, E.H.; Abramson, M.J.; Thien, F.C.K. Fatty acid levels and risk of asthma in young adults. Thorax 2004, 59, 105–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kompauer, I.; Demmelmair, H.; Koletzko, B.; Bolte, G.; Linseisen, J.; Heinrich, J. Association of fatty acids in serum phospholipids with lung function and bronchial hyperresponsiveness in adults. Eur. J. Epidemiol. 2008, 23, 175–190. [Google Scholar] [CrossRef]
- Mirsadraee, M.; Khashkhashi Moghaddam, S.; Saeedi, P.; Ghaffari, S. Effect of Borago officinalis extract on moderate persistent asthma: A phase two randomized, double blind, placebo-controlled clinical trial. Tanaffos 2016, 15, 168–174. [Google Scholar]
- Kanda, N.; Hoashi, T.; Saeki, H. Nutrition and atopic dermatitis. J. Nippon Med. Sch. 2021, 88, 171–177. [Google Scholar] [CrossRef]
- Simon, D.; Eng, P.A.; Borelli, S.; Kägi, R.; Zimmermann, C.; Zahner, C.; Drewe, J.; Hess, L.; Ferrari, G.; Lautenschlager, S.; et al. Gamma-linolenic acid levels correlate with clinical efficacy of evening primrose oil in patients with atopic dermatitis. Adv. Ther. 2014, 31, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Lin, H.; Gu, Y. Multiple roles of dihomo-γ-linolenic acid against proliferation diseases. Lipids Health Dis. 2012, 11, 25. [Google Scholar] [CrossRef] [Green Version]
- Kritz, H.; Sinzinger, H.; Lupattelli, G.; Virgolini, I.; Fitscha, P.; O’Grady, J. Prostaglandin E1 decreases human arterial accumulation of radiolabeled apo B-containing lipoproteins in vivo. Eur. J. Clin. Pharmacol. 1997, 52, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, H.; Williams, J.O.; Ferekidis, N.; Ismail, A.; Chan, Y.-H.; Michael, D.R.; Guschina, I.A.; Tyrrell, V.J.; O’Donnell, V.B.; Harwood, J.L.; et al. Dihomo-γ-linolenic acid inhibits several key cellular processes associated with atherosclerosis. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 2538–2550. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Li, H.; Dong, J.; Dong, Y.; Wang, C.-Z. Expression profile of polyunsaturated fatty acids in colorectal cancer. World J. Gastroenterol. 2015, 21, 2405–2412. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Shah, H.; Xu, Y.; Qian, S. Delta-5-desaturase: A novel therapeutic target for cancer management. Transl. Oncol. 2021, 14, 101207. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, Y.; Wang, T.; Shu, D.; Guo, P.; Miskimins, K.; Qian, S.Y. Inhibition of cancer migration and invasion by knocking down delta-5-desaturase in COX-2 overexpressed cancer cells. Redox Biol. 2017, 11, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Borghaei, H.; Borghaei, R.C.; Thornton, R.D.; Pease, E.; Laidlaw, W.; Mochan, E. DGLA discoordinately suppresses IL-1 induced metalloproteinase mRNA levels in human synovial fibroblasts. Inflamm. Res. 1997, 46 (Suppl. 2), S124–S126. [Google Scholar] [CrossRef]
- Pillinger, M.H.; Rosenthal, P.B.; Tolani, S.N.; Apsel, B.; Dinsell, V.; Greenberg, J.; Chan, E.S.L.; Gomez, P.F.; Abramson, S.B. Cyclooxygenase-2-derived E prostaglandins down-regulate matrix metalloproteinase-1 expression in fibroblast-like synoviocytes via inhibition of extracellular signal-regulated kinase activation. J. Immunol. 2003, 171, 6080–6089. [Google Scholar] [CrossRef] [Green Version]
- Höglund, N.; Nieminen, P.; Mustonen, A.-M.; Käkelä, R.; Tollis, S.; Koho, N.; Holopainen, M.; Ruhanen, H.; Mykkänen, A. Fatty acid fingerprints in bronchoalveolar lavage fluid and its extracellular vesicles reflect equine asthma severity. Unpublished work, 2023.
