Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review
Abstract
1. Introduction
- (i)
- contamination—bacteria are transferred into the wound from the surrounding environment,
- (ii)
- colonization—bacteria are replicated and start to adhere to the wound-forming biofilm, however without interfering with the ongoing wound healing process,
- (iii)
- infection—the replication rate of bacteria begins to overload the immune system leading to various consequences.
2. Design, Synthesis, and Properties of Antimicrobial Hydrogels
2.1. Hydrogels with Intrinsic Antimicrobial Activity
2.1.1. Polysaccharide Hydrogels
2.1.2. Antimicrobial Peptide Hydrogels
2.2. Hydrogels Loaded with Antimicrobial Agents
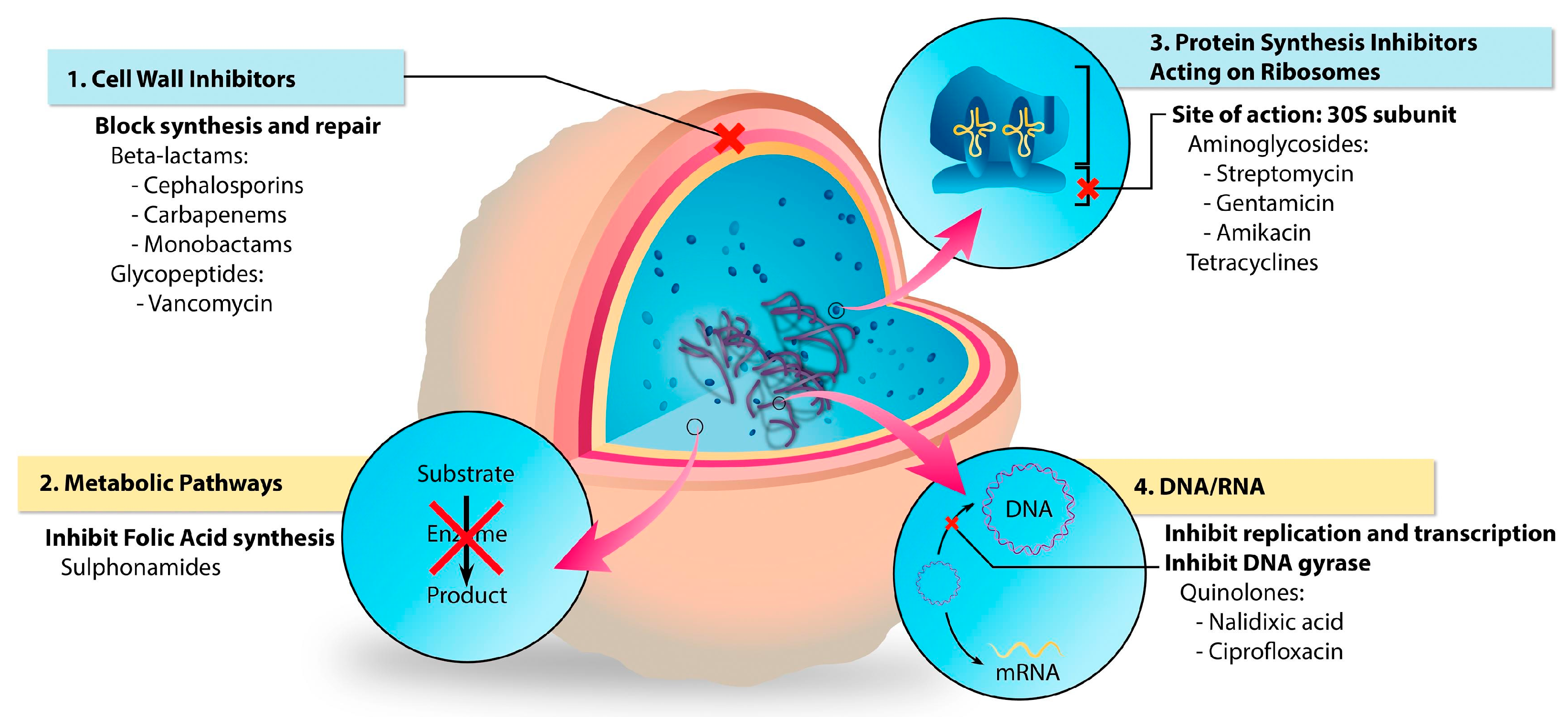
2.2.1. Hydrogels Loaded with Antibiotics
- Aminoglycosides (e.g., gentamicin, streptomycin) and tetracyclines (e.g., doxycycline, tetracycline hydrochloride) block the pathway of protein synthesis,
- Beta-lactams (e.g., ampicillin, ceftadizime, cefazolin) and glycopeptides (e.g., vancomycin) inhibit the synthesis of the bacterial cell wall,
- Sulphonamides (e.g., sulfadiazine) which interfere with the synthesis of the key metabolites,
- Quinolones (e.g., ciprofloxacin, levofloxacin) inhibit the synthesis of nucleic acids.
Gentamicin
Ciprofloxacin
Vancomycin
Amoxicillin
Other Antibiotics
2.2.2. Hydrogels Loaded with Biological Extracts or Natural Compounds
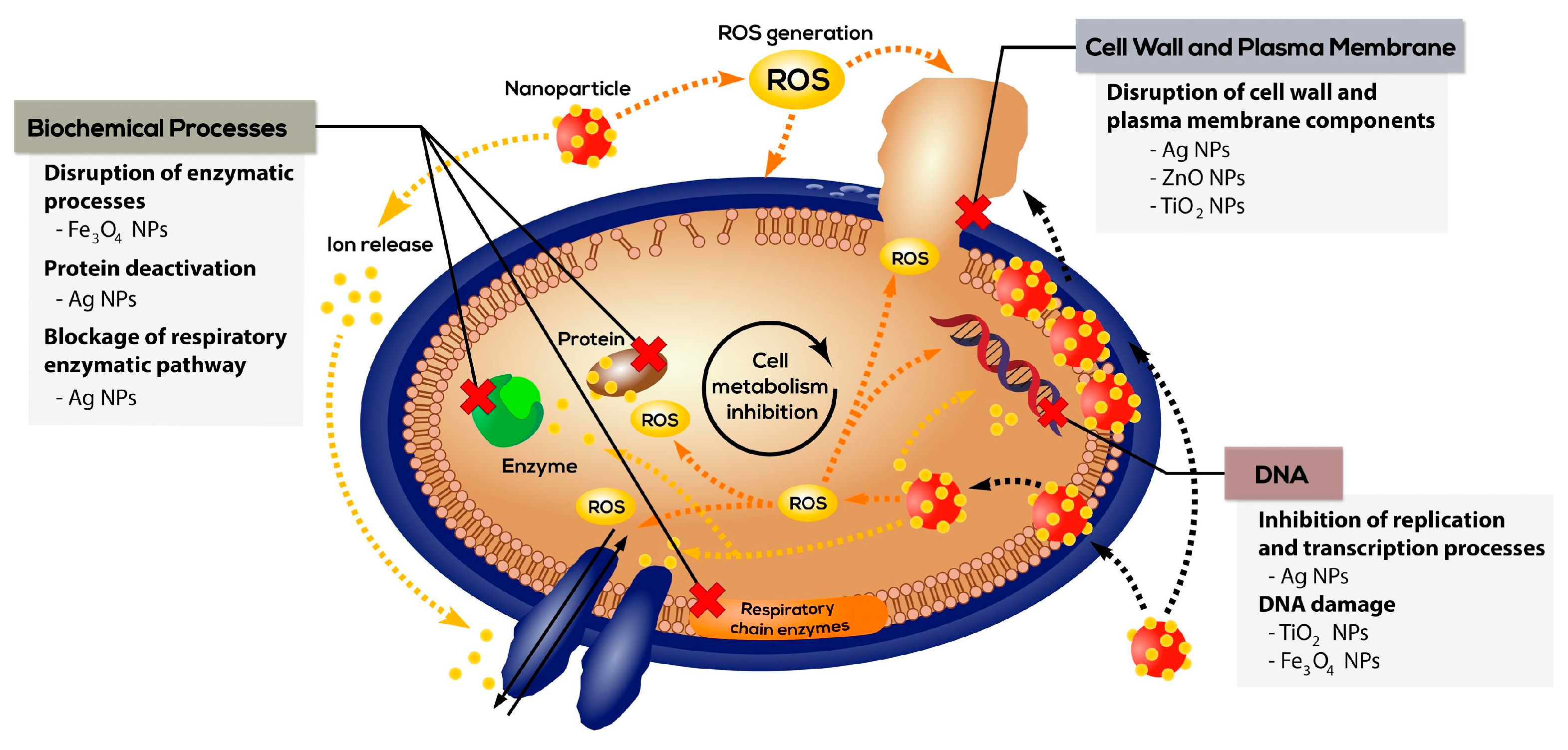
2.2.3. Hydrogels Loaded with Inorganic Particles
3. Conclusions and Future Perspectives
- (i)
- the lack of clinical animal models, as most studied experimental animal models are healthy young animals—there is little discussion on some old animals or animals with diseases,
- (ii)
- high susceptibility to damage during transport and storage, with possible drug leakage as well as deterioration of their structure and function.
