Human Decidual CD1a+ Dendritic Cells Undergo Functional Maturation Program Mediated by Gp96
Abstract
1. Introduction
2. Results
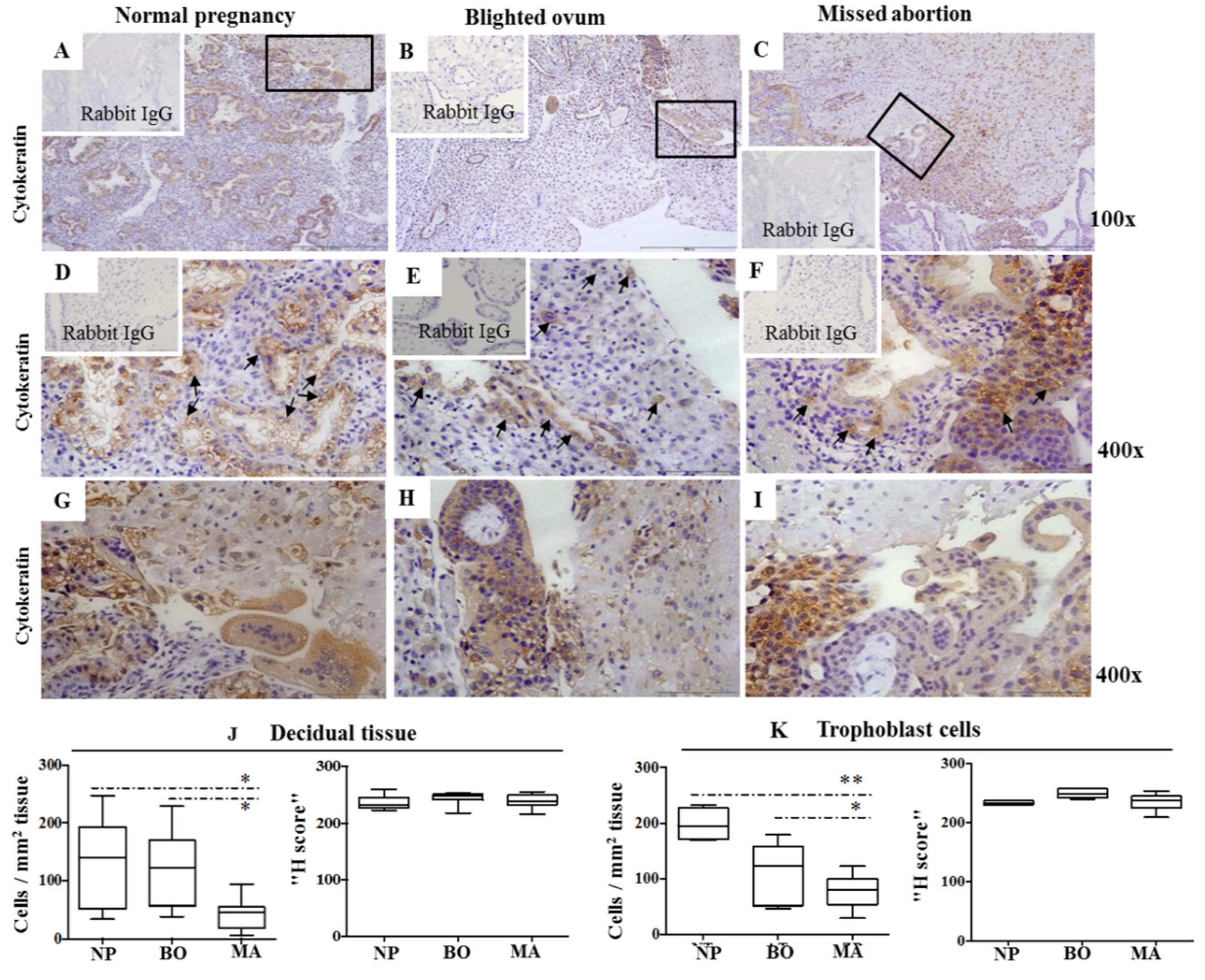
2.1. Cytokeratin Expression Pattern in Decidua of Normal and Pathological Pregnancies
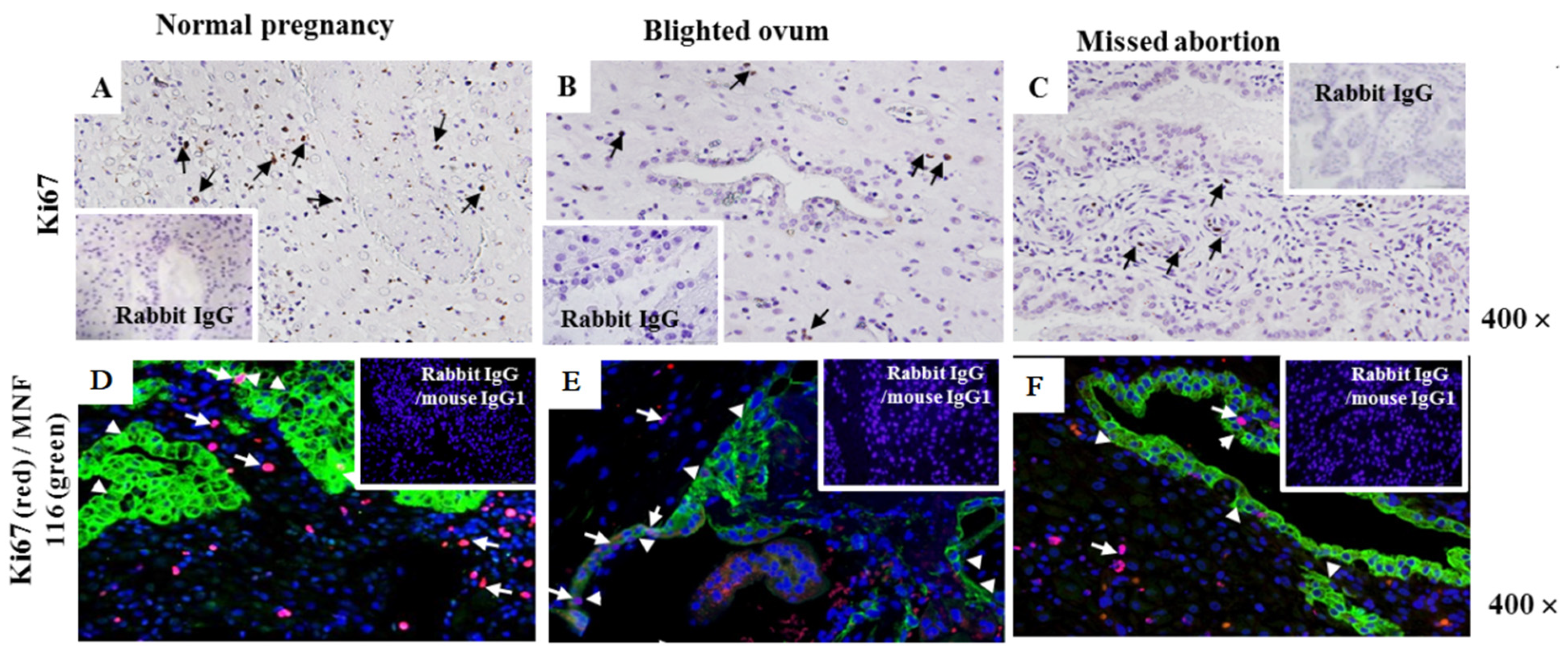
2.2. Assessment of Cell Proliferation Marker Ki-67 at the Maternal–Foetal Interface
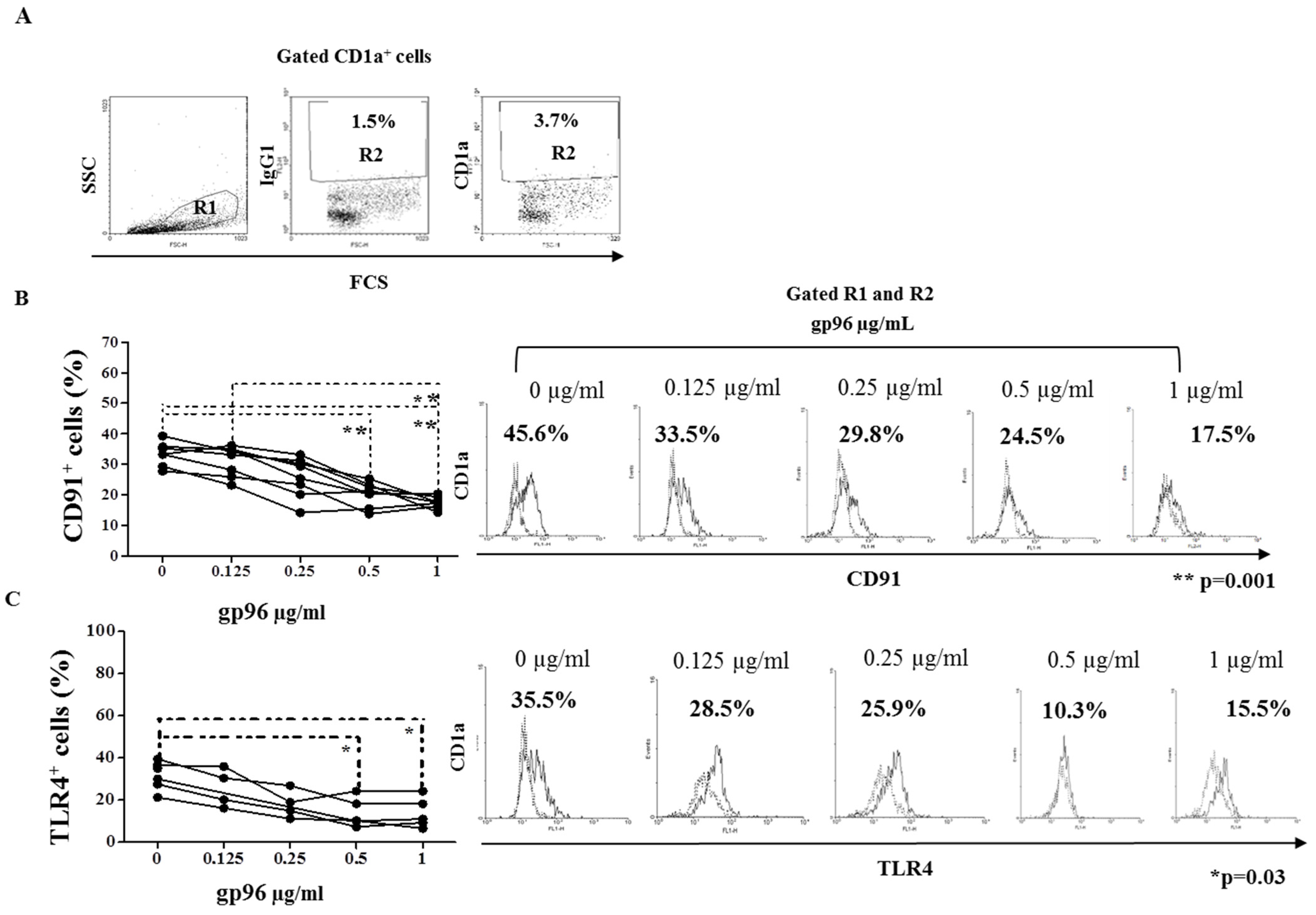
2.3. Gp96 Specifically Bind CD91 and TLR4 Receptors Expressed on Decidual CD1a+ Immature Dendritic Cells
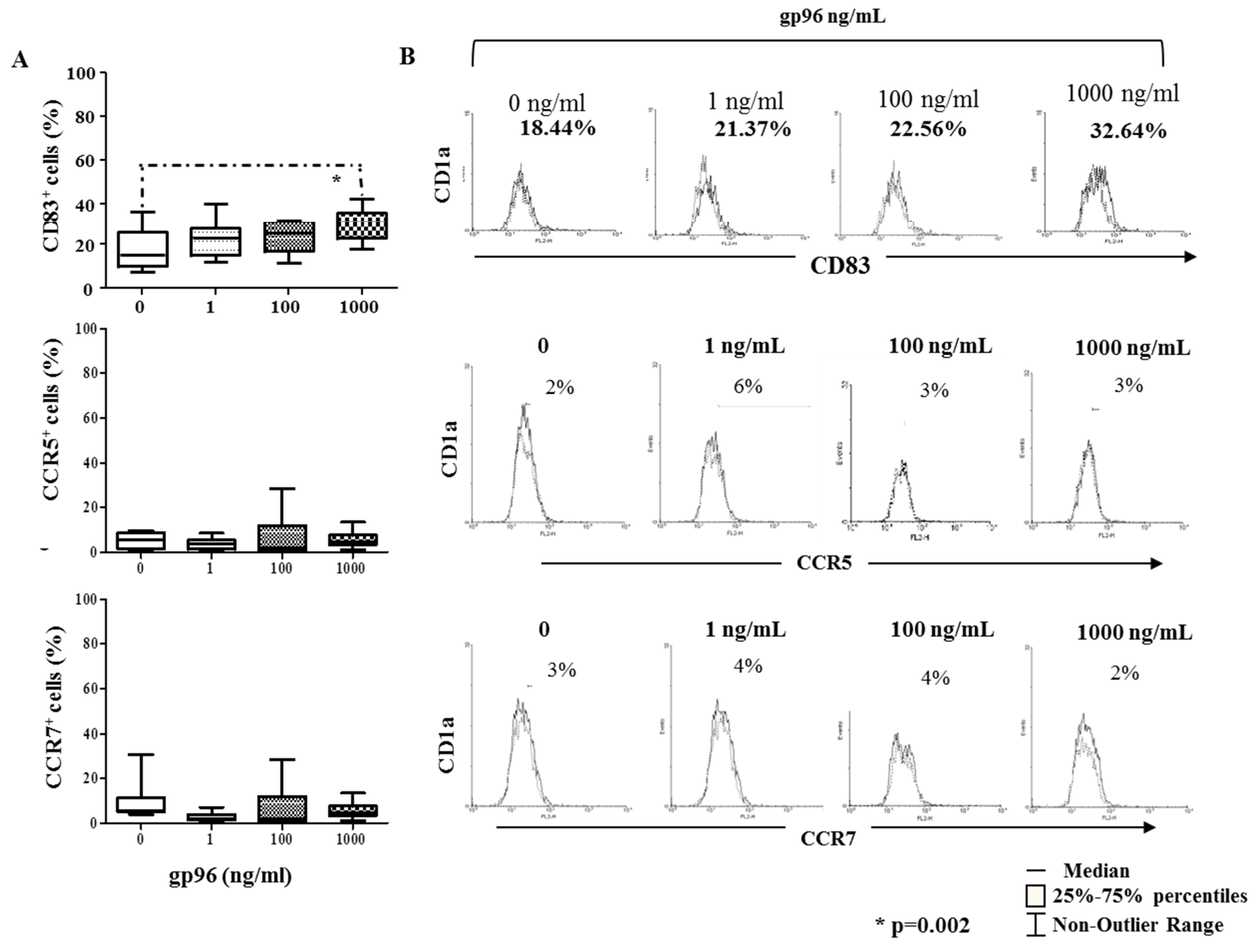
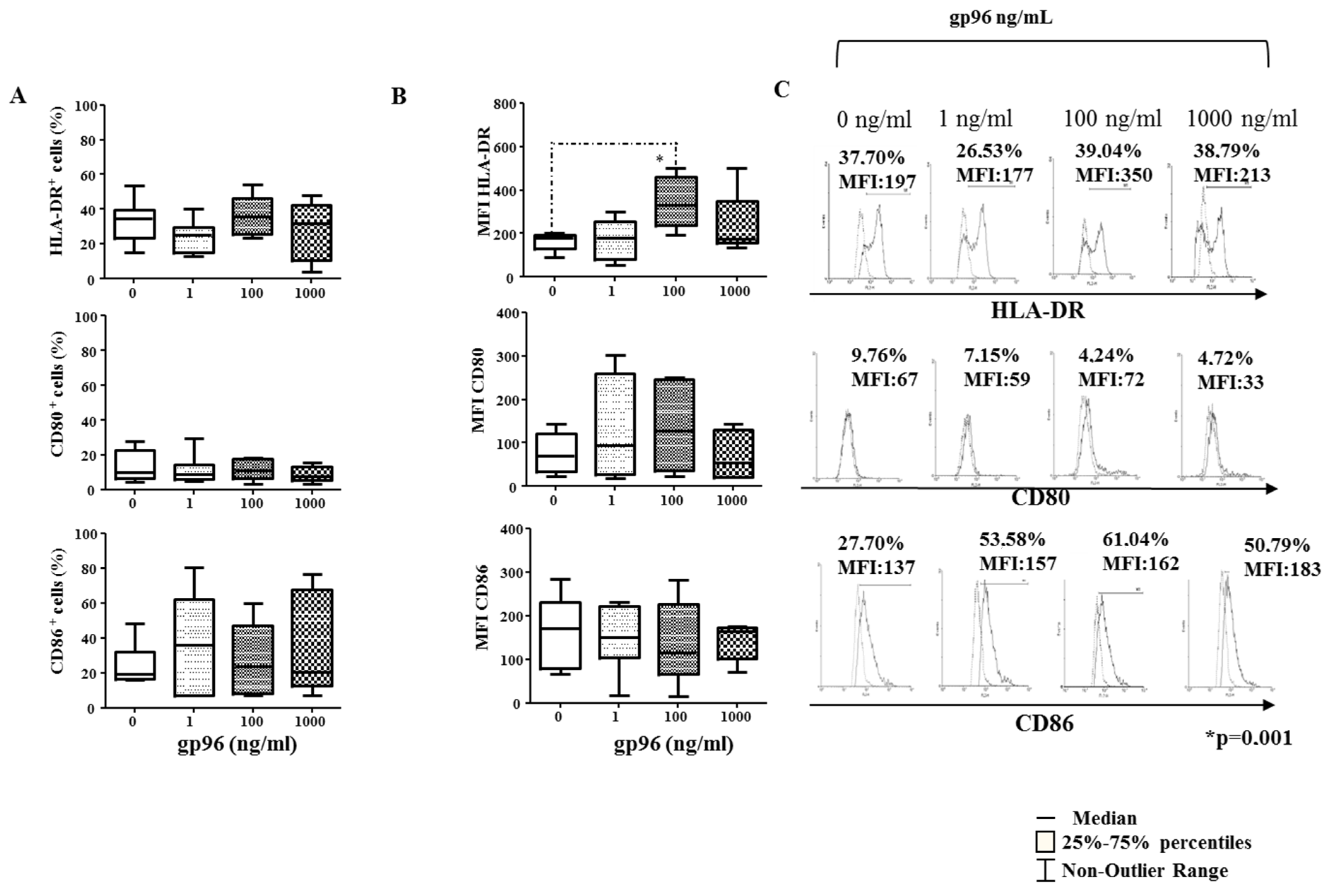
2.4. Gp96 Include Maturation of Decidual CD1a+ DCs
2.5. Gp96 Enhanced Expression of Pro-Inflammatory Cytokines on Decidual CD1a+ DCs
2.6. IL-15 Upregulate the Expression of gp96 in DMCs from Early Normal Pregnancy Decidua
3. Discussion
4. Materials and Methods
4.1. Human Tissue Samples
4.2. Immunohistology of Paraffin Embedded Tissue Sections
4.3. Immunofluorescence of Paraffin Embedded Tissue Sections
4.4. Isolation of Decidual Cells by Enzymatic Digestion
4.5. Binding Assay
4.6. Antigen Detection in DMCs Using Flow Cytometry
4.7. RNA Preparation and Real-Time Quantitative Polymerase Chain Reaction
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Scholz, C.; Toth, B.; Santoso, L.; Kuhn, C.; Franz, M.; Mayr, D.; Jeschke, U.; Friese, K.; Schiessl, B. Distribution and maturity of dendritic cells in diseases of insufficient placentation. Am. J. Reprod. Immunol. 2008, 60, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Laskarin, G.; Redzovic, A.; Medancic, S.S.; Rukavina, D. Regulation of NK-cell function by mucins via antigen-presenting cells. Med. Hypotheses 2010, 75, 541–543. [Google Scholar] [CrossRef]
- Alison, E.W.; Rupsha, F.; Cartwright, J.E. Extravillous trophoblast and decidual natural killer cells: A remodelling partnership. Hum. Reprod. 2012, 18, 458–471. [Google Scholar]