- Fussbroich, D.; Zimmermann, K.; Göpel, A.; Eickmeier, O.; Trischler, J.; Zielen, S.; Schubert, R.; Beermann, C. A specific combined long-chain polyunsaturated fatty acid supplementation reverses fatty acid profile alterations in a mouse model of chronic asthma. Lipids Health Dis. 2019, 18, 16. [Google Scholar] [CrossRef] [Green Version]
- Wasserman, M.A.; Griffin, R.L.; Marsalisi, F.B. Inhibition of bronchoconstriction by aerosols of prostaglandins E1 and E2. J. Pharmacol. Exp. Ther. 1980, 214, 68–73. [Google Scholar]
- Sweatman, W.J.F.; Collier, H.O.J. Effects of prostaglandins on human bronchial muscle. Nature 1968, 217, 69. [Google Scholar] [CrossRef]
- Henz, B.M.; Jablonska, S.; van de Kerkhof, P.C.M.; Stingl, G.; Blaszczyk, M.; Vandervalk, P.G.M.; Veenhuizen, R.; Muggli, R.; Raederstorff, D. Double-blind, multicentre analysis of the efficacy of borage oil in patients with atopic eczema. Br. J. Dermatol. 1999, 140, 685–688. [Google Scholar] [CrossRef]
- Vakharia, P.P.; Silverberg, J.I. New therapies for atopic dermatitis: Additional treatment classes. J. Am. Acad. Dermatol. 2018, 78 (Suppl. 1), S76–S83. [Google Scholar] [CrossRef]
- Balić, A.; Vlašić, D.; Žužul, K.; Marinović, B.; Bukvić Mokos, Z. Omega-3 versus omega-6 polyunsaturated fatty acids in the prevention and treatment of inflammatory skin diseases. Int. J. Mol. Sci. 2020, 21, 741. [Google Scholar] [CrossRef]
- Tanaka, T.; Kakutani, S.; Horikawa, C.; Kawashima, H.; Kiso, Y. Oral supplementation with dihomo-γ-linolenic acid (DGLA)-enriched oil increases serum DGLA content in healthy adults. Lipids 2012, 47, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Antimanon, S.; Anantayanon, J.; Wannawilai, S.; Khongto, B.; Laoteng, K. Physiological traits of dihomo-γ-linolenic acid production of the engineered Aspergillus oryzae by comparing mathematical models. Front. Microbiol. 2020, 11, 546230. [Google Scholar] [CrossRef] [PubMed]
- Jareonkitmongkol, S.; Sakuradani, E.; Shimizu, S. A novel ∆5-desaturase-defective mutant of Mortierella alpina 1S-4 and its dihomo-γ-linolenic acid productivity. Appl. Environ. Microbiol. 1993, 59, 4300–4304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawashima, H.; Akimoto, K.; Higashiyama, K.; Fujikawa, S.; Shimizu, S. Industrial production of dihomo-γ-linolenic acid by a Δ5 desaturase-defective mutant of Mortierella alpina 1S-4 fungus. J. Am. Oil Chem. Soc. 2000, 77, 1135–1138. [Google Scholar] [CrossRef]
- Abu-Ghosh, S.; Pal-Nath, D.; Markovitch, D.; Solovchenko, A.; Didi-Cohen, S.; Portugal, I.; Khozin-Goldberg, I.; Cohen, Z.; Boussiba, S. A novel source of dihomo-γ-linolenic acid: Possibilities and limitations of DGLA production in the high-density cultures of the Δ5 desaturase-mutant microalga Lobosphaera incisa. Eur. J. Lipid Sci. Technol. 2015, 117, 760–766. [Google Scholar] [CrossRef]
- Yazawa, H.; Iwahashi, H.; Kamisaka, Y.; Kimura, K.; Aki, T.; Ono, K.; Uemura, H. Heterologous production of dihomo-γ-linolenic acid in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2007, 73, 6965–6971. [Google Scholar] [CrossRef] [Green Version]
- Asadi-Samani, M.; Bahmani, M.; Rafieian-Kopaei, M. The chemical composition, botanical characteristic and biological activities of Borago officinalis: A review. Asian Pac. J. Trop. Med. 2014, 7 (Suppl. 1), S22–S28. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.M.; Swan, D.D.; Surette, M.E.; Stegner, J.; Chilton, T.; Fonteh, A.N.; Chilton, F.H. Dietary supplementation with γ-linolenic acid alters fatty acid content and eicosanoid production in healthy humans. J. Nutr. 1997, 127, 1435–1444. [Google Scholar] [CrossRef] [Green Version]
- Mustonen, A.-M.; Lehmonen, N.; Paakkonen, T.; Raekallio, M.; Käkelä, R.; Niemelä, T.; Mykkänen, A.; Sihvo, S.P.; Nieminen, P. Equine osteoarthritis modifies fatty acid signatures in synovial fluid and its extracellular vesicles. Arthritis Res. Ther. 2023. in revision. [Google Scholar]
