Neurons, Nose, and Neurodegenerative Diseases: Olfactory Function and Cognitive Impairment
Abstract
:1. Introduction
1.1. Odor and Smell Impairment
1.2. Olfactory Dysfunction in Neurodegenerative Diseases
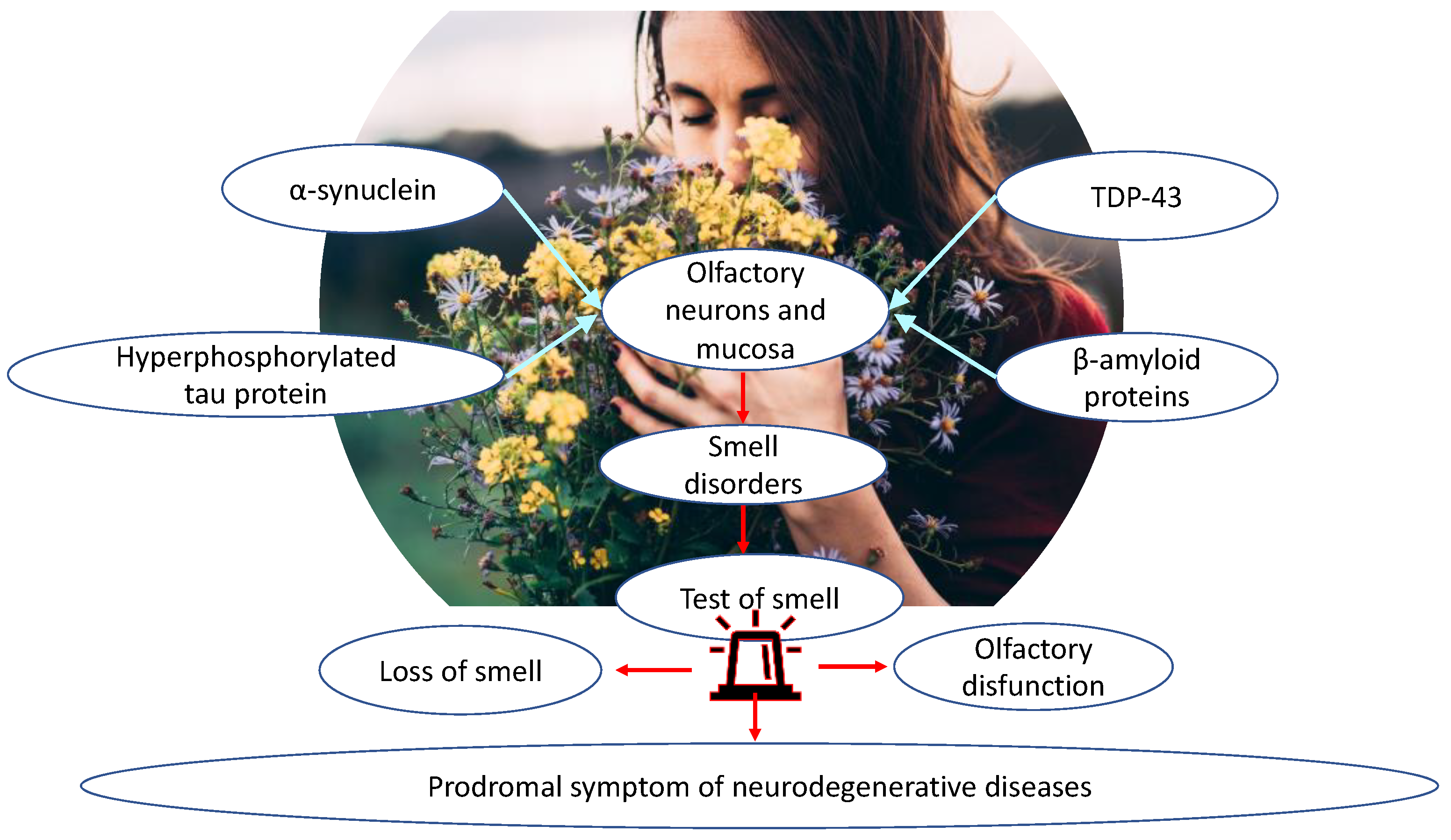
1.3. Olfactory Biomarkers
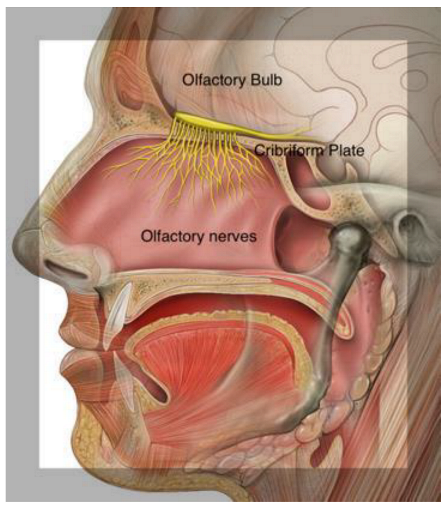
2. Olfactory Pathways
Olfactory Mucosa Molecular Mechanisms Neurobiology
3. Olfactory Tests for Smell Loss in Neurodegenerative Diseases
4. Loss of Smell and Aging
5. Smell and Cognitive Impairment in Neurodegenerative Diseases
5.1. Parkinson’s Disease
5.2. Mild Cognitive Impairment
5.3. Alzheimer’s Disease
5.4. Olfactory Function in Neuropsychiatric Disorders
6. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AMG | amygdala |
| CP | cribriform plate |
| DAT | dopamine transporter |
| DA | dopaminergic |
| EC | entorhinal cortex |
| fMRI | functional magnetic resonance imaging |
| GL | glomeruli |
| HDB | horizontal limb of the diagonal band of Broca |
| MCI | mild cognitive impairment |
| MDD | major depressive disorder |
| MDT | medial dorsal thalamus |
| MMSE | mini-mental status examination |
| OB | olfactive bulb |
| OE | olfactory epithelium |
| OFC | orbitofrontal cortex |
| ONL | olfactory nerve layer |
| OT | olfactory tract |
| ORNs | olfactory receptor neurons |
| PC | piriform cortex |
| PD | Parkinson’s disease |
References
- Ennis, M.; Hamilton, K.A.; Hayar, A. Handbook of Neurochemistry and Molecular Neurobiology: Sensory Neurochemistry; Neurochemistry of the Main Olfactory System; Springer: New York, NY, USA, 2007; pp. 137–204. [Google Scholar]
- Bryche, B.; St Albin, A.; Murri, S.; Lacôte, S.; Pulido, C.; Ar Gouilh, M.; Lesellier, S.; Servat, A.; Wasniewski, M.; Picard-Meyer, E.; et al. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav. Immun. 2020, 89, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Rey, N.L.; Wesson, D.W.; Brundin, P. The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol. Dis. 2018, 109, 226–248. [Google Scholar] [CrossRef] [PubMed]
- Bhatia-Dey, N.; Heinbockel, T. Neurological and Neuropsychiatric Disorders in Relation to Olfactory Dysfunction. In Sino-Nasal and Olfactory System Disorders; Heinbockel, T., Gendeh, B.S., Eds.; Intech Open: London, UK, 2020; Volume 7, p. 18. [Google Scholar]
- Hummel, T.; Landis, B.N.; Hüttenbrik, K.B. Smell and taste disorders. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2011, 10, Doc04. [Google Scholar] [PubMed]
- Doty, R.L.; Kamath, V. The influences of age on olfaction: A review. Front. Psychol. 2014, 5, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottaviano, G.; Zuccarello, D.; Frasson, G.; Scarpa, B.; Nardello, E.; Foresta, C.; Marioni, G.; Staffieri, A. Olfactory sensitivity and sexual desire in young adult and elderly men: An introductory investigation. Am. J. Rhinol. Allergy 2013, 27, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Sorokowska, A.; Sorokowski, P.; Havlicek, J. Body odor-based personality judgements: The effects of fraganced cosmetics. Front. Psychol. 2016, 7, 530. [Google Scholar] [CrossRef] [Green Version]
- Mullol, J.; Alobid, I.; Mariño-Sánchez, F.; Quintó, L.; de Haro, J.; Bernal-Sprekelsen, M.; Valero, A.; Picado, C.; Marin, C. Furthering the understanding of olfaction, prevalence of loss of smell and risk factors: A population-based survey (OLFACAT study). BMJ Open 2012, 2, e001256. [Google Scholar] [CrossRef] [Green Version]
- Doty, R.L. Olfactory dysfunction in neurodegenerative diseases: Is there a common pathological substrates? Lancet Neurol. 2017, 16, 478–488. [Google Scholar] [CrossRef]
- Frasnelli, J.; Hummel, T. Olfactory dysfunction, and daily life. Eur. Arch. Otorhinolaryngol. 2005, 262, 231–235. [Google Scholar] [CrossRef]
- Jaime-Lara, R.B.; Brooks, B.E.; Vizioli, C.; Chiles, M.; Nawal, N.; Ortiz-Figueroa, R.S.E.; Livinski, A.A.; Agarwal, K.; Colina-Prisco, C.; Iannarino, N.; et al. A systematic review of the biological mediators of fat taste and smell. Physiol. Rev. 2023, 103, 855–918. [Google Scholar] [CrossRef]
- Attems, J.; Wlaker, L.; Jellinger, K.A. Olfaction and aging: A mini-review. Gerontology 2015, 61, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.J.; Rawal, S.; Li, C.M.; Duffy, V.B. New chemosensory component in the US National Health and Nutrition Examination Survey (NHANES): First-year results for measured olfactory dysfunction. Rev. Endrocr. Metab. Disord. 2016, 17, 221–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hummel, T.; Nordin, S. Olfatory disorders and their consequences for quality of life. Acta Otolaryngol. 2005, 125, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Pence, T.S.; Reiter, E.R.; DiNardo, L.J.; Costanzo, R.M. Risk factors for hazardous events in olfactory-impaired patients. JAMA Otolaryngol. Head Neck Surg. 2014, 140, 951–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaume, F.; Quintó, L.; Alobid, J.; Mullol, J. Overuse of diagnostic tools and medications in acute rhinosinusitis in Spain: A population-based study (the PROSINUS study). BMJ Open 2018, 8, e018788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langdon, C.; Guillemany, J.M.; Valls, M.; Alobid, I.; Bartra, J.; Dávila, I.; del Cuvillo, A.; Ferrer, M.; Jáuregui, I.; Montoro, J.; et al. Allergic rhinitis causes loss of smell in children. Pediatr. Allergy Immunol. 2016, 27, 867–870. [Google Scholar] [CrossRef]
