Appressoria—Small but Incredibly Powerful Structures in Plant–Pathogen Interactions
Abstract
1. Introduction
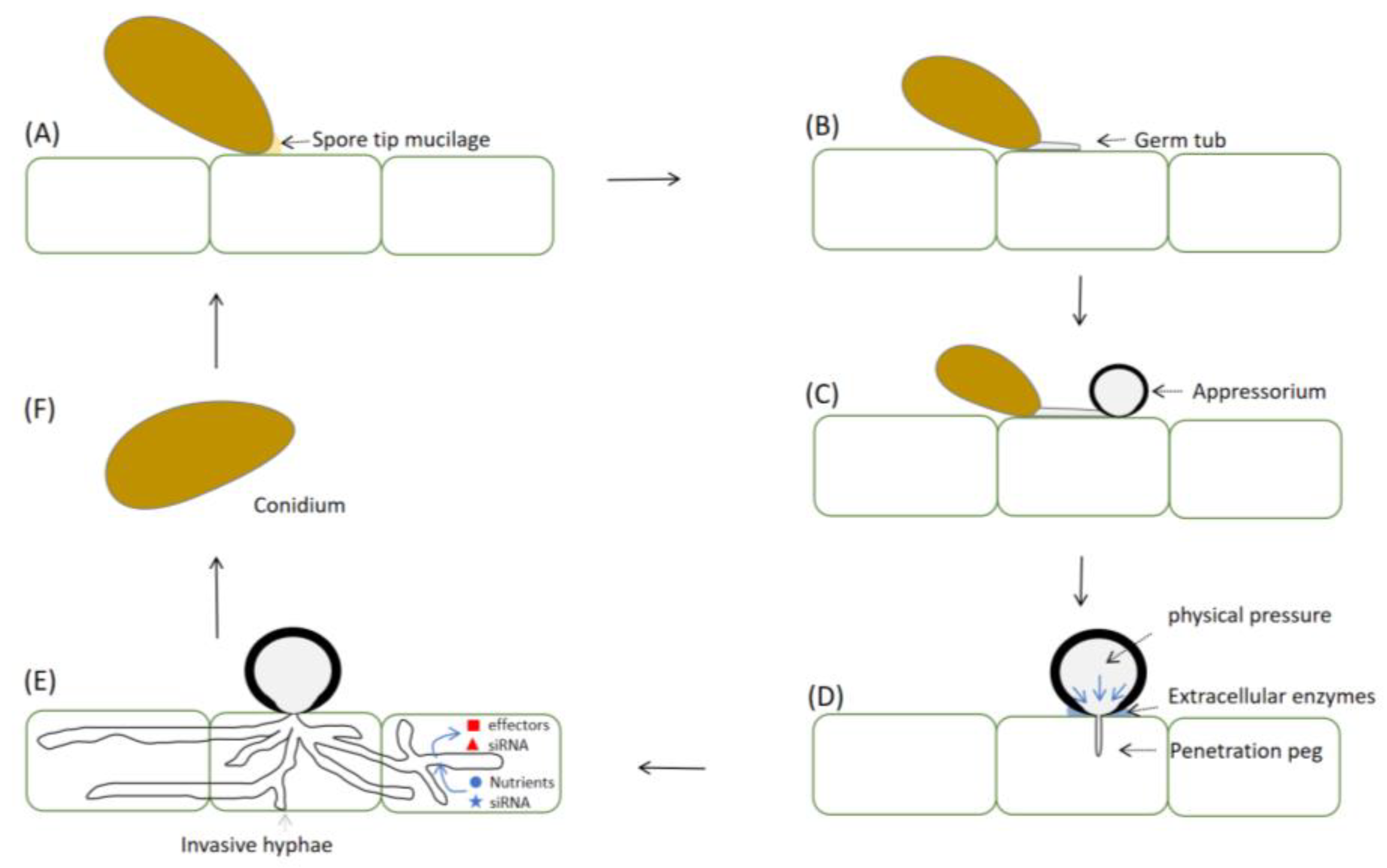
2. Appressoria Formation
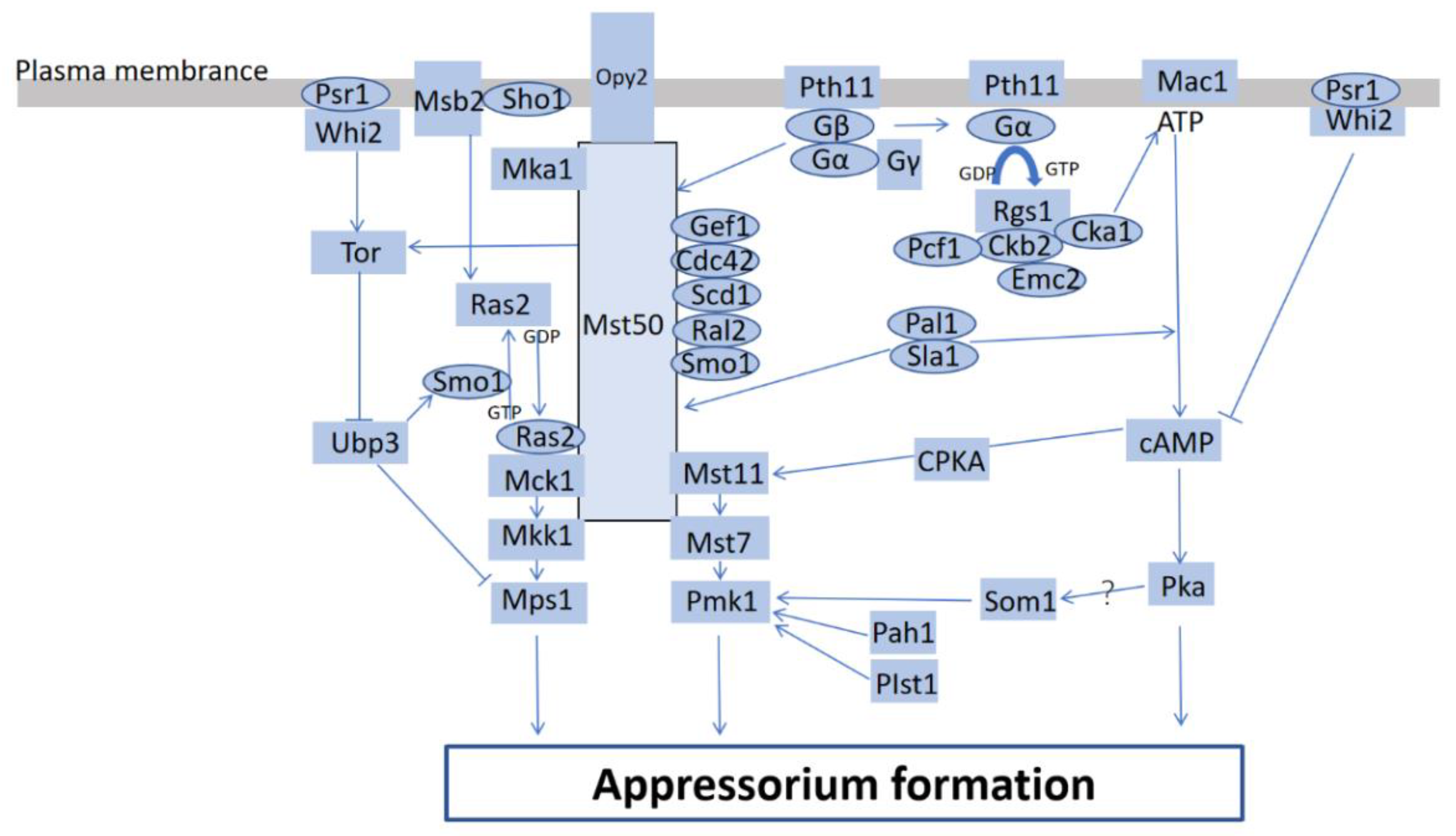
2.1. Magnaporthe oryzae
2.2. Colletotrichum
2.3. Other Fungi
3. Turgor Generation and Penetration
4. Factors Limiting Appressorium Formation and Their Potential Applications against Fungal Diseases of Plants
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A.; Heitman, J.; Howlett, B.J. Plant Pathogenic Fungi. Microbiol. Spectr. 2017, 5, 5.1.14. [Google Scholar] [CrossRef] [PubMed]
- Hamer, J.E.; Howard, R.J.; Chumley, F.G.; Valent, B. A mechanism for surface attachment in spores of a plant pathogenic fungus. Science 1988, 239, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Göhre, V.; Robatzek, S. Breaking the barriers: Microbial effector molecules subvert plant immunity. Annu. Rev. Phytopathol. 2008, 46, 189–215. [Google Scholar] [CrossRef] [PubMed]
- Talbot, N.J. On the trail of a cereal killer: Exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 2003, 57, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Ebbole, D.J. Magnaporthe as a model for understanding host-pathogen interactions. Annu. Rev. Phytopathol. 2007, 45, 437–456. [Google Scholar] [CrossRef] [PubMed]
- Emmett, R.W.; Parbery, D.G. Appressoria. Annu. Rev. Phytopathol. 1975, 13, 147–165. [Google Scholar] [CrossRef]
- Talbot, N.J. Appressoria. Curr. Biol. 2019, 29, R144–R146. [Google Scholar] [CrossRef]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-Microbe Interactions: Shaping the Evolution of the Plant Immune Response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef]
- Li, L.; Zhu, X.M.; Zhang, Y.R.; Cai, Y.Y.; Wang, J.Y.; Liu, M.Y.; Wang, J.Y.; Bao, J.D.; Lin, F.C. Research on the Molecular Interaction Mechanism between Plants and Pathogenic Fungi. Int. J. Mol. Sci. 2022, 23, 4658. [Google Scholar] [CrossRef]
- Shen, Y.; Liu, N.; Li, C.; Wang, X.; Xu, X.; Chen, W.; Xing, G.; Zheng, W. The early response during the interaction of fungal phytopathogen and host plant. Open Biol. 2017, 7, 170057. [Google Scholar] [CrossRef]
- Yang, W.; Li, S.P.; Cui, H.T.; Zou, S.H.; Wang, W. Molecular genetic mechanisms of interaction between host plants and pathogens. Hereditas 2020, 42, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Torre de la, A.; Castanheira, S.; Pérez-Martín, J. Incompatibility between proliferation and plant invasion is mediated by a regulator of appressorium formation in the corn smut fungus Ustilago maydis. Proc. Natl. Acad. Sci. USA 2020, 117, 30599–30609. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Suzuki, T.; Ikeda, K.; Jiang, S.; Hosogi, N.; Hyong, G.-S.; Hida, S.; Yamada, T.; Park, P. Extracellular matrix of Magnaporthe oryzae may have a role in host adhesion during fungal penetration and is digested by matrix metalloproteinases. J. Gen. Plant Pathol. 2007, 73, 388–398. [Google Scholar] [CrossRef]
- Deising, H.B.; Werner, S.; Wernitz, M. The role of fungal appressoria in plant infection. Microbes Infect. 2000, 2, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Liu, M.; Wang, Y.; Zou, Y.; You, Y.; Yang, L.; Hu, J.; Zhang, H.; Zheng, X.; et al. Magnaporthe oryzae auxiliary activity protein MoAa91 functions as chitin-binding protein to induce appressorium formation on artificial inductive surfaces and suppress plant immunity. mBio 2020, 11, e03304-19. [Google Scholar] [CrossRef]
- Yu, R.; Shen, X.; Liu, M.; Liu, X.; Yin, Z.; Li, X.; Feng, W.; Hu, J.; Zhang, H.; Zheng, X.; et al. The rice blast fungus MoRgs1 functioning in cAMP signaling and pathogenicity is regulated by casein kinase MoCk2 phosphorylation and modulated by membrane protein MoEmc2. PLoS Pathog. 2021, 17, e1009657. [Google Scholar] [CrossRef]
- Chen, D.; Hu, H.; He, W.; Zhang, S.; Tang, M.; Xiang, S.; Liu, C.; Cai, X.; Hendy, A.; Kamran, M.; et al. Endocytic protein Pal1 regulates appressorium formation and is required for full virulence of Magnaporthe oryzae. Mol. Plant Pathol. 2022, 23, 133–147. [Google Scholar] [CrossRef]
- Qu, Y.; Cao, H.; Huang, P.; Wang, J.; Liu, X.; Lu, J.; Lin, F.C. A kelch domain cell end protein, PoTea1, mediates cell polarization during appressorium morphogenesis in Pyricularia oryzae. Microbiol. Res. 2022, 259, 126999. [Google Scholar] [CrossRef]
- Yan, Y.; Tang, J.; Yuan, Q.; Liu, H.; Huang, J.; Hsiang, T.; Bao, C.; Zheng, L. Ornithine decarboxylase of the fungal pathogen Colletotrichum higginsianum plays an important role in regulating global metabolic pathways and virulence. Environ. Microbiol. 2022, 24, 1093–1116. [Google Scholar] [CrossRef]
- Fu, T.; Shin, J.H.; Lee, N.H.; Lee, K.H.; Kim, K.S. Mitogen-Activated Protein Kinase CsPMK1 Is Essential for Pepper Fruit Anthracnose by Colletotrichum scovillei. Front. Microbiol. 2022, 13, 770119. [Google Scholar] [CrossRef]