- Gulic, T.; Laskarin, G.; Redzovic, A.; Eminović, S.; Haller, H.; Rukavina, D. The significance of heat-shock protein gp96 and its receptors’ CD91 and Toll-like receptor 4 expression at the maternal foetal interface. Am. J. Reprod Immunol. 2013, 70, 10–23. [Google Scholar] [CrossRef]
- Gardner, L.; Moffett, A. Dendritic cells in the human decidua. Biol. Reprod. 2003, 69, 1438–1446. [Google Scholar] [CrossRef]
- Laskarin, G.; Cupurdija, K.; Tokmadzic, V.S.; Dorcic, D.; Dupor, J.; Juretic, K.; Strbo, N.; Crncic, T.B.; Marchezi, F.; Allavena, P.; et al. The presence of functional mannose receptor on macrophages at the maternal-fetal interface. Hum. Reprod. 2005, 20, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, U.; Schoppet, M.; McLellan, A.D.; Kapp, M.; Huppertz, H.I.; Kampgen, E.; Dietel, J. Human decidua contains potent immunostimulatory CD83+ dendritic cells. Am. J. Pathol. 2000, 157, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Laskarin, G.; Redzović, A.; Rubesa, Z.; Mantovani, A.; Allavena, P.; Haller, H.; Vlastelić, I.; Rukavina, D. Decidual natural killer cell tuning by autologous dendritic cells. Am. J. Reprod. Immunol. 2008, 59, 433–445. [Google Scholar] [CrossRef]
- Naderi, N.; Pourfathollah, A.A.; Alimoghaddam, K.; Moazzeni, S.M. Cord blood dendritic cells prevent the differentiation of naïve T-helper cells towards Th1 irrespective of their subtype. Clin. Exp. Med. 2009, 9, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Laskarin, G.; Redzovic, A.; Vlastelic, I.; Haller, H.; Medancic, S.S.; Solinas, G.; Rukavina, D. Tumor-associated glycoprotein (TAG-72) is a natural ligand for the C-type lectin-like domain that induces anti-inflammatory orientation of early pregnancy decidual CD1a+ dendritic cells. J. Reprod. Immunol. 