Circular RNAs as a Diagnostic and Therapeutic Target in Cardiovascular Diseases
Abstract
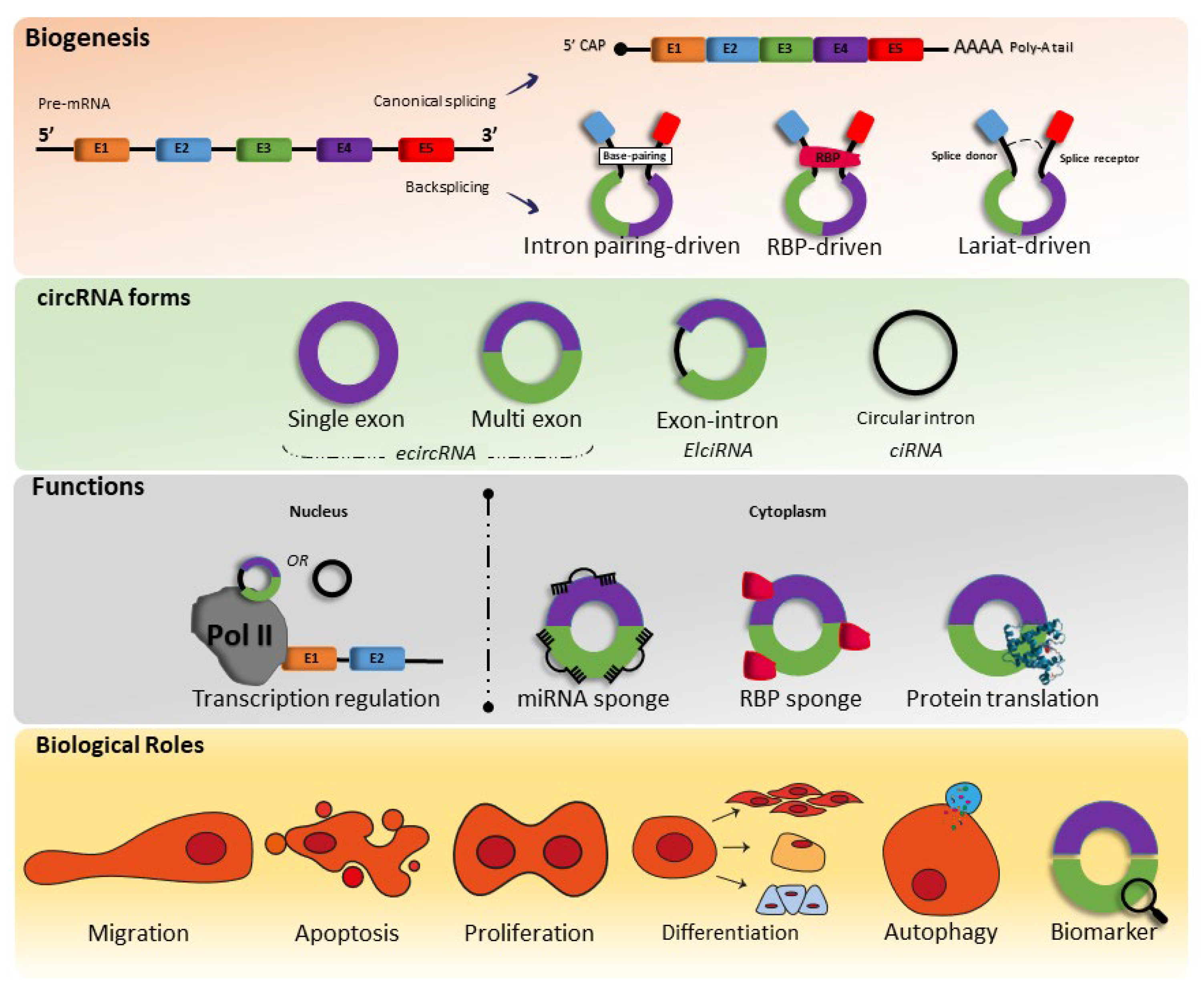
1. Biogenesis, Characteristics, and Function
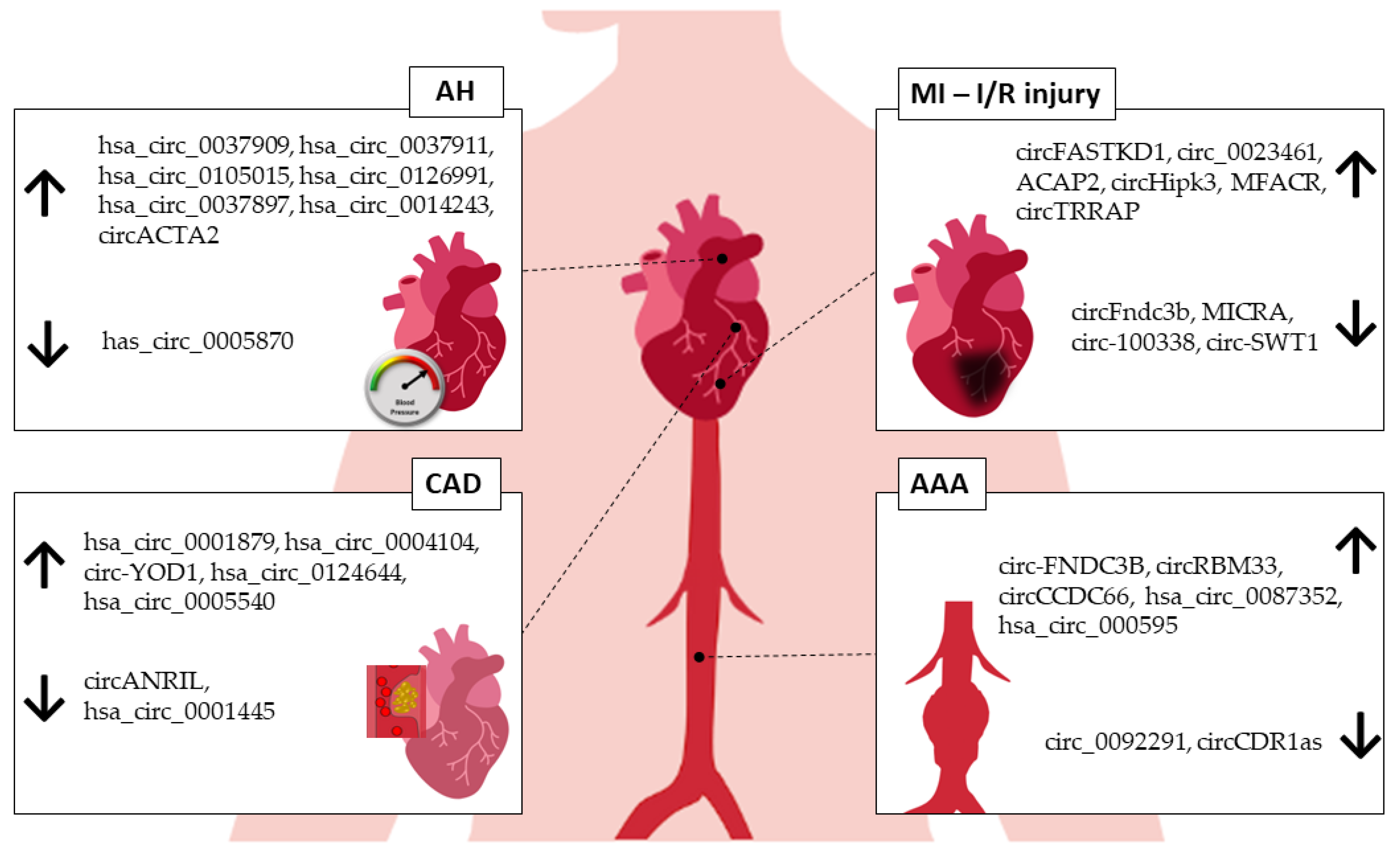
2. circRNA Expression in Cardiovascular Diseases
3. circRNAs in Arterial Hypertension
4. circRNAs in Myocardial Infarction
| Id | Host Gene | Species | Source | Expression | Action | Ref. |
|---|---|---|---|---|---|---|
| Cdr1as/CiRS-7 | CDR1 | Mouse | Cardiomyocytes | Up | Promotes CM apoptosis by sponging miR-7a. | [47] |
| Pig | Myocardium | Up | It is positively correlated with better left and right ventricle function and infarcted size decrease. | [48] | ||
| circFndc3b | FNDC3B | Mouse Human | Cardiomyocytes | Down | Limits ischemic injury via FUS/VEGF signaling. | [49] |
| circNfix | Nfix | Mouse | Cardiomyocytes | Up | Promotes cardiac regenerative repair after MI by suppressing Ybx1 degradation and increasing miR-214 activity. | [50] |
| circ-Ttc3 | TTC3 | Rat | Cardiomyocytes | Up | Promotes cardioprotection via miR-15b/Arl2 axis. | [51] |
| MICRA | ZNF609 | Human | Blood | Down | Biomarker. | [45] |
| circFASTKD1 | FASTKD1 | Human | HUVECs, HCMECs | Up | Its downregulation improved cardiac function after MI by enhancing angiogenesis via miR-106a/LATS1/2/YAP pathway. | [52] |
| circHelz | Helz | Mouse | Myocardium NMVCs | Up | Promotes pyroptosis resulting in myocardial injury via miR-133a-3p/NLRP3 axis. | [53] |
| circJARID2 | Jarid2 | Mouse | Cardiac tissues | Up | Apoptotic and inflammatory damage in CM promoted by miR-9-5p/BNIP3 axis. | [54] |
| circ-100338 | SNX27 | Human | HCAEC | Down | Induces angiogenesis in I/R injury by sponging miRNA-200a-3p. | [55] |
| circDLGAP4 | HECTD1 | Mouse | HUVECs | Down | Plays a role in apoptosis and cell migration via miR-143/HECTD1 axis. | [56] |
| circ-SWT1 | SWT1 | Human | HC AC16 cells | Down | Reduces apoptosis, oxidative stress, and endoplasmic reticulum stress via miR-192-5p/SOD2 axis. | [57] |
| circROBO2 | Robo2 | Mouse | Myocardial tissues | Up | circROBO2 knockdown reduces apoptosis by sponging miR-1184 and enhancing TRADD expression levels. | [58] |
| circRNA-101237 | CDK8 | Mouse | Cardiomyocytes | Up | Regulates CM apoptosis via let-7a-5p/IGF2BP3 axis. | [59] |
| circPAN3 | PAN3 | Rat | Cardiac tissues | Up | Promotes cardiac fibrosis after MI via miR-221/FoxO3/ATG7 axis. | [60] |
| circNCX1 | NCX1 | Mouse | Cardiomyocytes | Up | Promotes CM apoptosis via miR-133a-3p/CDIP1 regulatory pathway. | [61] |
| circRNA1615 | Copb1 | Mouse | Myocardial tissue | Down | Modulates autophagy by the miRNA152/3p/LRP6 molecular axis, reducing ferroptosis in MI mouse hearts. | [62] |
| circ_0023461 | ARAP1 | Human | HC AC16 cells | Up | Reduces hypoxia-induced dysfunction in cardiomyocytes via miR-370-3p/PDE4D axis. | [63] |
| circHipk3 | Hipk3 | Mouse | Cardiomyocyte | Up | Triggers CM proliferation and angiogenesis by miR-133a/Notch1 signaling path. | [43] |
| Mouse | Cardiomyocyte | Up | Induces tube formation, cell proliferation, and migration via miR-29a/VEGFA axis, stimulating cardiac angiogenesis after MI. | [44] | ||
| Human | HCM | Up | Induces CM apoptosis after I/R injury by sponging miRNA-124-3p. | [64] | ||
| circTLK1 | TLK1 | Mouse | Cardiomyocyte | Up | Promotes CM apoptosis via miR-214/RIPK1-mediated TNF signaling pathway. | [65] |
| MFACR | Smyd4 | Mouse | Cardiomyocyte | Up | Induces CM apoptosis via miR-652-3p/MTP18 axis. | [66] |
| Human | Plasma, HC AC16 cells | Up | Promotes CM apoptosis by downregulating miR-125b through methylation. | [67] | ||
| circUbe3a | Ube3a | Mouse | Cardiac tissue | Up | Exacerbates MI-induced myocardial fibrosis via miR-138-5p/RhoC axis. | [68] |
| circ_0060745 | Cse1l | Mouse | Cardiomyocyte | Up | Increases infarct size and impaired cardiac function after MI. | [69] |
| ACAP2 | ACAP2 | Human | Plasma, HC AC16 cells | Up | Induces CM apoptosis by promoting maturation of miR-532. | [70] |
| circCDYL | CDYL | Mouse | Cardiomyocyte | Down | Promotes CM proliferation through miR-4793-5p/APP pathway. | [71] |
| circTRRAP | TRRAP | Human | HC AC16 cells | Up | Increases inflammatory, apoptotic, and oxidative damage in CM via miR-370-3p/PAWR axis. | [72] |
| circMACF1 | Macf1 | Mouse | Cardiomyocyte | Down | Attenuates CM apoptosis via miR-500b-5p/EMP1 axis. | [73] |
| circACR | - | Mouse | Cardiomyocyte | Down | Promotes cardioprotection, decreasing myocardial infarction size, autophagy, and cell death via Pink1/FAM65B axis. | [74] |
| circMAT2B | MAT2B | Rat | H9c2 cells | Up | circMAT2B knockdown promotes anti-inflammatory and antiapoptotic role via miR-133/PI3K/AKT and Raf/MEK/ERK pathways. | [75] |
| circNFIB | Nfib | Mouse | Cardiac fibroblast | Down | Reduces cardiac fibrosis after MI by sponging miR-433. | [76] |
5. circRNAs in Coronary Artery Disease
