Alterations in Calcium Handling Are a Common Feature in an Arrhythmogenic Cardiomyopathy Cell Model Triggered by Desmosome Genes Loss
Abstract
1. Introduction
2. Results
2.1. Generation and Validation of Gene-Specific Knockout Clones PKP2, DSG2 and DSC2
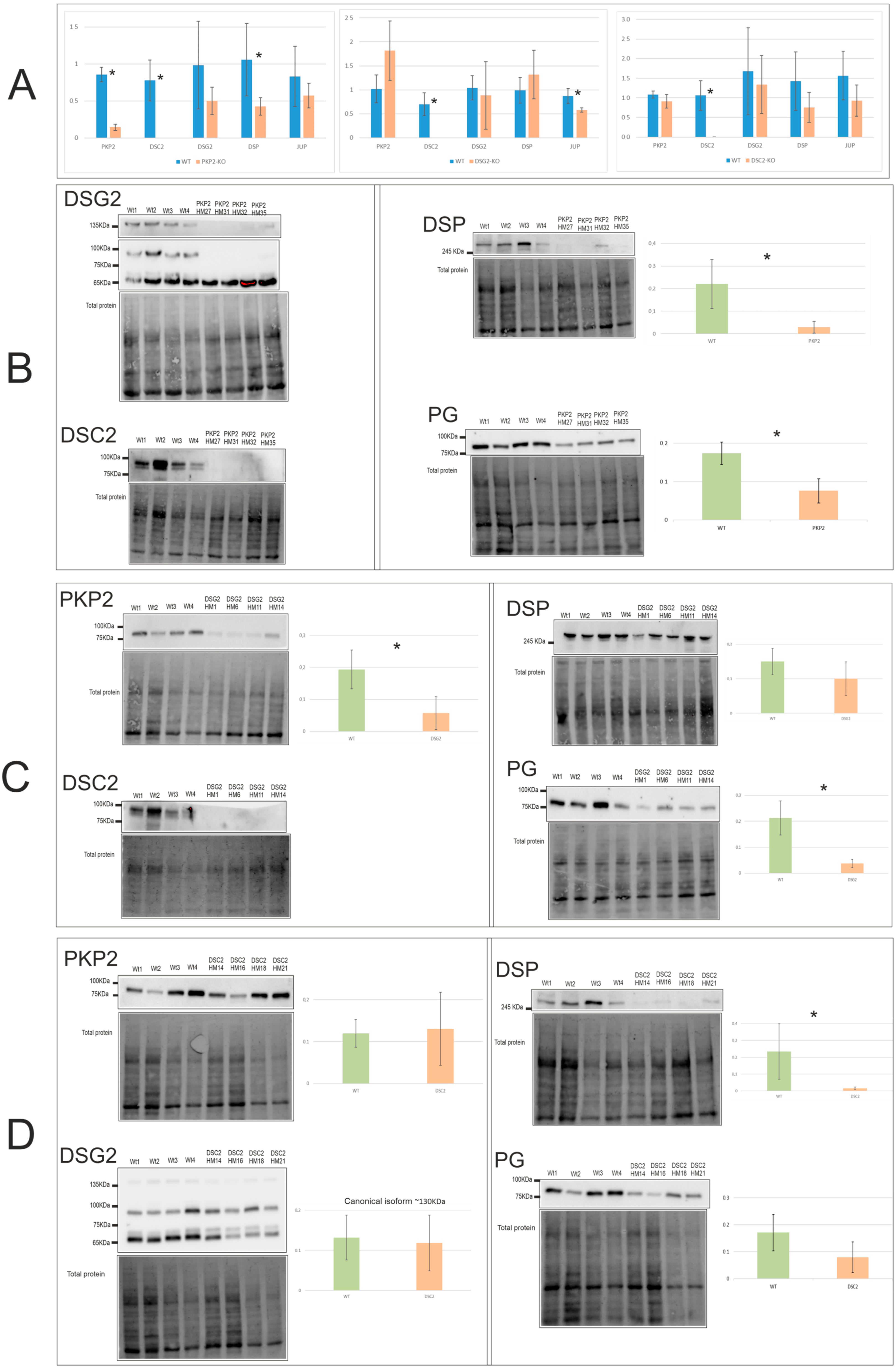
2.2. Alterations in the Expression Levels of Desmosomal Genes
2.3. Alterations in the Calcium Cycle
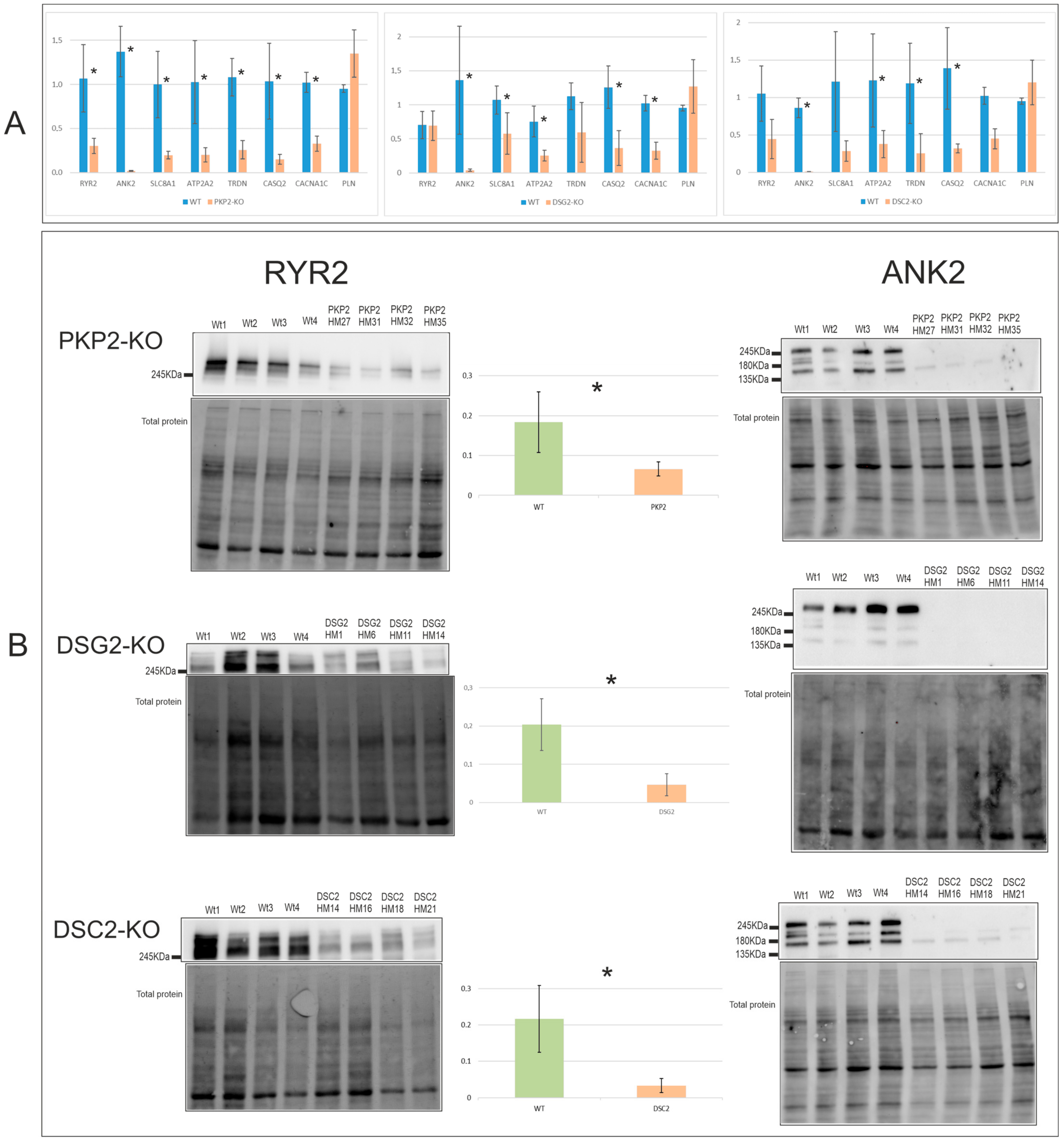
2.3.1. Expression Levels
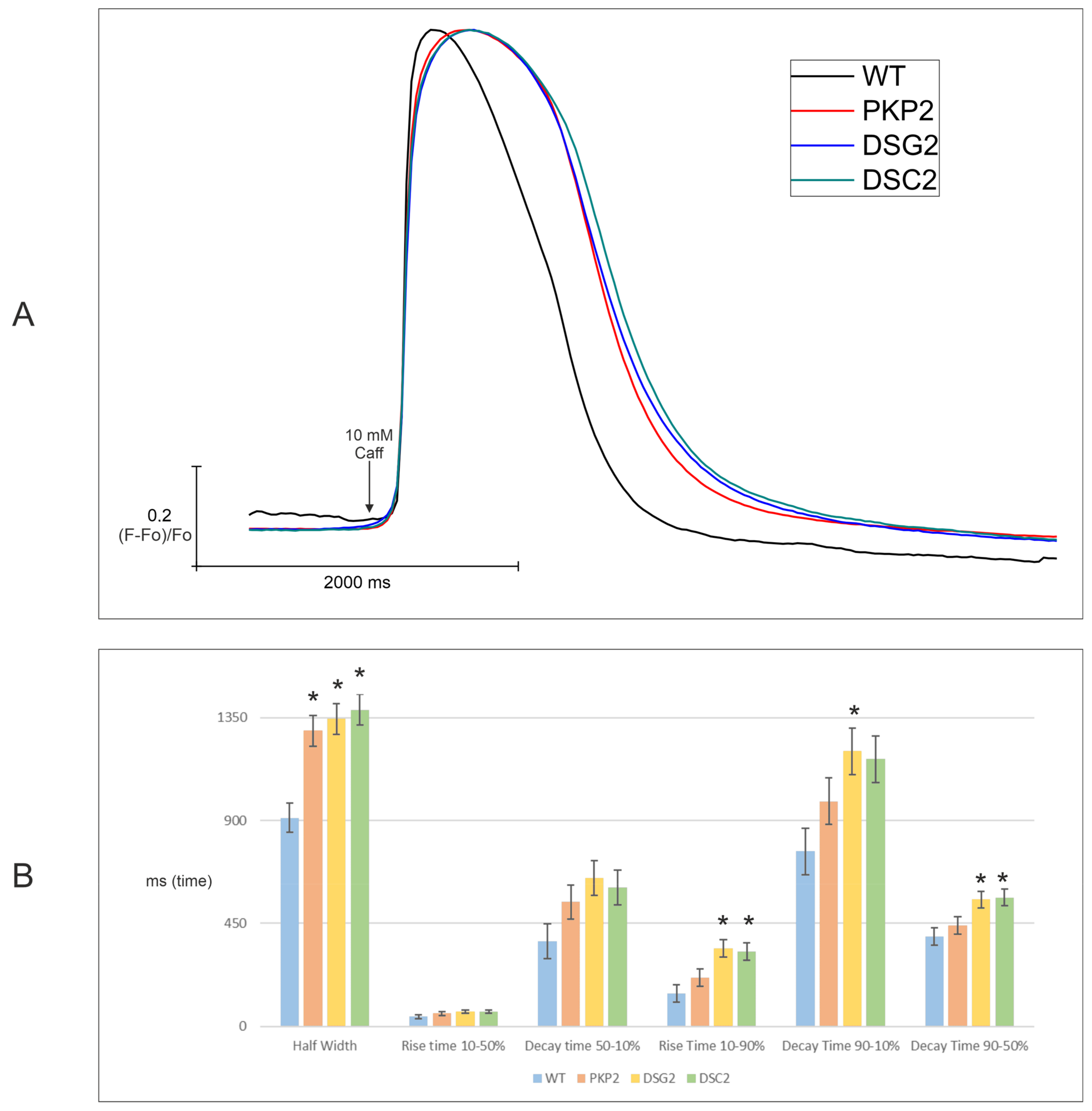
2.3.2. Calcium Imaging
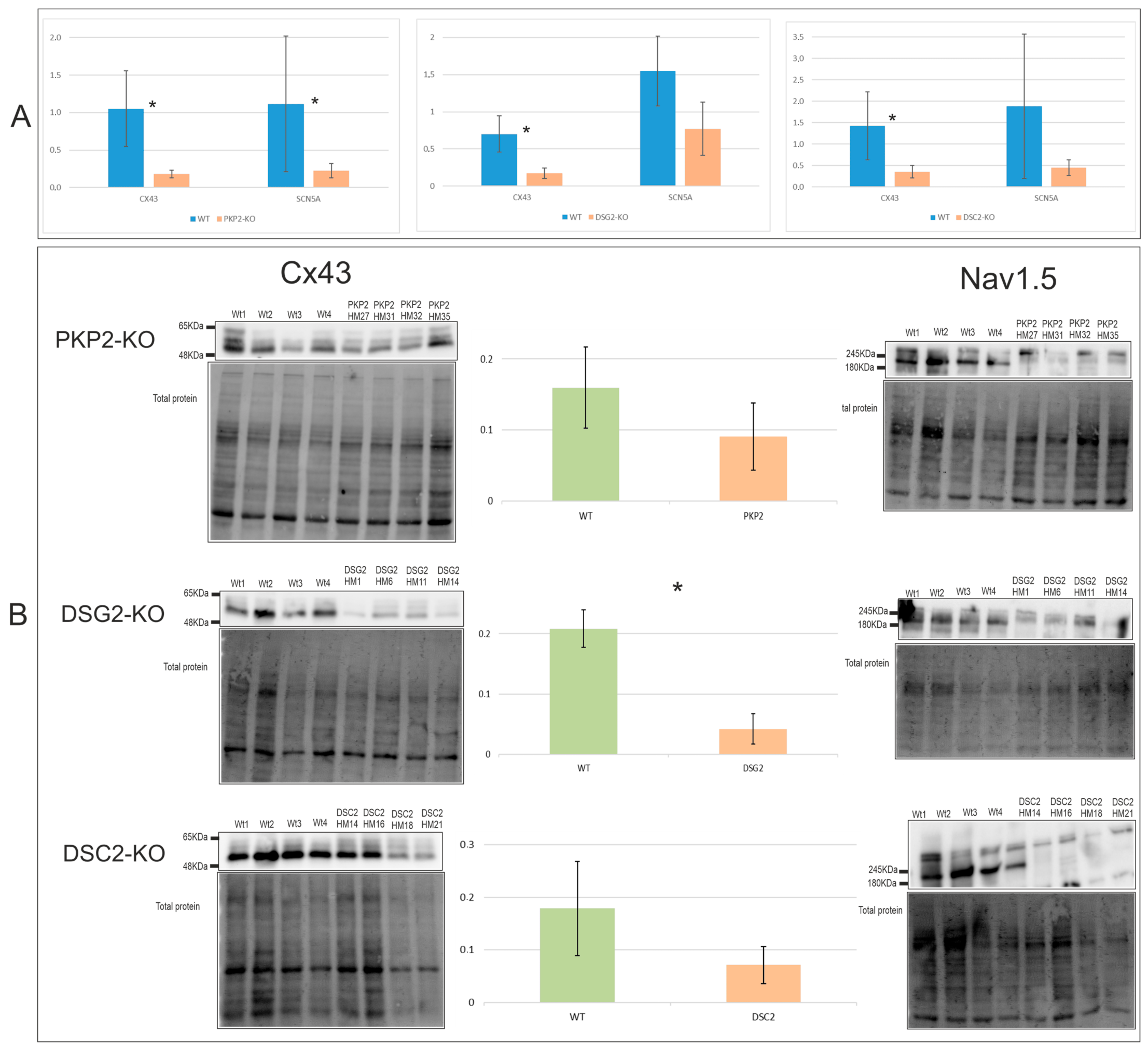
2.4. Alterations in Electrical Conduction Related Genes Cx43 and Nav1.5
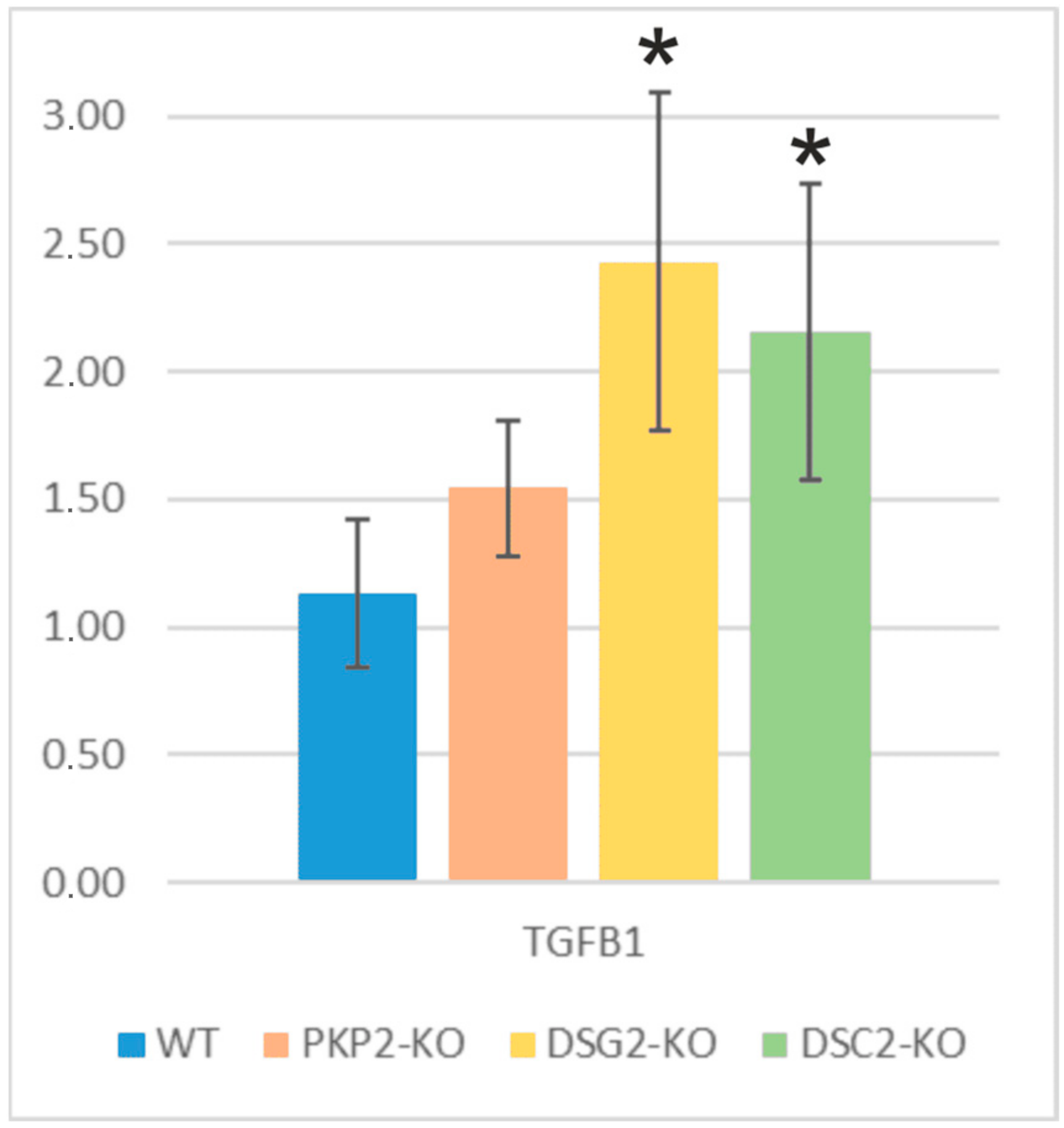
2.5. Fibrosis and Adipogenesis: Alterations in TGFB1 and PPARγ Genes
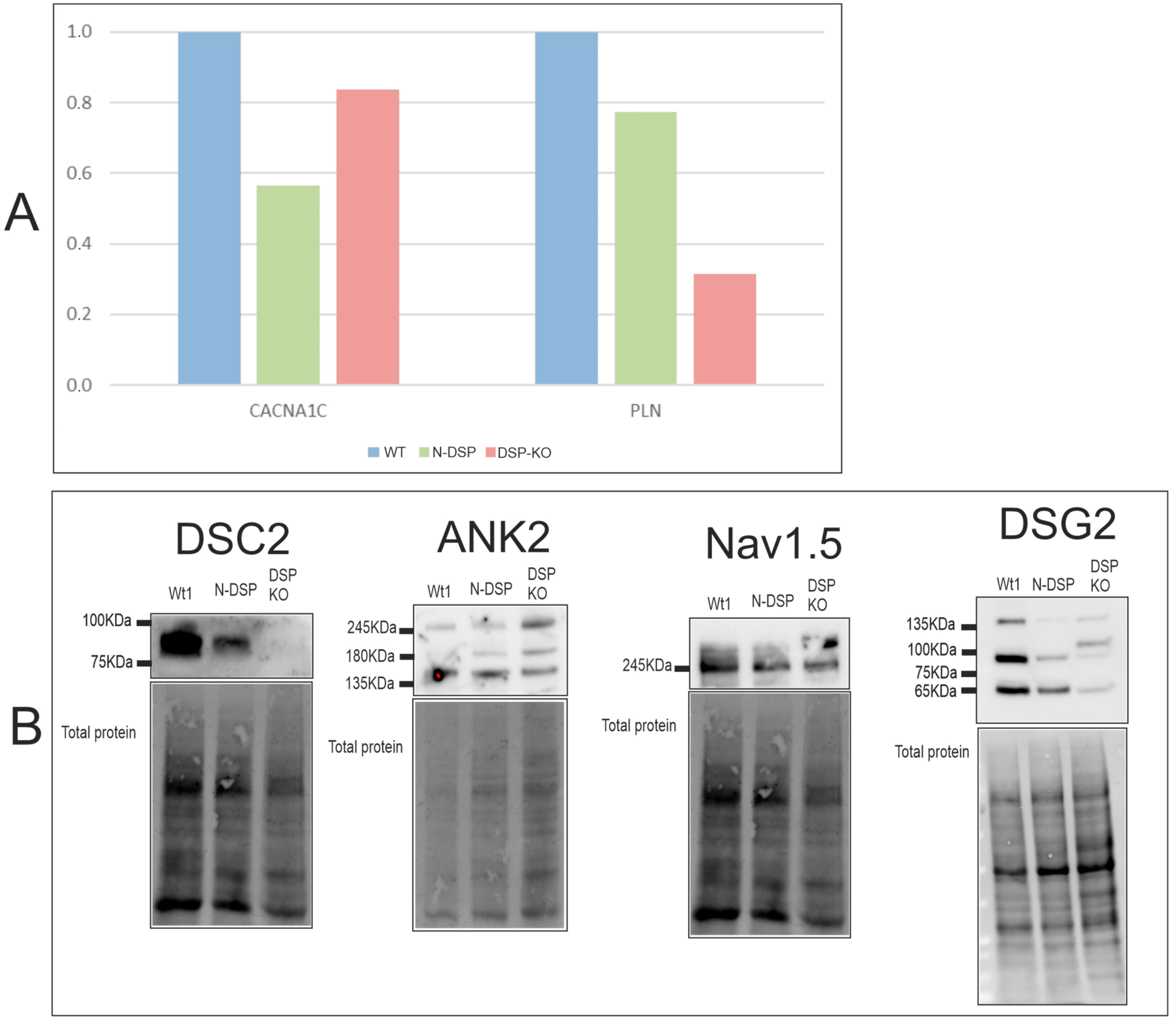
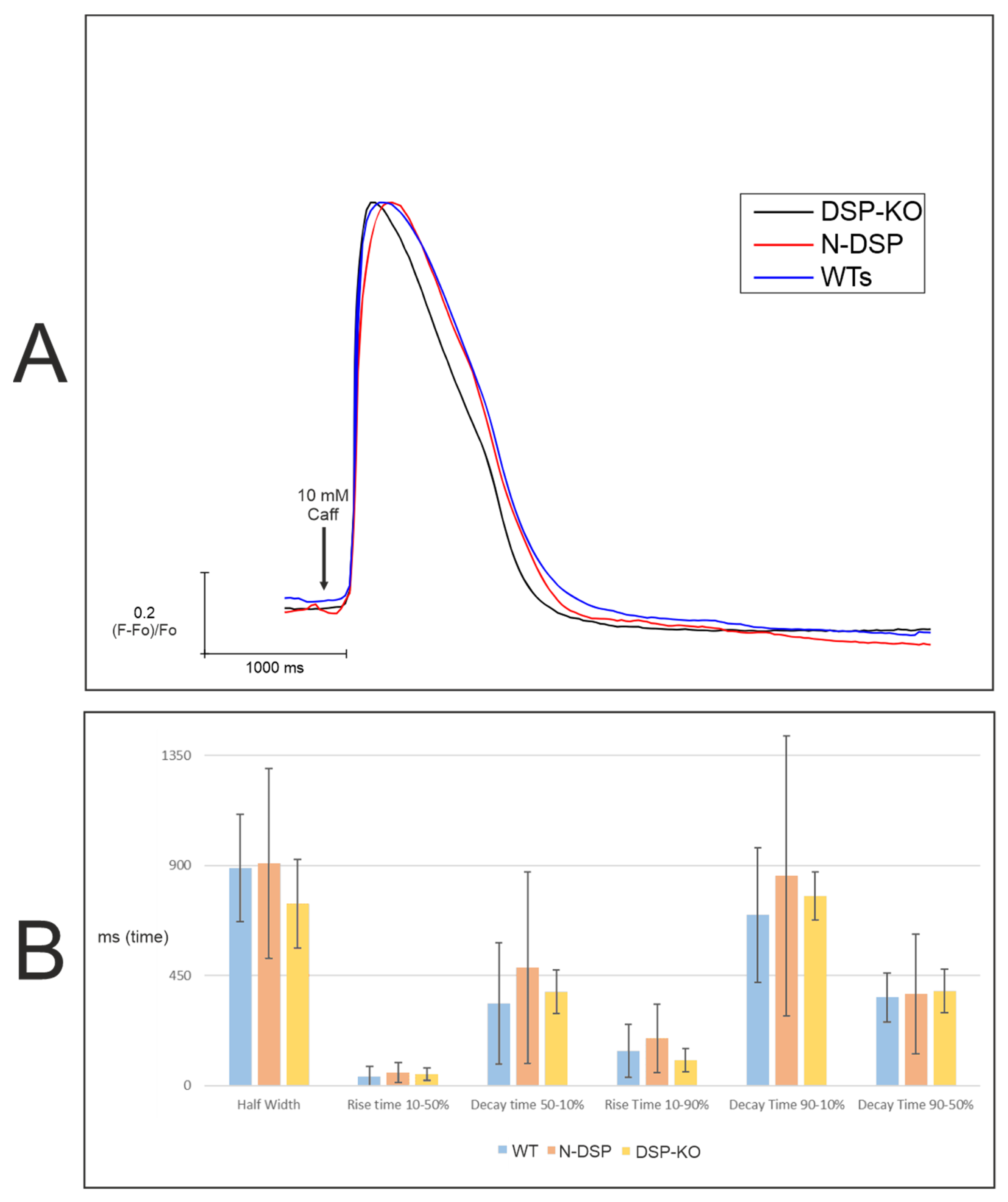
2.6. Molecular and Functional Evaluation of N-Truncated DSP and DSP-KO
2.6.1. Expression Levels
2.6.2. Calcium Imaging
3. Discussion
3.1. DSC2 Downregulation
3.2. Cx43 and Nav1.5 Downregulation
3.3. TGFB1 and PPARγ Upregulation
3.4. Downregulation in Calcium Handling Gene Expression
3.5. Slower Re-Uptake of Calcium with PKP2, DSG2, or DSC2 Loss
4. Materials and Methods
4.1. sgRNA Design and Cloning into the Cas9 px458 Vector
4.2. HL-1 Cell Culture and Electroporation
4.3. gDNA Extraction and Sanger Sequencing
4.4. RNA Extraction and Real-Time PCR (RT-PCR)
4.5. Protein Extraction and Western Blot (WB)
4.6. Calcium Imaging
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Corrado, D.; Basso, C.; Judge, D.P. Arrhythmogenic Cardiomyopathy. Circ. Res. 2017, 121, 784–802. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Xu, J.; Li, G.; Zhou, D.; Li, S.; Zhuang, B.; Chen, X.; Duan, X.; Li, L.; Fan, X.; et al. Arrhythmogenic Left Ventricular Cardiomyopathy: A Clinical and CMR Study. Sci. Rep. 2020, 10, 533. [Google Scholar] [CrossRef] [PubMed]
- Dalal, D.; James, C.; Tichnell, C.; Calkins, H. Arrhythmogenic Right Ventricular Dysplasia: A United States Experience. Heart Rhythm 2005, 2, S115. [Google Scholar] [CrossRef]
- Hoorntje, E.T.; te Rijdt, W.P.; James, C.A.; Pilichou, K.; Basso, C.; Judge, D.P.; Bezzina, C.R.; van Tintelen, J.P. Arrhythmogenic Cardiomyopathy: Pathology, Genetics, and Concepts in Pathogenesis. Cardiovasc. Res. 2017, 113, 1521–1531. [Google Scholar] [CrossRef]