- Kojima, K.; Kikuchi, T.; Takano, Y.; Oshiro, E.; Okuno, T. The mitogen-activated protein kinase gene MAF1 is essential for the early differentiation phase of appressorium formation in Colletotrichum lagenarium. Mol. Plant-Microbe Interact. 2002, 15, 1268–1276. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, D.; Tian, C. Mitogen-activated protein kinase cascade CgSte50-Ste11-Ste7-Mk1 regulates infection-related morphogenesis in the poplar anthracnose fungus Colletotrichum gloeosporioides. Microbiol. Res. 2021, 248, 126748. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Sun, P.; Sun, Q.; Li, R.; Qin, Z.; Sha, G.; Zhou, Y.; Bi, R.; Zhang, H.; Zheng, L.; et al. The MoPah1 phosphatidate phosphatase is involved in lipid metabolism, development, and pathogenesis in Magnaporthe oryzae. Mol. Plant Pathol. 2022, 23, 720–732. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, T.; Lv, Z.; Qin, M.; Qu, Z.; Zhang, Z.; Li, F.; Chen, D.; Zhang, X.; Chen, X.L.; et al. A Calcineurin Regulator MoRCN1 Is Important for Asexual Development, Stress Response, and Plant Infection of Magnaporthe oryzae. Front. Plant Sci. 2022, 13, 925645. [Google Scholar] [CrossRef]
- Fukada, F.; Kodama, S.; Nishiuchi, T.; Kajikawa, N.; Kubo, Y. Plant pathogenic fungi Colletotrichum and Magnaporthe share a common G(1) phase monitoring strategy for proper appressorium development. New Phytol. 2019, 222, 1909–1923. [Google Scholar] [CrossRef]
- Osés-Ruiz, M.; Sakulkoo, W.; Littlejohn, G.R.; Martin-Urdiroz, M.; Talbot, N.J. Two independent S-phase checkpoints regulate appressorium-mediated plant infection by the rice blast fungus Magnaporthe oryzae. Proc. Natl. Acad. Sci. USA 2017, 114, E237–E244. [Google Scholar] [CrossRef]
- Saunders, D.G.; Dagdas, Y.F.; Talbot, N.J. Spatial uncoupling of mitosis and cytokinesis during appressorium-mediated plant infection by the rice blast fungus Magnaporthe oryzae. Plant Cell 2010, 22, 2417–2428. [Google Scholar] [CrossRef]
- Castanheira, S.; Pérez-Martín, J. Appressorium formation in the corn smut fungus Ustilago maydis requires a G2 cell cycle arrest. Plant Signal. Behav. 2015, 10, e1001227. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Chen, X.L. The Redox Proteome of Thiol Proteins in the Rice Blast Fungus Magnaporthe oryzae. Front. Microbiol. 2021, 12, 648894. [Google Scholar] [CrossRef]
- Dean, R.; Van Kan, J.A.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Ryder, L.S.; Cruz-Mireles, N.; Molinari, C.; Eisermann, I.; Eseola, A.B.; Talbot, N.J. The appressorium at a glance. J. Cell Sci. 2022, 135. [Google Scholar] [CrossRef]
- Deng, S.; Xu, L.; Xu, Z.; Lv, W.; Chen, Z.; Yang, N.; Talbot, N.J.; Wang, Z. A putative PKA phosphorylation site S227 in MoSom1 is essential for infection-related morphogenesis and pathogenicity in Magnaporthe oryzae. Cell. Microbiol. 2021, 23, e13370. [Google Scholar] [CrossRef] [PubMed]
- Lambou, K.; Malagnac, F.; Barbisan, C.; Tharreau, D.; Lebrun, M.H.; Silar, P. The crucial role of the Pls1 tetraspanin during ascospore germination in Podospora anserina provides an example of the convergent evolution of morphogenetic processes in fungal plant pathogens and saprobes. Eukaryot. Cell 2008, 7, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Demoor, A.; Silar, P.; Brun, S. Appressorium: The Breakthrough in Dikarya. J. Fungi 2019, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Galhano, R.; Illana, A.; Ryder, L.S.; Rodríguez-Romero, J.; Demuez, M.; Badaruddin, M.; Martinez-Rocha, A.L.; Soanes, D.M.; Studholme, D.J.; Talbot, N.J.; et al. Tpc1 is an important Zn(II)2Cys6 transcriptional regulator required for polarized growth and virulence in the rice blast fungus. PLoS Pathog. 2017, 13, e1006516. [Google Scholar] [CrossRef]
- Cai, Y.Y.; Wang, J.Y.; Wu, X.Y.; Liang, S.; Zhu, X.M.; Li, L.; Lu, J.P.; Liu, X.H.; Lin, F.C. MoOpy2 is essential for fungal development, pathogenicity, and autophagy in Magnaporthe oryzae. Environ. Microbiol. 2022, 24, 1653–1671. [Google Scholar] [CrossRef]
- Lv, W.Y.; Xiao, Y.; Xu, Z.; Jiang, H.; Tong, Q.; Wang, Z.Y. The Paxillin MoPax1 Activates Mitogen-Activated Protein (MAP) Kinase Signaling Pathways and Autophagy through MAP Kinase Activator MoMka1 during Appressorium-Mediated Plant Infection by the Rice Blast Fungus Magnaporthe oryzae. mBio. 2022, 13, e0221822. [Google Scholar] [CrossRef]
- Qu, Y.; Wang, J.; Huang, P.; Liu, X.; Lu, J.; Lin, F.C. PoRal2 Is Involved in Appressorium Formation and Virulence via Pmk1 MAPK Pathways in the Rice Blast Fungus Pyricularia oryzae. Front. Plant Sci. 2021, 12, 702368. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Malmberg, R.L.; Momany, M. Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol. Biol. 2007, 7, 103. [Google Scholar] [CrossRef]
- Dulal, N.; Rogers, A.; Wang, Y.; Egan, M. Dynamic assembly of a higher-order septin structure during appressorium morphogenesis by the rice blast fungus. Fungal Genet. Biol. 2020, 140, 103385. [Google Scholar] [CrossRef]
- Dulal, N.; Rogers, A.M.; Proko, R.; Bieger, B.D.; Liyanage, R.; Krishnamurthi, V.R.; Wang, Y.; Egan, M.J. Turgor-dependent and coronin-mediated F-actin dynamics drive septin disc-to-ring remodeling in the blast fungus Magnaporthe oryzae. J. Cell Sci. 2021, 134, jcs251298. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, M.J.; Basiewicz, M.; Soanes, D.M.; Yan, X.; Ryder, L.S.; Csukai, M.; Oses-Ruiz, M.; Valent, B.; Talbot, N.J. Conidial Morphogenesis and Septin-Mediated Plant Infection Require Smo1, a Ras GTPase-Activating Protein in Magnaporthe oryzae. Genetics 2019, 211, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.C.; Zhang, W.W.; Zhang, S.L.; Yang, G.G.; Tan, L.Y.; Guo, M. Class I myosin mediated endocytosis and polarization growth is essential for pathogenicity of Magnaporthe oryzae. Appl. Microbiol. Biotechnol. 2021, 105, 7395–7410. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Meng, S.; Qiu, J.; Wang, C.; Shu, Y.; Luo, C.; Kou, Y. MoWhi2 regulates appressorium formation and pathogenicity via the MoTor signalling pathway in Magnaporthe oryzae. Mol. Plant Pathol. 2021, 22, 969–983. [Google Scholar] [CrossRef]