2011, 88, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Cools, N.; Petrizzo, A.; Smits, E.; Buonaguro, F.M.; Tornesello, M.L.; Berneman, Z.; Buonaguro, L. Dendritic cells in the pathogenesis and treatment of human diseases: A Janus Bifrons? Immunotherapy 2011, 3, 1203–1222. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P. Interaction of heat shock proteins with peptides and antigen presenting cells: Chaperoning of the innate and adaptive immune responses. Annu. Rev. Immunol. 2002, 20, 395–425. [Google Scholar] [CrossRef]
- Rupik, W.; Jasik, K.; Bembenek, J.; Widłak, W. The expression patterns of heat shock genes and proteins and their role during vertebrate’s development. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2011, 159, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, E.; Gehrmann, M.; Brunet, M.; Multhoff, G.; Garrido, C. Intracellular and extracellular functions of heat shock proteins: Repercussions in cancer therapy. J. Leukoc. Biol. 2007, 81, 15–27. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Mambula, S.S.; Gray, P.J. Extracellular heat shock proteins in cell signaling and immunity. Ann. N. Y. Acad. Sci. 2007, 1113, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Stanek, J.; Handwerger, S. Differential localization of heat shock proteins 90, 70, 60 and 27 in human decidua and placenta during pregnancy. Histochem. J. 1998, 30, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Binder, R.J.; Suto, R.; Anderson, K.M.; Srivastava, P.K. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int. Immunol. 2000, 12, 1539–1546. [Google Scholar] [CrossRef]
- Basu, S.; Binder, R.J.; Ramalingam, T.; Srivastava, P.K. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity 2001, 14, 303–313. [Google Scholar] [CrossRef]
- Neuer, A.; Spandorfer, S.; Giraldo, P.; Mele, C.; Liu, H.C.; Marzusch, K.; Kneissl, D.; Witkin, S.S. Immune Sensitization to the 60 kD Heat Shock Protein and Pregnancy Outcome. Infect. Dis. Obstet. Gynecol. 1997, 5, 154–157. [Google Scholar] [CrossRef]