- Mattesi, G.; Zorzi, A.; Corrado, D.; Cipriani, A. Natural History of Arrhythmogenic Cardiomyopathy. J. Clin. Med. 2020, 9, 878. [Google Scholar] [CrossRef]
- Najor, N.A. Desmosomes in Human Disease. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 51–70. [Google Scholar] [CrossRef]
- Corrado, D.; Van Tintelen, P.J.; McKenna, W.J.; Hauer, R.N.; Anastastakis, A.; Asimaki, A.; Calkins, H. Arrhythmogenic Right Ventricular Cardiomyopathy: Evaluation of the Current Diagnostic Criteria and Differential Diagnosis. Eur. Heart J. 2020, 41, 1414–1427. [Google Scholar] [CrossRef]
- Vermij, S.H.; Abriel, H.; van Veen, T.A.B. Refining the Molecular Organization of the Cardiac Intercalated Disc. Cardiovasc. Res. 2017, 113, 259–275. [Google Scholar] [CrossRef]
- Wilson, A.J.; Schoenauer, R.; Ehler, E.; Agarkova, I.; Bennett, P.M. Cardiomyocyte Growth and Sarcomerogenesis at the Intercalated Disc. Cell. Mol. Life Sci. 2014, 71, 165–181. [Google Scholar] [CrossRef]
- Ben-Haim, Y.; Asimaki, A.; Behr, E.R. Brugada Syndrome and Arrhythmogenic Cardiomyopathy: Overlapping Disorders of the Connexome? EP Eur. 2021, 23, 653–664. [Google Scholar] [CrossRef]
- Agullo-Pascual, E.; Cerrone, M.; Delmar, M. Arrhythmogenic Cardiomyopathy and Brugada Syndrome: Diseases of the Connexome. FEBS Lett. 2014, 588, 1322–1330. [Google Scholar] [CrossRef] [PubMed]
- Austin, K.M.; Trembley, M.A.; Chandler, S.F.; Sanders, S.P.; Saffitz, J.E.; Abrams, D.J.; Pu, W.T. Molecular Mechanisms of Arrhythmogenic Cardiomyopathy. Nat. Rev. Cardiol. 2019, 16, 519–537. [Google Scholar] [CrossRef] [PubMed]
- Gerull, B.; Brodehl, A. Genetic Animal Models for Arrhythmogenic Cardiomyopathy. Front. Physiol. 2020, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Sommariva, E.; Stadiotti, I.; Perrucci, G.L.; Tondo, C.; Pompilio, G. Cell Models of Arrhythmogenic Cardiomyopathy: Advances and Opportunities. Dis. Model. Mech. 2017, 10, 823–835. [Google Scholar] [CrossRef]
- Claycomb, W.C.; Lanson, N.A.; Stallworth, B.S.; Egeland, D.B.; Delcarpio, J.B.; Bahinski, A.; Izzo, N.J. HL-1 Cells: A Cardiac Muscle Cell Line That Contracts and Retains Phenotypic Characteristics of the Adult Cardiomyocyte. Proc. Natl. Acad. Sci. USA 1998, 95, 2979–2984. [Google Scholar] [CrossRef]