| Id | Host Gene | Species | Source | Expression | Action | Ref. |
|---|---|---|---|---|---|---|
| hsa_circ_0001879 | NIPSNAP3A | Human | Peripheral blood | Up | Biomarker. | [82] |
| hsa_circ_0004104 | SPARC | Human | Peripheral blood | Up | Biomarker. Its upregulation might contribute to the pathogenesis of atherosclerosis. | |
| circ-YOD1 | YOD1 | Human | Blood/HASMCs | Up | Biomarker. | [84] |
| hsa_circ_0124644 | ROBO2 | Human | Peripheral blood | Up | Biomarker. | [83] |
| circEsyt2 | Esyt2 | Mouse | Aortae tissue | Up | Enhances cell migration and proliferation and inhibits apoptosis and differentiation in VSMC. | [80] |
| circANRIL | ANRIL | Human | Peripheral blood | Down | Promotes atheroprotection by increasing apoptosis and inhibiting macrophages proliferation. | [81] |
| hsa_circ_0001445 | SMARCA5 | Human | Plasma | Down | Biomarker. | [85] |
| hsa_circ_0005540 | MCTP1 | Human | Plasma | Up | Biomarker. | [86] |
6. circRNAs in Abdominal Aortic Aneurysm
7. Exercise-Related circRNAs in the Cardiovascular System
8. Conclusions
9. Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Sanger, H.L.; Klotz, G.; Riesner, D.; Gross, H.J.; Kleinschmidt, A.K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc. Natl. Acad. Sci. USA 1976, 73, 3852–3856. [Google Scholar] [CrossRef]
- Tan, W.L.W.; Lim, B.T.S.; Anene-Nzelu, C.G.O.; Ackers-Johnson, M.; Dashi, A.; See, K.; Tiang, Z.; Lee, D.P.; Chua, W.W.; Luu, T.D.A.; et al. A Landscape of Circular RNA Expression in the Human Heart. Cardiovasc. Res. 2017, 113, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Werfel, S.; Nothjunge, S.; Schwarzmayr, T.; Strom, T.-M.; Meitinger, T.; Engelhardt, S. Characterization of circular RNAs in human, mouse and rat hearts. J. Mol. Cell. Cardiol. 2016, 98, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Huo, C.; Ding, N.; Li, J.; Xiao, J.; Lin, X.; Cai, B.; Zhang, Y.; Xu, J. Dynamic Organization of lncRNA and Circular RNA Regulators Collectively Controlled Cardiac Differentiation in Humans. eBioMedicine 2017, 24, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Weng, X.; Zhao, Y.; Chen, W.; Gan, T.; Xu, D. Circular RNAs in Cardiovascular Disease: An Overview. BioMed Res. Int. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zheng, Q.; Bao, C.; Li, S.; Guo, W.; Zhao, J.; Chen, D.; Gu, J.; He, X.; Huang, S. Circular RNA is enriched and stable in exosomes: A promising biomarker for cancer diagnosis. Cell Res. 2015, 25, 981–984. [Google Scholar] [CrossRef]
- Vo, J.N.; Cieslik, M.; Zhang, Y.; Shukla, S.; Xiao, L.; Zhang, Y.; Wu, Y.-M.; Dhanasekaran, S.M.; Engelke, C.G.; Cao, X.; et al. The Landscape of Circular RNA in Cancer. Cell 2019, 176, 869–881.e813. [Google Scholar] [CrossRef]
- Zhang, Y.; Xue, W.; Li, X.; Zhang, J.; Chen, S.; Zhang, J.-L.; Yang, L.; Chen, L.-L. The Biogenesis of Nascent Circular RNAs. Cell Rep. 2016, 15, 611–624. [Google Scholar] [CrossRef]
- Liang, D.; Wilusz, J.E. Short intronic repeat sequences facilitate circular RNA production. Genes Dev. 2014, 28, 2233–2247. [Google Scholar] [CrossRef]
- LiLi, Z.; Huang, C.; Bao, C.; Chen, L.; Lin, M.; Wang, X.; Zhong, G.; Yu, B.; Hu, W.; Dai, L.; et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat. Struct. Mol. Biol. 2015, 22, 256–264. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.-O.; Chen, T.; Xiang, J.-F.; Yin, Q.-F.; Xing, Y.-H.; Zhu, S.; Yang, L.; Chen, L.-L. Circular Intronic Long Noncoding RNAs. Mol. Cell 2013, 51, 792–806. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef]
- Ebert, M.S.; Neilson, J.R.; Sharp, P.A. MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian cells. Nat. Methods 2007, 4, 721–726. [Google Scholar] [CrossRef]
- World Health Organization. World Health Statistics 2021: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2021; ISBN 9789240027053. [Google Scholar]
- Lim, T.B.; Lavenniah, A.; Foo, R.S.-Y. Circles in the heart and cardiovascular system. Cardiovasc. Res. 2020, 116, 269–278. [Google Scholar] [CrossRef]
- Su, Q.; Lv, X. Revealing New Landscape of Cardiovascular Disease through Circular RNA-MiRNA-MRNA Axis. Genomics 2020, 112, 1680–1685. [Google Scholar] [CrossRef]
- Li, B.; Li, Y.; Hu, L.; Liu, Y.; Zhou, Q.; Wang, M.; An, Y.; Li, P. Role of Circular RNAs in the Pathogenesis of Cardiovascular Disease. J. Cardiovasc. Transl. Res. 2020, 13, 572–583. [Google Scholar] [CrossRef]
- Xia, S.; Feng, J.; Lei, L.; Hu, J.; Xia, L.; Wang, J.; Xiang, Y.; Liu, L.; Zhong, S.; Han, L.; et al. Comprehensive characterization of tissue-specific circular RNAs in the human and mouse genomes. Brief. Bioinform. 2017, 18, 984–992. [Google Scholar] [CrossRef]
- Misir, S.; Wu, N.; Yang, B.B. Specific expression and functions of circular RNAs. Cell Death Differ. 2022, 29, 481–491. [Google Scholar] [CrossRef]