- (iii)
- Frequent lack of matching between hydrogel degradation rate, active ingredient release rate, and the wound regeneration rate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barczak, M.; Borowski, P.; Gila-Vilchez, C.; Alaminos, M.; González-Caballero, F.; López-López, M.T. Revealing importance of particles’ surface functionalization on the properties of magnetic alginate hydrogels. Carbohydr. Polym. 2020, 247, 116747. [Google Scholar] [CrossRef]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef]
- Fan, D.-Y.; Tian, Y.; Liu, Z.-J. Injectable Hydrogels for Localized Cancer Therapy. Front. Chem. 2019, 7, 675. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing Hydrogels for Controlled Drug Delivery. Nat. Rev. Mater. 2016, 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Park, K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2012, 64, 49–60. [Google Scholar] [CrossRef]
- Akbari, A.; Padervand, M.; Jalilian, E.; Seidi, F. Sodium alginate-halloysite nanotube gel beads as potential delivery system for sunitinib malate anticancer compound. Mater. Lett. 2020, 274, 128038. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef] [PubMed]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine 2010, 5, 469–484. [Google Scholar] [CrossRef]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Li, J.; Wu, C.; Chu, P.K.; Gelinsky, M. 3D printing of hydrogels: Rational design strategies and emerging biomedical applications. Mater. Sci. Eng. R Rep. 2020, 140, 100543. [Google Scholar] [CrossRef]
- Taneja, H.; Salodkar, S.M.; Parmar, A.S.; Chaudhary, S. Hydrogel based 3D printing: Bio ink for tissue engineering. J. Mol. Liq. 2022, 367, 120390. [Google Scholar] [CrossRef]
- Advincula, R.C.; Dizon, J.R.C.; Caldona, E.B.; Viers, R.A.; Siacor, F.D.C.; Maalihan, R.D.; Espera, A.H. On the progress of 3D-printed hydrogels for tissue engineering. MRS Commun. 2021, 11, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Fatimi, A.; Okoro, O.V.; Podstawczyk, D.; Siminska-Stanny, J.; Shavandi, A. Natural Hydrogel-Based Bio-Inks for 3D Bioprinting in Tissue Engineering: A Review. Gels 2022, 8, 179. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, J.; Wang, D.; Zhang, J.; Yang, L.; Sun, S.; Liang, Y. 3D Bioprinting of Hydrogels for Cartilage Tissue Engineering. Gels 2021, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Buenger, D.; Topuz, F.; Groll, J. Hydrogels in sensing applications. Prog. Polym. Sci. 2012, 37, 1678–1719. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A Nanostructured Conductive Hydrogels-Based Biosensor Platform for Human Metabolite Detection. Nano Lett. 2015, 15, 1146–1151. [Google Scholar] [CrossRef]
- Ulijn, R.V.; Bibi, N.; Jayawarna, V.; Thornton, P.; Todd, S.J.; Mart, R.; Smith, A.; Gough, J.E. Bioresponsive hydrogels. Mater. Today 2007, 10, 40–48. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In Situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef]
- Griffin, D.R.; Weaver, W.M.; Scumpia, P.O.; Di Carlo, D.; Segura, T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015, 14, 737–744. [Google Scholar] [CrossRef]
- Liu, S.; Guo, W. Anti-Biofouling and Healable Materials: Preparation, Mechanisms, and Biomedical Applications. Adv. Funct. Mater. 2018, 28, 1800596. [Google Scholar] [CrossRef]
- Guan, G.; Zhang, Q.; Jiang, Z.; Liu, J.; Wan, J.; Jin, P.; Lv, Q. Multifunctional Silk Fibroin Methacryloyl Microneedle for Diabetic Wound Healing. Small 2022, 18, 2203064. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Wang, X.; Zhu, Y.; Su, W.; Lv, Q.; Li, D. Antimicrobial hydrogel microspheres for protein capture and wound healing. Mater. Des. 2022, 215, 110478. [Google Scholar] [CrossRef]
- Stashak, T.S.; Farstvedt, E.; Othic, A. Update on wound dressings: Indications and best use. Clin. Tech. Equine Pr. 2004, 3, 148–163. [Google Scholar] [CrossRef]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Bsc, C.E.D.; Wilson, M.J.; E Hill, K.; Stephens, P.; Hill, C.M.; Harding, K.G.; Thomas, D.W.; Fds, C.M.H.; Frcs, K.G.H. Use of molecular techniques to study microbial diversity in the skin: Chronic wounds reevaluated. Wound Repair Regen. 2001, 9, 332–340. [Google Scholar] [CrossRef]
- Salleh, N.A.B.M.; Tanaka, Y.; Sutarlie, L.; Su, X. Detecting bacterial infections in wounds: A review of biosensors and wearable sensors in comparison with conventional laboratory methods. Anal. 2022, 147, 1756–1776. [Google Scholar] [CrossRef] [PubMed]
- Bertesteanu, S.; Triaridis, S.; Stankovic, M.; Lazar, V.; Chifiriuc, M.C.; Vlad, M.; Grigore, R. Polymicrobial wound infections: Pathophysiology and current therapeutic approaches. Int. J. Pharm. 2014, 463, 119–126. [Google Scholar] [CrossRef]
- Bessa, L.J.; Fazii, P.; Di Giulio, M.; Cellini, L. Bacterial isolates from infected wounds and their antibiotic susceptibility pattern: Some remarks about wound infection. Int. Wound J. 2013, 12, 47–52. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Yazdi, M.K.; Azarfam, M.Y.; Zare, M.; Ramsey, J.D.; Seidi, F.; Saeb, M.R.; Ramakrishna, S.; Mozafari, M. Injectable Cell-Laden Hydrogels for Tissue Engineering: Recent Advances and Future Opportunities. Tissue Eng. Part A 2021, 27, 821–843. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R.; Daniel, E.-A.-K. Chitin and Chitosan: Production and Application of Versatile Bio-medical Nanomaterials. Int. J. Adv. Res. 2016, 4, 411. [Google Scholar]
- Sahariah, P.; Másson, M. Antimicrobial Chitosan and Chitosan Derivatives: A Review of the Structure–Activity Relationship. Biomacromolecules 2017, 18, 3846–3868. [Google Scholar] [CrossRef]
- Chen, Y.-L.; Chou, C.-C. Factors affecting the susceptibility of Staphylococcus aureus CCRC 12657 to water soluble lactose chitosan derivative. Food Microbiol. 2005, 22, 29–35. [Google Scholar] [CrossRef]
- Roller, S.; Covill, N. The antifungal properties of chitosan in laboratory media and apple juice. Int. J. Food Microbiol. 1999, 47, 67–77. [Google Scholar] [CrossRef]
- Chien, R.-C.; Yen, M.-T.; Mau, J.-L. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef]
- Khalil, A.M.; Abdel-Monem, R.A.; Darwesh, O.M.; Hashim, A.I.; Nada, A.A.; Rabie, S.T. Synthesis, Characterization, and Evaluation of Antimicrobial Activities of Chitosan and Carboxymethyl Chitosan Schiff-Base/Silver Nanoparticles. J. Chem. 2017, 2017, 1434320. [Google Scholar] [CrossRef]
- Kingkaew, J.; Kirdponpattara, S.; Sanchavanakit, N.; Pavasant, P.; Phisalaphong, M. Effect of molecular weight of chitosan on antimicrobial properties and tissue compatibility of chitosan-impregnated bacterial cellulose films. Biotechnol. Bioprocess Eng. 2014, 19, 534–544. [Google Scholar] [CrossRef]
- Li, J.; Zhuang, S. Antibacterial activity of chitosan and its derivatives and their interaction mechanism with bacteria: Current state and perspectives. Eur. Polym. J. 2020, 138, 109984. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.; Nemeş, N.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-Seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Kumar, A.B.V.; Varadaraj, M.C.; Gowda, L.R.; Tharanathan, R.N. Characterization of chito-oligosaccharides prepared by chitosanolysis with the aid of papain and Pronase, and their bactericidal action against Bacillus cereus and Escherichia coli. Biochem. J. 2005, 391, 167–175. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.D.; Assis, O.B. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Chung, Y.-C.; Wang, H.L.; Chen, Y.M.; Li, S.L. Effect of abiotic factors on the antibacterial activity of chitosan against waterborne pathogens. Bioresour. Technol. 2003, 88, 179–184. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, J.; Ran, L.; Yu, K.; Lu, B.; Lan, G.; Dai, F.; Lu, F. An injectable self-healing hydrogel with adhesive and antibacterial properties effectively promotes wound healing. Carbohydr. Polym. 2018, 201, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Hu, X.-H.; Chu, L.-Q.; Jia, S.-R.; Xie, Y.-Y.; Zhong, C. Development of bacterial cellulose/chitosan based semi-interpenetrating hydrogels with improved mechanical and antibacterial properties. Int. J. Biol. Macromol. 2018, 122, 380–387. [Google Scholar] [CrossRef]