- Langdon, C.; Lehrer, E.; Berenguer, J.; Laxe, S.; Alobid, I.; Quintó, L.; Mariño-Sánchez, F.; Bernabeu, M.; Marin, C.; Mullol, J. Olfactory training in posttraumatic smell impairment: Mild improvement in threshold performances-results from a randomized controlled study. J. Neurotrauma. 2018, 35, 2641–2652. [Google Scholar] [CrossRef]
- Xie, L.; Hu, L. Research progress in the early diagnosis of Parkinson’s disease. Neurol. Sci. 2022, 43, 6225–6231. [Google Scholar] [CrossRef]
- Bongianni, M.; Catalan, M.; Perra, D.; Fontana, E.; Janes, F.; Bertolotti, C.; Sacchetto, L.; Capaldi, S.; Tagliapietra, M.; Polverino, P.; et al. Olfactory swab sampling optimization for α-synuclein aggregate detection in patients with Parkinson’s disease. Transl. Neurodegener. 2022, 11, 37. [Google Scholar] [CrossRef]
- Bushdid, C.; Magnasco, M.O.; Vosshall, L.B.; Keller, A. Humans can discriminate more than 1 trillion olfactory stimuli. Science 2014, 343, 1370–1372. [Google Scholar] [CrossRef]
- Hummel, T.; Whitcroft, K.L.; Andrews, P.; Altundag, A.; Cinghi, C.; Costanzo, R.M.; Damm, M.; Frasnelli, J.; Gudziol, H.; Gupta, N.; et al. Position paper on olfactory dysfunction. Rhinology 2017, 56, 1–30. [Google Scholar]
- Lafaille-Magnan, M.E.; Poirier, J.; Etienne, P.; Tremblay-Mercier, J.; Frenette, J.; Rosa-Neto, P.; Breitner, J.C.S.; PREVENT-AD Research Group. Odor identification as a biomarker of preclinical AD in older adults at risk. Neurology 2017, 89, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, M.R.; Amrutkar, C.V.; Ahah, H.C.; Benedict, R.H.; Rajakrishnan, S.; Doody, R.S.; Yan, L.; Szigeti, K. Validation of olfactory deficit as a biomarker of Alzheimer disease. Neurol. Clin. Pract. 2017, 7, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morley, J.F.; Duda, J.E. Olfaction as a biomarker in Parkinson’s disease. Biomark Med. 2010, 4, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Bowman, G.L. Biomarkers for early detection of Parkinson’s disease: A scent of consistency with olfactory dysfunction. Neurology 2017, 89, 1432–1434. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, Y.; Liu, K.; Han, X.; Liu, R.; Ren, Y.; Cong, L.; Zhang, Q.; Hou, T.; Song, L.; et al. Anosmia, mild cognitive impairment, and biomarkers of brain aging in older adults. Alzheimer’s Dement. 2022, 1–13. [Google Scholar] [CrossRef]
- D’Andrea, F.; Tischler, V.; Dening, T.; Churchill, A. Olfactory stimulation for people with dementia: A rapid review. Dementia 2022, 21, 1800–1824. [Google Scholar] [CrossRef]
- Joussain, P.; Thevenet, M.; Rouby, C.; Bensafi, M. Effect of Aging on Hedonic Appreciation of Pleasant and Unpleasant Odors. PLoS ONE 2013, 8, e61376. [Google Scholar] [CrossRef] [Green Version]
- Joussain, P.; Rouby, C.; Bensafi, M. A pleasant familiar odor influences perceived stress and peripheral nervous system activity during normal aging. Front. Psychol. 2014, 5, 113. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Boesveldt, S.C.; de Graaf, C.; de Wijk, R.A. Dynamics of autonomic nervous system responses and facial expressions to odors. Front. Psychol. 2014, 5, 110. [Google Scholar] [CrossRef] [Green Version]
- Su, C.Y.; Menuz, K.; Carlson, J.R. Olfactory Perception: Receptors, Cells, and Circuits. Cell 2009, 139, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Gottfried, J.A.; Smith, A.P.R.; Rugg, M.D.; Dolan, R.J. Remembrance of odors past: Human olfactory cortex in cross-modal recognition memory. Neuron 2004, 42, 687–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marin, C.; Vilas, D.; Langdon, C.; Alobid, I.; López-Chacón, M.; Haehner, A.; Hummel, T.; Mullol, J. Olfactory dysfunction in neurodegenerative diseases. Curr. Allergy Asthma Rep. 2018, 18, 42. [Google Scholar] [CrossRef]
- Bathini, P.; Brai, E.; Auber, L.A. Olfactory dysfunction in the pathophysiological continuum of dementia. Ageing Res. Rev. 2019, 55, 100956. [Google Scholar] [CrossRef] [Green Version]
- Ciofalo, A.; Cavaliere, C.; Masieri, S.; Di Chicco, A.; Fatuzzo, I.; Lo Re, F.; Baroncelli, S.; Begvarfaj, E.; Adduci, A.; Mezzaroma, I.; et al. Long-Term Subjective and Objective Assessment of Smell and Taste in COVID-19. Cells 2022, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Kobal, G.; Hummel, T.; Sekinger, B.; Baez, S.; Roscher, S.; Wolf, S. “Sniffin Sticks”: Screening of olfactory performance. Rhinology 1996, 34, 222–226. [Google Scholar] [PubMed]
- Haehner, A.; Tosch, C.; Wolz, M.; Klingelhoefer, L.; Fauser, M.; Storc, A.; Reichmann, H.; Hummel, T. Olfactory training in patients with Parkinson’s disease. PLoS ONE 2013, 8, e61680. [Google Scholar] [CrossRef] [Green Version]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. “Sniffin” sticks’: Olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem. Senses 1997, 22, 39–52. [Google Scholar] [CrossRef]