- Witkin, S.S. Immunity to heat shock proteins and pregnancy outcome. Infect. Dis. Obstet. Gynecol. 1999, 7, 35–38. [Google Scholar] [CrossRef]
- Redzovic, A.; Gulic, T.; Laskarin, G.; Eminovic, S.; Haller, H.; Rukavina, D. Heat-shock proteins 70 induce pro-inflammatory maturation program in decidual CD1a(+) dendritic cells. Am. J. Reprod. Immunol. 2015, 74, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Muttukrishna, S.; Swer, M.; Suri, S.; Jamil, A.; Calleja-Agius, J.; Gangooly, S.; Ludlow, H.; Jurkovic, D.; Jauniaux, E. Soluble Flt-1 and PlGF: New markers of early pregnancy loss? PLoS ONE 2011, 6, 18041. [Google Scholar] [CrossRef] [PubMed]
- Lambrinoudaki, I.V.; Augoulea, A.; Christodoulakos, G.E.; Economou, E.V.; Kaparos, G.; Kontoravdis, A.; Papadias, C.; Creatsas, G. Measurable serum markers of oxidative stress response in women with endometriosis. Fertil. Steril. 2009, 91, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Livingston, J.C.; Ahokas, R.; Haddad, B.; Sibai, B.M.; Awaads, R. Heat shock protein 70 is not increased in women with severe preeclampsia. Hypertens. Pregnancy 2002, 21, 123–126. [Google Scholar] [CrossRef]
- Gelber, S.E.; Bongiovanni, A.M.; Jean-Pierre, C.; Linhares, I.M.; Skupski, D.W.; Witkin, S.S. Antibodies to the 70 kDa heat shock protein in midtrimester amniotic fluid and intraamniotic immunity. Am. J. Obstet. Gynecol. 2007, 197, 278. [Google Scholar] [CrossRef] [PubMed]
- Ziegert, M.; Witkin, S.S.; Sziller, I.; Alexander, H.; Brylla, E.; Härtig, W. Heat shock proteins and heat shock protein-antibody complexes in placental tissues. Infect. Dis. Obstet. Gynecol. 1999, 7, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Pockley, A.G.; Muthana, M.; Calderwood, S.K. The dual immunoregulatory roles of stress proteins. Trends Biochem. Sci. 2008, 33, 71–79. [Google Scholar] [CrossRef]