- Zhong, Y.; Xiao, H.; Seidi, F.; Jin, Y. Natural Polymer-Based Antimicrobial Hydrogels without Synthetic Antibiotics as Wound Dressings. Biomacromolecules 2020, 21, 2983–3006. [Google Scholar] [CrossRef]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Han, K.; Bai, Q.; Wu, W.; Sun, N.; Cui, N.; Lu, T. Gelatin-based adhesive hydrogel with self-healing, hemostasis, and electrical conductivity. Int. J. Biol. Macromol. 2021, 183, 2142–2151. [Google Scholar] [CrossRef]
- Watson, A.L.; Eckhart, K.E.; Wolf, M.E.; Sydlik, S.A. Hyaluronic Acid-Based Antibacterial Hydrogels for Use as Wound Dressings. ACS Appl. Bio Mater. 2022, 5, 5608–5616. [Google Scholar] [CrossRef]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2011, 11, 37–51. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 453. [Google Scholar] [CrossRef]
- Bai, Q.; Zheng, C.; Chen, W.; Sun, N.; Gao, Q.; Liu, J.; Hu, F.; Sahu, P.; Yan, X.; Zhang, Y.; et al. Current challenges and future applications of antibacterial nanomaterials and chitosan hydrogel in burn wound healing. Mater. Adv. 2022, 3, 6707–6727. [Google Scholar] [CrossRef]
- Carratalá, J.V.; Serna, N.; Villaverde, A.; Vázquez, E.; Ferrer-Miralles, N. Nanostructured antimicrobial peptides: The last push towards clinics. Biotechnol. Adv. 2020, 44, 107603. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.-Y.; Li, Q.; Feng, L.-B.; Hu, S.-X.; Zhang, S.-Q.; Li, C.-X.; Zhang, X.-B. Injectable Antimicrobial Hydrogels with Antimicrobial Peptide and Sanguinarine Controlled Release Ability for Preventing Bacterial Infections. Am. J. Transl. Res. 2021, 13, 12614. [Google Scholar] [PubMed]
- Wei, G.; Su, Z.; Reynolds, N.P.; Arosio, P.; Hamley, I.W.; Gazit, E.; Mezzenga, R. Self-assembling peptide and protein amyloids: From structure to tailored function in nanotechnology. Chem. Soc. Rev. 2017, 46, 4661–4708. [Google Scholar] [CrossRef]
- Schnaider, L.; Brahmachari, S.; Schmidt, N.W.; Mensa, B.; Shaham-Niv, S.; Bychenko, D.; Adler-Abramovich, L.; Shimon, L.J.W.; Kolusheva, S.; DeGrado, W.F.; et al. Self-assembling dipeptide antibacterial nanostructures with membrane disrupting activity. Nat. Commun. 2017, 8, 1365. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, A.P.; Draper, E.R.; Gilmore, B.F.; Laverty, G. Ultrashort self-assembling Fmoc-peptide gelators for anti-infective biomaterial applications. J. Pept. Sci. 2017, 23, 131–140. [Google Scholar] [CrossRef]
- Porter, S.L.; Coulter, S.M.; Pentlavalli, S.; Thompson, T.P.; Laverty, G. Self-assembling diphenylalanine peptide nanotubes selectively eradicate bacterial biofilm infection. Acta Biomater. 2018, 77, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Shome, A.; Das, D.; Das, P.K. Hydrogelation Through Self-Assembly of Fmoc-Peptide Functionalized Cationic Amphiphiles: Potent Antibacterial Agent. J. Phys. Chem. B 2010, 114, 4407–4415. [Google Scholar] [CrossRef] [PubMed]
- Mwangi, J.; Hao, X.; Lai, R.; Zhang, Z.-Y. Antimicrobial peptides: New hope in the war against multidrug resistance. Zool. Res. 2019, 40, 488–505. [Google Scholar] [CrossRef]
- Veiga, A.S.; Schneider, J.P. Antimicrobial hydrogels for the treatment of infection. Biopolymers 2013, 100, 637–644. [Google Scholar] [CrossRef]
- Kohanski, M.A.; Dwyer, D.J.; Collins, J.J. How antibiotics kill bacteria: From targets to networks. Nat. Rev. Microbiol. 2010, 8, 423–435. [Google Scholar] [CrossRef]
- Matica, M.A.; Aachmann, F.L.; Tøndervik, A.; Sletta, H.; Ostafe, V. Chitosan as a Wound Dressing Starting Material: Antimicrobial Properties and Mode of Action. Int. J. Mol. Sci. 2019, 20, 5889. [Google Scholar] [CrossRef]
- Ng, V.W.; Chan, J.M.; Sardon, H.; Ono, R.J.; García, J.M.; Yang, Y.Y.; Hedrick, J.L. Antimicrobial hydrogels: A new weapon in the arsenal against multidrug-resistant infections. Adv. Drug Deliv. Rev. 2014, 78, 46–62. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, W.; Xu, Q.; Zheng, Y. Progress in Antibacterial Hydrogel Dressing. Gels 2022, 8, 503. [Google Scholar] [CrossRef]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef]
- Sharma, P.K.; Halder, M.; Srivastava, U.; Singh, Y. Antibacterial PEG-Chitosan Hydrogels for Controlled Antibiotic/Protein Delivery. ACS Appl. Bio Mater. 2019, 2, 5313–5322. [Google Scholar] [CrossRef] [PubMed]
- Bal-Öztürk, A.; Özkahraman, B.; Özbaş, Z.; Yaşayan, G.; Tamahkar, E.; Alarçin, E. Advancements and future directions in the antibacterial wound dressings—A review. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 109, 703–716. [Google Scholar] [CrossRef]
- Simões, D.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P.; Mendonça, A.G.; Correia, I.J. Recent advances on antimicrobial wound dressing: A review. Eur. J. Pharm. Biopharm. 2018, 127, 130–141. [Google Scholar] [CrossRef]
- Germovsek, E.; I Barker, C.; Sharland, M. What do I need to know about aminoglycoside antibiotics. Arch. Dis. Child.-Educ. Pr. Ed. 2016, 102, 89–93. [Google Scholar] [CrossRef]
- Hodiamont, C.J.; Broek, A.K.V.D.; de Vroom, S.L.; Prins, J.M.; Mathôt, R.A.A.; van Hest, R.M. Clinical Pharmacokinetics of Gentamicin in Various Patient Populations and Consequences for Optimal Dosing for Gram-Negative Infections: An Updated Review. Clin. Pharmacokinet. 2022, 61, 1075–1094. [Google Scholar] [CrossRef]
- Wang, N.; Luo, J.; Deng, F.; Huang, Y.; Zhou, H. Antibiotic Combination Therapy: A Strategy to Overcome Bacterial Resistance to Aminoglycoside Antibiotics. Front. Pharmacol. 2022, 13, 553. [Google Scholar] [CrossRef]
- Baquero, F.; Levin, B.R. Proximate and ultimate causes of the bactericidal action of antibiotics. Nat. Rev. Genet. 2020, 19, 123–132. [Google Scholar] [CrossRef]
- Zhu, Y.; Hao, W.; Wang, X.; Ouyang, J.; Deng, X.; Yu, H.; Wang, Y. Antimicrobial peptides, conventional antibiotics, and their synergistic utility for the treatment of drug-resistant infections. Med. Res. Rev. 2022, 42, 1377–1422. [Google Scholar] [CrossRef]
- Tiwari, A.; Sharma, P.; Vishwamitra, B.; Singh, G. Review on Surface Treatment for Implant Infection via Gentamicin and Antibiotic Releasing Coatings. Coatings 2021, 11, 1006. [Google Scholar] [CrossRef]
- Xu, C.; Akakuru, O.U.; Ma, X.; Zheng, J.; Zheng, J.; Wu, A. Nanoparticle-Based Wound Dressing: Recent Progress in the Detection and Therapy of Bacterial Infections. Bioconjug. Chem. 2020, 31, 1708–1723. [Google Scholar] [CrossRef]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef]
- Hu, J.; Quan, Y.; Lai, Y.; Zheng, Z.; Hu, Z.; Wang, X.; Dai, T.; Zhang, Q.; Cheng, Y. A smart aminoglycoside hydrogel with tunable gel degradation, on-demand drug release, and high antibacterial activity. J. Control. Release 2017, 247, 145–152. [Google Scholar] [CrossRef]
- Zilberman, M.; Egozi, D.; Shemesh, M.; Keren, A.; Mazor, E.; Baranes-Zeevi, M.; Goldstein, N.; Berdicevsky, I.; Gilhar, A.; Ullmann, Y. Hybrid wound dressings with controlled release of antibiotics: Structure-release profile effects and in vivo study in a guinea pig burn model. Acta Biomater. 2015, 22, 155–163. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Hu, X.; Liang, J.; Fan, Y.; Zhang, X. Superabsorbent polysaccharide hydrogels based on pullulan derivate as antibacterial release wound dressing. J. Biomed. Mater. Res. Part A 2011, 98A, 31–39. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hadisi, Z.; Ismail, A.F.; Aziz, M.; Akbari, M.; Berto, F.; Chen, X. B, In vitro and in vivo evaluation of chitosan-alginate/gentamicin wound dressing nanofibrous with high antibacterial performance. Polym. Test. 2020, 82, 106298. [Google Scholar] [CrossRef]
- Lungu, I.-A.; Moldovan, O.-L.; Biriș, V.; Rusu, A. Fluoroquinolones Hybrid Molecules as Promising Antibacterial Agents in the Fight against Antibacterial Resistance. Pharmaceutics 2022, 14, 1749. [Google Scholar] [CrossRef]
- Kanth, S.; Puttaiahgowda, Y.M.; Gupta, S.; T, S. Recent advancements and perspective of ciprofloxacin-based antimicrobial polymers. J. Biomater. Sci. Polym. Ed. 2022, 1–34. [Google Scholar] [CrossRef]
- Kothari, A.; Jain, N.; Kumar, S.K.; Kumar, A.; Kaushal, K.; Kaur, S.; Pandey, A.; Gaurav, A.; Omar, B.J. Potential Synergistic Antibiotic Combinations against Fluoroquinolone-Resistant Pseudomonas aeruginosa. Pharmaceuticals 2022, 15, 243. [Google Scholar] [CrossRef]
- Sharma, D.; Patel, R.P.; Zaidi, S.T.R.; Sarker, M.R.; Lean, Q.Y.; Ming, L.C. Interplay of the Quality of Ciprofloxacin and Antibiotic Resistance in Developing Countries. Front. Pharmacol. 2017, 8, 546. [Google Scholar] [CrossRef]