- Gong, B.; Xu, J.Z.; Tong, W. Landscape of circRNAs Across 11 Organs and 4 Ages in Fischer 344 Rats. Chem. Res. Toxicol. 2020, 34, 240–246. [Google Scholar] [CrossRef]
- van Heesch, S.; Witte, F.; Schneider-Lunitz, V.; Schulz, J.F.; Adami, E.; Faber, A.B.; Kirchner, M.; Maatz, H.; Blachut, S.; Sandmann, C.-L.; et al. The Translational Landscape of the Human Heart. Cell 2019, 178, 242–260.e29. [Google Scholar] [CrossRef]
- Pamudurti, N.R.; Bartok, O.; Jens, M.; Ashwal-Fluss, R.; Stottmeister, C.; Ruhe, L.; Hanan, M.; Wyler, E.; Perez-Hernandez, D.; Ramberger, E.; et al. Translation of CircRNAs. Mol. Cell 2017, 66, 9–21.e7. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, S.; Sudano, I.; Kokubo, Y.; Sulaica, E.M. Arterial hypertension. Lancet 2021, 398, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, Y.; Dong, Z.; Song, J.; Zhang, Z.; Liang, L.; Liu, X.; Sun, L.; Li, X.; Zhang, M.; et al. Expression Profiles of Circular RNA in Aortic Vascular Tissues of Spontaneously Hypertensive Rats. Front. Cardiovasc. Med. 2021, 8, 814402. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zheng, B.; Zhang, X.-H.; Nie, Z.-Y.; Yu, J.; Zhang, H.; Wang, D.-D.; Shi, B.; Bai, Y.; Yang, Z.; et al. circACTA2 mediates Ang II-induced VSMC senescence by modulation of the interaction of ILF3 with CDK4 mRNA. Aging 2021, 13, 11610–11628. [Google Scholar] [CrossRef]
- Zhang, J.-R.; Sun, H.-J. LncRNAs and circular RNAs as endothelial cell messengers in hypertension: Mechanism insights and therapeutic potential. Mol. Biol. Rep. 2020, 47, 5535–5547. [Google Scholar] [CrossRef] [PubMed]
- Weiser-Evans, M.C.M. Smooth Muscle Differentiation Control Comes Full Circle. Circ. Res. 2017, 121, 591–593. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, Z.; Zheng, B.; Zhang, X.-H.; Zhang, M.-L.; Zhao, X.-S.; Zhao, H.-Y.; Suzuki, T.; Wen, J.K. A Novel Regulatory Mechanism of Smooth Muscle α-Actin Expression by NRG-1/circACTA2/miR-548f-5p AxisNovelty and Significance. Circ. Res. 2017, 121, 628–635. [Google Scholar] [CrossRef]
- Bao, X.; He, X.; Zheng, S.; Sun, J.; Luo, Y.; Tan, R.; Zhao, J.; Zhong, F.; Zhang, L. Up-regulation of circular RNA hsa_circ_0037909 promotes essential hypertension. J. Clin. Lab. Anal. 2019, 33, e22853. [Google Scholar] [CrossRef]
- Bao, X.; Zheng, S.; Mao, S.; Gu, T.; Liu, S.; Sun, J.; Zhang, L. A potential risk factor of essential hypertension in case-control study: Circular RNA hsa_circ_0037911. Biochem. Biophys. Res. Commun. 2018, 498, 789–794. [Google Scholar] [CrossRef]
- Cheng, X.; Joe, B. Circular RNAs in rat models of cardiovascular and renal diseases. Physiol. Genom. 2017, 49, 484–490. [Google Scholar] [CrossRef]
- He, X.; Bao, X.; Tao, Z.; Sun, J.; Zheng, S.; Zhong, F.; Zhang, L. The microarray identification circular RNA hsa_circ_0105015 up-regulated involving inflammation pathway in essential hypertension. J. Clin. Lab. Anal. 2021, 35, e23603. [Google Scholar] [CrossRef]
- Liu, L.; Gu, T.; Bao, X.; Zheng, S.; Zhao, J.; Zhang, L. Microarray Profiling of Circular RNA Identifies hsa_circ_0126991 as a Potential Risk Factor for Essential Hypertension. Cytogenet. Genome Res. 2019, 157, 203–212. [Google Scholar] [CrossRef]
- Tao, Z.; Zheng, S.; He, X.; Sun, J.; He, C.; Zhang, L. Hsa_circ_0037897 may be a risk factor for essential hypertension via hsa-miR-145-5p. Clin. Exp. Hypertens. 2021, 43, 281–286. [Google Scholar] [CrossRef]
- Zheng, S.; Gu, T.; Bao, X.; Sun, J.; Zhao, J.; Zhang, T.; Zhang, L. Circular RNA hsa_circ_0014243 may serve as a diagnostic biomarker for essential hypertension. Exp. Ther. Med. 2018, 17, 1728–1736. [Google Scholar] [CrossRef]
- Wu, N.; Jin, L.; Cai, J. Profiling and bioinformatics analyses reveal differential circular RNA expression in hypertensive patients. Clin. Exp. Hypertens. 2017, 39, 454–459. [Google Scholar] [CrossRef]
- Anderson, J.L.; Morrow, D.A. Acute myocardial infarction. New Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef]
- Wen, Z.-J.; Xin, H.; Wang, Y.-C.; Liu, H.-W.; Gao, Y.-Y.; Zhang, Y.-F. Emerging roles of circRNAs in the pathological process of myocardial infarction. Mol. Ther. Nucleic Acids 2021, 26, 828–848. [Google Scholar] [CrossRef]
- Gong, C.; Zhou, X.; Lai, S.; Wang, L.; Liu, J. Long Noncoding RNA/Circular RNA-MiRNA-MRNA Axes in Ischemia-Reperfusion Injury. Biomed. Res. Int. 2020, 2020, 8838524. [Google Scholar] [CrossRef]
- Marinescu, M.; Lazar, A.; Marta, M.M.; Cozma, A.; Catana, C. Non-Coding RNAs: Prevention, Diagnosis, and Treatment in Myocardial Ischemia—Reperfusion Injury. Int. J. Mol. Sci. 2022, 23, 2728. [Google Scholar] [CrossRef]
- Huang, F.; Mai, J.; Chen, J.; He, Y.; Chen, X. Non-coding RNAs modulate autophagy in myocardial ischemia-reperfusion injury: A systematic review. J. Cardiothorac. Surg. 2021, 16, 140. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, B.; Bin, X.; Xie, C.; Li, B.; Liu, O.; Tang, Z. CircHIPK3: Key Player in Pathophysiology and Potential Diagnostic and Therapeutic Tool. Front. Med. 2021, 8, 615417. [Google Scholar] [CrossRef] [PubMed]