- Almeida, M.B.; Nascimento, J.L.; Herculano, A.M.; Crespo-López, M.E. Molecular chaperones: Toward new therapeutic tools. Biomed. Pharmacother. 2011, 65, 239–243. [Google Scholar] [CrossRef]
- Matsutake, T.; Sawamura, T.; Srivastava, P.K. High efficiency CD91- and LOX-1-mediatedre-presentation of gp96-chaperoned peptides by MHC II molecules. Cancer Immun. 2010, 10, 1–7. [Google Scholar]
- Chen, T.; Guo, J.; Han, C.; Yang, M.; Cao, X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J. Immunol. 2009, 182, 1449–1459. [Google Scholar] [CrossRef]
- Boyman, O.; Conrad, C.; Dudli, C.; Kielhorn, E.; Nickoloff, B.J.; Nestle, F.O. Activation of dendritic antigen-presenting cells expressing common heat shock protein receptor CD91 during induction of psoriasis. Br. J. Dermatol. 2005, 152, 1211–1218. [Google Scholar] [CrossRef]
- Aosai, F.; Rodriguez Pena, M.S.; Mun, H.S.; Fang, H.; Mitsunaga, T.; Norose, K.; Kang, H.K.; Bae, Y.S.; Yano, A. Toxoplasma gondii-derived heat shock protein 70 stimulates maturation of murine bone marrow-derived dendritic cells via Toll-like receptor 4. Cell Stress Chaperones 2006, 11, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Abe, S.; Kodaira, K.; Yukawa, T.; Hozawa, S.; Mochizuki, H.; Kurosawa, M. Heat shock protein 70 gene polymorphisms in Japanese patients with aspirin-exacerbated respiratory disease. J. Investig. Med. 2013, 61, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Sozzani, S.; Allavena, P.; D’Amico, G.; Luini, W.; Bianchi, G.; Kataura, M.; Imai, T.; Yoshie, O.; Bonecchi, R.; Mantovani, A. Differential regulation of chemokine receptors during dendritic cell maturation: A model for their trafficking properties. J. Immunol. 1998, 161, 1083–1086. [Google Scholar] [CrossRef]
- Drake, P.M.; Gunn, M.D.; Charo, I.F.; Tsou, C.L.; Zhou, Y.; Huang, L.; Fisher, S.J. Human Placental Cytotrophoblasts Attract Monocytes and CD56bright Natural Killer Cells via the Actions of Monocyte Inflammatory Protein 1α. J. Exp. Med. 2001, 193, 1199–1212. [Google Scholar] [CrossRef]