- Quarmley, M.; Moberg, P.J.; Mechanic-Hamilton, D.; Kabadi, S.; Arnold, S.E.; Wolk, D.A.; Roalf, D.R. Odor identification screening improves diagnostic classification in incipient alzheimer’s disease. J. Alzheimer’s Dis. 2016, 55, 1497–1507. [Google Scholar] [CrossRef] [Green Version]
- Doty, R.L.; Marcus, A.; Lee, W.W. Development of the 12-item cross-cultural smell identification test (CC-SIT). Laryngoscope 1996, 106, 353–356. [Google Scholar] [CrossRef]
- Rodriguez-Violante, M.; Glonzalez-Latapi, P.; Camacho-Ordoñez, A.; Martínez-Ramírez, D.; Morales-Briceño, H.; Cervantes-Arriaga, A. Low specificity and sensitivity of smell identification testing for the diagnosis of Parkinson’s disease. Arq. Neuropsiquiatry 2014, 72, 33–37. [Google Scholar] [CrossRef]
- Schubert, C.R.; Carmichael, L.L.; Murphy, C.; Klein, B.E.K.; Klein, R.; Cruickshanks, K.J. Olfaction and the 5-Year incidence of cognitive impairment in an epidemiological study of older adults. J. Am. Geriatr. Soc. 2008, 56, 1517–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M. The odor stick identification test for the japanese (OSIT-J): Clinical suitability for patients suffering from olfactory disturbance. Chem. Senses 2005, 30, i216–i217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardesin, A.; Alobid, I.; Benítez, P.; Sierra, E.; de Haro, J.; Bernal-Sprekelsen, M.; Picado, C.; Mullol, J. Barcelona Smell Test-24 (BAST-24): Validation and smell characteristics in the healthy Spanish population. Rhinology 2006, 44, 83–89. [Google Scholar]
- Iijima, M.; Kobayakawa, T.; Saito, S.; Osawa, M.; Tsutsumo, Y.; Hashimoto, S.; Iwata, M. Smell identification in Japanese Parkinson’s Disease patients: Using the odor stick identification test for Japanese subjects. Intern. Med. 2008, 47, 1887–1892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maremmani, C.; Rossi, G.; Tambasco, N.; Fattori, B.; Pieroni, A.; Ramat, S.; Napolitano, A.; Vanni, P.; Serra, P.; Piersanti, P.; et al. The validity and reliability of the Italian olfactory identification test (IOIT) in healthy subjects and in Parkinson’s disease patients. Parkinsonism Relat. Disord. 2012, 18, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kim, H.R.; Min, H.J.; Kim, K.S.; Jin, J.C.; Han, D.H. A novel olfactory threshold test for screening cognitive decline among elderly people. PLoS ONE 2021, 16, e0254357. [Google Scholar] [CrossRef]
- Parma, V.; Hannum, M.E.; O’Leary, M.; Pellegrino, R.; Rawson, N.E.; Reed, D.R.; Dalton, P.H. SCENTinel 1.0: Development of a Rapid Test to Screen for Smell Loss. Chem. Senses 2021, 46, bjab012. [Google Scholar] [CrossRef]
- Oleszkiewicz, A.; Abriat, A.; Doelz, G.; Azema, E.; Hummel, T. Beyond olfaction: Beneficial effects of olfactory training extend to aging-related cognitive decline. Behav. Neurosci. 2021, 135, 732–740. [Google Scholar] [CrossRef]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From discoveries in ageing research to therapeutics for healthy ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef] [Green Version]
- Cherry, J.A.; Baum, M.J. Sex differences in main olfactory system pathways involved in psychosexual function. Genes Brain Behav. 2020, 19, e12618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Zong, G.; Doty, R.L.; Sun, Q. Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: A cross-sectional study. BMJ Open 2016, 6, e013246. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Pinto, A.V.; Santos, R.M.; Coutinho, R.A.; Oliveira, L.M.; Santos, G.B.; Alho, A.T.L.; Leite, R.E.P.; Farfel, J.M.; Suemoto, C.K.; Grinberg, L.T.; et al. Sexual dimorphism in the human olfactory bulb: Females have more neurons and glial cells than males. PLoS ONE 2014, 9, e111733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvand, M.; Rankin, C.H. Is There a Shared Etiology of Olfactory Impairments in Normal Aging and Neurodegenerative Disease? J. Alzheimer’s Dis. 2020, 73, 1–21. [Google Scholar] [CrossRef]
- Murphy, C.; Nordin, S.; Acosta, L. Odor learning, recall, and recognition memory in young and elderly adults. Neuropsychology 1997, 11, 126–137. [Google Scholar] [CrossRef]
- Suzuki, Y.; Critchley, H.D.; Suckling, J.; Fukuda, R.; Williams, S.C.; Andrew, C.; Howard, R.; Ouldred, E.; Bryant, C.; Swift, C.G.; et al. Functional magnetic resonance imaging of odor identification: The effect of aging. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M756–M760. [Google Scholar] [CrossRef] [Green Version]
- Ferdon, S.; Murphy, C. The cerebellum and olfaction in the aging brain: A functional magnetic resonance imaging study. Neuroimage 2003, 20, 12–21. [Google Scholar] [CrossRef]
- Buschhüter, D.; Smitka, M.; Puschmann, S.; Gerber, J.C.; Abolmaali, N.D.; Hummel, T. Correlation between olfactory bulb volume and olfactory function. Neuroimage 2008, 42, 498–502. [Google Scholar] [CrossRef]