- Huang, P.; Li, Y.; Wang, J.; Wang, Q.; Huang, Z.; Liu, X.; Lin, F.; Lu, J. Casein Kinase 2 Mediates Degradation of Transcription Factor Pcf1 during Appressorium Formation in the Rice Blast Fungus. J. Fungi 2022, 8, 144. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Xiang, S.; He, W.; Tang, M.; Zhang, S.; Chen, D.; Zhang, X.; Liu, C.; Li, G.; Xing, J.; et al. Deubiquitinase Ubp3 regulates ribophagy and deubiquitinates Smo1 for appressorium-mediated infection by Magnaporthe oryzae. Mol. Plant Pathol. 2022, 23, 832–844. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.; Xu, Z.; Wang, C.; Lv, W.; Yue, X.; Xu, L.; Tang, S.; Dai, H.; Wang, Z. The putative deubiquitinating enzyme MoUbp4 is required for infection-related morphogenesis and pathogenicity in the rice blast fungus Magnaporthe oryzae. Curr. Genet. 2020, 66, 561–576. [Google Scholar] [CrossRef]
- Yang, J.; Chen, D.J.; Matar, K.A.O.; Zheng, T.H.; Zhao, Q.Q.; Xie, Y.M.; Gao, X.Q.; Li, M.Y.; Wang, B.H.; Lu, G.D. The deubiquitinating enzyme MoUbp8 is required for infection-related development, pathogenicity, and carbon catabolite repression in Magnaporthe oryzae. Appl. Microbiol. Blot. 2020, 104, 5081–5094. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Yin, Z.; You, Y.; Zou, Y.; Liu, M.; He, Y.; Zhang, H.; Zheng, X.; Zhang, Z.; et al. MicroRNA-like milR236, regulated by transcription factor MoMsn2, targets histone acetyltransferase MoHat1 to play a role in appressorium formation and virulence of the rice blast fungus Magnaporthe oryzae. Fungal Genet. Biol. 2020, 137, 103349. [Google Scholar] [CrossRef]
- Li, C.; Cao, S.; Zhang, C.; Zhang, Y.; Zhang, Q.; Xu, J.-R.; Wang, C. MoCDC14 is important for septation during conidiation and appressorium formation in Magnaporthe oryzae. Mol. Plant Pathol. 2018, 19, 328–340. [Google Scholar] [CrossRef]
- Reza, M.H.; Sanyal, K. Defective in utilizing glutathione 3, DUG3, is required for conidiation and host infection in the rice blast fungus Magnaporthe oryzae. MicroPubl. Biol, 2022; online. [Google Scholar] [CrossRef]
- Liu, Y.; Pagac, M.; Yang, F.; Patkar, R.N.; Naqvi, N.I. Fungal Jasmonate as a Novel Morphogenetic Signal for Pathogenesis. J. Fungi 2021, 7, 693. [Google Scholar] [CrossRef] [PubMed]
- Falter, C.; Reumann, S. The essential role of fungal peroxisomes in plant infection. Mol. Plant Pathol 2022, 23, 781–794. [Google Scholar] [CrossRef] [PubMed]
- Sangappillai, V.; Nadarajah, K. Fatty acid synthase beta dehydratase in the lipid biosynthesis pathway is required for conidiogenesis, pigmentation and appressorium formation in Magnaporthe oryzae S6. Int. J. Mol. Sci. 2020, 21, 7224. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Zhang, Z.; Wang, Y.; Liu, M.; Jiang, H.; Chai, R.; Mao, X.; Qiu, H.; Liu, F.; et al. MoPex19, which is essential for maintenance of peroxisomal structure and woronin bodies, is required for metabolism and development in the rice blast fungus. PLoS ONE 2014, 9, e85252. [Google Scholar] [CrossRef]
- Kuroki, M.; Shiga, Y.; Narukawa-Nara, M.; Arazoe, T.; Kamakura, T. Extremely Low Concentrations of Acetic Acid Stimulate Cell Differentiation in Rice Blast Fungus. iScience 2020, 23, 100786. [Google Scholar] [CrossRef]
- Lin, L.; Cao, J.; Du, A.; An, Q.; Chen, X.; Yuan, S.; Batool, W.; Shabbir, A.; Zhang, D.; Wang, Z.; et al. eIF3k Domain-Containing Protein Regulates Conidiogenesis, Appressorium Turgor, Virulence, Stress Tolerance, and Physiological and Pathogenic Development of Magnaporthe oryzae Oryzae. Front. Plant Sci. 2021, 12, 748120. [Google Scholar] [CrossRef]
- Aron, O.; Wang, M.; Lin, L.; Batool, W.; Lin, B.; Shabbir, A.; Wang, Z.; Tang, W. MoGLN2 is important for vegetative growth, conidiogenesis, maintenance of cell wall integrity and pathogenesis of Magnaporthe oryzae. J. Fungi 2021, 7, 463. [Google Scholar] [CrossRef]
- Zhang, P.; Fang, Z.; Song, Y.; Wang, S.; Bao, L.; Liu, M.; Dang, Y.; Wei, Y.; Zhang, S.H. Aspartate Transaminase AST2 Involved in Sporulation and Necrotrophic Pathogenesis in the Hemibiotrophs Magnaporthe oryzae and Colletotrichum graminicola. Front. Microbiol. 2022, 13, 864866. [Google Scholar] [CrossRef]
- Aron, O.; Wang, M.; Mabeche, A.W.; Wajjiha, B.; Li, M.; Yang, S.; You, H.; Cai, Y.; Zhang, T.; Li, Y.; et al. MoCpa1-mediated arginine biosynthesis is crucial for fungal growth, conidiation, and plant infection of Magnaporthe oryzae. Appl. Microbiol. Biotechnol. 2021, 105, 5915–5929. [Google Scholar] [CrossRef]
- Zhou, W.; Shi, W.; Xu, X.-W.; Li, Z.-G.; Yin, C.-F.; Peng, J.-B.; Pan, S.; Chen, X.-L.; Zhao, W.-S.; Zhang, Y.; et al. Glutamate synthase MoGlt1-mediated glutamate homeostasis is important for autophagy, virulence and conidiation in the rice blast fungus. Mol. Plant Pathol. 2018, 19, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.; Sanyal, K. Loss of nucleosome assembly protein 1 affects growth and appressorium structure in blast fungus Magnaporthe oryzae. MicroPubl. Biol. 2022; online. [Google Scholar] [CrossRef]