- Fang, H.; Wu, Y.; Huang, X.; Wang, W.; Ang, B.; Cao, X.; Wan, T. Toll-like receptor 4 (TLR4) is essential for Hsp70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J. Biol. Chem. 2011, 286, 30393–30400. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, H.; Michimata, T.; Hayakawa, S.; Tanebe, K.; Sakai, M.; Fujimura, M.; Matsushima, K.; Saito, S. A Th2 chemokine, TARC, produced by trophoblasts and endometrial gland cells, regulates the infiltration of CCR4+ T lymphocytes into human decidua at early pregnancy. J. Reprod. Immunol. 2002, 48, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sones, J.L.; Lob, H.E.; Isroff, C.E.; Davisson, R.L. The Role of Decidual Natural Killer Cells, Interleukin-15 and Interferon-γ in Placental Development and Preeclampsia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R490–R492. [Google Scholar] [CrossRef]
- Mariee, N.; Li, T.C.; Laird, S.M. Expression of leukaemia inhibitory factor and interleukin 15 in endometrium of women with recurrent implantation failure after IVF; correlation with the number of endometrial natural killer cells. Hum. Reprod. 2012, 27, 1946–1954. [Google Scholar] [CrossRef]
- Veljkovic Vujaklija, D.; Dominovic, M.; Gulic, T.; Mahmutefendic, H.; Haller, H.; Saito, S.; Rukavina, D. Granulysin expression and the interplay of granulysin and perforin at the maternal-fetal interface. J. Reprod. Immunol. 2013, 97, 186–196. [Google Scholar] [CrossRef]
- Nakashima, A.; Shiozaki, A.; Myojo, S.; Ito, M.; Tatematsu, M.; Sakai, M.; Takamori, Y.; Ogawa, K.; Nagata, K.; Saito, S. Granulysin produced by uterine natural killer cells induces apoptosis of extravillous trophoblasts in spontaneous abortion. Am. J. Pathol. 2008, 173, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Gulic, T.; Laskarin, G.; Dominovic, M.; Glavan Gacanin, L.; Babarovic, E.; Haller, H.; Rukavina, D. Potential role of heat-shock protein 70 and interleukin-15 in the pathogenesis of threatened spontaneous abortions. Am. J. Reprod. Immunol. 2016, 76, 126–136. [Google Scholar] [CrossRef] [PubMed]