- Si, X.; Zheng, H.; Wei, G.; Li, M.; Li, W.; Wang, H.; Guo, H.; Sun, J.; Li, C.; Zhong, S.; et al. circRNA Hipk3 Induces Cardiac Regeneration after Myocardial Infarction in Mice by Binding to Notch1 and miR-133a. Mol. Ther. Nucleic Acids 2020, 21, 636–655. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, R.; Shen, C.; Liu, W.; Yuan, J.; Li, C.; Deng, W.; Wang, Z.; Zhang, W.; Ge, J.; et al. Exosomal CircHIPK3 Released from Hypoxia-Induced Cardiomyocytes Regulates Cardiac Angiogenesis after Myocardial Infarction. Oxidative Med. Cell. Longev. 2020, 2020, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Somoza, A.; Zhang, L.; Vausort, M.; Devaux, Y. The circular RNA MICRA for risk stratification after myocardial infarction. IJC Heart Vasc. 2017, 17, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Vausort, M.; Salgado-Somoza, A.; Zhang, L.; Leszek, P.; Scholz, M.; Teren, A.; Burkhardt, R.; Thiery, J.; Wagner, D.R.; Devaux, Y. Myocardial Infarction-Associated Circular RNA Predicting Left Ventricular Dysfunction. J. Am. Coll. Cardiol. 2016, 68, 1247–1248. [Google Scholar] [CrossRef]
- Geng, H.-H.; Li, R.; Su, Y.-M.; Xiao-Ping, J.; Pan, M.; Cai, X.-X.; Ji, X.-P. The Circular RNA Cdr1as Promotes Myocardial Infarction by Mediating the Regulation of miR-7a on Its Target Genes Expression. PLoS ONE 2016, 11, e0151753. [Google Scholar] [CrossRef]
- Mester-Tonczar, J.; Winkler, J.; Einzinger, P.; Hasimbegovic, E.; Kastner, N.; Lukovic, D.; Zlabinger, K.; Spannbauer, A.; Traxler, D.; Batkai, S.; et al. Association between Circular RNA CDR1as and Post-Infarction Cardiac Function in Pig Ischemic Heart Failure: Influence of the Anti-Fibrotic Natural Compounds Bufalin and Lycorine. Biomolecules 2020, 10, 1180. [Google Scholar] [CrossRef]
- Garikipati, V.N.S.; Verma, S.K.; Cheng, Z.; Liang, D.; Truongcao, M.M.; Cimini, M.; Yue, Y.; Huang, G.; Wang, C.; Benedict, C.; et al. Circular RNA CircFndc3b modulates cardiac repair after myocardial infarction via FUS/VEGF-A axis. Nat. Commun. 2019, 10, 4317. [Google Scholar] [CrossRef]
- Huang, S.; Li, X.; Zheng, H.; Si, X.; Li, B.; Wei, G.; Li, C.; Chen, Y.; Chen, Y.; Liao, W.; et al. Loss of Super-Enhancer-Regulated circRNA Nfix Induces Cardiac Regeneration After Myocardial Infarction in Adult Mice. Circulation 2019, 139, 2857–2876. [Google Scholar] [CrossRef]
- Cai, L.; Qi, B.; Wu, X.; Peng, S.; Zhou, G.; Wei, Y.; Xu, J.; Chen, S.; Liu, S. Circular RNA Ttc3 regulates cardiac function after myocardial infarction by sponging miR-15b. J. Mol. Cell. Cardiol. 2019, 130, 10–22. [Google Scholar] [CrossRef]
- Gao, W.-Q.; Hu, X.-M.; Zhang, Q.; Yang, L.; Lv, X.-Z.; Chen, S.; Wu, P.; Duan, D.-W.; Lang, Y.-H.; Ning, M.; et al. Downregulation of circFASTKD1 ameliorates myocardial infarction by promoting angiogenesis. Aging 2021, 13, 3588–3604. [Google Scholar] [CrossRef]
- Bian, Y.; Pang, P.; Li, X.; Yu, S.; Wang, X.; Liu, K.; Ju, J.; Wu, H.; Gao, Y.; Liu, Q.; et al. CircHelz activates NLRP3 inflammasome to promote myocardial injury by sponging miR-133a-3p in mouse ischemic heart. J. Mol. Cell. Cardiol. 2021, 158, 128–139. [Google Scholar] [CrossRef]
- Cai, X.; Li, B.; Wang, Y.; Zhu, H.; Zhang, P.; Jiang, P.; Yang, X.; Sun, J.; Hong, L.; Shao, L. CircJARID2 Regulates Hypoxia-Induced Injury in H9c2 Cells by Affecting miR-9-5p–Mediated BNIP3. J. Cardiovasc. Pharmacol. 2021, 78, e77–e85. [Google Scholar] [CrossRef]
- Chang, H.; Li, Z.-B.; Wu, J.-Y.; Zhang, L. Circ-100338 Induces Angiogenesis after Myocardial Ischemia-Reperfusion Injury by Sponging MiR-200a-3p. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6323–6332. [Google Scholar] [CrossRef]
- Chen, L.; Luo, W.; Zhang, W.; Chu, H.; Wang, J.; Dai, X.; Cheng, Y.; Zhu, T.; Chao, J. circDLPAG4/HECTD1 mediates ischaemia/reperfusion injury in endothelial cells via ER stress. RNA Biol. 2020, 17, 240–253. [Google Scholar] [CrossRef]
- Chen, S.; Sun, L.; Hao, M.; Liu, X. Circ-SWT1 Ameliorates H2O2-Induced Apoptosis, Oxidative Stress and Endoplasmic Reticulum Stress in Cardiomyocytes via miR-192-5p/SOD2 Axis. Cardiovasc. Toxicol. 2022, 22, 378–389. [Google Scholar] [CrossRef]
- Chen, T.-P.; Zhang, N.-J.; Wang, H.-J.; Hu, S.-G.; Geng, X. Knockdown of circROBO2 attenuates acute myocardial infarction through regulating the miR-1184/TRADD axis. Mol. Med. 2021, 27, 21. [Google Scholar] [CrossRef]
- Gan, J.; Yuan, J.; Liu, Y.; Lu, Z.; Xue, Y.; Shi, L.; Zeng, H. Circular RNA_101237 mediates anoxia/reoxygenation injury by targeting let-7a-5p/IGF2BP3 in cardiomyocytes. Int. J. Mol. Med. 2020, 45, 451–460. [Google Scholar] [CrossRef]