- Gong, Z.; Ning, N.; Li, Z.; Xie, X.; Wilson, R.A.; Liu, W. Two Magnaporthe appressoria-specific (MAS) proteins, MoMas3 and MoMas5, are required for suppressing host innate immunity and promoting biotrophic growth in rice cells. Mol. Plant Pathol. 2022, 23, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.M.; Li, L.; Cai, Y.Y.; Wu, X.Y.; Shi, H.B.; Liang, S.; Qu, Y.M.; Naqvi, N.I.; Del Poeta, M.; Dong, B.; et al. A VASt-domain protein regulates autophagy, membrane tension, and sterol homeostasis in rice blast fungus. Autophagy 2021, 17, 2939–2961. [Google Scholar] [CrossRef]
- Sun, L.; Qian, H.; Wu, M.; Zhao, W.; Liu, M.; Wei, Y.; Zhu, X.; Li, L.; Lu, J.; Lin, F.; et al. A Subunit of ESCRT-III, MoIst1, Is Involved in Fungal Development, Pathogenicity, and Autophagy in Magnaporthe oryzae. Front. Plant Sci. 2022, 13, 845139. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Wei, Y.; Zhang, P.; Liu, X.; Li, X.; Wang, S.; Liang, H.; Zhang, S.H. The Bicarbonate Transporter (MoAE4) Localized on Both Cytomembrane and Tonoplast Promotes Pathogenesis in Magnaporthe oryzae. J. Fungi 2021, 7, 955. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, H.; Wei, Y.; Zhang, P.; Dang, Y.; Li, G.; Zhang, S.H. Alternative Splicing of MoPTEN Is Important for Growth and Pathogenesis in Magnaporthe oryzae. Front. Microbiol. 2021, 12, 715773. [Google Scholar] [CrossRef]
- Ohara, A.; Tashika, Y.; Abe, A.; Sone, T. The DNA damage signal transducer ortholog Mop53BP1 is required for proper appressorium differentiation and pathogenicity in Pyricularia oryzae. J. Gen. Plant Pathol. 2018, 84, 176–188. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Li, C.; Zeng, Y.; Tan, X.; Zhang, D.; Liu, Y. MoPer1 is required for growth, conidiogenesis, and pathogenicity in Magnaporthe oryzae. Rice 2018, 11, 64. [Google Scholar] [CrossRef]
- Hong, Y.H.; Cai, R.L.; Guo, J.Y.; Zhong, Z.H.; Bao, J.D.; Wang, Z.H.; Chen, X.F.; Zhou, J.; Lu, G.D. Carbon catabolite repressor MoCreA is required for the asexual development and pathogenicity of the rice blast fungus. Fungal Genet. Biol. 2021, 146. [Google Scholar] [CrossRef]
- Ying, S.M.; Zhang, Z.; Zhang, Y.N.; Hao, Z.N.; Chai, R.Y.; Qiu, H.P.; Wang, Y.L.; Zhu, X.M.; Wang, J.Y.; Sun, G.C.; et al. MoDHX35, a DEAH-Box Protein, Is Required for Appressoria Formation and Full Virulence of the Rice Blast Fungus, Magnaporthe oryzae. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, S.R.; Lin, L.L.; Chen, X.M.; Abdul, W.; Lin, Y.H.; Otieno, F.J.; Shabbir, A.; Batool, W.; Zhang, Y.Q.; Tang, W.; et al. Disruption of putative short-chain acyl-CoA dehydrogenases compromised free radical scavenging, conidiogenesis, and pathogenesis of Magnaporthe oryzae. Fungal Genet. Biol. 2019, 127, 23–34. [Google Scholar] [CrossRef]
- Shabbir, A.; Batool, W.; Yu, D.; Lin, L.L.; An, Q.L.; Chen, X.M.; Guo, H.Y.; Yuan, S.S.; Malota, S.; Wang, Z.H.; et al. Magnaporthe oryzae Chloroplast Targeting Endo-beta-1,4-Xylanase I MoXYL1A Regulates Conidiation, Appressorium Maturation and Virulence of the Rice Blast Fungus. Rice 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Egan, M.J.; Wang, Z.Y.; Jones, M.A.; Smirnoff, N.; Talbot, N.J. Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc. Natl. Acad. Sci. USA 2007, 104, 11772–11777. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z. A double-edged sword: Reactive oxygen species (ROS) during the rice blast fungus and host interaction. FEBS. J 2021, 289, 5505–5515. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Shim, S.H. The fungus Colletotrichum as a source for bioactive secondary metabolites. Arch. Pharm. Res. 2019, 42, 735–753. [Google Scholar] [CrossRef]
- He, P.; Wang, Y.; Wang, X.; Zhang, X.; Tian, C. The mitogen-activated protein kinase CgMK1 governs appressorium formation, melanin synthesis, and plant infection of Colletotrichum gloeosporioides. Front. Microbiol. 2017, 8, 2216. [Google Scholar] [CrossRef]
- Wang, X.; Lu, D.; Tian, C. Mucin Msb2 cooperates with the transmembrane protein Sho1 in various plant surface signal sensing and pathogenic processes in the poplar anthracnose fungus Colletotrichum gloeosporioides. Mol. Plant Pathol. 2021, 22, 1553–1573. [Google Scholar] [CrossRef]
- Fang, Y.-L.; Xia, L.-M.; Wang, P.; Zhu, L.-H.; Ye, J.-R.; Huang, L. The MAPKKK CgMck1 Is Required for Cell Wall Integrity, Appressorium Development, and Pathogenicity in Colletotrichum gloeosporioides. Genes 2018, 9, 543. [Google Scholar] [CrossRef]
- Liu, N.; Wang, W.; He, C.; Luo, H.; An, B.; Wang, Q. NADPH Oxidases Play a Role in Pathogenicity via the Regulation of F-Actin Organization in Colletotrichum gloeosporioides. Front. Cell. Infect. Microbiol. 2022, 12, 845133. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Liang, Y.; Wang, Y.; Tian, C. A Cdc42 homolog in Colletotrichum gloeosporioides regulates morphological development and is required for ROS-mediated plant infection. Curr. Genet. 2018, 64, 1153–1169. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Wang, T.; Zhou, G.; Ren, W.; Duan, X.; Gao, L.; Chen, J.; Xu, L.; Zhu, P. Ethylene promotes expression of the appressorium- and pathogenicity-related genes via GPCR- and MAPK-dependent manners in Colletotrichum gloeosporioides. J. Fungi 2022, 8, 570. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, D.; Tian, C. CgEnd3 regulates endocytosis, appressorium formation, and virulence in the poplar anthracnose fungus Colletotrichum gloeosporioides. Int. J. Mol. Sci. 2021, 22, 4029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; An, B.; Wang, W.; Zhang, B.; He, C.; Luo, H.; Wang, Q. Actin-bundling protein fimbrin regulates pathogenicity via organizing F-actin dynamics during appressorium development in Colletotrichum gloeosporioides. Mol. Plant Pathol. 2022, 23, 1472–1486. [Google Scholar] [CrossRef]