| Primary Antibodies | |||
|---|---|---|---|
| Specificity | Clone | Provider | Concentration |
| Cytokeratin | MNF 116 (mouse IgG1) | DAKO, Carpinteria, CA, USA | 1:100 |
| Cytokeratin | Polyclonal (rabbit IgG) | Abcam, Cambridge, UK | 1:100 |
| Ki-67 | SP6 (rabbit IgG1) | Abcam, Cambridge, UK | 1:100 |
| CD1a (FITC, PE) | HI149 (mouse IgG1) | BD Biosciences, San Diego, CA, USA | 20 μL/106 cells |
| TLR4 (FITC) | 76B357.1(mouse IgG2b) | Abcam, Cambridge, UK | 2.5 μg/106 cells |
| CD91 (PE) | A2MR-α2 (mouse IgG1) | BD Biosci. San Diego, CA, USA | 20 μL/106 cells |
| CD83 (PE, PE-Cy5) | HB15e (mouse IgG1) | BD Biosciences, San Diego, CA, USA | 20 μL/106 cells |
| CCR5 (PE) | 2D7 (mouse IgG2a) | BD Biosciences, San Diego, CA, USA | 20 μL/106 cells |
| CCR7 (PE) | 3D12 (rat IgG2a) | BD Biosciences, San Diego, CA, USA | 20 μL/106 cells |
| CD80 (PE) | L307.4 (mouse IgG1) | BD Biosciences, San Diego, CA, USA | 20 μL/106 cells |
| C86 (PE) | IT2.2 (mouse IgG1) | BD Biosciences, San Diego, CA, USA | 20 μL/106 cells |
| HLA-DR (PE) | G46-6 (mouse IgG2a) | BD Biosciences, San Diego, CA, USA | 20 μL/106 cells |
| IL-4 (PE) | 8D4-8 (mouse IgG1) | BD Pharmingen, San Jose, CA, USA | 0.25 μg/106 cells |
| IFN-γ (PE) | B27 (mouse IgG1) | BD Pharmingen, San Jose, CA, USA | 0.5 μg/106 cells |
| IL15 (PE) | 34559 (mouse IgG1) | R&D Systems, Minneapolis, MN, USA | 10 μL/106 cells |
| CCL3 (PE) | 93342 (mouse IgG2b) | R&D Systems, Minneapolis, MN, USA | 20 μL/106 cells |
| CCL17 (PE) | 54015 (mouse IgG1) | R&D Systems, Minneapolis, MN, USA | 20 μL/106 cells |
| CCL22 (PE) | 57203 (mouse IgG2b) | R&D Systems, Minneapolis, MN, USA | 20 μL/106 cells |