- Li, F.; Long, T.-Y.; Bi, S.-S.; Sheikh, S.A.; Zhang, C.-L. circPAN3 exerts a profibrotic role via sponging miR-221 through FoxO3/ATG7-activated autophagy in a rat model of myocardial infarction. Life Sci. 2020, 257, 118015. [Google Scholar] [CrossRef]
- Li, M.; Ding, W.; Tariq, M.A.; Chang, W.; Zhang, X.; Xu, W.; Hou, L.; Wang, Y.; Wang, J. A circular transcript of ncx1 gene mediates ischemic myocardial injury by targeting miR-133a-3p. Theranostics 2018, 8, 5855–5869. [Google Scholar] [CrossRef]
- Li, R.-L.; Fan, C.-H.; Gong, S.-Y.; Kang, S. Effect and Mechanism of LRP6 on Cardiac Myocyte Ferroptosis in Myocardial Infarction. Oxidative Med. Cell. Longev. 2021, 2021, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Li, B.; Jiang, L.; Liu, Z.; Wu, F.; Zhang, Y.; Liu, J.; Duan, W. circ_0023461 Silencing Protects Cardiomyocytes from Hypoxia-Induced Dysfunction through Targeting miR-370-3p/PDE4D Signaling. Oxidative Med. Cell. Longev. 2021, 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Pan, C.-L.; Jiang, G.-X.; Zhang, Y.-M.; Zhang, Z. CircHIPK3 aggravates myocardial ischemia-reperfusion injury by binding to miRNA-124-3p. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 10107–10114. [Google Scholar] [CrossRef]
- Song, Y.-F.; Zhao, L.; Wang, B.-C.; Sun, J.-J.; Hu, J.-L.; Zhu, X.-L.; Zhao, J.; Zheng, D.-K.; Ge, Z.-W. The circular RNA TLK1 exacerbates myocardial ischemia/reperfusion injury via targeting miR-214/RIPK1 through TNF signaling pathway. Free Radic. Biol. Med. 2020, 155, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Gan, T.-Y.; Li, N.; Liu, C.-Y.; Zhou, L.-Y.; Gao, J.-N.; Chen, C.; Yan, K.-W.; Ponnusamy, M.; Zhang, Y.-H.; et al. Circular RNA mediates cardiomyocyte death via miRNA-dependent upregulation of MTP18 expression. Cell Death Differ. 2017, 24, 1111–1120. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, L.; Deng, W.; Jiang, M. CircRNA MFACR Is Upregulated in Myocardial Infarction and Downregulates miR-125b to Promote Cardiomyocyte Apoptosis Induced by Hypoxia. J. Cardiovasc. Pharmacol. 2021, 78, 802–808. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Zhao, R.; Qiu, Z.; Shen, C.; Wang, Z.; Liu, W.; Zhang, W.; Ge, J.; Shi, B. CircUbe3a from M2 macrophage-derived small extracellular vesicles mediates myocardial fibrosis after acute myocardial infarction. Theranostics 2021, 11, 6315–6333. [Google Scholar] [CrossRef]
- Zhai, C.; Qian, G.; Wu, H.; Pan, H.; Xie, S.; Sun, Z.; Shao, P.; Tang, G.; Hu, H.; Zhang, S. Knockdown of circ_0060745 alleviates acute myocardial infarction by suppressing NF-κB activation. J. Cell. Mol. Med. 2020, 24, 12401–12410. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, Y.; Zhang, J.; Wang, J.; He, J.; Zhang, Z.; Liu, F. CircRNA ACAP2 Is Overexpressed in Myocardial Infarction and Promotes the Maturation of miR-532 to Induce the Apoptosis of Cardiomyocyte. J. Cardiovasc. Pharmacol. 2021, 78, 247–252. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Z.; Cheng, Q.; Wang, Z.; Lv, X.; Wang, Z.; Li, N. Circular RNA (CircRNA) CDYL Induces Myocardial Regeneration by CeRNA after Myocardial Infarction. Med. Sci. Monit. 2020, 26, e923188-1–e923188-8. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Wang, J.; Chen, H.; He, R.; Wu, H. CircTRRAP Knockdown Has Cardioprotective Function in Cardiomyocytes via the Signal Regulation of miR-370-3p/PAWR Axis. Cardiovasc. Ther. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Zhao, B.; Li, G.; Peng, J.; Ren, L.; Lei, L.; Ye, H.; Wang, Z.; Zhao, S. CircMACF1 Attenuates Acute Myocardial Infarction Through MiR-500b-5p-EMP1 Axis. J. Cardiovasc. Trans. Res. Cell 2020, 14, 161–172. [Google Scholar] [CrossRef]
- Zhou, L.-Y.; Zhai, M.; Huang, Y.; Xu, S.; An, T.; Wang, Y.-H.; Zhang, R.-C.; Liu, C.-Y.; Dong, Y.-H.; Wang, M.; et al. The circular RNA ACR attenuates myocardial ischemia/reperfusion injury by suppressing autophagy via modulation of the Pink1/ FAM65B pathway. Cell Death Differ. 2019, 26, 1299–1315. [Google Scholar] [CrossRef]
- Zhu, Y.; Zou, C.; Jia, Y.; Zhang, H.; Ma, X.; Zhang, J. Knockdown of circular RNA circMAT2B reduces oxygen-glucose deprivation-induced inflammatory injury in H9c2 cells through up-regulating miR-133. Cell Cycle 2020, 19, 2622–2630. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, W.; Yang, T.; Meng, X.; Jiang, Z.; Tao, L.; Wang, L. Upregulation of Circular RNA CircNFIB Attenuates Cardiac Fibrosis by Sponging miR-433. Front. Genet. 2019, 10, 564. [Google Scholar] [CrossRef]
- Nowbar, A.N.; Gitto, M.; Howard, J.P.; Francis, D.P.; Al-Lamee, R. Mortality from Ischemic Heart Disease: Analysis of Data from the World Health Organization and Coronary Artery Disease Risk Factors from NCD Risk Factor Collaboration. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005375. [Google Scholar] [CrossRef]
- Malakar, A.K.; Choudhury, D.; Halder, B.; Paul, P.; Uddin, A.; Chakraborty, S. A review on coronary artery disease, its risk factors, and therapeutics. J. Cell. Physiol. 2019, 234, 16812–16823. [Google Scholar] [CrossRef]