- Cerrone, M.; Montnach, J.; Lin, X.; Zhao, Y.-T.; Zhang, M.; Agullo-Pascual, E.; Leo-Macias, A.; Alvarado, F.J.; Dolgalev, I.; Karathanos, T.V.; et al. Plakophilin-2 Is Required for Transcription of Genes That Control Calcium Cycling and Cardiac Rhythm. Nat. Commun. 2017, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Lyon, A.; van Opbergen, C.J.M.; Delmar, M.; Heijman, J.; van Veen, T.A.B. In Silico Identification of Disrupted Myocardial Calcium Homeostasis as Proarrhythmic Trigger in Arrhythmogenic Cardiomyopathy. Front. Physiol. 2021, 12, 732573. [Google Scholar] [CrossRef]
- Kim, J.-C.; Pérez-Hernández, M.; Alvarado, F.J.; Maurya, S.R.; Montnach, J.; Yin, Y.; Zhang, M.; Lin, X.; Vasquez, C.; Heguy, A.; et al. Disruption of Ca 2+i Homeostasis and Connexin 43 Hemichannel Function in the Right Ventricle Precedes Overt Arrhythmogenic Cardiomyopathy in Plakophilin-2–Deficient Mice. Circulation 2019, 140, 1015–1030. [Google Scholar] [CrossRef]
- van Opbergen, C.J.M.; Noorman, M.; Pfenniger, A.; Copier, J.S.; Vermij, S.H.; Li, Z.; van der Nagel, R.; Zhang, M.; de Bakker, J.M.T.; Glass, A.M.; et al. Plakophilin-2 Haploinsufficiency Causes Calcium Handling Deficits and Modulates the Cardiac Response Towards Stress. Int. J. Mol. Sci. 2019, 20, 4076. [Google Scholar] [CrossRef]
- Oxford, E.M.; Musa, H.; Maass, K.; Coombs, W.; Taffet, S.M.; Delmar, M. Connexin43 Remodeling Caused by Inhibition of Plakophilin-2 Expression in Cardiac Cells. Circ. Res. 2007, 101, 703–711. [Google Scholar] [CrossRef]
- Fidler, L.M.; Wilson, G.J.; Liu, F.; Cui, X.; Scherer, S.W.; Taylor, G.P.; Hamilton, R.M. Abnormal Connexin43 in Arrhythmogenic Right Ventricular Cardiomyopathy Caused by Plakophilin-2 Mutations. J. Cell. Mol. Med. 2009, 13, 4219–4228. [Google Scholar] [CrossRef] [PubMed]
- Vallverdú-Prats, M.; Brugada, R.; Alcalde, M. Premature Termination Codon in 5′ Region of Desmoplakin and Plakoglobin Genes May Escape Nonsense-Mediated Decay through the Reinitiation of Translation. Int. J. Mol. Sci. 2022, 23, 656. [Google Scholar] [CrossRef] [PubMed]