- Mazal, P.P.; Haehner, A.; Hummel, T. Relation of the volume of the olfactory bulb to psychophysical measures of olfactory function. Eur. Arch. Otorhinolaryngol. 2016, 273, 1–7. [Google Scholar] [CrossRef]
- Papazian, E.J.; Pinto, J.M. Olfactory loss and aging: Connections with health and well-being. Chem. Senses 2021, 46, bjab045. [Google Scholar] [CrossRef]
- Wilson, R.S.; Schneider, J.A.; Arnold, S.E.; Tang, Y.; Boyle, P.A.; Benner, D.A. Olfactory identification and incidence of mild cognitive impairment in older age. Arch. Gen. Psychiatry 2007, 64, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Mackay-Sim, A.; Johnston, A.N.; Owen, C.; Burne, T.H. Olfactory ability in the healthy population: Reassessing presbyosmia. Chem. Senses 2006, 31, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. Olfactory system: Functional organization and involvement in neurodegenerative disease. Neurology 2010, 75, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- Acebes, A.; Martín-Peña, A.; Chevalier, V.; Ferrús, A. Synapse loss in olfactory local interneurons modifies perception. J. Neurosci. 2011, 31, 2734–2745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barresi, M.; Ciurleo, R.; Giacoppo, S.; Foti, C.V.; Celi, D.; Bramanti, P.; Marino, S. Evaluation of olfactory dysfunction in neurodegenerative diseases. J. Neurol. Sci. 2012, 323, 16–24. [Google Scholar] [CrossRef]
- Djordjevic, J.; Jones-Gotman, M.; De Sousa, K.; Chertkow, H. Olfaction in patients with mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 2008, 29, 693–706. [Google Scholar] [CrossRef]
- Doty, R.L.; Hawkes, C.H. Chemosensory dysfunction in neurodegenerative diseases. Handb. Clin. Neurol. 2019, 164, 325–360. [Google Scholar]
- Alotaibi, M.; Lessard-Beaudoin, M.; Busch, K.; Loudghi, A.; Gaudreau, P.; Graham, R.K. Olfactory Dysfunction Associated with Cognitive Decline in an Elderly Population. Exp Aging Res. 2022, 22, 1–16. [Google Scholar] [CrossRef]
- Sedghizadeh, M.J.; Hojjati, H.; Ezzatdoost, K.; Aghajan, H.; Vahabi, Z.; Tarighatnia, H. Olfactory response as a marker for Alzheimer’s disease: Evidence from perceptual and frontal lobe oscillation coherence deficit. PLoS ONE 2020, 15, e0243535. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson’ disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Brozzetti, L.; Sacchetto, L.; Cecchini, M.P.; Avesani, A.; Perra, D.; Bongianni, M.; Portioli, C.; Scupoli, M.; Ghetti, B.; Monaco, S.; et al. Neurodegeneration-Associated Proteins in Human Olfactory Neurons Collected by Nasal Brushing. Front. Neurosci. 2020, 14, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franks, K.H.; Chuah, M.I.; King, A.E.; Vickers, J.C. Connectivity of Pathology: The Olfactory System as a Model for Network-Driven Mechanisms of Alzheimer’s Disease Pathogenesis. Front. Aging Neurosci. 2015, 7, 234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacyna, R.R.; Han, S.D.; Wroblewski, K.E.; McClintock, M.K.; Pinto, J.M. Rapid olfactory decline during aging predicts dementia and GMV loss in AD brain regions. Alzheimers Dement. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fullard, M.E.; Morley, J.F.; Duda, J.E. Olfactory Dysfunction as an Early Biomarker in Parkinson’s Disease. Neurosci. Bull. 2017, 33, 515–525. [Google Scholar] [CrossRef]
- Johansen, K.K.; Waro, B.J.; Aasly, J.O. Olfactory dysfunction in sporadic Parkinson’s disease and LRRK2 carriers. Acta Neurol. Scand. 2014, 129, 300–306. [Google Scholar] [CrossRef]
- Saunders-Pullman, R.; Mirelman, A.; Wang, C.; Alcalay, R.N.; Luciano, M.S.; Ortega, R.; Raymond, D.; Mejia-Santana, H.; Ozelius, L.; Clark, L.; et al. Olfactory identification in LRRK2 G2019S mutation carriers: A relevant marker? Ann. Clin. Transl. Neurol. 2014, 1, 670–678. [Google Scholar] [CrossRef] [Green Version]
- Beavan, M.; McNeill, A.; Proukakis, C.H.; Hughes, D.A.; Mehta, A.; Schapira, A.H.V. Evolution of prodromal clinical markers of Parkinson disease in a glucocerebrosidase mutation positive cohort. JAMA Neurol. 2015, 72, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Chase, B.A.; Markopoulou, K. Olfactory Dysfunction in Familial and Sporadic Parkinson’s Disease. Front Neurol. 2020, 11, 447. [Google Scholar] [CrossRef]
- Doty, R.L. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 2012, 8, 329–339. [Google Scholar] [CrossRef]
- Wattendorf, E.; Welge-Lüssen, A.; Fiedler, K.; Bilecen, D.; Wolfensberger, M.; Fuhr, P.; Hummel, T.; Westermann, B. Olfactory impairment predicts brain atrophy in Parkinson’s disease. J. Neurosci. 2009, 29, 15410–15413. [Google Scholar] [CrossRef] [Green Version]