- Cao, Q.; Guo, Z.; Du, S.; Ling, H.; Song, C. Circular RNAs in the pathogenesis of atherosclerosis. Life Sci. 2020, 255, 117837. [Google Scholar] [CrossRef]
- Gong, X.; Tian, M.; Cao, N.; Yang, P.; Xu, Z.; Zheng, S.; Liao, Q.; Chen, C.; Zeng, C.; Jose, P.A.; et al. Circular RNA circEsyt2 regulates vascular smooth muscle cell remodeling via splicing regulation. J. Clin. Investig. 2021, 131, e147031. [Google Scholar] [CrossRef]
- Holdt, L.M.; Stahringer, A.; Sass, K.; Pichler, G.; Kulak, N.A.; Wilfert, W.; Kohlmaier, A.; Herbst, A.; Northoff, B.H.; Nicolaou, A.; et al. Circular non-coding RNA ANRIL modulates ribosomal RNA maturation and atherosclerosis in humans. Nat. Commun. 2016, 7, 12429. [Google Scholar] [CrossRef]
- Wang, L.; Shen, C.; Wang, Y.; Zou, T.; Zhu, H.; Lu, X.; Li, L.; Yang, B.; Chen, J.; Chen, S.; et al. Identification of circular RNA Hsa_circ_0001879 and Hsa_circ_0004104 as novel biomarkers for coronary artery disease. Atherosclerosis 2019, 286, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, X.; Gao, C.; Jian, D.; Hao, P.; Rao, L.; Li, M. Peripheral blood circular RNA hsa_circ_0124644 can be used as a diagnostic biomarker of coronary artery disease. Sci. Rep. 2017, 7, 39918. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Yin, R.-X.; Zhang, Q.-H.; Liao, P.-J.; Wang, Y.; Nie, R.-J.; Li, H. A novel circRNA-miRNA-mRNA network identifies circ-YOD1 as a biomarker for coronary artery disease. Sci. Rep. 2019, 9, 18314. [Google Scholar] [CrossRef] [PubMed]
- Vilades, D.; Martínez-Camblor, P.; Ferrero-Gregori, A.; Bär, C.; Lu, D.; Xiao, K.; Vea, À.; Nasarre, L.; Vega, J.S.; Leta, R.; et al. Plasma circular RNA hsa_circ_0001445 and coronary artery disease: Performance as a biomarker. FASEB J. 2020, 34, 4403–4414. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-P.; Pan, Y.-H.; Cai, M.-Y.; Cen, J.-M.; Chen, C.; Zheng, L.; Liu, X.; Xiong, X.-D. Plasma-Derived Exosomal Circular RNA hsa_circ_0005540 as a Novel Diagnostic Biomarker for Coronary Artery Disease. Dis. Markers 2020, 2020, 1–7. [Google Scholar] [CrossRef]
- DeRoo, E.; Stranz, A.; Yang, H.; Hsieh, M.; Se, C.; Zhou, T. Endothelial Dysfunction in the Pathogenesis of Abdominal Aortic Aneurysm. Biomolecules 2022, 12, 509. [Google Scholar] [CrossRef]
- Sampson, U.K.A.; Norman, P.E.; Fowkes, F.G.R.; Aboyans, V.; Song, Y.; Harrell, F.E.H., Jr.; Forouzanfar, M.H.; Naghavi, M.; Denenberg, J.O.; McDermott, M.M.; et al. Global and Regional Burden of Aortic Dissection and Aneurysms: Mortality Trends in 21 World Regions, 1990 to 2010. Glob. Heart 2014, 9, 171–180.e10. [Google Scholar] [CrossRef]
- Kessler, V.; Klopf, J.; Eilenberg, W.; Neumayer, C.; Brostjan, C. AAA Revisited: A Comprehensive Review of Risk Factors, Management, and Hallmarks of Pathogenesis. Biomedicines 2022, 10, 94. [Google Scholar] [CrossRef]
- Wang, Z.; You, Y.; Yin, Z.; Bao, Q.; Lei, S.; Yu, J.; Xie, C.; Ye, F.; Xie, X. Burden of Aortic Aneurysm and Its Attributable Risk Factors from 1990 to 2019: An Analysis of the Global Burden of Disease Study 2019. Front. Cardiovasc. Med. 2022, 9, 901225. [Google Scholar] [CrossRef]
- Bath, M.F.; Gokani, V.J.; Sidloff, D.A.; Jones, L.R.; Choke, E.; Sayers, R.D.; Bown, M.J. Systematic review of cardiovascular disease and cardiovascular death in patients with a small abdominal aortic aneurysm. Br. J. Surg. 2015, 102, 866–872. [Google Scholar] [CrossRef]
- Haller, S.J.; Azarbal, A.F.; Rugonyi, S. Predictors of Abdominal Aortic Aneurysm Risks. Bioengineering 2020, 7, 79. [Google Scholar] [CrossRef]
- Miyake, T.; Miyake, T.; Kurashiki, T.; Morishita, R. Molecular Pharmacological Approaches for Treating Abdominal Aortic Aneurysm. Ann. Vasc. Dis. 2019, 12, 137–146. [Google Scholar] [CrossRef]
- Zhou, M.; Shi, Z.; Cai, L.; Li, X.; Ding, Y.; Xie, T.; Fu, W. Circular RNA expression profile and its potential regulative role in human abdominal aortic aneurysm. BMC Cardiovasc. Disord. 2020, 20, 70. [Google Scholar] [CrossRef]
- Kuivaniemi, H.; Ryer, E.J.; Elmore, J.R.; Tromp, G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev. Cardiovasc. Ther. 2015, 13, 975–987. [Google Scholar] [CrossRef]
- Di Gregoli, K.; Anuar, N.N.M.; Bianco, R.; White, S.J.; Newby, A.C.; George, S.J.; Johnson, J.L. MicroRNA-181b Controls Atherosclerosis and Aneurysms Through Regulation of TIMP-3 and Elastin. Circ. Res. 2017, 120, 49–65. [Google Scholar] [CrossRef]
- Sun, X.; He, S.; Wara, A.K.M.; Icli, B.; Shvartz, E.; Tesmenitsky, Y.; Belkin, N.; Li, D.; Blackwell, T.S.; Sukhova, G.K.; et al. Systemic Delivery of MicroRNA-181b Inhibits Nuclear Factor-κB Activation, Vascular Inflammation, and Atherosclerosis in Apolipoprotein E–Deficient Mice. Circ. Res. 2014, 114, 32–40. [Google Scholar] [CrossRef]