- Gao, G.; Jiang, Y.-W.; Jia, H.-R.; Wu, F.-G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials 2018, 188, 83–95. [Google Scholar] [CrossRef]
- Millanao, A.R.; Mora, A.Y.; Villagra, N.A.; Bucarey, S.A.; Hidalgo, A.A. Biological Effects of Quinolones: A Family of Broad-Spectrum Antimicrobial Agents. Molecules 2021, 26, 7153. [Google Scholar] [CrossRef]
- Chrzanowska, A.; Struga, M.; Roszkowski, P.; Koliński, M.; Kmiecik, S.; Jałbrzykowska, K.; Zabost, A.; Stefańska, J.; Augustynowicz-Kopeć, E.; Wrzosek, M.; et al. The Effect of Conjugation of Ciprofloxacin and Moxifloxacin with Fatty Acids on Their Antibacterial and Anticancer Activity. Int. J. Mol. Sci. 2022, 23, 6261. [Google Scholar] [CrossRef]
- Aldred, K.J.; Kerns, R.J.; Osheroff, N. Mechanism of Quinolone Action and Resistance. Biochemistry 2014, 53, 1565–1574. [Google Scholar] [CrossRef]
- Fan, Y.; Lüchow, M.; Zhang, Y.; Lin, J.; Fortuin, L.; Mohanty, S.; Brauner, A.; Malkoch, M. Nanogel Encapsulated Hydrogels as Advanced Wound Dressings for the Controlled Delivery of Antibiotics. Adv. Funct. Mater. 2020, 31, 2006453. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Pacheco, G.; Islan, G.A.; Alvarez, V.A.; Barud, H.S.; Castro, G.R. Chitosan-bacterial cellulose patch of ciprofloxacin for wound dressing: Preparation and characterization studies. Int. J. Biol. Macromol. 2019, 147, 1136–1145. [Google Scholar] [CrossRef]
- Hanna, D.H.; Saad, G.R. Encapsulation of ciprofloxacin within modified xanthan gum- chitosan based hydrogel for drug delivery. Bioorganic Chem. 2018, 84, 115–124. [Google Scholar] [CrossRef]
- Khalil, I.A.; Saleh, B.; Ibrahim, D.M.; Jumelle, C.; Yung, A.; Dana, R.; Annabi, N. Ciprofloxacin-loaded bioadhesive hydrogels for ocular applications. Biomater. Sci. 2020, 8, 5196–5209. [Google Scholar] [CrossRef]
- Marchesan, S.; Qu, Y.; Waddington, L.J.; Easton, C.D.; Glattauer, V.; Lithgow, T.J.; McLean, K.M.; Forsythe, J.S.; Hartley, P.G. Self-assembly of ciprofloxacin and a tripeptide into an antimicrobial nanostructured hydrogel. Biomaterials 2013, 34, 3678–3687. [Google Scholar] [CrossRef] [PubMed]
- Berini, F.; Orlandi, V.; Gornati, R.; Bernardini, G.; Marinelli, F. Nanoantibiotics to fight multidrug resistant infections by Gram-positive bacteria: Hope or reality. Biotechnol. Adv. 2022, 57. [Google Scholar] [CrossRef]
- Biondi, S.; Chugunova, E.; Panunzio, M. From Natural Products to Drugs. Stud. Nat. Prod. Chem. 2016, 50, 249–297. [Google Scholar] [CrossRef]
- Zeng, D.; Debabov, D.; Hartsell, T.L.; Cano, R.J.; Adams, S.; Schuyler, J.A.; McMillan, R.; Pace, J.L. Approved Glycopeptide Antibacterial Drugs: Mechanism of Action and Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a026989. [Google Scholar] [CrossRef]
- Boot, W.; Schmid, T.; D’Este, M.; Guillaume, O.; Foster, A.; Decosterd, L.; Richards, R.G.; Eglin, D.; Zeiter, S.; Moriarty, T.F. A Hyaluronic Acid Hydrogel Loaded with Gentamicin and Vancomycin Successfully Eradicates Chronic Methicillin-Resistant Staphylococcus aureus Orthopedic Infection in a Sheep Model. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef]
- del Olmo, J.A.; Pérez-Álvarez, L.; Martínez, V.S.; Cid, S.B.; González, R.P.; Vilas-Vilela, J.L.; Alonso, J.M. Drug Delivery from Hyaluronic Acid–BDDE Injectable Hydrogels for Antibacterial and Anti-Inflammatory Applications. Gels 2022, 8, 223. [Google Scholar] [CrossRef]
- Posadowska, U.; Brzychczy-Wloch, M.; Pamula, E. Injectable gellan gum-based nanoparticles-loaded system for the local delivery of vancomycin in osteomyelitis treatment. J. Mater. Sci. Mater. Med. 2015, 27, 1–9. [Google Scholar] [CrossRef]
- Myers, S.A.; Bennett, T. XPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1–4. [Google Scholar]
- Chang, C.H.; Lin, Y.H.; Yeh, C.L.; Chen, Y.C.; Chiou, S.F.; Hsu, Y.M.; Chen, Y.S.; Wang, C.C. Nanoparticles Incorporated in PH-Sensitive Hydrogels as Amoxicillin Delivery for Eradication of Helicobacter Pylori. Biomacromolecules 2010, 11, 133–142. [Google Scholar] [CrossRef]
- Torres-Figueroa, A.V.; Pérez-Martínez, C.J.; del Castillo-Castro, T.; Bolado-Martínez, E.; Corella-Madueño, M.A.G.; García-Alegría, A.M.; Lara-Ceniceros, T.E.; Armenta-Villegas, L. Composite Hydrogel of Poly(acrylamide) and Starch as Potential System for Controlled Release of Amoxicillin and Inhibition of Bacterial Growth. J. Chem. 2020, 2020, 5860487. [Google Scholar] [CrossRef]
- Khan, Y.A.; Ozaltin, K.; Bernal-Ballen, A.; Di Martino, A. Chitosan-alginate hydrogels for simultaneous and sustained releases of ciprofloxacin, amoxicillin and vancomycin for combination therapy. J. Drug Deliv. Sci. Technol. 2020, 61, 102126. [Google Scholar] [CrossRef]
- del Olmo, J.A.; Alonso, J.M.; Sáez-Martínez, V.; Benito-Cid, S.; Moreno-Benítez, I.; Bengoa-Larrauri, M.; Pérez-González, R.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Self-healing, antibacterial and anti-inflammatory chitosan-PEG hydrogels for ulcerated skin wound healing and drug delivery. Biomater. Adv. 2022, 139, 212992. [Google Scholar] [CrossRef] [PubMed]
- del Olmo, J.A.; Pérez-Álvarez, L.; Sáez-Martínez, V.; Benito-Cid, S.; Ruiz-Rubio, L.; Pérez-González, R.; Vilas-Vilela, J.L.; Alonso, J.M. Wound healing and antibacterial chitosan-genipin hydrogels with controlled drug delivery for synergistic anti-inflammatory activity. Int. J. Biol. Macromol. 2022, 203, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, A.S.; Afifi, S.A.; Elkhodairy, K.A. Newly Developed Topical Cefotaxime Sodium Hydrogels: Antibacterial Activity and In Vivo Evaluation. BioMed Res. Int. 2016, 2016, 6525163. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; O’Connell, C.D.; Cometta, S.; Hutmacher, D.W.; Meinert, C.; Bock, N. Gelatin Methacryloyl Hydrogels for the Localized Delivery of Cefazolin. Polymers 2021, 13, 3960. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Aslam, Z.; Ullah, S.; Shah, M.R. Synthesis of pH responsive, photocrosslinked gelatin-based hydrogel system for control release of ceftriaxone. Chem. Phys. Lipids 2021, 238, 105101. [Google Scholar] [CrossRef] [PubMed]
- Candido, J.D.C.; Conceição, N.A.; Moreira, A.P.D.; Calçada, L.A.; Araújo, L.S.; dos Santos, R.A.; Middea, A.; Luchese, R.; Prudencio, E.R.; Castro, R.N.; et al. Alginate hydrogels incorporating neomycin or propolis as potential dressings for diabetic ulcers: Structure, swelling, and antimicrobial barrier properties. Polym. Adv. Technol. 2019, 30, 2623–2635. [Google Scholar] [CrossRef]
- Zhong, Y.; Seidi, F.; Li, C.; Wan, Z.; Jin, Y.; Song, J.; Xiao, H. Antimicrobial/Biocompatible Hydrogels Dual-Reinforced by Cellulose as Ultrastretchable and Rapid Self-Healing Wound Dressing. Biomacromolecules 2021, 22, 1654–1663. [Google Scholar] [CrossRef]
- Cheng, Y.; Chang, Y.; Ko, Y.; Liu, C.J. Sustained release of levofloxacin from thermosensitive chitosan-based hydrogel for the treatment of postoperative endophthalmitis. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 108, 8–13. [Google Scholar] [CrossRef]
- Chang, Y.-F.; Cheng, Y.-H.; Ko, Y.-C.; Chiou, S.-H.; Liu, C.J.-L. Development of topical chitosan/ β-glycerophosphate-based hydrogel loaded with levofloxacin in the treatment of keratitis: An ex-vivo study. Heliyon 2021, 8, e08697. [Google Scholar] [CrossRef]
- Montanari, E.; D’Arrigo, G.; Di Meo, C.; Virga, A.; Coviello, T.; Passariello, C.; Matricardi, P. Chasing bacteria within the cells using levofloxacin-loaded hyaluronic acid nanohydrogels. Eur. J. Pharm. Biopharm. 2014, 87, 518–523. [Google Scholar] [CrossRef]
- Panahi, Y.; Gharekhani, A.; Hamishehkar, H.; Zakeri-Milani, P.; Gharekhani, H. Stomach-Specific Drug Delivery of Clarithromycin Using aSemi Interpenetrating Polymeric Network Hydrogel Made of Montmorillonite and Chitosan: Synthesis, Characterization and InVitro Drug Release Study. Adv. Pharm. Bull. 2019, 9, 159–173. [Google Scholar] [CrossRef]
- Moghaddam, R.H.; Dadfarnia, S.; Shabani, A.M.H.; Amraei, R.; Moghaddam, Z.H. Doxycycline drug delivery using hydrogels of O-carboxymethyl chitosan conjugated with caffeic acid and its composite with polyacrylamide synthesized by electron beam irradiation. Int. J. Biol. Macromol. 2020, 154, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, B.; Li, M.; He, J.; Yin, Z.; Guo, B. Injectable Antimicrobial Conductive Hydrogels for Wound Disinfection and Infectious Wound Healing. Biomacromolecules 2020, 21, 1841–1852. [Google Scholar] [CrossRef] [PubMed]