- Landolfi, A.; Picillo, M.; Pellecchia, M.T.; Troisi, J.; Amboni, M.; Barone, P.; Erro, R. Screening performances of an 8-item UPSIT Italian version in the diagnosis of Parkinson’s disease. Neurol Sci. 2022, 19, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Brodoehl, S.; Klingner, C.; Volk, G.F.; Bitter, T.; Witte, O.W.; Redecker, C. Decreased olfactory bulb volume in idiopathic Parkinson’s disease detected by 3.0-Tesla magnetic resonance imaging. Mov. Disord. 2012, 27, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Tan, H.Y.; Wu, Z.H.; Sun, C.P.; He, J.X.; Li, X.C.; Shao, M. Imaging of olfactory bulb and gray matter volumes in brain areas associated with olfactory function in patients with Parkinson’s disease and multiple system atrophy. Eur. J. Radiol. 2014, 83, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gu, C.Z.; Su, J.B.; Zhu, L.H.; Zhou, Y.; Huang, H.Y.; Liu, C.F. Changes in olfactory bulb volume in Parkinson’s disease: A systematic review and meta-analysis. PLoS ONE 2016, 11, e0149286. [Google Scholar] [CrossRef]
- Bohnen, N.I.; Müller, M.L. In vivo neurochemical imaging of olfactory dysfunction in Parkinson’s disease. J. Neural Transm. 2013, 120, 571–576. [Google Scholar] [CrossRef]
- Saramago, I.; Franceschi, A.M. Olfactory Dysfunction in Neurodegenerative Disease. Top. Magn. Reson. Imaging 2021, 30, 167–172. [Google Scholar] [CrossRef]
- Hawkes, C. Olfaction in neurodegenerative disorder. Adv. Otorhinolaryngol. 2006, 63, 133–151. [Google Scholar]
- Attems, J.; Walker, L.; Jellinger, K.A. Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol. 2014, 127, 459–475. [Google Scholar] [CrossRef]
- Velayudhan, L. Smell identfication function and Alzheimer’s disease: A selective review. Curr. Opin. Psychiatry 2015, 28, 173–179. [Google Scholar] [CrossRef]
- Cavaco, S.; Goncalves, A.; Mendes, A.; Vila-Cha, N.; Moreira, I.; Fernandes, J.; Damásio, J.; Teixeira-Pinto, A.; Lima, A.B. Abnormal olfaction in Parkinson’s disease is related to faster disease progression. Behav. Neurol. 2015, 2015, 976589. [Google Scholar] [CrossRef] [Green Version]
- Tanik, N.; Serin, H.I.; Celikbilek, A.; Inan, L.E.; Gundogdu, F. Associations of olfactory bulb and depth of olfactory sulcus with basal ganglia and hippocampus in patients with Parkinson’s disease. Neurosci. Lett. 2016, 620, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Sunwoo, M.K.; Ham, J.H.; Lee, J.J.; Lee, P.H.; Sohn, Y.H. Apathy and olfactory dysfunction in early Parkinson’s disease. J. Mov. Disord. 2015, 8, 21–25. [Google Scholar] [CrossRef]
- Wang, J.; You, H.; Liu, J.F.; Ni, D.F.; Zhang, Z.X.; Guan, J. Association of olfactory bulb volume and olfactory sulcus depth with olfactory function in patients with Parkinson disease. AJNR Am. J. Neuroradiol. 2011, 32, 677–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, R.C. Mild Cognitive Impairment: Transition from Aging to Alzheimer’s Disease. In Alzheimer’s Disease; Iqbal, K., Sisodia, S.S., Winblad, B., Eds.; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Devanand, D.P.; Michaels-Marston, K.S.; Liu, X.; Pelton, G.H.; Padilla, M.; Marder, K.; Bell, K.; Stern, Y.; Mayeux, R. Olfactory Deficits in Patients With Mild Cognitive Impairment Predict Alzheimer’s Disease at Follow-Up. Am. J. Psychiatry 2000, 157, 1399–1405. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Tardiff, S.; Dye, C.; Arrighi, H.M. Rate of conversion from prodromal Alzheimer’s disease to Alzheimer’s dementia: A systematic review o the literature. Dement. Geriatr. Cogn. Disord. Extra. 2013, 3, 320–332. [Google Scholar] [CrossRef]
- Apostolova, L.G.; Dutton, R.A.; Dinov, I.D.; Hayashi, K.M.; Toga, A.W.; Cummings, J.L.; Thompson, P.M. Conversion of Mild Cognitive Impairment to Alzheimer Disease Predicted by Hippocampal Atrophy Maps. Arch. Neurol. 2006, 63, 693. [Google Scholar] [CrossRef] [Green Version]
- Jobin, B.; Boller, B.; Frasnelli, J. Volumetry of Olfactory Structures in Mild Cognitive Impairment and Alzheimer’s Disease: A Systematic Review and a Meta-Analysis. Brain Sci. 2021, 11, 1010. [Google Scholar] [CrossRef]
- Rajan, K.B.; Weuve, J.; Banes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef]
- Wimo, A.; Winblad, B.; Aguero-Torres, H.; von Strauss, E. The Magnitude of Dementia Occurrence in the World. Alzheimer Dis. Assoc. Disord. 2003, 17, 63–67. [Google Scholar] [CrossRef]
- Ubeda-Bañon, I.; Saiz-Sanchez, D.; Flores-Cuadrado, A.; Rioja-Corroto, E.; Gonzalez-Rodriguez, M.; Villar-Conde, S.; Astillero-Lopez, V.; Cabello-de la Rosa, J.P.; Gallardo-Alcañiz, M.J.; Vaamonde-Gamo, J.; et al. The human olfactory system in two proteinopathies: Alzheimer’s and Parkinson’s diseases. Transl. Neurodegener. 2020, 9, 22. [Google Scholar] [CrossRef]
- Hasan, S.; Adler, C.H.; Zhang, N.; Serrano, G.E.; Sue, L.I.; Shill, H.A.; Mehta, S.H.; Beach, T.G.; Driver-Dunckley, E.D. Olfactory Dysfunction in Incidental Lewy Body Disease and Parkinson’s Disease: An Update. Innov. Clin. Neurosci. 2022, 19, 19–23. [Google Scholar] [PubMed]
- Zou, Y.M.; Lu, D.; Liu, L.P.; Zhang, H.H.; Zhou, Y.Y. Olfactory dysfunction in Alzheimer’s disease. Neuropsychiatry Dis. Treat. 2016, 12, 869–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C. Olfactory and other sensory impairments in Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Waldton, S. Clinical observations of impaired cranial nerve function in senile dementia. Acta Psychiatr. Scand. 1974, 50, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Devanand, D.P.; Liu, X.; Tabert, M.H.; Pradhaban, G.; Cuasay, K.; Bell, K.; de Leon, M.J.; Doty, R.L.; Stern, Y.; Pelton, G.H. Combining early markers strongly conversion from mild cognitive impairment to Alzheimer’s disease. Biol. Psychiatry 2008, 64, 871–879. [Google Scholar] [CrossRef] [Green Version]