- Si, K.; Lu, D.; Tian, J. Integrated analysis and the identification of a circRNA-miRNA-mRNA network in the progression of abdominal aortic aneurysm. PeerJ 2021, 9, e12682. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, H.; Bian, C.; Chen, C.; Tu, S.; Guo, J.; Wu, Y.; Böckler, D.; Zhang, J. Circular RNA Expression: Its Potential Regulation and Function in Abdominal Aortic Aneurysms. Oxidative Med. Cell. Longev. 2021, 2021, 1–21. [Google Scholar] [CrossRef]
- He, X.; Li, X.; Han, Y.; Chen, G.; Xu, T.; Cai, D.; Sun, Y.; Wang, S.; Lai, Y.; Teng, Z.; et al. CircRNA Chordc1 protects mice from abdominal aortic aneurysm by contributing to the phenotype and growth of vascular smooth muscle cells. Mol. Ther. Nucleic Acids 2022, 27, 81–98. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, Z.; Xiao, L.; Li, W.; Wang, Z.; Duan, Z.; Li, X. Identification of Circ-FNDC3B, an Overexpressed circRNA in Abdominal Aortic Aneurysm, as a Regulator of Vascular Smooth Muscle Cells. Int. Heart J. 2021, 62, 21–186. [Google Scholar] [CrossRef]
- Lv, P.; Yin, Y.-J.; Kong, P.; Cao, L.; Xi, H.; Wang, N.; Yang, H.-C.; Lv, Y.-H.; Chen, N.; Wang, R.; et al. SM22α Loss Contributes to Apoptosis of Vascular Smooth Muscle Cells via Macrophage-Derived circRasGEF1B. Oxidative Med. Cell. Longev. 2021, 2021, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Yang, X.; Han, F.; Wang, H. Circ_0092291 attenuates angiotensin II–induced cell damages in human aortic vascular smooth muscle cells via mediating the miR-626/COL4A1 signal axis. J. Physiol. Biochem. 2022, 78, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yang, Y.; Sun, Y.; Wei, G.; Zheng, H.; Chen, Y.; Cai, D.; Li, C.; Ma, Y.; Lin, Z.; et al. Circular RNA Cdyl promotes abdominal aortic aneurysm formation by inducing M1 macrophage polarization and M1-type inflammation. Mol. Ther. 2022, 30, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yuan, Q.; Zhao, W.; Zhou, W. Circular RNA RBM33 contributes to extracellular matrix degradation via miR-4268/EPHB2 axis in abdominal aortic aneurysm. PeerJ 2021, 9, e12232. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, Z.; Meng, G.; Hua, L. Circular RNA CCDC66 facilitates abdominal aortic aneurysm through the overexpression of CCDC66. Cell Biochem. Funct. 2020, 38, 830–838. [Google Scholar] [CrossRef]
- Yue, J.; Zhu, T.; Yang, J.; Si, Y.; Xu, X.; Fang, Y.; Fu, W. CircCBFB-mediated miR-28-5p facilitates abdominal aortic aneurysm via LYPD3 and GRIA4. Life Sci. 2020, 253, 117533. [Google Scholar] [CrossRef]
- Ma, X.; Xu, J.; Lu, Q.; Feng, X.; Liu, J.; Cui, C.; Song, C. Hsa_circ_0087352 promotes the inflammatory response of macrophages in abdominal aortic aneurysm by adsorbing hsa-miR-149-5p. Int. Immunopharmacol. 2022, 107, 108691. [Google Scholar] [CrossRef]
- Zhao, F.; Chen, T.; Jiang, N. CDR1as/miR-7/CKAP4 axis contributes to the pathogenesis of abdominal aortic aneurysm by regulating the proliferation and apoptosis of primary vascular smooth muscle cells. Exp. Ther. Med. 2020, 19, 3760–3766. [Google Scholar] [CrossRef]
- Zheng, C.; Niu, H.; Li, M.; Zhang, H.; Yang, Z.; Tian, L.; Wu, Z.; Li, D.; Chen, X. Cyclic RNA has-circ-000595 regulates apoptosis of aortic smooth muscle cells. Mol. Med. Rep. 2015, 12, 6656–6662. [Google Scholar] [CrossRef]
- Correia, C.C.M.; Rodrigues, L.F.; Pelozin, B.R.D.A.; Oliveira, E.M.; Fernandes, T. Long Non-Coding RNAs in Cardiovascular Diseases: Potential Function as Biomarkers and Therapeutic Targets of Exercise Training. Non-Coding RNA 2021, 7, 65. [Google Scholar] [CrossRef]
- Wang, L.; Lv, Y.; Li, G.; Xiao, J. MicroRNAs in heart and circulation during physical exercise. J. Sport Health Sci. 2018, 7, 433–441. [Google Scholar] [CrossRef]
- Guo, M.; Qiu, J.; Shen, F.; Wang, S.; Yu, J.; Zuo, H.; Yao, J.; Xu, S.; Hu, T.; Wang, D.; et al. Comprehensive analysis of circular RNA profiles in skeletal muscles of aging mice and after aerobic exercise intervention. Aging 2020, 12, 5071–5090. [Google Scholar] [CrossRef]
- Niu, Y.; Wan, C.; Zhang, J.; Zhang, S.; Zhao, Z.; Zhu, L.; Wang, X.; Ren, X.; Wang, J.; Lei, P. Aerobic exercise improves VCI through circRIMS2/miR-186/BDNF-mediated neuronal apoptosis. Mol. Med. 2021, 27, 4. [Google Scholar] [CrossRef]
- Meinecke, A.; Mitzka, S.; Just, A.; Cushman, S.; Stojanović, S.D.; Xiao, K.; Mooren, F.C.; Fiedler, J.; Thum, T. Cardiac endurance training alters plasma profiles of circular RNA MBOAT2. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H13–H21. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, C.; Zhang, R.; Yan, J.; Li, M.; Ma, S.; Chen, K.; Chen, L.; Liu, J.; Xiu, J.; et al. Circ-Ddx60 contributes to the antihypertrophic memory of exercise hypertrophic preconditioning. J. Adv. Res. 2022, in press. [Google Scholar] [CrossRef]