- Vite, A.; Gandjbakhch, E.; Prost, C.; Fressart, V.; Fouret, P.; Neyroud, N.; Gary, F.; Donal, E.; Varnous, S.; Fontaine, G.; et al. Desmosomal Cadherins Are Decreased in Explanted Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy Patient Hearts. PLoS ONE 2013, 8, e75082. [Google Scholar] [CrossRef] [PubMed]
- Akdis, D.; Medeiros-Domingo, A.; Gaertner-Rommel, A.; Kast, J.I.; Enseleit, F.; Bode, P.; Klingel, K.; Kandolf, R.; Renois, F.; Andreoletti, L.; et al. Myocardial Expression Profiles of Candidate Molecules in Patients with Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia Compared to Those with Dilated Cardiomyopathy and Healthy Controls. Heart Rhythm 2016, 13, 731–741. [Google Scholar] [CrossRef]
- Chen, S.N.; Gurha, P.; Lombardi, R.; Ruggiero, A.; Willerson, J.T.; Marian, A.J. The Hippo Pathway Is Activated and Is a Causal Mechanism for Adipogenesis in Arrhythmogenic Cardiomyopathy. Circ. Res. 2014, 114, 454–468. [Google Scholar] [CrossRef]
- Cerrone, M.; Lin, X.; Zhang, M.; Agullo-Pascual, E.; Pfenniger, A.; Chkourko Gusky, H.; Novelli, V.; Kim, C.; Tirasawadichai, T.; Judge, D.P.; et al. Missense Mutations in Plakophilin-2 Cause Sodium Current Deficit and Associate With a Brugada Syndrome Phenotype. Circulation 2014, 129, 1092–1103. [Google Scholar] [CrossRef]
- Khudiakov, A.; Zaytseva, A.; Perepelina, K.; Smolina, N.; Pervunina, T.; Vasichkina, E.; Karpushev, A.; Tomilin, A.; Malashicheva, A.; Kostareva, A. Sodium Current Abnormalities and Deregulation of Wnt/β-Catenin Signaling in IPSC-Derived Cardiomyocytes Generated from Patient with Arrhythmogenic Cardiomyopathy Harboring Compound Genetic Variants in Plakophilin 2 Gene. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165915. [Google Scholar] [CrossRef]
- Sato, P.Y.; Musa, H.; Coombs, W.; Guerrero-Serna, G.; Patino, G.A.; Taffet, S.M.; Isom, L.L.; Delmar, M. Loss of Plakophilin-2 Expression Leads to Decreased Sodium Current and Slower Conduction Velocity in Cultured Cardiac Myocytes. Circ. Res. 2009, 105, 523–526. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, C.; Rao, F.; Modi, R.M.; Zhu, J.; Liu, X.; Mai, L.; Tan, H.; Yu, X.; Lin, Q.; et al. Silencing of Desmoplakin Decreases Connexin43/Nav1.5 Expression and Sodium Current in HL-1 Cardiomyocytes. Mol. Med. Rep. 2013, 8, 780–786. [Google Scholar] [CrossRef]