- Hederos, C.-A.; Berg, A. Epogam evening primrose oil treatment in atopic dermatitis and asthma. Arch. Dis. Child. 1996, 75, 494–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawashima, H.; Tateishi, N.; Shiraishi, A.; Teraoka, N.; Tanaka, T.; Tanaka, A.; Matsuda, H.; Kiso, Y. Oral administration of dihomo-γ-linolenic acid prevents development of atopic dermatitis in NC/Nga mice. Lipids 2008, 43, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Ziboh, V.A.; Naguwa, S.; Vang, K.; Wineinger, J.; Morrissey, B.M.; Watnik, M.; Gershwin, M.E. Suppression of leukotriene B4 generation by ex-vivo neutrophils isolated from asthma patients on dietary supplementation with gammalinolenic acid-containing borage oil: Possible implication in asthma. Clin. Dev. Immunol. 2004, 11, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariamenatu, A.H.; Abdu, E.M. Overconsumption of omega-6 polyunsaturated fatty acids (PUFAs) versus deficiency of omega-3 PUFAs in modern-day diets: The disturbing factor for their “balanced antagonistic metabolic functions” in the human body. J. Lipids 2021, 2021, 8848161. [Google Scholar] [CrossRef]
- Veselinovic, M.; Vasiljevic, D.; Vucic, V.; Arsic, A.; Petrovic, S.; Tomic-Lucic, A.; Savic, M.; Zivanovic, S.; Stojic, V.; Jakovljevic, V. Clinical benefits of n-3 PUFA and γ-linolenic acid in patients with rheumatoid arthritis. Nutrients 2017, 9, 325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serhiyenko, V.A.; Serhiyenko, A.A. Diabetic cardiac autonomic neuropathy: Do we have any treatment perspectives? World J. Diabetes 2015, 6, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Meuronen, T.; Lankinen, M.A.; Kolmert, J.; de Mello Laaksonen, V.; Sallinen, T.; Ågren, J.; Virtanen, K.A.; Laakso, M.; Wheelock, C.E.; Pihlajamäki, J.; et al. The FADS1 rs174550 genotype modifies the n-3 and n-6 PUFA and lipid mediator responses to a high alpha-linolenic acid and high linoleic acid diets. Mol. Nutr. Food Res. 2022, 66, e2200351. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Al-Khudairy, L.; Abdelhamid, A.S.; Rees, K.; Brainard, J.S.; Brown, T.J.; Ajabnoor, S.M.; O’Brien, A.T.; Winstanley, L.E.; Donaldson, D.H.; et al. Omega-6 fats for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2018, 7, CD011094. [Google Scholar] [CrossRef] [Green Version]
- Abidi, A.; Gupta, S.; Agarwal, M.; Bhalla, H.L.; Saluja, M. Evaluation of efficacy of curcumin as an add-on therapy in patients of bronchial asthma. J. Clin. Diagn. Res. 2014, 8, HC19–HC24. [Google Scholar] [CrossRef]
- Harmon, S.D.; Kaduce, T.L.; Manuel, T.D.; Spector, A.A. Effect of the ∆6-desaturase inhibitor SC-26196 on PUFA metabolism in human cells. Lipids 2003, 38, 469–476. [Google Scholar] [CrossRef]
- He, C.; Qu, X.; Wan, J.; Rong, R.; Huang, L.; Cai, C.; Zhou, K.; Gu, Y.; Qian, S.Y.; Kang, J.X. Inhibiting delta-6 desaturase activity suppresses tumor growth in mice. PLoS ONE 2012, 7, e47567. [Google Scholar] [CrossRef]
- Wang, J.; Liang, H.; Sun, M.; Zhang, L.; Xu, H.; Liu, W.; Li, Y.; Zhou, Y.; Li, Y.; Li, M. Delta-6-desaturase inhibitor enhances radiation therapy in glioblastoma in vitro and in vivo. Cancer Manag. Res. 2018, 10, 6779–6790. [Google Scholar] [CrossRef]
- Wachira, J.K.; Larson, M.K.; Harris, W.S. N-3 fatty acids affect haemostasis but do not increase the risk of bleeding: Clinical observations and mechanistic insights. Br. J. Nutr. 2014, 111, 1652–1662. [Google Scholar] [CrossRef] [Green Version]
- Takagahara, S.; Shinohara, H.; Itokawa, S.; Satomi, Y.; Ando, A.; Yamamoto, T.; Suzuki, H.; Fujimoto, T.; Kubo, K.; Ikeda, S. A novel orally available delta-5 desaturase inhibitor prevents atherosclerotic lesions accompanied by changes in fatty acid composition and eicosanoid production in ApoE knockout mice. J. Pharmacol. Exp. Ther. 2019, 371, 290–298. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustonen, A.-M.; Nieminen, P. Dihomo-γ-Linolenic Acid (20:3n-6)—Metabolism, Derivatives, and Potential Significance in Chronic Inflammation. Int. J. Mol. Sci. 2023, 24, 2116. https://doi.org/10.3390/ijms24032116
Mustonen A-M, Nieminen P. Dihomo-γ-Linolenic Acid (20:3n-6)—Metabolism, Derivatives, and Potential Significance in Chronic Inflammation. International Journal of Molecular Sciences. 2023; 24(3):2116. https://doi.org/10.3390/ijms24032116
Chicago/Turabian StyleMustonen, Anne-Mari, and Petteri Nieminen. 2023. "Dihomo-γ-Linolenic Acid (20:3n-6)—Metabolism, Derivatives, and Potential Significance in Chronic Inflammation" International Journal of Molecular Sciences 24, no. 3: 2116. https://doi.org/10.3390/ijms24032116
APA StyleMustonen, A.-M., & Nieminen, P. (2023). Dihomo-γ-Linolenic Acid (20:3n-6)—Metabolism, Derivatives, and Potential Significance in Chronic Inflammation. International Journal of Molecular Sciences, 24(3), 2116. https://doi.org/10.3390/ijms24032116