- Roberts, R.O.; Christianson, T.J.; Kremers, W.K.; Mielke, M.M.; Machulda, M.M.; Vassilaki, M.; Alhurani, R.E.; Geda, Y.E.; Knopman, D.S.; Petersen, R.C. Association between olfactory dysfunction and amnestic mild cognitive impairment and alzheimer disease dementia. JAMA Neurol. 2016, 73, 93–101. [Google Scholar] [CrossRef] [Green Version]
- Haehner, A.; Chen, B.; Espin, M.; Haussmann, R.; Matthes, C.; Desser, D.; Loessner, L.; Brandt, M.D.; Donix, M.; Hummel, T. Training with Odors Impacts Hippocampal Thickness in Patients with Mild Cognitive Impairment. J. Alzheimer’s Dis. 2022, 88, 743–755. [Google Scholar] [CrossRef]
- Silva, M.M.E.; Mercer, P.B.S.; Witt, M.C.Z.; Pessoa, R.R. Olfactory dysfunction in Alzheimer’s disease Systematic review and meta-analysis. Dement. Neuropsychol. 2018, 12, 123–132. [Google Scholar] [CrossRef]
- Ter Laak, H.J.; Renkawek, K.; van Workum, F.P. The olfactory bulb in Alzheimer disease: A morphologic study of neuron loss, tangles, and senile plaques in relation to olfaction. Alzheimer Dis. Assoc. Disord. 1994, 8, 38–48. [Google Scholar] [CrossRef]
- Murphy, C.; Jernigan, T.L.; Fennema-Notestine, C. Left hippocampal volume loss in Alzheimer’s disease is reflected in performance on odor identification: A structural MRI study. J. Int. Neuropsychol. Soc. 2003, 9, 459–471. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Eslinger, P.J.; Doty, R.L.; Zimmerman, E.K.; Grunfeld, R.; Sun, X.; Meadowcroft, M.D.; Connor, J.R.; Price, J.L.; Smith, M.B.; et al. Olfactory deficit detected by fMRI in early Alzheimer’s disease. Brain Res. 2010, 1357, 184–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelley, B.J.; Petersen, R.C. Alzheimer’s Disease and Mild Cognitive Impairment. Neurol. Clin. 2007, 25, 577–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewers, M.; Walsh, C.; Trojanowski, J.Q.; Shaw, L.M.; Petersen, R.C.; Jack, C.R., Jr.; Feldman, H.H.; Bokde, A.L.W.; Alexander, G.E.; Scheltens, P.; et al. Prediction of conversion from mild cognitive impairment to Alzheimer’s disease dementia based upon biomarkers and neuropsychological test performance. Neurobiol. Aging 2012, 33, 1203–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritchie, C.; Smailagic, N.; Noel-Storr, A.H.; Takwoingi, Y.; Flicker, L.; Mason, S.E.; McShane, R. Plasma and cerebrospinal fluid amyloid beta for the diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2014, 2014, Cd008782. [Google Scholar] [CrossRef]
- Ayala-Grosso, C.A.; Pieruzzini, R.; Diaz-Solano, D.; Wittig, O.; Abrante, L.; Vargas, L.; Cardier, J. Amyloid-aβ Peptide in olfactory mucosa and mesenchymal stromal cells of mild cognitive impairment and Alzheimer’s disease patients. Brain Pathol. 2015, 25, 136–145. [Google Scholar] [CrossRef]
- Attems, J.; Jellinger, K.A. Olfactory tau pathology in Alzheimer’s disease and mild cognitive impairment. Clin. Neuropathol. 2006, 25, 265–271. [Google Scholar]
- Klein, J.; Yan, X.; Johnson, A.; Tomljanovic, A.; Zou, J.; Polly, K.; Honig, L.S.; Brickman, A.M.; Stern, Y.; Devanand, D.P.; et al. Olfactory impairment is related to tau pathology and neuroinflammation in Alzheimer’s disease. J. Alzheimer’s Dis. 2021, 80, 1051–1065. [Google Scholar] [CrossRef]
- Yan, Y.; Aierken, A.; Wang, C.; Song, D.; Ni, J.; Wang, Z.; Quan, Z.; Qing, H. A potential biomarker of preclinical Alzheimer’s disease: The olfactory dysfunction and its pathogenesis-based neural circuitry impairments. Neurosci. Biobehav. Rev. 2022, 132, 857–869. [Google Scholar] [CrossRef]
- Vasavada, M.M.; Wang, J.; Eslinger, P.J.; Gill, D.J.; Sun, X.; Karunanayaka, P.; Yang, Q.X. Olfactory Cortex Degeneration in Alzheimer’s Disease and Mild Cognitive Impairment. J. Alzheimer’s Dis. 2015, 45, 947–958. [Google Scholar] [CrossRef]
- Ryo, Y.; Takeuchi, M.; Ueda, N.; Ohi, K.; Kihara, H.; Shimada, T.; Uehara, T.; Kawasaki, Y. Olfactory function in neuropsychiatric disorders. Psychiatry Res. 2017, 252, 175–179. [Google Scholar] [CrossRef]
- Taalman, H.; Wallace, C.; Milev, R. Olfactory Functioning and Depression: A Systematic Review. Front. Psychiatry 2017, 8, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucco, G.M.; Bollini, F. Odour recognition memory and odour identification in patients with mild and severe major depressive disorders. Psychiatry Res. 2011, 190, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.; Soler, Z.M.; Nguyen, S.A.; Muus, J.S.; Schlosser, R.J. The association between olfaction and depression: A systematic review. Chem. Senses 2016, 41, 479–486. [Google Scholar] [CrossRef] [Green Version]