- Kam, C.Y.; Dubash, A.D.; Magistrati, E.; Polo, S.; Satchell, K.J.F.; Sheikh, F.; Lampe, P.D.; Green, K.J. Desmoplakin Maintains Gap Junctions by Inhibiting Ras/MAPK and Lysosomal Degradation of Connexin-43. J. Cell Biol. 2018, 217, 3219–3235. [Google Scholar] [CrossRef]
- Patel, D.M.; Dubash, A.D.; Kreitzer, G.; Green, K.J. Disease Mutations in Desmoplakin Inhibit Cx43 Membrane Targeting Mediated by Desmoplakin–EB1 Interactions. J. Cell Biol. 2014, 206, 779–797. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Finlay, M.; Ahmed, A.K.; Ciaccio, E.J.; Asimaki, A.; Saffitz, J.E.; Quarta, G.; Nobles, M.; Syrris, P.; Chaubey, S.; et al. Electrophysiological Abnormalities Precede Overt Structural Changes in Arrhythmogenic Right Ventricular Cardiomyopathy Due to Mutations in Desmoplakin-A Combined Murine and Human Study. Eur. Heart J. 2012, 33, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Gehmlich, K.; Syrris, P.; Reimann, M.; Asimaki, A.; Ehler, E.; Evans, A.; Quarta, G.; Pantazis, A.; Saffitz, J.E.; McKenna, W.J. Molecular Changes in the Heart of a Severe Case of Arrhythmogenic Right Ventricular Cardiomyopathy Caused by a Desmoglein-2 Null Allele. Cardiovasc. Pathol. 2012, 21, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Gehmlich, K.; Syrris, P.; Peskett, E.; Evans, A.; Ehler, E.; Asimaki, A.; Anastasakis, A.; Tsatsopoulou, A.; Vouliotis, A.-I.; Stefanadis, C.; et al. Mechanistic Insights into Arrhythmogenic Right Ventricular Cardiomyopathy Caused by Desmocollin-2 Mutations. Cardiovasc. Res. 2011, 90, 77–87. [Google Scholar] [CrossRef]
- Dubash, A.D.; Kam, C.Y.; Aguado, B.A.; Patel, D.M.; Delmar, M.; Shea, L.D.; Green, K.J. Plakophilin-2 Loss Promotes TGF-Β1/P38 MAPK-Dependent Fibrotic Gene Expression in Cardiomyocytes. J. Cell Biol. 2016, 212, 425–438. [Google Scholar] [CrossRef]
- Djouadi, F.; Lecarpentier, Y.; Hebert, J.-L.; Charron, P.; Bastin, J.; Coirault, C. A Potential Link between Peroxisome Proliferator-Activated Receptor Signalling and the Pathogenesis of Arrhythmogenic Right Ventricular Cardiomyopathy. Cardiovasc. Res. 2009, 84, 83–90. [Google Scholar] [CrossRef]
- Caspi, O.; Huber, I.; Gepstein, A.; Arbel, G.; Maizels, L.; Boulos, M.; Gepstein, L. Modeling of Arrhythmogenic Right Ventricular Cardiomyopathy With Human Induced Pluripotent Stem Cells. Circ. Cardiovasc. Genet. 2013, 6, 557–568. [Google Scholar] [CrossRef]