- Mushtaq, A.; Tariq, M.; Ahmed, M.; Zhou, Z.; Ali, I.; Mahmood, R.T. Carbamoyl phosphate synthase subunit CgCPS1 is necessary for virulence and to regulate stress tolerance in Colletotrichum gloeosporioides. Plant Pathol. J. 2021, 37, 232–242. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, M.; Xu, C.; Zhou, Z. Functional analysis of the pathogenicity-related gene CgNVF1 in Colletotrichum gloeosporioides. Acta Phuys. Sin. 2018, 48, 810–816. [Google Scholar]
- Xu, S.; Ke, Z.; Zhang, K.; Liu, Z.; Li, X. Biological function of a regulator of G-protein signaling CgRGS3 in Colletotrichum gloeosporioides. Acta Phuys. Sin. 2018, 45, 827–835. [Google Scholar]
- Zhang, J.; Wang, M.; Chi, F.; Xu, J.; Zhou, Z. Cloning and functional analysis of CgCMK1 in Colletotrichum gloeosporioides. Acta Pharmacol. Sin. 2020, 50, 40–48. [Google Scholar]
- Mahto, B.K.; Singh, A.; Pareek, M.; Rajam, M.V.; Dhar-Ray, S.; Reddy, P.M. Host-induced silencing of the Colletotrichum gloeosporioides conidial morphology 1 gene (CgCOM1) confers resistance against Anthracnose disease in chilli and tomato. Plant Mol. Biol. 2020, 104, 381–395. [Google Scholar] [CrossRef]
- Zhong, C.; Xiao, C.; Zhang, H.; Pu, J.; Wu, Q.; Liu, Y.; Liu, X. Sequence Characteristics of Laccase Gene Cglac3 and Its Expression in Two Infection-Related Gene Mutants from Colletotrichum gloeosporioides on Mango. Chin. J. Trop. Crops 2020, 41, 1202–1207. [Google Scholar]
- Liu, S.K.; Wang, Q.N.; Liu, N.; Luo, H.L.; He, C.Z.; An, B. The histone deacetylase HOS2 controls pathogenicity through regulation of melanin biosynthesis and appressorium formation in Colletotrichum gloeosporioides. Phytopathol. Res. 2022, 4. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhou, Z.S.; Wu, J.Y.; Ji, Z.R.; Zhang, J.X. Comparative transcriptome analysis reveals significant differences in gene expression between appressoria and hyphae in Colletotrichum gloeosporioides. Gene 2018, 670, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wu, J.; Wang, M.; Zhang, J. ABC protein CgABCF2 is required for asexual and sexual development, appressorial formation and plant infection in Colletotrichum gloeosporioides. Microb. Pathog. 2017, 110, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, X.; Shi, T.; Li, C.; Huang, G. The Colletotrichum gloeosporioides perilipin homologue CAP 20 regulates functional appressorial formation and fungal virulence. J. Phytopathol. 2018, 166, 216–225. [Google Scholar] [CrossRef]
- Li, C.; Sun, W.; Cao, S.; Hou, R.; Li, X.; Ming, L.; Kan, J.; Zhao, Y.; Liu, F. The CfMK1 Gene Regulates Reproduction, Appressorium Formation, and Pathogenesis in a Pear Anthracnose-Causing Fungus. J. Fungi 2022, 8, 77. [Google Scholar] [CrossRef]
- Lee, N.H.; Fu, T.; Shin, J.H.; Song, Y.W.; Jang, D.C.; Kim, K.S. The Small GTPase CsRAC1 Is Important for Fungal Development and Pepper Anthracnose in Colletotrichum scovillei. Plant Pathol. J. 2021, 37, 607–618. [Google Scholar] [CrossRef]
- Shin, J.-H.; Park, B.-S.; Kim, K.S. The CsSTE50 Adaptor Protein in Mitogen-Activated Protein Kinase Cascades Is Essential for Pepper Anthracnose Disease of Colletotrichum scovillei. Plant Pathol. J. 2022, 38, 593–602. [Google Scholar] [CrossRef]
- Shin, J.H.; Kim, H.Y.; Fu, T.; Lee, K.H.; Kim, K.S. CsPOM1, a DYRK Family Kinase, Plays Diverse Roles in Fungal Development, Virulence, and Stress Tolerance in the Anthracnose Pathogen Colletotrichum scovillei. Front. Cell. Infect. Microbiol. 2022, 12, 861915. [Google Scholar] [CrossRef]
- Song, M.; Fang, S.; Li, Z.; Wang, N.; Li, X.; Liu, W.; Zhang, Y.; Lin, C.; Miao, W. CsAtf1, a bZIP transcription factor, is involved in fludioxonil sensitivity and virulence in the rubber tree anthracnose fungus Colletotrichum siamense. Fungal Genet. Biol. 2022, 158, 103649. [Google Scholar] [CrossRef]
- Yokoyama, A.; Izumitsu, K.; Sumita, T.; Tanaka, C.; Irie, T.; Suzuki, K. Homeobox transcription factor CoHox3 is essential for appressorium formation in the plant pathogenic fungus Colletotrichum orbiculare. Mycoscience 2018, 59, 353–362. [Google Scholar] [CrossRef]
- Kodama, S.; Nishiuchi, T.; Kubo, Y. Colletotrichum orbiculare MTF4 Is a Key Transcription Factor Downstream of MOR Essential for Plant Signal-Dependent Appressorium Development and Pathogenesis. Mol. Plant-Microbe Interact. 2019, 32, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Y.; Sun, J.Y.; Gao, Y.B.; Liu, K.X.; Yuan, M.Y.; Gao, W.D.; Wang, F.; Fu, D.D.; Chen, N.; Xiao, S.Q.; et al. The key iron assimilation genesClFTR1,ClNPS6were crucial for virulence of Curvularia lunatavia initiating its appressorium formation and virulence factors. Environ. Microbiol. 2021, 23, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Gao, W.; Sun, J.; Mao, X.; Liu, K.; Xu, J.; Fu, D.; Yuan, M.; Wang, H.; Chen, N.; et al. NADPH Oxidase ClNOX2 Regulates Melanin-Mediated Development and Virulence in Curvularia lunata. Mol. Plant-Microbe Interact. 2020, 33, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Wang, Q.; Zhu, H.; Wang, J.; Shen, S.; Liu, N.; Hao, Z.; Dong, J. Identification and Expression Pattern Analysis of Septin Gene Family of Setosphaeria turcica. Sci. Agric. Sin. 2020, 53, 5017–5026. [Google Scholar] [CrossRef]