- Özkahraman, B.; Tamahkar, E.; Idil, N.; Suloglu, A.K.; Perçin, I. Evaluation of hyaluronic acid nanoparticle embedded chitosan–gelatin hydrogels for antibiotic release. Drug Dev. Res. 2020, 82, 241–250. [Google Scholar] [CrossRef]
- Delir, S.; Sirousazar, M.; Kheiri, F. Clindamycin releasing bionanocomposite hydrogels as potential wound dressings for the treatment of infected wounds. J. Biomater. Sci. Polym. Ed. 2020, 31, 1489–1514. [Google Scholar] [CrossRef]
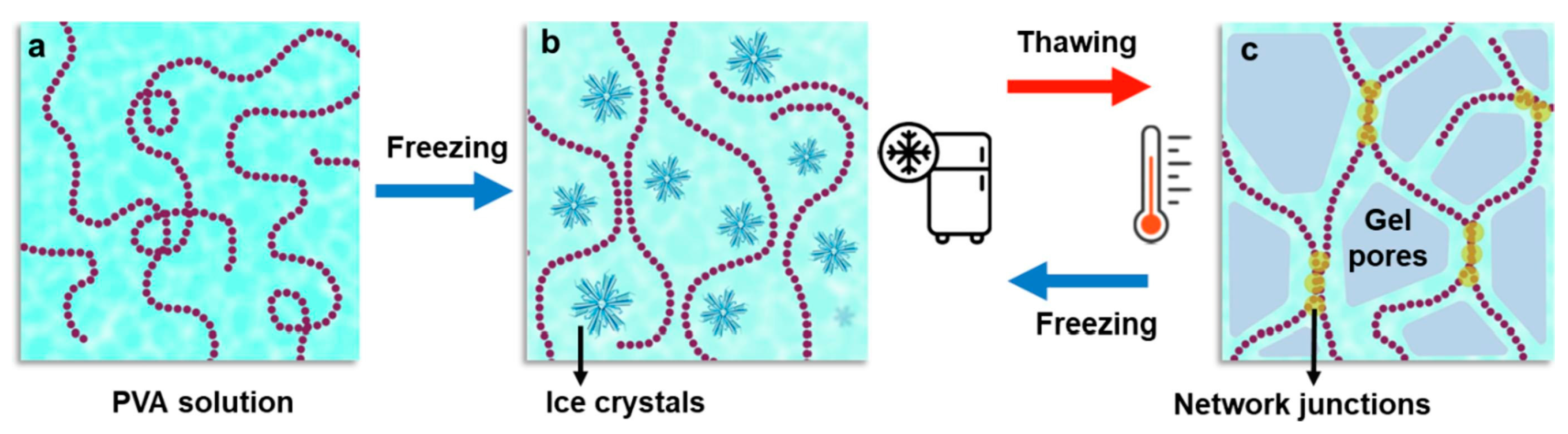
- Adelnia, H.; Ensandoost, R.; Moonshi, S.S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Qing, X.; He, G.; Liu, Z.; Yin, Y.; Cai, W.; Fan, L.; Fardim, P. Preparation and properties of polyvinyl alcohol/N–succinyl chitosan/lincomycin composite antibacterial hydrogels for wound dressing. Carbohydr. Polym. 2021, 261, 117875. [Google Scholar] [CrossRef]
- Spížek, J.; Řezanka, T. Lincosamides: Chemical structure, biosynthesis, mechanism of action, resistance, and applications. Biochem. Pharmacol. 2017, 133, 20–28. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, D.; Quan, L.; Chai, H.; Sui, X.; Wang, B.; Xu, H.; Mao, Z. Mussel-inspired adhesive gelatin-polyacrylamide hydrogel wound dressing loaded with tetracycline hydrochloride to enhance complete skin regeneration. Soft Matter 2021, 18, 662–674. [Google Scholar] [CrossRef]
- Motasadizadeh, H.; Tavakoli, M.; Damoogh, S.; Mottaghitalab, F.; Gholami, M.; Atyabi, F.; Farokhi, M.; Dinarvand, R. Dual drug delivery system of teicoplanin and phenamil based on pH-sensitive silk fibroin/sodium alginate hydrogel scaffold for treating chronic bone infection. Biomater. Adv. 2022, 139, 213032. [Google Scholar] [CrossRef]
- Roska, T.P.; Sartini; Mudjahid, M.; Marzaman, A.N.F.; Datu, N.N.P.; Permana, A.D. Development of chloramphenicol wound dressing protein-based microparticles in chitosan hydrogel system for improved effectiveness of dermal wound therapy. Biomater. Adv. 2022, 143, 213175. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, F.; Long, L.; Kong, Q.; Luo, R.; Wang, Y. Dual-responsive injectable hydrogels encapsulating drug-loaded micelles for on-demand antimicrobial activity and accelerated wound healing. J. Control. Release 2020, 324, 204–217. [Google Scholar] [CrossRef]
- Zagra, L.; Gallazzi, E.; Romanò, D.; Scarponi, S.; Romanò, C. Two-stage cementless hip revision for peri-prosthetic infection with an antibacterial hydrogel coating: Results of a comparative series. Int. Orthop. 2018, 43, 111–115. [Google Scholar] [CrossRef]
- Drago, L.; Boot, W.; Dimas, K.; Malizos, K.; Hänsch, G.M.; Stuyck, J.; Gawlitta, D.; Romanò, C.L. Does Implant Coating with Antibacterial-Loaded Hydrogel Reduce Bacterial Colonization and Biofilm Formation In Vitro. Clin. Orthop. Relat. Res. 2014, 472, 3311–3323. [Google Scholar] [CrossRef]
- Malizos, K.; Blauth, M.; Danita, A.; Capuano, N.; Mezzoprete, R.; Logoluso, N.; Drago, L.; Romanò, C.L. Fast-resorbable antibiotic-loaded hydrogel coating to reduce post-surgical infection after internal osteosynthesis: A multicenter randomized controlled trial. J. Orthop. Traumatol. 2017, 18, 159–169. [Google Scholar] [CrossRef]
- Hu, J.; Zheng, Z.; Liu, C.; Hu, Q.; Cai, X.; Xiao, J.; Cheng, Y. A pH-responsive hydrogel with potent antibacterial activity against both aerobic and anaerobic pathogens. Biomater. Sci. 2018, 7, 581–584. [Google Scholar] [CrossRef]
- Micale, N.; Citarella, A.; Molonia, M.S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Hydrogels for the Delivery of Plant-Derived (Poly)Phenols. Molecules 2020, 25, 3254. [Google Scholar] [CrossRef]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial hydrogels: Promising materials for medical application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef]
- Nafee, N.; Youssef, A.; El-Gowelli, H.; Asem, H.; Kandil, S. Antibiotic-free nanotherapeutics: Hypericin nanoparticles thereof for improved in vitro and in vivo antimicrobial photodynamic therapy and wound healing. Int. J. Pharm. 2013, 454, 249–258. [Google Scholar] [CrossRef]
- Lai, W.-F.; Rogach, A.L. Hydrogel-Based Materials for Delivery of Herbal Medicines. ACS Appl. Mater. Interfaces 2017, 9, 11309–11320. [Google Scholar] [CrossRef]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Hemeg, H.A.; Moussa, I.M.; Ibrahim, S.; Dawoud, T.M.; Alhaji, J.H.; Mubarak, A.S.; Kabli, S.A.; Alsubki, R.A.; Tawfik, A.M.; Marouf, S.A. Antimicrobial effect of different herbal plant extracts against different microbial population. Saudi J. Biol. Sci. 2020, 27, 3221–3227. [Google Scholar] [CrossRef]
- Chummun, I.; Bekah, D.; Goonoo, N.; Bhaw-Luximon, A. Assessing the mechanisms of action of natural molecules/extracts for phase-directed wound healing in hydrogel scaffolds. RSC Med. Chem. 2021, 12, 1476–1490. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; te Velde, A.A.; de Boer, L.; Speijer, D.; Vandenbroucke-Grauls, M.J.C.; Zaat, S.A.J. How honey kills bacteria. FASEB J. 2010, 24, 2576–2582. [Google Scholar] [CrossRef]
- Yusof, N.; Hafiza, A.A.; Zohdi, R.M.; Bakar, Z.A. Development of honey hydrogel dressing for enhanced wound healing. Radiat. Phys. Chem. 2007, 76, 1767–1770. [Google Scholar] [CrossRef]
- Zhong, Y.; Seidi, F.; Wang, Y.; Zheng, L.; Jin, Y.; Xiao, H. Injectable chitosan hydrogels tailored with antibacterial and antioxidant dual functions for regenerative wound healing. Carbohydr. Polym. 2022, 298, 120103. [Google Scholar] [CrossRef]
- Feng, X.; Hou, X.; Cui, C.; Sun, S.; Sadik, S.; Wu, S.; Zhou, F. Mechanical and antibacterial properties of tannic acid-encapsulated carboxymethyl chitosan/polyvinyl alcohol hydrogels. Eng. Regen. 2021, 2, 57–62. [Google Scholar] [CrossRef]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Cai, K.; Zhang, B.; Tang, S.; Zhang, W.; Liu, W. Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing. Burn. Trauma 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Gheorghita, D.; Grosu, E.; Robu, A.; Ditu, L.M.; Deleanu, I.M.; Pircalabioru, G.G.; Raiciu, A.-D.; Bita, A.-I.; Antoniac, A.; Antoniac, V.I. Essential Oils as Antimicrobial Active Substances in Wound Dressings. Materials 2022, 15, 6923. [Google Scholar] [CrossRef]
- Mahmood, H.; Khan, I.U.; Asif, M.; Khan, R.U.; Asghar, S.; Khalid, I.; Khalid, S.H.; Irfan, M.; Rehman, F.; Shahzad, Y.; et al. In vitro and in vivo evaluation of gellan gum hydrogel films: Assessing the co impact of therapeutic oils and ofloxacin on wound healing. Int. J. Biol. Macromol. 2020, 166, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Low, W.L.; Kenward, M.; Cairul, M.; Amin, I.M.; Martin, C.; Nahar, L.; Basar, N.; Sarker, S.D.; Adams, J.D. Ionically Crosslinked Chitosan Hydrogels for the Controlled Release of Antimicrobial Essential Oils and Metal Ions for Wound Management Applications. Medicines 2016, 3, 8. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Altaf, F.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Akram, M.A.; Safdar, A.; Butt, M.S.; Noor, T.; Sher, F. Synthesis and Characterization of PVA/Starch Hydrogel Membranes Incorporating Essential Oils Aimed to Be Used in Wound Dressing Applications. J. Polym. Environ. 2021, 29, 156–174. [Google Scholar] [CrossRef]
- Lu, G.; Shen, X.; Xiao, D.; Rong, L.; Mao, Z.; Wang, B.; Sui, X.; Zhao, M.; Feng, X. Antibacterial thyme oil-loaded zwitterionic emulsion hydrogels. J. Mater. Chem. B 2022, 10, 2691–2698. [Google Scholar] [CrossRef]
- Jiji, S.; Udhayakumar, S.; Rose, C.; Muralidharan, C.; Kadirvelu, K. Thymol enriched bacterial cellulose hydrogel as effective material for third degree burn wound repair. Int. J. Biol. Macromol. 2018, 122, 452–460. [Google Scholar] [CrossRef]
- Najafloo, R.; Behyari, M.; Imani, R.; Nour, S. A mini-review of Thymol incorporated materials: Applications in antibacterial wound dressing. J. Drug Deliv. Sci. Technol. 2020, 60, 101904. [Google Scholar] [CrossRef]
- Alven, S.; Nqoro, X.; Aderibigbe, B.A. Polymer-Based Materials Loaded with Curcumin for Wound Healing Applications. Polymers 2020, 12, 2286. [Google Scholar] [CrossRef]
- Miguel, S.P.; Sequeira, R.S.; Moreira, A.F.; Cabral, C.S.; Mendonça, A.G.; Ferreira, P.; Correia, I.J. An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process. Eur. J. Pharm. Biopharm. 2019, 139, 1–22. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, M.; Xu, M.; Miao, F.; Merzougui, C.; Zhang, X.; Wei, Y.; Chen, W.; Huang, D. The fabrication of antibacterial hydrogels for wound healing. Eur. Polym. J. 2021, 146, 110268. [Google Scholar] [CrossRef]