- Reisqs, J.; Moreau, A.; Charrabi, A.; Sleiman, Y.; Meli, A.C.; Millat, G.; Briand, V.; Beauverger, P.; Richard, S.; Chevalier, P. The PPARγ Pathway Determines Electrophysiological Remodelling and Arrhythmia Risks in DSC2 Arrhythmogenic Cardiomyopathy. Clin. Transl. Med. 2022, 12, 10765. [Google Scholar] [CrossRef]
- Hawthorne, R.N.; Blazeski, A.; Lowenthal, J.; Kannan, S.; Teuben, R.; DiSilvestre, D.; Morrissette-McAlmon, J.; Saffitz, J.E.; Boheler, K.R.; James, C.A.; et al. Altered Electrical, Biomolecular, and Immunologic Phenotypes in a Novel Patient-Derived Stem Cell Model of Desmoglein-2 Mutant ARVC. J. Clin. Med. 2021, 10, 3061. [Google Scholar] [CrossRef]
- The UniProt Consortium; Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Agivetova, R.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; et al. UniProt: The Universal Protein Knowledgebase in 2021. Nucleic Acids Res. 2021, 49, D480–D489. [Google Scholar] [CrossRef]
- Camors, E.; Mohler, P.J.; Bers, D.M.; Despa, S. Ankyrin-B Reduction Enhances Ca Spark-Mediated SR Ca Release Promoting Cardiac Myocyte Arrhythmic Activity. J. Mol. Cell. Cardiol. 2012, 52, 1240–1248. [Google Scholar] [CrossRef] [PubMed]
- Skogestad, J.; Aronsen, J.M.; Tovsrud, N.; Wanichawan, P.; Hougen, K.; Stokke, M.K.; Carlson, C.R.; Sjaastad, I.; Sejersted, O.M.; Swift, F. Coupling of the Na+/K+-ATPase to Ankyrin B Controls Na+/Ca2+ Exchanger Activity in Cardiomyocytes. Cardiovasc. Res. 2020, 116, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D. Calcium in the Heart: From Physiology to Disease: Calcium in the Heart: From Physiology to Disease. Exp. Physiol. 2014, 99, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.A.; Caldwell, J.L.; Kistamás, K.; Trafford, A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017, 121, 181–195. [Google Scholar] [CrossRef]
- Rehm, H.L. ClinGen—The Clinical Genome Resource. N. Engl. J. Med. 2015, 372, 2235–2242. [Google Scholar] [CrossRef]