- Li, T.; Zhu, H.; Wei, N.; Long, F.; Wu, J.; Zhang, Y.; Dong, J.; Shen, S.; Hao, Z. The Expression Pattern and Interaction Analysis of the Homologues of Splicing Factor SC35 in Setosphaeria turcica. Sci. Agric. Sin. 2021, 54, 733–743. [Google Scholar]
- Sumita, T.; Izumitsu, K.; Shigeyoshi, S.; Gotoh, S.; Yoshida, H.; Tsuji, K.; Yoshida, H.; Kitade, Y.; Tanaka, C. An adaptor protein BmSte50 interacts with BmSte11 MAPKKK and is involved in host infection, conidiation, melanization, and sexual development in Bipolaris maydis. Mycoscience 2020, 61, 85–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Yu, D.; Peng, Y.; Min, H.; Lai, Z. Copper Ions are Required for Cochliobolus heterostrophus in Appressorium Formation and Virulence on Maize. Phytopathology 2020, 110, 494–504. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, P.; Cao, B.; Ma, S.; Li, R.; Wang, X.; Zhao, A. An APSES Transcription Factor Xbp1 Is Required for Sclerotial Development, Appressoria Formation, and Pathogenicity in Ciboria shiraiana. Front. Microbiol. 2021, 12, 739686. [Google Scholar] [CrossRef]
- Zhu, P.; Zhang, S.; Li, R.; Liu, C.; Fan, W.; Hu, T.; Zhao, A. Host-Induced Gene Silencing of a G Protein α Subunit Gene CsGpa1 Involved in Pathogen Appressoria Formation and Virulence Improves Tobacco Resistance to Ciboria shiraiana. J. Fungi 2021, 7, 1053. [Google Scholar] [CrossRef]
- Bolton, M.D.; Thomma, B.P.; Nelson, B.D. Sclerotinia sclerotiorum (Lib.) de Bary: Biology and molecular traits of a cosmopolitan pathogen. Mol. Plant Pathol. 2006, 7, 1–16. [Google Scholar] [CrossRef]
- Li, M.; Liang, X.; Rollins, J.A. Sclerotinia sclerotiorum γ-glutamyl transpeptidase (Ss-Ggt1) is required for regulating glutathione accumulation and development of sclerotia and compound appressoria. Mol. Plant Microbe Interact. 2012, 25, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Choquer, M.; Rascle, C.; Gonçalves, I.R.; de Vallée, A.; Ribot, C.; Loisel, E.; Smilevski, P.; Ferria, J.; Savadogo, M.; Souibgui, E.; et al. The infection cushion of Botrytis cinerea: A fungal ‘weapon’ of plant-biomass destruction. Environ. Microbiol. 2021, 23, 2293–2314. [Google Scholar] [CrossRef] [PubMed]
- Mentges, M.; Glasenapp, A.; Boenisch, M.; Malz, S.; Henrissat, B.; Frandsen, R.J.N.; Güldener, U.; Münsterkötter, M.; Bormann, J.; Lebrun, M.H.; et al. Infection cushions of Fusarium graminearum are fungal arsenals for wheat infection. Mol. Plant Pathol. 2020, 21, 1070–1087. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Yu, H.; Cong, J.; Xiao, K.; Zhang, X.; Liu, J.; Zhang, Y.; Pan, H. Transcription factor SsFoxE3 activating SsAtg8 is critical for sclerotia, compound appressoria formation, and pathogenicity in Sclerotinia sclerotiorum. Mol. Plant Pathol. 2022, 23, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chen, Y.; Wu, Z.; Yang, N.; Rana, K.; Meng, X.; Liu, B.; Wan, H.; Qian, W. SsCox17, a copper chaperone, is required for pathogenic process and oxidative stress tolerance of Sclerotinia sclerotiorum. Plant Sci. 2022, 322, 111345. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Y.; Yan, B.; Liao, H.; Dong, M.; Meng, X.; Wan, H.; Qian, W. Host-Induced Gene Silencing of a Multifunction Gene Sscnd1 Enhances Plant Resistance Against Sclerotinia sclerotiorum. Front. Microbiol. 2021, 12, 693334. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Lai, W.; Huang, K.; Li, Y.; Wang, Z.; Chen, X.; Wang, A. SsATG8 and SsNBR1 mediated-autophagy is required for fungal development, proteasomal stress response and virulence in Sclerotinia sclerotiorum. Fungal Genet. Biol. 2021, 157, 103632. [Google Scholar] [CrossRef]
- Yu, P.L.; Rollins, J.A. The cAMP-dependent protein kinase A pathway perturbs autophagy and plays important roles in development and virulence of Sclerotinia sclerotiorum. Fungal Biol. 2022, 126, 20–34. [Google Scholar] [CrossRef]
- Cong, J.; Xiao, K.; Jiao, W.; Zhang, C.; Zhang, X.; Liu, J.; Zhang, Y.; Pan, H. The coupling between cell wall integrity mediated by MAPK KINASES And SsFkh1 is involved in sclerotia formation and pathogenicity of Sclerotinia sclerotiorum. Front. Microbiol. 2022, 13, 816091. [Google Scholar] [CrossRef]
- Chethana, K.W.T.; Jayawardena, R.S.; Chen, Y.J.; Konta, S.; Tibpromma, S.; Abeywickrama, P.D.; Gomdola, D.; Balasuriya, A.; Xu, J.; Lumyong, S.; et al. Diversity and Function of Appressoria. Pathogens 2021, 10, 746. [Google Scholar] [CrossRef]
- Green, J.R.; Carver, T.; Gurr, S.J. The formation and function of infection and feeding structures. In The Powdery Mildews; American Phytopathological Society Press: Saint Paul, MN, USA, 2002; pp. 66–82. [Google Scholar]
- Konta, S.; Hongsanan, S.; Tibpromma, S.; Thongbai, B.; Boonmee, S. An advance in the endophyte story: Oxydothidaceae fam. nov. with six new species of Oxydothis. Mycosphere 2016, 7, 1425–1446. [Google Scholar] [CrossRef]
- Cook, R.; Braun, U.; Beales, P.A. Development of appressoria on conidial germ tubes of Erysiphe species. Mycoscience 2011, 52, 183–197. [Google Scholar] [CrossRef]