- Orasji, S.S.S.; Mulder, J.L.; de Bruijn, S.F.T.M.; Wirtz, P.W. Olfactory dysfunction in behavioral variant frontotemporal dementia. Clin. Neurol. Neurosurg. 2016, 141, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Carnemolla, S.E.; Hsieh, J.W.; Sipione, R.; Landis, B.N.; Kumfor, F.; Piguet, O.; Manuel, A.L. Olfactory dysfunction in frontotemporal dementia and psychiatric disorders: A systematic review. Neurosci. Biobehav. Rev. 2020, 118, 588–611. [Google Scholar] [CrossRef] [PubMed]
- Fagundo, A.B.; Jimenez-Murcia, S.; Giner-Bartolome, C.; Islam, M.A.; de la Torre, R.; Pastor, A.; Casanueva, F.F.; Crujeiras, A.B.; Granero, R.; Baños, R.; et al. Modulation of higher-order olfaction components on executive functions in humans. PLoS ONE 2015, 10, e0130319. [Google Scholar] [CrossRef] [Green Version]
- Frasnelli, J.; Lundstrom, J.N.; Boyle, J.A.; Djordjevic, J.; Zatorre, R.J.; Jones-Gotman, M. Neuroanatomical correlates of olfactory performance. Exp. Brain Res. 2010, 201, 1–11. [Google Scholar] [CrossRef]
- Witoonpanich, P.; Cash, D.M.; Shakespeare, T.J.; Yong, K.X.; Nicholas, J.M.; Omar, R.; Crutch, S.J.; Rossor, M.N.; Warren, J.D. Olfactory impairment in posterior cortical atrophy. J. Neurol. Neurosurg. Psychiatry 2013, 84, 588–590. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.D.; Pelavin, P.E.; Shenton, M.E.; Chilakamarri, P.; McCarley, R.W.; Nestor, P.G.; Levitt, J.J. Olfactory sulcal depth and olfactory bulb volume in patients with schizophrenia: An MRI study. Brain Imaging Behav. 2011, 5, 252–261. [Google Scholar] [CrossRef]
- Doty, R.L.; Hawkes, C.H.; Good, K.P.; Duda, J.E. Odor perception and neuropathology in neurodegenerative diseases and schizophrenia. In Handbook of Olfaction and Gustation, 3rd ed.; Doty, R.L., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 3, pp. 403–452. [Google Scholar]
- Son, G.; Jahanshahi, A.; Yoo, S.J.; Boonstra, J.T.; Hopkins, D.A.; Steinbusch, H.W.M.; Moon, C. Olfactory neuropathology in Alzheimer’s disease: A sign of ongoing neurodegeneration. BMB Rep. 2021, 54, 295–304. [Google Scholar] [CrossRef]
- Hsieh, J.W.; Keller, A.; Wong, M.; Jiang, R.S.; Vosshall, L.B. SMELL-S and SMELL-R: Olfactory tests not influenced by odor-specific insensitivity or prior olfactory experience. Proc. Natl. Acad. Sci. USA 2017, 114, 11275–11284. [Google Scholar]
- Sinding, C.; Puschmann, L.; Hummel, T. Is the age-related loss in olfactory sensitivity similar for light and heavy molecules? Chem. Senses 2014, 39, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Poletti, S.C.; Michel, E.; Hummel, T. Olfactory Training Using Heavy and Light Weight Molecule Odors. Perception 2017, 46, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Han, J.E.; Frasnelli, J.; Zeighami, Y.; Larcher, K.; Boyle, J.; McConnell, T.; Malik, S.; Jones-Gotman, M.; Dagher, A. Ghrelin Enhances Food Odor Conditioning in Healthy Humans: An fMRI Study. Cell Rep. 2018, 25, 2643–2652.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dan, X.; Wechter, N.; Gray, S.; Mohanty, J.G.; Croteau, D.L.; Bohr, V.A. Olfactory dysfunction in aging and neurodegenerative diseases. Ageing Res. Rev. 2021, 70, 101416. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.C.G.; Silva, F.G.; Costa, L.O.P.; Freitas, S.M.S.F. Smell tests to distinguish Parkinson’s disease from other neurological disorders: A systematic review and meta-analysis. Expert Rev. Neurother. 2021, 21, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Parker, G.; Paterson, A.; Romano, M.; Graham, R. Altered Sensory Phenomena Experienced in Bipolar Disorder. Am. J. Psychiatry 2017, 174, 1146–1150. [Google Scholar] [CrossRef]
- Cha, H.; Kim, S.; Seo, M.S.; Kim, H.S. Effects of olfactory stimulation on cognitive function and behavior problems in older adults with dementia: A systematic literature review. Geriatr Nurs. 2021, 42, 1210–1217. [Google Scholar] [CrossRef]
- Yahiaoui-Doktor, M.; Luck, T.; Riedel-Heller, S.G.; Loeffler, M.; Wirkner, K.; Engel, C. Olfactory function is associated with cognitive performance: Results from the population-based LIFE-Adult-Study. Alzheimers Res. Ther. 2019, 11, 43. [Google Scholar] [CrossRef] [Green Version]
- Winchester, R.L.; Martyn, K. Could Early Identification of Changes in Olfactory Function Be an Indicator of Preclinical Neurodegenerative Disease? A Systematic Review. Neurol. Ther. 2020, 9, 243–263. [Google Scholar] [CrossRef]
- Barbato, C.; Di Certo, M.G.; Gabanella, F.; Petrella, C.; Fiore, M.; Passananti, C.; Colizza, A.; Cavalcanti, L.; Ralli, M.; Greco, A.; et al. Staying tuned for post-COVID-19 syndrome: Looking for new research to sniff out. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5318–5321. [Google Scholar] [CrossRef] [PubMed]
- Butowt, R.; Bilinska, K.; von Bartheld, C.S. Olfactory dysfunction in COVID-19: New insights into the underlying mechanisms. Trends Neurosci. 2023, 46, 75–90. [Google Scholar] [CrossRef] [PubMed]
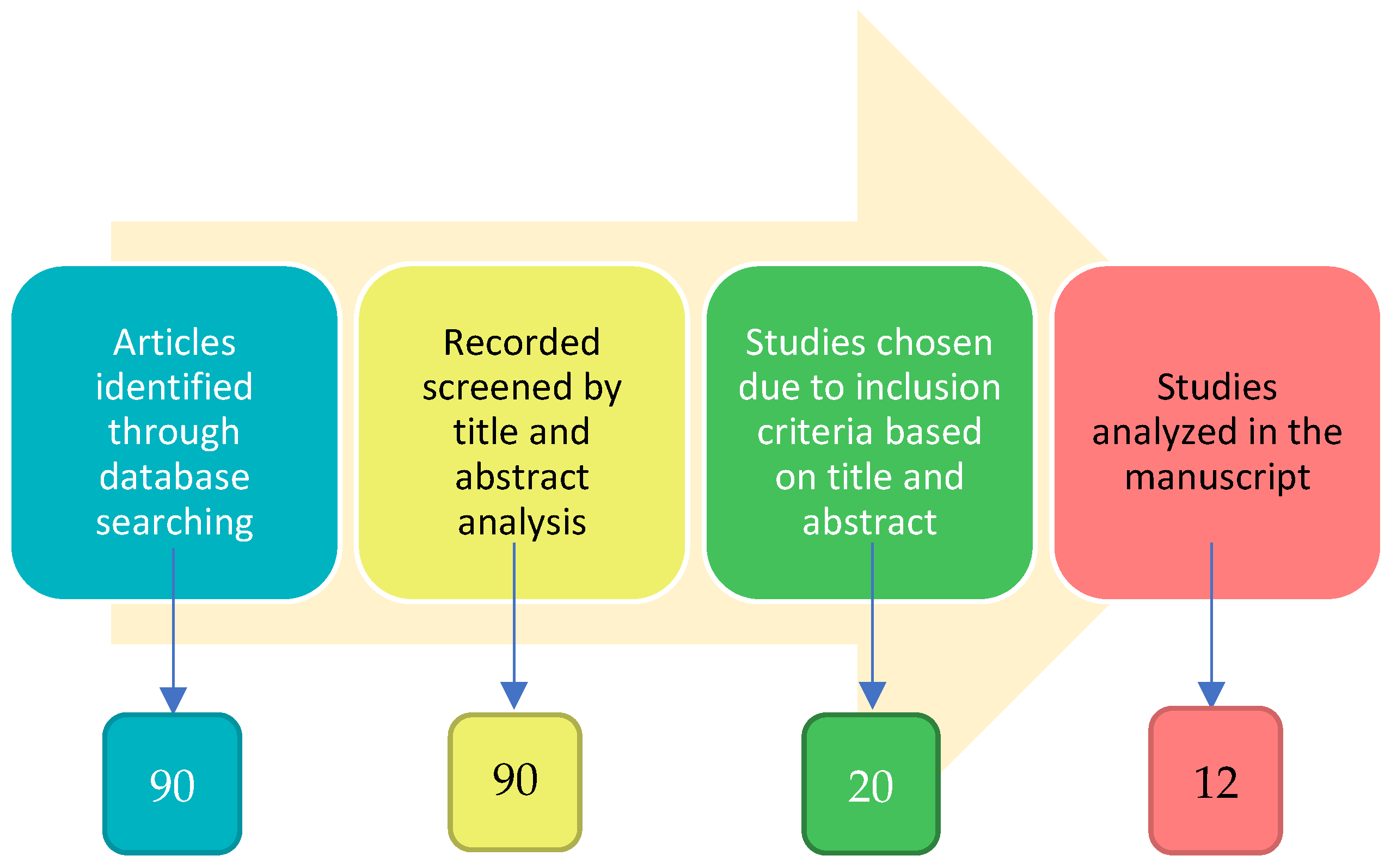

| Year | Country | Study | Purpose |
|---|---|---|---|
| 2013 | USA | Observational Study | Outline how progressive changes in olfaction may be used as a biomarker of cholinergic denervation and cognitive decline in Parkinson’s Disease patients |