- Kant, V.; Gopal, A.; Pathak, N.N.; Kumar, P.; Tandan, S.K.; Kumar, D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2014, 20, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Merrell, J.G.; McLaughlin, S.W.; Tie, L.; Laurencin, C.T.; Chen, A.F.; Nair, L.S. Curcumin loaded poly (ε-caprolactone) nanofibers: Diabetic wound dressing with antioxidant and anti-inflammatory properties. Clin. Exp. Pharmacol. Physiol. 2009, 36, 1149–1156. [Google Scholar] [CrossRef]
- Fathollahipour, S.; Koosha, M.; Tavakoli, J.; Maziarfar, S.; Mehrabadi, J.F. Erythromycin releasing PVA/sucrose and PVA/honey hydrogels as wound dressings with antibacterial activity and enhanced bio-adhesion. Iran. J. Pharm. Res. IJPR 2020, 19, 448–464. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Arce, E.; Chávez-Soto, M.A.; Herrera-Arellano, A.; Arzate, S.; Agüero, J.; Feria-Romero, I.A.; Cruz-Guzmán, A.; Lozoya, X. Therapeutic effectiveness of a Mimosa tenuiflora cortex extract in venous leg ulceration treatment. J. Ethnopharmacol. 2007, 109, 523–528. [Google Scholar] [CrossRef]
- Koneru, A.; Dharmalingam, K.; Anandalakshmi, R. Cellulose based nanocomposite hydrogel films consisting of sodium carboxymethylcellulose–grapefruit seed extract nanoparticles for potential wound healing applications. Int. J. Biol. Macromol. 2020, 148, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Polat, T.G.; Duman, O.; Tunç, S. Agar/κ-carrageenan/montmorillonite nanocomposite hydrogels for wound dressing applications. Int. J. Biol. Macromol. 2020, 164, 4591–4602. [Google Scholar] [CrossRef]
- Yegappan, R.; Selvaprithiviraj, V.; Amirthalingam, S.; Jayakumar, R. Carrageenan based hydrogels for drug delivery, tissue engineering and wound healing. Carbohydr. Polym. 2018, 198, 385–400. [Google Scholar] [CrossRef]
- Azizi, S.; Mohamad, R.; Rahim, R.A.; Mohammadinejad, R.; Bin Ariff, A. Hydrogel beads bio-nanocomposite based on Kappa-Carrageenan and green synthesized silver nanoparticles for biomedical applications. Int. J. Biol. Macromol. 2017, 104, 423–431. [Google Scholar] [CrossRef]
- Stompor-Gorący, M.; Bajek-Bil, A.; Machaczka, M. Chrysin: Perspectives on Contemporary Status and Future Possibilities as Pro-Health Agent. Nutrients 2021, 13, 2038. [Google Scholar] [CrossRef] [PubMed]
- Pushpavalli, G.; Kalaiarasi, P.; Veeramani, C.; Pugalendi, K.V. Effect of chrysin on hepatoprotective and antioxidant status in d-galactosamine-induced hepatitis in rats. Eur. J. Pharmacol. 2010, 631, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Cho, H. Modulation of the activity of pro-inflammatory enzymes, COX-2 and iNOS, by chrysin derivatives. Pharmacol. Res. 2004, 49, 37–43. [Google Scholar] [CrossRef]
- Gupta, P.; Sheikh, A.; Abourehab, M.A.S.; Kesharwani, P. Amelioration of Full-Thickness Wound Using Hesperidin Loaded Dendrimer-Based Hydrogel Bandages. Biosensors 2022, 12, 462. [Google Scholar] [CrossRef]
- Bagher, Z.; Ehterami, A.; Safdel, M.H.; Khastar, H.; Semiari, H.; Asefnejad, A.; Davachi, S.M.; Mirzaii, M.; Salehi, M. Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. J. Drug Deliv. Sci. Technol. 2019, 55, 101379. [Google Scholar] [CrossRef]
- Politano, A.D.; Campbell, K.T.; Rosenberger, L.H.; Sawyer, R.G. Use of Silver in the Prevention and Treatment of Infections: Silver Review. Surg. Infect. 2013, 14, 8–20. [Google Scholar] [CrossRef]
- Ricketts, C.R.; Lowbury, E.J.L.; Lawrence, J.C.; Hall, M.; Wilkins, M.D. Mechanism of Prophylaxis by Silver Compounds against Infection of Burns. BMJ 1970, 2, 444–446. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.L.; Yang, Q.; Davis, S.; Sampson, E.M.; I Azeke, J.; Hamad, A.; Schultz, G.S. Antimicrobial dressing efficacy against mature Pseudomonas aeruginosa biofilm on porcine skin explants. Int. Wound J. 2013, 12, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Pangli, H.; Vatanpour, S.; Hortamani, S.; Jalili, R.; Ghahary, A. Incorporation of Silver Nanoparticles in Hydrogel Matrices for Controlling Wound Infection. J. Burn. Care Res. 2020, 42, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Stankic, S.; Suman, S.; Haque, F.; Vidic, J. Pure and multi metal oxide nanoparticles: Synthesis, antibacterial and cytotoxic properties. J. Nanobiotechnol. 2016, 14, 73. [Google Scholar] [CrossRef]
- Gedik, G.; Aksit, A.; Engin, B.; Paksu, U. Production of Metal Oxide Containing Antibacterial Coated Textile Material and Investigation of the Mechanism of Action. Fibers Polym. 2018, 19, 2548–2563. [Google Scholar] [CrossRef]
- Singh, A.; Dubey, A.K. Various Biomaterials and Techniques for Improving Antibacterial Response. ACS Appl. Biol. Mater. 2018, 1, 3–20. [Google Scholar] [CrossRef]
- Godoy-Gallardo, M.; Eckhard, U.; Delgado, L.M.; de Roo Puente, Y.J.; Hoyos-Nogués, M.; Gil, F.J.; Perez, R.A. Antibacterial approaches in tissue engineering using metal ions and nanoparticles: From mechanisms to applications. Bioact. Mater. 2021, 6, 4470–4490. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Turner, T.D. Interactive dressings used in the management of human soft tissue injuries and their potential in veterinary practice. Vet. Dermatol. 1997, 8, 235–242. [Google Scholar] [CrossRef]
- El-Mekkawi, D.M.; Selim, M.M.; Hamdi, N.; Hassan, S.; Ezzat, A. Studies on the influence of the physicochemical characteristics of nanostructured copper, zinc and magnesium oxides on their antibacterial activities. J. Environ. Chem. Eng. 2018, 6, 5608–5615. [Google Scholar] [CrossRef]
- Malachová, K.; Praus, P.; Rybková, Z.; Kozák, O. Antibacterial and antifungal activities of silver, copper and zinc montmorillonites. Appl. Clay Sci. 2011, 53, 642–645. [Google Scholar] [CrossRef]
- Nešović, K.; Mišković-Stanković, V. A comprehensive review of the polymer-based hydrogels with electrochemically synthesized silver nanoparticles for wound dressing applications. Polym. Eng. Sci. 2020, 60, 1393–1419. [Google Scholar] [CrossRef]
- Piras, C.C.; Mahon, C.S.; Smith, D.K. Self-Assembled Supramolecular Hybrid Hydrogel Beads Loaded with Silver Nanoparticles for Antimicrobial Applications. Chem.–A Eur. J. 2020, 26, 8452–8457. [Google Scholar] [CrossRef]
- Xie, Y.; Yue, L.; Zheng, Y.; Zhao, L.; Liang, C.; He, W.; Liu, Z.; Sun, Y.; Yang, Y. The antibacterial stability of poly(dopamine) in-situ reduction and chelation nano-Ag based on bacterial cellulose network template. Appl. Surf. Sci. 2019, 491, 383–394. [Google Scholar] [CrossRef]
- Li, J.; Zheng, J.; Yu, Y.; Su, Z.; Zhang, L.; Chen, X. Facile synthesis of rGO–MoS2–Ag nanocomposites with long-term antimicrobial activities. Nanotechnology 2020, 31, 125101. [Google Scholar] [CrossRef]
- Łysakowska, M.E.; Ciebiada-Adamiec, A.; Klimek, L.; Sienkiewicz, M. The activity of silver nanoparticles (Axonnite) on clinical and environmental strains of Acinetobacter spp. Burns 2015, 41, 364–371. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Ahsan, A.; Farooq, M.A. Therapeutic potential of green synthesized silver nanoparticles loaded PVA hydrogel patches for wound healing. J. Drug Deliv. Sci. Technol. 2019, 54, 101308. [Google Scholar] [CrossRef]
- Nguyen, N.T.-P.; Nguyen, L.V.-H.; Thanh, N.T.; Van Toi, V.; Quyen, T.N.; Tran, P.A.; Wang, H.-M.D.; Nguyen, T.-H. Stabilization of silver nanoparticles in chitosan and gelatin hydrogel and its applications. Mater. Lett. 2019, 248, 241–245. [Google Scholar] [CrossRef]
- Rescignano, N.; Hernandez, R.; Lopez, L.D.; Calvillo, I.; Kenny, J.M.; Mijangos, C. Preparation of alginate hydrogels containing silver nanoparticles: A facile approach for antibacterial applications. Polym. Int. 2016, 65, 921–926. [Google Scholar] [CrossRef]
- Kumar, A.; Behl, T.; Chadha, S. Synthesis of physically crosslinked PVA/Chitosan loaded silver nanoparticles hydrogels with tunable mechanical properties and antibacterial effects. Int. J. Biol. Macromol. 2020, 149, 1262–1274. [Google Scholar] [CrossRef]
- Urzedo, A.L.; Gonçalves, M.C.; Nascimento, M.H.M.; Lombello, C.B.; Nakazato, G.; Seabra, A.B. Cytotoxicity and Anti-bacterial Activity of Alginate Hydrogel Containing Nitric Oxide Donor and Silver Nanoparticles for Topical Applications. ACS Biomater. Sci. Eng. 2020, 6, 2117–2134. [Google Scholar] [CrossRef]