- Lubbe, C.M.; Denman, S.; Cannon, P.F.; Groenewald, J.Z.; Lamprecht, S.C.; Crous, P.W. Characterization of Colletotrichum species associated with diseases of Proteaceae. Mycologia 2004, 96, 1268–1279. [Google Scholar] [CrossRef] [PubMed]
- Monkhung, S.; Takamatsu, S.; To-Anun, C.J.A.J.O.B. Molecular and morphological characterization of Phyllactinia cassiae-fistulae (Erysiphaceae; Ascomycota) from Thailand. AJB 2013, 12, 109–114. [Google Scholar] [CrossRef]
- Paulitz, T.C.; Adams, K. Composition and distribution of pythium communities in wheat fields in eastern washington state. Phytopathology 2003, 93, 867–873. [Google Scholar] [CrossRef]
- Wilson, R.A.; Talbot, N.J. Under pressure: Investigating the biology of plant infection by Magnaporthe oryzae. Nat. Rev. Microbiol. 2009, 7, 185–195. [Google Scholar] [CrossRef]
- Arya, G.C.; Cohen, H. The Multifaceted Roles of Fungal Cutinases during Infection. J. Fungi 2022, 8, 199. [Google Scholar] [CrossRef]
- Tucker, S.L.; Talbot, N.J. Surface attachment and pre-penetration stage development by plant pathogenic fungi. Annu. Rev. Phytopathol. 2001, 39, 385–417. [Google Scholar] [CrossRef]
- Eisermann, I.; Weihmann, F.; Krijger, J.J.; Kroling, C.; Hause, G.; Menzel, M.; Pienkny, S.; Kiesow, A.; Deising, H.B.; Wirsel, S.G.R. Two genes in a pathogenicity gene cluster encoding secreted proteins are required for appressorial penetration and infection of the maize anthracnose fungus Colletotrichum graminicola. Environ. Microbiol. 2019, 21, 4773–4791. [Google Scholar] [CrossRef]
- Howard, R.J.; Ferrari, M.A.; Roach, D.H.; Money, N.P. Penetration of hard substrates by a fungus employing enormous turgor pressures. Proc. Natl. Acad. Sci. USA 1991, 88, 11281–11284. [Google Scholar] [CrossRef]
- Chumley, F.; Valent, B. Genetic analysis of melanin-deficient, nonpathogenic mutants of Magnaporthe grisea. Mol. Plant-Microbe Interact. 1990, 3, 135–143. [Google Scholar] [CrossRef]
- Howard, R.J.; Ferrari, M.A. Role of melanin in appressorium function. Exp. Mycol. 1989, 13, 403–418. [Google Scholar] [CrossRef]
- Joke, C.D.J.; McCormack, B.J.; Smirnoff, N.; Talbot, N.J. Glycerol generates turgor in rice blast. Nature 1997, 389, 244–245. [Google Scholar] [CrossRef]
- Thines, E.; Eilbert, F.; Sterner, O.; Anke, H. Signal transduction leading to appressorium formation in germinating conidia of Magnaporthe grisea: Effects of second messengers diacylglycerols, ceramides and sphingomyelin. FEMS Microbiol. Lett. 1997, 156, 91–94. [Google Scholar] [CrossRef]
- Ryder, L.S.; Dagdas, Y.F.; Kershaw, M.J.; Venkataraman, C.; Madzvamuse, A.; Yan, X.; Cruz-Mireles, N.; Soanes, D.M.; Oses-Ruiz, M.; Styles, V.; et al. A sensor kinase controls turgor-driven plant infection by the rice blast fungus. Nature 2019, 574, 423–427. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Liang, S.; Zhang, Y.R.; Lu, J.P.; Lin, F.C.; Liu, X.H. MoSec61 beta, the beta subunit of Sec61, is involved in fungal development and pathogenicity, plant immunity, and ER-phagy in Magnaporthe oryzae. Virulence 2020, 11, 1685–1700. [Google Scholar] [CrossRef]
- Ludwig, N.; Löhrer, M.; Hempel, M.; Mathea, S.; Schliebner, I.; Menzel, M.; Kiesow, A.; Schaffrath, U.; Deising, H.B.; Horbach, R. Melanin is not required for turgor generation but enhances cell-wall rigidity in appressoria of the corn pathogen Colletotrichum graminicola. Mol. Plant Microbe Interact. 2014, 27, 315–327. [Google Scholar] [CrossRef]
- Wang, T.; Ren, D.D.; Guo, H.; Chen, X.; Zhu, P.K.; Nie, H.Z.; Xu, L. CgSCD1 Is Essential for Melanin Biosynthesis and Pathogenicity of Colletotrichum gloeosporioides. Pathogens 2020, 9. [Google Scholar] [CrossRef]
- Wang, X.; Lu, D.; Tian, C. Analysis of melanin biosynthesis in the plant pathogenic fungus Colletotrichum gloeosporioides. Fungal Biol. 2021, 125, 679–692. [Google Scholar] [CrossRef]
- Zhu, S.Y.; Yan, Y.X.; Qu, Y.M.; Wang, J.; Feng, X.X.; Liu, X.H.; Lin, F.C.; Lu, J.P. Role refinement of melanin synthesis genes by gene knockout reveals their functional diversity in Pyricularia oryzae strains. Microbiol. Res. 2021, 242. [Google Scholar] [CrossRef]
- Lin, S.Y.; Okuda, S.; Ikeda, K.; Okuno, T.; Takano, Y. LAC2 encoding a secreted laccase is involved in appressorial melanization and conidial pigmentation in Colletotrichum orbiculare. Mol. Plant-Microbe Interact. 2012, 25, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Loehrer, M.; Botterweck, J.; Jahnke, J.; Mahlmann, D.M.; Gaetgens, J.; Oldiges, M.; Horbach, R.; Deising, H.; Schaffrath, U. In vivo assessment by Mach-Zehnder double-beam interferometry of the invasive force exerted by the Asian soybean rust fungus (Phakopsora pachyrhizi). New Phytol. 2014, 203, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Ryder, L.S.; Talbot, N.J. Regulation of appressorium development in pathogenic fungi. Curr. Opin. Plant Biol. 2015, 26, 8–13. [Google Scholar] [CrossRef]
- Pryce-Jones, E.; Carver, T.I.M.; Gurr, S.J. The roles of cellulase enzymes and mechanical force in host penetration by Erysiphe graminis f.sp. hordei. Physiol. Mol. Plant Pathol. 1999, 55, 175–182. [Google Scholar] [CrossRef]