- Haghighi, K.; Kolokathis, F.; Gramolini, A.O.; Waggoner, J.R.; Pater, L.; Lynch, R.A.; Fan, G.-C.; Tsiapras, D.; Parekh, R.R.; Dorn, G.W.; et al. A Mutation in the Human Phospholamban Gene, Deleting Arginine 14, Results in Lethal, Hereditary Cardiomyopathy. Proc. Natl. Acad. Sci. USA 2006, 103, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Vostrikov, V.V.; Soller, K.J.; Ha, K.N.; Gopinath, T.; Veglia, G. Effects of Naturally Occurring Arginine 14 Deletion on Phospholamban Conformational Dynamics and Membrane Interactions. Biochim. Biophys. Acta (BBA) Biomembr. 2015, 1848, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Karakikes, I.; Stillitano, F.; Nonnenmacher, M.; Tzimas, C.; Sanoudou, D.; Termglinchan, V.; Kong, C.-W.; Rushing, S.; Hansen, J.; Ceholski, D.; et al. Correction of Human Phospholamban R14del Mutation Associated with Cardiomyopathy Using Targeted Nucleases and Combination Therapy. Nat. Commun. 2015, 6, 6955. [Google Scholar] [CrossRef]
- Kamel, S.M.; van Opbergen, C.J.M.; Koopman, C.D.; Verkerk, A.O.; Boukens, B.J.D.; de Jonge, B.; Onderwater, Y.L.; van Alebeek, E.; Chocron, S.; Polidoro Pontalti, C.; et al. Istaroxime Treatment Ameliorates Calcium Dysregulation in a Zebrafish Model of Phospholamban R14del Cardiomyopathy. Nat. Commun. 2021, 12, 7151. [Google Scholar] [CrossRef] [PubMed]
- Zhihao, L.; Jingyu, N.; Lan, L.; Michael, S.; Rui, G.; Xiyun, B.; Xiaozhi, L.; Guanwei, F. SERCA2a: A Key Protein in the Ca2+ Cycle of the Heart Failure. Heart Fail. Rev. 2020, 25, 523–535. [Google Scholar] [CrossRef]
- Jaski, B.E.; Jessup, M.L.; Mancini, D.M.; Cappola, T.P.; Pauly, D.F.; Greenberg, B.; Borow, K.; Dittrich, H.; Zsebo, K.M.; Hajjar, R.J. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID Trial), a First-in-Human Phase 1/2 Clinical Trial. J. Card. Fail. 2009, 15, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Jessup, M.; Greenberg, B.; Mancini, D.; Cappola, T.; Pauly, D.F.; Jaski, B.; Yaroshinsky, A.; Zsebo, K.M.; Dittrich, H.; Hajjar, R.J. Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): A Phase 2 Trial of Intracoronary Gene Therapy of Sarcoplasmic Reticulum Ca 2+ -ATPase in Patients With Advanced Heart Failure. Circulation 2011, 124, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, B.; Yaroshinsky, A.; Zsebo, K.M.; Butler, J.; Felker, G.M.; Voors, A.A.; Rudy, J.J.; Wagner, K.; Hajjar, R.J. Design of a Phase 2b Trial of Intracoronary Administration of AAV1/SERCA2a in Patients With Advanced Heart Failure. JACC: Heart Fail. 2014, 2, 84–92. [Google Scholar] [CrossRef]