- Mohamed, R.R.; Sabaa, M.W. Synthesis and characterization of antimicrobial crosslinked carboxymethyl chitosan nanoparticles loaded with silver. Int. J. Biol. Macromol. 2014, 69, 95–99. [Google Scholar] [CrossRef]
- Chen, K.; Wang, F.; Liu, S.; Wu, X.; Xu, L.; Zhang, D. In situ reduction of silver nanoparticles by sodium alginate to obtain silver-loaded composite wound dressing with enhanced mechanical and antimicrobial property. Int. J. Biol. Macromol. 2020, 148, 501–509. [Google Scholar] [CrossRef]
- Li, M.; Jiang, X.; Wang, D.; Xu, Z.; Yang, M. In situ reduction of silver nanoparticles in the lignin based hydrogel for enhanced antibacterial application. Colloids Surfaces B Biointerfaces 2019, 177, 370–376. [Google Scholar] [CrossRef]
- Velmurugan, P.; Anbalagan, K.; Manosathyadevan, M.; Lee, K.-J.; Cho, M.; Lee, S.-M.; Park, J.-H.; Oh, S.-G.; Bang, K.-S.; Oh, B.-T. Green synthesis of silver and gold nanoparticles using Zingiber officinale root extract and antibacterial activity of silver nanoparticles against food pathogens. Bioprocess Biosyst. Eng. 2014, 37, 1935–1943. [Google Scholar] [CrossRef]
- Chen, Y.; Qiu, N.; Ma, B.; Hu, G.; Oh, D.-H.; Fu, X. Preparation and characterization of a novel antibacterial hydrogel based on thiolated ovalbumin/gelatin with silver ions. Innov. Food Sci. Emerg. Technol. 2022, 78, 103007. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, Y.; Wang, L.; Xu, L.; Zhai, M.; Wei, S. Radiation synthesis and characterization of nanosilver/gelatin/carboxymethyl chitosan hydrogel. Radiat. Phys. Chem. 2012, 81, 553–560. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Guo, Z.; Xiao, Y.; Zhang, Y.; Sun, X.; Zhe, T.; Cao, Y.; Wang, L.; Lu, Q.; et al. Silver nanoparticle-embedded hydrogel as a photothermal platform for combating bacterial infections. Chem. Eng. J. 2019, 382, 122990. [Google Scholar] [CrossRef]
- Reithofer, M.R.; Lakshmanan, A.; Ping, A.T.; Chin, J.M.; Hauser, C.A. In situ synthesis of size-controlled, stable silver nanoparticles within ultrashort peptide hydrogels and their anti-bacterial properties. Biomaterials 2014, 35, 7535–7542. [Google Scholar] [CrossRef]
- Shrivastava, S.; Bera, T.; Roy, A.; Singh, G.; Ramachandrarao, P.; Dash, D. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology 2007, 18, 225103. [Google Scholar] [CrossRef]
- Anisha, B.; Biswas, R.; Chennazhi, K.; Jayakumar, R. Chitosan-hyaluronic acid/nano silver composite sponges for drug resistant bacteria infected diabetic wounds. Int. J. Biol. Macromol. 2013, 62, 310–320. [Google Scholar] [CrossRef]
- Ruffo, M.; Parisi, O.I.; Dattilo, M.; Patitucci, F.; Malivindi, R.; Pezzi, V.; Tzanov, T.; Puoci, F. Synthesis and evaluation of wound healing properties of hydro-diab hydrogel loaded with green-synthetized AGNPS: In vitro and in ex vivo studies. Drug Deliv. Transl. Res. 2022, 12, 1881–1894. [Google Scholar] [CrossRef]
- Boonkaew, B.; Suwanpreuksa, P.; Cuttle, L.; Barber, P.M.; Supaphol, P. Hydrogels containing silver nanoparticles for burn wounds show antimicrobial activity without cytotoxicity. J. Appl. Polym. Sci. 2013, 131. [Google Scholar] [CrossRef]
- Adhya, A.; Bain, J.; Dutta, G.; Hazra, A.; Majumdar, B.; Ray, O.; Ray, S.; Adhikari, S. Healing of burn wounds by topical treatment: A randomized controlled comparison between silver sulfadiazine and nano-crystalline silver. J. Basic Clin. Pharm. 2015, 6, 29–34. [Google Scholar] [CrossRef]
- Gunasekaran, T.; Nigusse, T.; Dhanaraju, M.D. Silver Nanoparticles as Real Topical Bullets for Wound Healing. J. Am. Coll. Clin. Wound Spec. 2011, 3, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Boonkaew, B.; Kempf, M.; Kimble, R.; Supaphol, P.; Cuttle, L. Antimicrobial efficacy of a novel silver hydrogel dressing compared to two common silver burn wound dressings: Acticoat™ and PolyMem Silver®. Burns 2014, 40, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Mi, F.-L.; Wu, Y.-B.; Shyu, S.-S.; Schoung, J.-Y.; Huang, Y.-B.; Tsai, Y.-H.; Hao, J.-Y. Control of wound infections using a bilayer chitosan wound dressing with sustainable antibiotic delivery. J. Biomed. Mater. Res. 2001, 59, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Talodthaisong, C.; Boonta, W.; Thammawithan, S.; Patramanon, R.; Kamonsutthipaijit, N.; Hutchison, J.; Kulchat, S. Composite guar gum-silver nanoparticle hydrogels as self-healing, injectable, and antibacterial biomaterials. Mater. Today Commun. 2020, 24, 100992. [Google Scholar] [CrossRef]
- Palem, R.R.; Rao, K.M.; Kang, T.J. Self-healable and dual-functional guar gum-grafted-polyacrylamidoglycolic acid-based hydrogels with nano-silver for wound dressings. Carbohydr. Polym. 2019, 223, 115074. [Google Scholar] [CrossRef]
- Raho, R.; Nguyen, N.-Y.; Zhang, N.; Jiang, W.; Sannino, A.; Liu, H.; Pollini, M.; Paladini, F. Photo-assisted green synthesis of silver doped silk fibroin/carboxymethyl cellulose nanocomposite hydrogels for biomedical applications. Mater. Sci. Eng. C 2019, 107, 110219. [Google Scholar] [CrossRef]
- Liu, R.; Dai, L.; Si, C.; Zeng, Z. Antibacterial and hemostatic hydrogel via nanocomposite from cellulose nanofibers. Carbohydr. Polym. 2018, 195, 63–70. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ueda, K.; Ogasawara, K.; Kanetaka, H.; Narushima, T. In vitro evaluation of Ag-containing calcium phosphates: Effectiveness of Ag-incorporated β-tricalcium phosphate. Mater. Sci. Eng. C 2017, 75, 926–933. [Google Scholar] [CrossRef]
- Haidari, H.; Kopecki, Z.; Bright, R.; Cowin, A.J.; Garg, S.; Goswami, N.; Vasilev, K. Ultrasmall AgNP-Impregnated Bio-compatible Hydrogel with Highly Effective Biofilm Elimination Properties. ACS Appl. Mater. Interfaces 2020, 12, 41011–41025. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Huang, J.; Wu, X.; Ren, Y.; Li, Z.; Ren, J. Controlled release of silver ions from AgNPs using a hydrogel based on konjac glucomannan and chitosan for infected wounds. Int. J. Biol. Macromol. 2020, 149, 148–157. [Google Scholar] [CrossRef]
- Haidari, H.; Kopecki, Z.; Sutton, A.; Garg, S.; Cowin, A.; Vasilev, K. pH-Responsive “Smart” Hydrogel for Controlled Delivery of Silver Nanoparticles to Infected Wounds. Antibiotics 2021, 10, 49. [Google Scholar] [CrossRef]
- Brown, A.N.; Smith, K.; Samuels, T.A.; Lu, J.; Obare, S.O.; Scott, M.E. Nanoparticles Functionalized with Ampicillin Destroy Multiple-Antibiotic-Resistant Isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and Methicillin-Resistant Staphylococcus aureus. Appl. Environ. Microbiol. 2012, 78, 2768–2774. [Google Scholar] [CrossRef]
- Daniel-Da-Silva, A.L.; Salgueiro, A.M.; Trindade, T. Effects of Au nanoparticles on thermoresponsive genipin-crosslinked gelatin hydrogels. Gold Bull. 2013, 46, 25–33. [Google Scholar] [CrossRef]
- Ribeiro, M.; Ferraz, M.P.; Monteiro, F.J.; Fernandes, M.H.; Beppu, M.M.; Mantione, D.; Sardon, H. Antibacterial silk fibroin/nanohydroxyapatite hydrogels with silver and gold nanoparticles for bone regeneration. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.R.; Varaprasad, K.; Reddy, N.N.; Raju, K.M. Fabrication of Au and Ag Bi-metallic nanocomposite for antimicrobial applications. J. Appl. Polym. Sci. 2012, 125, 1357–1362. [Google Scholar] [CrossRef]
- Varaprasad, K.; Reddy, G.S.M.; Jayaramudu, J.; Sadiku, R.; Ramam, K.; Ray, S.S. Development of microbial resistant Carbopol nanocomposite hydrogels via a green process. Biomater. Sci. 2013, 2, 257–263. [Google Scholar] [CrossRef]
- Rigo, C.; Ferroni, L.; Tocco, I.; Roman, M.; Munivrana, I.; Gardin, C.; Cairns, W.R.L.; Vindigni, V.; Azzena, B.; Barbante, C.; et al. Active Silver Nanoparticles for Wound Healing. Int. J. Mol. Sci. 2013, 14, 4817–4840. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Mahajan, V.K.; Mehta, K.S.; Chauhan, P.S. Zinc Therapy in Dermatology: A Review. Dermatol. Res. Pract. 2014, 2014, 709152. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tang, W.; Liu, J.; Han, Y.; Yan, Q.; Dong, Y.; Liu, X.; Yang, D.; Ma, G.; Cao, H. A novel sprayable thermosensitive hydrogel coupled with zinc modified metformin promotes the healing of skin wound. Bioact. Mater. 2023, 20, 610–626. [Google Scholar] [CrossRef] [PubMed]