| Secondary Antibodies | |||
| Anti-mouse IgG Alexa Fluor 488 | goat IgG (H+L) | Molecular Probes, OR, USA | 1:500 |
| Anti-rabbit IgG Alexa Fluor 594 | goat IgG(H+L) | Molecular Probes, OR, USA | 1:500 |
| Control Antibodies | |||
| IgG1 (FITC, PE) | MOPC-21 (mouse IgG1) | BD Biosciences, San Diego, CA, USA | 20 μL/106 cells |
| IgG2a (FITC, PE) | G155-178 (mouse IgG2a) | BD Biosciences, San Diego, CA, USA | 20 μL/106 cells |
| IgG2b (FITC, PE) | 27–35 (mouse IgG2b) | BD Biosciences, San Diego, CA, USA | 20 μL/106 cells |
| IgG2a (PE) | R35-95 (rat IgG2a) | BD Biosciences, San Diego, CA, USA | 20 μL/106 cells |
| IgG1 unconjugated | X40 (mouse IgG1) | BD Pharmingen, San Diego, CA, USA | dilution 1:100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulic, T.; Laskarin, G.; Glavan, L.; Grubić Kezele, T.; Haller, H.; Rukavina, D. Human Decidual CD1a+ Dendritic Cells Undergo Functional Maturation Program Mediated by Gp96. Int. J. Mol. Sci. 2023, 24, 2278. https://doi.org/10.3390/ijms24032278
Gulic T, Laskarin G, Glavan L, Grubić Kezele T, Haller H, Rukavina D. Human Decidual CD1a+ Dendritic Cells Undergo Functional Maturation Program Mediated by Gp96. International Journal of Molecular Sciences. 2023; 24(3):2278. https://doi.org/10.3390/ijms24032278
Chicago/Turabian StyleGulic, Tamara, Gordana Laskarin, Lana Glavan, Tanja Grubić Kezele, Herman Haller, and Daniel Rukavina. 2023. "Human Decidual CD1a+ Dendritic Cells Undergo Functional Maturation Program Mediated by Gp96" International Journal of Molecular Sciences 24, no. 3: 2278. https://doi.org/10.3390/ijms24032278
APA StyleGulic, T., Laskarin, G., Glavan, L., Grubić Kezele, T., Haller, H., & Rukavina, D. (2023). Human Decidual CD1a+ Dendritic Cells Undergo Functional Maturation Program Mediated by Gp96. International Journal of Molecular Sciences, 24(3), 2278. https://doi.org/10.3390/ijms24032278