- Hulot, J.-S.; Salem, J.-E.; Redheuil, A.; Collet, J.-P.; Varnous, S.; Jourdain, P.; Logeart, D.; Gandjbakhch, E.; Bernard, C.; Hatem, S.N.; et al. Effect of Intracoronary Administration of AAV1/SERCA2a on Ventricular Remodelling in Patients with Advanced Systolic Heart Failure: Results from the AGENT-HF Randomized Phase 2 Trial: SERCA2a Gene Therapy in Heart Failure. Eur. J. Heart Fail. 2017, 19, 1534–1541. [Google Scholar] [CrossRef] [PubMed]
- Image Lab Software. Bio-Rad. Available online: https://www.bio-rad.com/es-es/product/image-lab-software?ID=KRE6P5E8Z (accessed on 1 June 2022).
- Sikkel, M.B.; Francis, D.P.; Howard, J.; Gordon, F.; Rowlands, C.; Peters, N.S.; Lyon, A.R.; Harding, S.E.; MacLeod, K.T. Hierarchical Statistical Techniques Are Necessary to Draw Reliable Conclusions from Analysis of Isolated Cardiomyocyte Studies. Cardiovasc. Res. 2017, 113, 1743–1752. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallverdú-Prats, M.; Carreras, D.; Pérez, G.J.; Campuzano, O.; Brugada, R.; Alcalde, M. Alterations in Calcium Handling Are a Common Feature in an Arrhythmogenic Cardiomyopathy Cell Model Triggered by Desmosome Genes Loss. Int. J. Mol. Sci. 2023, 24, 2109. https://doi.org/10.3390/ijms24032109
Vallverdú-Prats M, Carreras D, Pérez GJ, Campuzano O, Brugada R, Alcalde M. Alterations in Calcium Handling Are a Common Feature in an Arrhythmogenic Cardiomyopathy Cell Model Triggered by Desmosome Genes Loss. International Journal of Molecular Sciences. 2023; 24(3):2109. https://doi.org/10.3390/ijms24032109
Chicago/Turabian StyleVallverdú-Prats, Marta, David Carreras, Guillermo J. Pérez, Oscar Campuzano, Ramon Brugada, and Mireia Alcalde. 2023. "Alterations in Calcium Handling Are a Common Feature in an Arrhythmogenic Cardiomyopathy Cell Model Triggered by Desmosome Genes Loss" International Journal of Molecular Sciences 24, no. 3: 2109. https://doi.org/10.3390/ijms24032109
APA StyleVallverdú-Prats, M., Carreras, D., Pérez, G. J., Campuzano, O., Brugada, R., & Alcalde, M. (2023). Alterations in Calcium Handling Are a Common Feature in an Arrhythmogenic Cardiomyopathy Cell Model Triggered by Desmosome Genes Loss. International Journal of Molecular Sciences, 24(3), 2109. https://doi.org/10.3390/ijms24032109