- Neacsu, I.-A.; Melente, A.E.; Holban, A.-M.; Ficai, A.; Ditu, L.-M.; Kamerzan, C.-M.; Tihăuan, B.M.; Nicoara, A.I.; Bezirtzoglou, E.; Chifiriuc, M.-C.; et al. Novel hydrogels based on collagen and ZnO nanoparticles with antibacterial activity for improved wound dressings. Romanian Biotechnol. Lett. 2019, 24, 317–323. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, S.; Zhong, L.; Wang, B.; Wang, H.; Hong, F. Zn2+-loaded TOBC nanofiber-reinforced biomimetic calcium alginate hydrogel for antibacterial wound dressing. Int. J. Biol. Macromol. 2019, 143, 235–242. [Google Scholar] [CrossRef]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Majumder, S.; Ranjan Dahiya, U.; Yadav, S.; Sharma, P.; Ghosh, D.; Rao, G.K.; Rawat, V.; Kumar, G.; Kumar, A.; Srivastava, C.M. Zinc Oxide Nanoparticles Functionalized on Hydrogel Grafted Silk Fibroin Fabrics as Efficient Composite Dressing. Biomolecules 2020, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, Y.; Wang, X.; Peng, J.; Xu, Y.; Chang, J. Multifunctional Hydrogels Prepared by Dual Ion Cross-Linking for Chronic Wound Healing. ACS Appl. Mater. Interfaces 2017, 9, 16054–16062. [Google Scholar] [CrossRef]
- Man, E.; Lamprou, D.; Easdon, C.; McLellan, I.; Yiu, H.H.P.; Hoskins, C. Exploration of Dual Ionic Cross-Linked Alginate Hydrogels Via Cations of Varying Valences towards Wound Healing. Polymers 2022, 14, 5192. [Google Scholar] [CrossRef]
- Baram, N.; Starosvetsky, D.; Starosvetsky, J.; Epshtein, M.; Armon, R.; Ein-Eli, Y. Photocatalytic inactivation of microorganisms using nanotubular TiO2. Appl. Catal. B Environ. 2011, 101, 212–219. [Google Scholar] [CrossRef]
- Dalrymple, O.K.; Stefanakos, E.; Trotz, M.A.; Goswami, D.Y. A review of the mechanisms and modeling of photocatalytic disinfection. Appl. Catal. B Environ. 2010, 98, 27–38. [Google Scholar] [CrossRef]
- Yuan, W.; Ji, J.; Fu, J.; Shen, J. A facile method to construct hybrid multilayered films as a strong and multifunctional antibacterial coating. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 85B, 556–563. [Google Scholar] [CrossRef]
- Kong, Y.; Hou, Z.; Zhou, L.; Zhang, P.; Ouyang, Y.; Wang, P.; Chen, Y.; Luo, X. Injectable Self-Healing Hydrogels Containing CuS Nanoparticles with Abilities of Hemostasis, Antibacterial activity, and Promoting Wound Healing. ACS Biomater. Sci. Eng. 2020, 7, 335–349. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, L.; Yuan, T.; Zhang, Q.; Fan, H. Asymmetric polyurethane membrane with In Situ-generated nano-TiO2 as wound dressing. J. Appl. Polym. Sci. 2010, 119, 1532–1541. [Google Scholar] [CrossRef]
- Díaz-Visurraga, J.; Meléndrez, M.; García, A.; Paulraj, M.; Cárdenas, G. Semitransparent chitosan-TiO2 nanotubes composite film for food package applications. J. Appl. Polym. Sci. 2010, 116, 3503–3515. [Google Scholar] [CrossRef]
- Qian, T.; Su, H.; Tan, T. The bactericidal and mildew-proof activity of a TiO2–chitosan composite. J. Photochem. Photobiol. A Chem. 2011, 218, 130–136. [Google Scholar] [CrossRef]
- He, L.; Liu, Y.; Mustapha, A.; Lin, M. Antifungal activity of zinc oxide nanoparticles against Botrytis cinerea and Penicillium expansum. Microbiol. Res. 2011, 166, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Yu, R.; Li, B.; Somasundaran, P.; Chandran, K. Stresses exerted by ZnO, CeO2 and anatase TiO2 nanoparticles on the Nitrosomonas europaea. J. Colloid Interface Sci. 2010, 348, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Schwegmann, H.; Feitz, A.; Frimmel, F.H. Influence of the zeta potential on the sorption and toxicity of iron oxide nanoparticles on S. cerevisiae and E. coli. J. Colloid Interface Sci. 2010, 347, 43–48. [Google Scholar] [CrossRef]
- Akhavan, O. Lasting antibacterial activities of Ag–TiO2/Ag/a-TiO2 nanocomposite thin film photocatalysts under solar light irradiation. J. Colloid Interface Sci. 2009, 336, 117–124. [Google Scholar] [CrossRef]
- Karunakaran, C.; Abiramasundari, G.; Gomathisankar, P.; Manikandan, G.; Anandi, V. Cu-doped TiO2 nanoparticles for photocatalytic disinfection of bacteria under visible light. J. Colloid Interface Sci. 2010, 352, 68–74. [Google Scholar] [CrossRef]
- Giavaresi, G.; Torricelli, P.; Fornasari, P.; Giardino, R.; Barbucci, R.; Leone, G. Blood vessel formation after soft-tissue implantation of hyaluronan-based hydrogel supplemented with copper ions. Biomaterials 2005, 26, 3001–3008. [Google Scholar] [CrossRef]
- Villanueva, M.E.; Diez, A.M.D.R.; González, J.A.; Pérez, C.J.; Orrego, M.; Piehl, L.; Teves, S.; Copello, G.J. Antimicrobial Activity of Starch Hydrogel Incorporated with Copper Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 16280–16288. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Yang, C.; Zhang, L.; Li, S.; Shi, S.; Wang, R.; Zhang, X.; Yue, T.; Sun, J.; Wang, J. Copper metal–organic frameworks loaded on chitosan film for the efficient inhibition of bacteria and local infection therapy. Nanoscale 2019, 11, 11830–11838. [Google Scholar] [CrossRef]
- Madzovska-Malagurski, I.; Sekulic, M.V.; Kostic, D.; Levic, S. Towards antimicrobial yet bioactive Cu-alginate hydrogels. Biomed. Mater. 2016, 11, 35015. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Pyarasani, R.D.; Reddy, K.K.; Akbari-Fakhrabadi, A.; Carrasco-Sánchez, V.; Amalraj, J. Hydroxypropyl methylcellulose-copper nanoparticle and its nanocomposite hydrogel films for antibacterial application. Carbohydr. Polym. 2020, 254, 117302. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.; Bajpai, A.K.; Bajpai, J.; Sharma, A. Antimicrobial poly(vinyl alcohol) cryogel-copper nanocomposites for possible applications in biomedical fields. Des. Monomers Polym. 2015, 18, 385–400. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Varaprasad, K.; Reddy, K.K.; Pyarasani, R.D.; Akbari-Fakhrabadi, A.; Amalraj, J. Chitosan-pluronic based Cu nanocomposite hydrogels for prototype antimicrobial applications. Int. J. Biol. Macromol. 2019, 143, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Wang, M.-H. Antimicrobial and Wound Healing Properties of FeO Fabricated Chitosan/PVA Nanocomposite Sponge. Antibiotics 2021, 10, 524. [Google Scholar] [CrossRef]
- Chircov, C.; Bejenaru, I.T.; Nicoară, A.I.; Bîrcă, A.C.; Oprea, O.C.; Tihăuan, B. Chitosan-Dextran-Glycerol Hydrogels Loaded with Iron Oxide Nanoparticles for Wound Dressing Applications. Pharmaceutics 2022, 14, 2620. [Google Scholar] [CrossRef]
- Pandey, D.K.; Kuddushi, M.; Kumar, A.; Singh, D.K. Iron oxide nanoparticles loaded smart hybrid hydrogel for anti-inflammatory drug delivery: Preparation and characterizations. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 650, 129631. [Google Scholar] [CrossRef]
- Ahmadi, M.; Monji, D.; Taromi, F.A. Bio-inspired surface modification of iron oxide nanoparticles for active stabilization in hydrogels. Soft Matter 2020, 17, 955–964. [Google Scholar] [CrossRef]
- Vukajlovic, D.; Bretcanu, O.; Novakovic, K. Fabrication and characterization of two types of bone composites made of chitosan-genipin hydrogel and Bioglass 45S5. Open Ceram. 2021, 8, 100174. [Google Scholar] [CrossRef]
- Rammal, H.; GhavamiNejad, A.; Erdem, A.; Mbeleck, R.; Nematollahi, M.; Diltemiz, S.E.; Alem, H.; Darabi, M.A.; Ertas, Y.N.; Caterson, E.J.; et al. Advances in biomedical applications of self-healing hydrogels. Mater. Chem. Front. 2021, 5, 4368–4400. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapusta, O.; Jarosz, A.; Stadnik, K.; Giannakoudakis, D.A.; Barczyński, B.; Barczak, M. Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review. Int. J. Mol. Sci. 2023, 24, 2191. https://doi.org/10.3390/ijms24032191
Kapusta O, Jarosz A, Stadnik K, Giannakoudakis DA, Barczyński B, Barczak M. Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review. International Journal of Molecular Sciences. 2023; 24(3):2191. https://doi.org/10.3390/ijms24032191
Chicago/Turabian StyleKapusta, Oliwia, Anna Jarosz, Katarzyna Stadnik, Dimitrios A. Giannakoudakis, Bartłomiej Barczyński, and Mariusz Barczak. 2023. "Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review" International Journal of Molecular Sciences 24, no. 3: 2191. https://doi.org/10.3390/ijms24032191
APA StyleKapusta, O., Jarosz, A., Stadnik, K., Giannakoudakis, D. A., Barczyński, B., & Barczak, M. (2023). Antimicrobial Natural Hydrogels in Biomedicine: Properties, Applications, and Challenges—A Concise Review. International Journal of Molecular Sciences, 24(3), 2191. https://doi.org/10.3390/ijms24032191