| 2014 | UK | Review | Review of potential biomarkers of prodromal Dementia with Lewy bodies |
| 2016 | USA | Observational Study | Standardized tests of odor identification ability may provide a useful tool to improve diagnostic and predictive accuracy for cognitive decline |
| 2017 | USA | Review | Potential for olfaction as a biomarker for early or differential diagnosis and prognosis in Parkinson’s Disease |
| 2018 | Spain | Review | Olfactory function measurements in neurodegenerative diseases |
| 2018 | China | Review | Review of studies about markers of imaging and neurophysiological, genetic, cognitive, autonomic function of Rapid Eye Movement and their predictive value for neurodegenerative diseases |
| 2019 | Germany; USA | Review | Identification of new marker for Prodromal Parkinson’s Disease |
| 2020 | Canada | Meta Analysis | Verify whether the presence of Subjective Cognitive Decline is associated with a decrease in olfactory identification ability |
| 2020 | Austria; Germany; France | Review | Assemble current knowledge from different medical fields on olfactory/gustatory dysfunction |
| 2022 | China | Review | Review of the olfactory evaluation of Alzheimer’s Disease model mice |
| 2022 | UK | Review | Review of the recent research from longitudinal research studies in isolated Rapid Eye Movement |
| 2022 | Canada | Review | Review of the recent research from translational research studies in Drug Delivery N2B Neurological and Psychiatric Illnesses |
| Test | Type | Response Mode | Parameters Test |
|---|---|---|---|
| University of Pennsylvania Smell Identification Test (UPSIT) | Scratch-and-sniff micro-encapsulated odorant strips (40 items) | Four option words for each odorant | Identification |
| Sniffin’ Sticks Test (SST) | 12 or 16 smell identifications pen-like | Four option words depicting scent object | Threshold, identification, discrimination |
| Brief Smell Identification Test (B-SIT) | 12 test items derived from UPSIT | Four response alternative words | Identification |
| San Diego Odor Identification Test (SDOIT) | Common natural odors in opaque jars | Options with pictures | Identification |
| Scandinavian Odor Identification Test (SOIT) | Odors culturally validated by Scandinavian people | Four alternatives | Identification |
| Barcellona Smell test-24 (BAST24) | 24 odors scoring test detection | Four option words for each odorant | Forced choice Smell detection, Identification |
| Odor Stick Identification Test for Japan (OSIT-J) | 13 odors scoring test | Four option words for each odorant | Smell detection |
| The Italian Olfactory Identification Test (IOIT) | 33 micro-encapsulated odorants | Four possible answers | Identification |
| Visual Analog Scale (VAS) | 10-cm line, both ends of which have statements of the maximal and minimal extremes | Marking the line at the appropriate point between the two extreme statements, defined as “anosmia” and “normal” | Identification |
| Sniff Bubble Rosy Smell | PEA, which has Rosy smell, from 1, highest concentration, to 7, lowest concentration | Score from minimum to maximum (anosmia-normal) | Identification |
| SCENTinel 1.0 | Flower odor (Givaudan; perfume compound with 2-phenylethanol [CAS No. 60-12-8] as the main components | “First attempt” is a four-alternative forced choice. “Second attempt” is a three-alternative forced choice, intensity range: 1–100 | Odor detection, intensity, and identification |
 | Neuropathology hallmarks Olfactory mucosa:
Tauopathy and α-Synucleinopathy in:
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatuzzo, I.; Niccolini, G.F.; Zoccali, F.; Cavalcanti, L.; Bellizzi, M.G.; Riccardi, G.; de Vincentiis, M.; Fiore, M.; Petrella, C.; Minni, A.; et al. Neurons, Nose, and Neurodegenerative Diseases: Olfactory Function and Cognitive Impairment. Int. J. Mol. Sci. 2023, 24, 2117. https://doi.org/10.3390/ijms24032117
Fatuzzo I, Niccolini GF, Zoccali F, Cavalcanti L, Bellizzi MG, Riccardi G, de Vincentiis M, Fiore M, Petrella C, Minni A, et al. Neurons, Nose, and Neurodegenerative Diseases: Olfactory Function and Cognitive Impairment. International Journal of Molecular Sciences. 2023; 24(3):2117. https://doi.org/10.3390/ijms24032117
Chicago/Turabian StyleFatuzzo, Irene, Giovanni Francesco Niccolini, Federica Zoccali, Luca Cavalcanti, Mario Giuseppe Bellizzi, Gabriele Riccardi, Marco de Vincentiis, Marco Fiore, Carla Petrella, Antonio Minni, and et al. 2023. "Neurons, Nose, and Neurodegenerative Diseases: Olfactory Function and Cognitive Impairment" International Journal of Molecular Sciences 24, no. 3: 2117. https://doi.org/10.3390/ijms24032117
APA StyleFatuzzo, I., Niccolini, G. F., Zoccali, F., Cavalcanti, L., Bellizzi, M. G., Riccardi, G., de Vincentiis, M., Fiore, M., Petrella, C., Minni, A., & Barbato, C. (2023). Neurons, Nose, and Neurodegenerative Diseases: Olfactory Function and Cognitive Impairment. International Journal of Molecular Sciences, 24(3), 2117. https://doi.org/10.3390/ijms24032117