- Lin, H.; Zhu, Y.; Zheng, C.; Hu, D.; Ma, S.; Chen, L.; Wang, Q.; Chen, Z.; Xie, J.; Yan, Y.; et al. Antihypertrophic Memory After Regression of Exercise-Induced Physiological Myocardial Hypertrophy Is Mediated by the Long Noncoding RNA Mhrt779. Circulation 2021, 143, 2277–2292. [Google Scholar] [CrossRef]
- Yu, Z.; Huang, Q.; Zhang, Q.; Wu, H.; Zhong, Z. CircRNAs open a new era in the study of cardiovascular disease (Review). Int. J. Mol. Med. 2021, 47, 49–64. [Google Scholar] [CrossRef]
| Id | Host Gene | Species | Source | Expression | Action | Ref. |
|---|---|---|---|---|---|---|
| hsa_circ_0037909 | GSPT1 | Human | Blood, HAECs, HUVECs | Up | Sponges hsa-miR-637, possibly impacting in LDL and serum creatinine concentrations. | [29] |
| hsa_circ_0037911 | GSPT1 | Human | Blood | Up | Biomarker. | [30] |
| rno_circ_006016 | Erc2 | Rat | Kidney | Down | Controls blood pressure by multiple circRNA–miRNA–gene interactions. | [31] |
| hsa_circ_0105015 | GSPT1 | Human | Blood Endothelial cells | Up | Promotes target hsa-miR-637 to activate the inflammatory pathway. | [32] |
| hsa_circ_0126991 | SEPT11 | Human | Blood | Up | Biomarker. | [33] |
| rno_circ_0009197 | Cdh23 | Rat | Aortic vascular tissues | Down | Aortic circRNAs play potential roles in regulating hypertensive vascular remodeling and dysfunction. | [24] |
| rno_circ_0005818 | Dnajc1 | Up | ||||
| rno_circ_0005304 | - | Up | ||||
| rno_circ_0005506 | Ryr2 | Up | ||||
| rno_circ_0009301 | - | Up | ||||
| circACTA2 | ACTA2 | Human | Artery tissues | Up | Promotes vascular smooth muscle cell senescence by targeting circACTA2/ILF3/CDK4 axis. | [25] |
| Human | HASMCs | Up | Regulates α-SMA expression. | [28] | ||
| hsa_circ_0037897 | GSPT1 | Human | Blood | Up | May be involved in hypertension by sponging hsa-miR-145-5p. | [34] |
| hsa_circ_0014243 | CHTOP | Human | Blood | Up | Plays a crucial role in genesis and development of hypertension. Could be used as biomarker. | [35] |
| has_circ_0005870 | SETD2 | Human | Plasma | Down | Biomarker. | [36] |
| Id | Host Gene | Species | Source | Expression | Action | Ref. |
|---|---|---|---|---|---|---|
| circChordc1 | Chordc1 | Mouse | Abdominal aorta | Down | Improves VSMCs growth, suppressing aneurysm formation and reducing the risk of rupture by inducing vimentin degradation. | [100] |
| circ-FNDC3B | FNDC3B | Human | Aortic tissue | Up | Promotes VSMCs inflammation and oxidative stress through miR-143-3p/ADAM10 axis, leading to the development of AAA. | [101] |
| circRasGEF1B | Rasgef1b | Mouse | Abdominal aorta | Up | circRasGEF1B-ZFP36 axis mediates macrophage-induced VSMC apoptosis in an Sm22α-/- mice AAA model. | [102] |
| circ_0092291 | EIF2S2 | Human | Blood HAVSMC | Down | Inhibits AAA-associated cell damage via circ_0092291/miR-626/COL4A1 axis. | [103] |
| circCdyl | Cdyl | Mouse | Abdominal aorta | Up | Promotes vascular inflammation through M1-type macrophages polarization, inducing AAA formation. | [104] |
| circRBM33 | RBM33 | Human | Abdominal aorta | Up | Promotes ECM degradation in aorta-isolated VSMCs via the miR-4268/EPHB2 axis. | [105] |
| circCCDC66 | CCDC6 6 | Human | VSMCs | Up | Upregulates its host gene, modulating VSMCs proliferation and apoptosis and facilitating AAA development. | [106] |
| circCBFB | CBFB | Human | HAVSMC | - | Its suppression increased the expression of miR-28-5p, promoting apoptosis of VSMCs. | [107] |
| hsa_circ_0087352 | UBQLN1 | Human | THP-1 cells HAVSMC | Up | Promotes inflammatory response in macrophages, enhancing the expression and secretion of IL-6 and TNF-α by sponging hsa-miR-149-5p. | [108] |
| CircCDR1as | CDR1 | Human | Aortic tissues VSMCs | Down | Regulates VSMC apoptosis and proliferation via circCDR1as/miR-7/CKAP4 axis. | [109] |
| hsa_circ_000595 | - | Human | Aortic tissues HAVSMC | Up | Its knockdown decreased apoptosis in HAVSMC. | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joaquim, V.H.A.; Pereira, N.P.; Fernandes, T.; Oliveira, E.M. Circular RNAs as a Diagnostic and Therapeutic Target in Cardiovascular Diseases. Int. J. Mol. Sci. 2023, 24, 2125. https://doi.org/10.3390/ijms24032125
Joaquim VHA, Pereira NP, Fernandes T, Oliveira EM. Circular RNAs as a Diagnostic and Therapeutic Target in Cardiovascular Diseases. International Journal of Molecular Sciences. 2023; 24(3):2125. https://doi.org/10.3390/ijms24032125
Chicago/Turabian StyleJoaquim, Victor Hugo Antonio, Noemy Pinto Pereira, Tiago Fernandes, and Edilamar Menezes Oliveira. 2023. "Circular RNAs as a Diagnostic and Therapeutic Target in Cardiovascular Diseases" International Journal of Molecular Sciences 24, no. 3: 2125. https://doi.org/10.3390/ijms24032125
APA StyleJoaquim, V. H. A., Pereira, N. P., Fernandes, T., & Oliveira, E. M. (2023). Circular RNAs as a Diagnostic and Therapeutic Target in Cardiovascular Diseases. International Journal of Molecular Sciences, 24(3), 2125. https://doi.org/10.3390/ijms24032125