- Kankanala, P.; Czymmek, K.; Valent, B. Roles for rice membrane dynamics and plasmodesmata during biotrophic invasion by the blast fungus. Plant Cell 2007, 19, 706–724. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Y.; He, L.; Zhu, J.; Wu, B.; Liu, F.; Mu, W. Activity of the Novel Fungicide Mefentrifluconazole Against Colletotrichum scovillei. Plant Dis. 2021, 105, 1522–1530. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.; Wang, X.; Zhong, L.; Shi, G.; Xu, Y.; Li, Y.; Li, R.; Huang, Y.; Ye, X.; et al. A novel β-1,3-glucanase Gns6 from rice possesses antifungal activity against Magnaporthe oryzae. J. Plant Physiol. 2021, 265, 153493. [Google Scholar] [CrossRef]
- Wu, X.; Chen, Y.; Li, C.; Zhang, X.; Tan, X.; Lv, L.; Liu, Y.; Zhang, D. GroEL protein from the potential biocontrol agent Rhodopseudomonas palustris enhances resistance to rice blast disease. Pest Manag. Sci. 2021, 77, 5445–5453. [Google Scholar] [CrossRef]
- Mondal, S. Preparation, properties and applications of nanocellulosic materials. Carbohydr. Polym. 2017, 163, 301–316. [Google Scholar] [CrossRef]
- Saito, H.; Yamashita, Y.; Sakata, N.; Ishiga, T.; Shiraishi, N.; Usuki, G.; Nguyen, V.T.; Yamamura, E.; Ishiga, Y. Covering soybean leaves with cellulose nanofiber changes leaf surface hydrophobicity and confers resistance against Phakopsora pachyrhizi. Front. Plant Sci. 2021, 12, 726565. [Google Scholar] [CrossRef]
- Barilli, E.; Agudo, F.J.; Masi, M.; Nocera, P.; Evidente, A.; Rubiales, D. Anthraquinones and their analogues as potential biocontrol agents of rust and powdery mildew diseases of field crops. Pest Manag. Sci. 2022, 78, 3489–3497. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Ye, H.; Shen, Q.; Jiang, X.; Cui, G.; Gu, W.; Zhang, L.H.; Naqvi, N.I.; Deng, Y.Z. Tangeretin inhibits fungal ferroptosis to suppress rice blast. J. Integr. Plant Biol. 2021, 63, 2136–2149. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.K.; Chakraborty, M.; Rahman, M.; Gupta, D.R.; Mahmud, N.U.; Rahat, A.A.M.; Sarker, A.; Hannan, M.A.; Rahman, M.M.; Akanda, A.M.; et al. Marine Natural Product Antimycin A Suppresses Wheat Blast Disease Caused by Magnaporthe oryzae Triticum. J. Fungi 2022, 8, 618. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhao, L.; Dong, Y.; Chen, W.; Li, C.; Gao, X.; Chen, R.; Li, L.; Xu, Z. The antagonistic mechanism of Bacillus velezensis ZW10 against rice blast disease: Evaluation of ZW10 as a potential biopesticide. PLoS ONE 2021, 16, e0256807. [Google Scholar] [CrossRef] [PubMed]
- Kgosi, V.T.; Tingting, B.; Ying, Z.; Liu, H. Anti-Fungal Analysis of Bacillus subtilis DL76 on Conidiation, Appressorium Formation, Growth, Multiple Stress Response, and Pathogenicity in Magnaporthe oryzae. Int. J. Mol. Sci. 2022, 23, 5314. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, Y.; Lu, K.; Chen, R.; Jiang, J. Bacillus subtilis KLBMPGC81 suppresses appressorium-mediated plant infection by altering the cell wall integrity signaling pathway and multiple cell biological processes in Magnaporthe oryzae. Front. Cell. Infect. Microbiol. 2022, 12, 983757. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, B.S.; Kim, H.Y.; Lee, K.H.; Kim, K.S. Antagonistic and Plant Growth-Promoting Effects of Bacillus velezensis BS1 Isolated from Rhizosphere Soil in a Pepper Field. Plant Pathol. J. 2021, 37, 307–314. [Google Scholar] [CrossRef]
- Chai, C.H.; Hong, C.F.; Huang, J.W. Identification and characterization of a multifunctional biocontrol agent, Streptomyces griseorubiginosus LJS06, against cucumber anthracnose. Front. Microbiol. 2022, 13, 923276. [Google Scholar] [CrossRef]
- Xie, D.; Cai, X.; Yang, C.; Xie, L.; Qin, G.; Zhang, M.; Huang, Y.; Gong, G.; Chang, X.; Chen, H. Studies on the control effect of Bacillus subtilis on wheat powdery mildew. Pest Manag. Sci. 2021, 77, 4375–4382. [Google Scholar] [CrossRef]
- Dean, R.A. Signal pathways and appressorium morphogenesis. Annu. Rev. Phytopathol. 1997, 35, 211–234. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; Mazzola, M. Diversity and natural functions of antibiotics produced by beneficial and plant pathogenic bacteria. Annu. Rev. Phytopathol. 2012, 50, 403–424. [Google Scholar] [CrossRef] [PubMed]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, S. Insect Pathogenic Fungi: Genomics, Molecular Interactions, and Genetic Improvements. Annu. Rev. Entomol. 2017, 62, 73–90. [Google Scholar] [CrossRef]
- Ahrén, D.; Tholander, M.; Fekete, C.; Rajashekar, B.; Friman, E.; Johansson, T.; Tunlid, A. Comparison of gene expression in trap cells and vegetative hyphae of the nematophagous fungus Monacrosporium haptotylum. Microbiology 2005, 151, 789–803. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, T.-T.; Li, G.-H.; Zhao, P.-J. Appressoria—Small but Incredibly Powerful Structures in Plant–Pathogen Interactions. Int. J. Mol. Sci. 2023, 24, 2141. https://doi.org/10.3390/ijms24032141
Shi T-T, Li G-H, Zhao P-J. Appressoria—Small but Incredibly Powerful Structures in Plant–Pathogen Interactions. International Journal of Molecular Sciences. 2023; 24(3):2141. https://doi.org/10.3390/ijms24032141
Chicago/Turabian StyleShi, Ting-Ting, Guo-Hong Li, and Pei-Ji Zhao. 2023. "Appressoria—Small but Incredibly Powerful Structures in Plant–Pathogen Interactions" International Journal of Molecular Sciences 24, no. 3: 2141. https://doi.org/10.3390/ijms24032141
APA StyleShi, T.-T., Li, G.-H., & Zhao, P.-J. (2023). Appressoria—Small but Incredibly Powerful Structures in Plant–Pathogen Interactions. International Journal of Molecular Sciences, 24(3), 2141. https://doi.org/10.3390/ijms24032141





